Abstract
In the search of significant biological agents for the pathogenic causing deformities, four bidentate heterocyclic Schiff base ligands and their sixteen Co(II), Ni(II), Cu(II) and Zn(II) metal complexes were derived by the condensation of 3,4-dihydro-2H-1,5-benzodioxepin-7-amine with salicylaldehyde derivatives/2-hydroxy-1-naphthaldehyde. Further, the synthesized compounds were characterized by numerous analytical techniques such as NMR, FT-IR, UV–Vis, SEM, EDAX, mass spectrometry, ESR, powder XRD, TGA, magnetic susceptibility, elemental analysis, magnetic susceptibility and molar conductance measurement for structural elucidation. The antioxidant ability of the compounds was examined by DPPH and ABTS assays while the antimicrobial (against Staphylococcus aureus, Bacillus subtilis, Escherichia coli, Pseudomonas aeruginosa, Rhizopus oryzae and Candida albicans microbial strains) and anti-inflammatory activities of the synthesized compounds were evaluated by serial dilution and egg albumin assays, respectively. The results of pharmacological activities showed that the ligand HL4 (4) and Cu(II) complexes have significant antioxidant activity while the ligand HL2 (2) and Zn(II) complexes have excellent antimicrobial and anti-inflammatory activities because of their dependency on electron donating and electron withdrawing groups, respectively. Further, the cytotoxic study was carried out for the most potent antimicrobial and anti-inflammatory HL2 (2), HL3 (3) ligands and their (9–16) metal complexes on Vero cell lines using calorimetric assay which revealed that the complex (12) is lesser cytotoxic than the other tested compounds. After that, the less cytotoxic and more potential antimicrobial Zn(II) complex (12) and its ligand HL2 (2) were assessed by molecular docking study to know the interaction modes and binding affinity of these compounds with the active sites of Staphylococcus aureus S1: DHFR, Escherichia coli DNA gyrase B and Candida albicans sterol-14-alpha-demethylase enzymes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The progress of medicinal chemistry begun in eighteenth century in enthusiastic environment. Many researchers attempted to synthesize and design pharmaceutical agents [1, 2] and bioactive compounds that require biological importance on living system and human body. These compounds are called ‘drugs’ which have a positive biological response on living organism. The main moto of the medicinal chemistry [3] is to examine the interaction of drugs with cells by focusing on biochemical reaction. Numerous compounds are used as drugs in pharmaceutical industry which have good therapeutic index and less cytotoxic; and used to cure many pathogenic ailments.
The pathogenic microorganisms are dangerous for living beings because as they cause mortality and morbidity in all around the world. Therefore, the bioactive agents such as plant extracts were used for fighting against various microbial diseases [4] until the discovery of penicillin (1928) and sulfa drugs (1930). Vitamin C or ascorbic acid is used as antioxidant drug [5] and aspirin is used on broad scale in current medicinal industries as the anti-inflammatory agent [6] to remove harmful elements and damaged tissues from the body to relieve organisms from various illness. The synthetic, natural and semi-synthetic agents with a particular mechanism are able to alter in the physiological and metabolic levels by including modification on cell walls. But the excessive use of these agents has negative impact on the functioning of the body which leads to aging, cell damages, organs failure and sometime major cause of death. Furthermore, the development of potential antipathogen is currently unmet in heath cares. Hence, there is a significant need to synthesize potential pharmaceutical agents which cure the pathogenic deformities with very minimum disadvantages. So, the synthesize of a significant drug to cure the microorganism-based diseases with minimum cytotoxicity is a main objective of the present study which is based on the coordination compounds of Schiff base ligands.
Coordination complexes are a significant class of inorganic compounds which plays an important role in medicinal chemistry because they have numerous properties like denticity, flexibility, reactivity etc. The current researches deal with the coordination compounds [7, 8] of transition metals because these are essential for many biological processes and available in electron rich constituents such as nucleic acid, proteins, enzymes and carbohydrates etc. Therefore, it is necessary to research on the metal ions-based drugs, with the aim to synthesize less toxic drugs for biological systems [9]. The research in bio-inorganic chemistry has good argument to deal with Schiff base ligands-based transition metal compounds [10]. Schiff base ligands are backbone of many fields like analytical, biological, organic and inorganic industries because they have numerous features such as chelation, chemical sensors, structure modifications, reactivity etc. The activity of the ligands improved on complexation with transition metal ions due to the behavior of groups attached in the moiety, solubility, cell permeability, enzymatic action etc. Therefore, the study of Schiff base ligand-based transition metal complexes is point of interest in the medicinal field because in the pharmaceutical industries, the widespread applicability of bioactive compounds has gained extreme propulsion of the use of transition metal complexes through the corroborative amalgamation of organic ligands as well as inorganic metal ions which offer various prospects for acquiring the potency to target the multiple biological targets in the medicinal field.
Among the transition metal complexes, Co(II), Ni(II), Cu(II), Zn(II) metal complexes have attracted the researcher due to structural flexibility, enzyme inhibitory properties, photochromic effects, penetrating power, cheating ability, good therapeutic index etc. which alter the biological response of any drug. Further, the selection of Co(II), Ni(II), Cu(II), Zn(II) metal ions in various hydrolytic enzymatic cycles among all forms of life especially carboxypeptidase, carbonic anhydrase and alcohol dehydrogenase etc. is no doubt indispensable owning to its Lewis acidity, redox inactiveness, rapid ligand exchange capability as well as flexible coordination environment. Therefore, the Co(II), Ni(II), Cu(II), Zn(II) complexes of Schiff base ligands is broadly studied in medicinal industries which have significant biological applications such as anticancer [11, 12], antifungal [13], antioxidant [14], antibacterial [15, 16], antiviral [17, 18], antituberculosis [19], anti-inflammatory [20, 21], anti-HIV [22, 23] antimalarial [24], antiparasitic [25, 26] etc.
By analyzing the significant biological importance of transition metal(II) complexes of Schiff base ligands, the synthesize of four heterocyclic ligands were carried out by condensing 3,4-dihydro-2H-benzo[b][1,4]dioxepin-7-amine (3,4-dihydro-2H-1,5-benzodioxepin-7-amine) with salicylaldehyde derivatives/2-hydroxy-1-naphthaldehyde, further which treated with Co(II), Ni(II), Cu(II) and Zn(II) acetates to form their sixteen metal(II) complexes. The characterization of the compounds was done via numerous spectroscopic and physical techniques like NMR, FT-IR, ESR, SEM, UV–Vis, mass spectrometry, molar conductance, magnetic susceptibility measurements, powder XRD, TGA and elemental analysis (CHN). The synthesized compounds were also elucidated for their in vitro biological properties against antioxidant, antimicrobial and anti-inflammatory activities. The antioxidant activity of the compounds was performed by DPPH and ABTS assays using ascorbic acid (standard drug). The antimicrobial evaluation of the synthesized compounds was assessed against six microbial strains by serial dilution methodology. Then, anti-inflammatory ability of the compounds was examined by egg albumin assay. Further, the in vitro cytotoxicity was evaluated for the more potent antimicrobial and anti-inflammatory ligands HL2 (2), HL3 (3) and their metal complexes on Vero cells to check their toxicity level. In the recent time, the molecular docking study is highly supporting the biological ability of the compounds, therefore, the molecular docking study was implemented to examine the binding energy and interaction modes of the highly potent microbial compounds (complex (12) and ligand (2)) with the S. aureus S1: DHFR (2W9S), E. coli DNA gyrase B (4DUH) and C. albicans sterol-14-alpha-demethylase (5TZ1) enzymes.
Experimental
Materials
In the current research work, all the used reagents and chemicals, i.e. 3,4-dihydro-2H-benzo[b][1,4]dioxepin-7-amine (3,4-dihydro-2H-1,5-benzodioxepin-7-amine) (99%), 5-bromosalicylaldehyde (98%), 3,5-dichlorosalicylaldehyde (99%), 3-methoxy-5-nitrosalicylaldehyde (99%), 2-hydroxy-1-naphthaldehyde (98%), cobalt(II) acetate tetrahydrate, nickel(II) acetate tetrahydrate, copper(II) acetate monohydrate and zinc(II) acetate dihydrate (≥ 98%) were of AR grade and highly pure therefore employed as such for experimental work which were purchased from Sigma-Aldrich firm.
Instruments for physical measurements
Fourier transform infrared spectra of the compounds as KBr pellets were assessed in the range of 4000–400 cm−1 on Perkin Elmer BX II spectrometer. The 13C and 1H NMR spectral data of Zn(II) metal complexes and their ligands were observed in CDCl3 and DMSO-d6 solvent on the Avance III 400 MHz:Bruker NMR instrument using tetramethylsilane (reference). The Thermo Scientific FLASH 2000 analyzer was used to obtain the elemental analysis (CHN). The ESR spectra of copper(II) complexes were observed on JES-FA200 ESR spectrometer with X-band using 3000 gauss magnetic field and TCNE (tetracyanoethylene) as standard. Molar conductivity of the 1 × 10−3 M concentration of ligands and their respective complexes in DMF solvent was assessed at room temperature by a Systronics conductivity bridge model-306. The thermogravimetric analysis was carried out at 10 °C/min heating rate by using alumina as a standard on Perkin Elmer Diamond TG/DTA thermogravimetric analyzer in atmosphere of highly pure argon having 20 mL/min flow rate. The hot-stage Gallenkamp apparatus was used to record the melting point of the compounds in open capillaries. The mass spectrometry analysis was evaluated on SCIEX Triple TOF 5600 spectrometer using acetonitrile as a solvent. UV–Vis spectra of the compounds were recorded on UV–Vis–NIR Varian Cary 5000 spectrometer at standard temperature in THF. Powder XRD of the ligands and their metal compplexes was evaluated by Rigaku Miniflex-II with Cu-Kα (1.54 Å) radiation. The value of magnetic susceptibility of the compounds was evaluated by Guoy’s method taking a vibrating sample magnetometer and Hg[Co(SCN)4] as calibrant at room temperature. SEM and EDAX were recorded on JEOL 7610F plus instrument and the images of micrographs were obtained at nitrogen atmosphere.
Protocol for preparation of Schiff base ligands (HL1-HL4) (1–4)
The heterocyclic ligands (1–4) were synthesized by dissolving 3,4-dihydro-2H-benzo[b][1,4]dioxepin-7-amine (3,4-dihydro-2H-1,5-benzodioxepin-7-amine) (0.825 g, 5.0 mmol) and 5-bromosalicylaldehyde (1.005 g, 5.0 mmol)/3,5-dichlorosalicylaldehyde (0.955 g, 5.0 mmol)/3-methoxy-5-nitrosalicylaldehyde (0.985 g, 5.0 mmol)/2-hydroxy-1-naphthaldehyde (0.860 g, 5.0 mmol) in 30 mL methanol which refluxed for 5–6 h by adding 0.1 mL of glacial acetic acid. TLC was used to check the completion of reaction. After cooling, the colored precipitates were obtained at ambient temperature which were filtered, washed with hexane and finally recrystallized by methanol to purify the compounds.
Protocol for preparation of transition metal(II) complexes [M(L1–4)2(H2O)2] (5–20)
The metal(II) complexes were prepared by dissolving Co(II) acetate tetrahydrate (0.249 g), Ni(II) acetate tetrahydrate (0.248 g), Cu(II) acetate monohydrate (0.199 g), Zn(II) acetate dihydrate (0.219 g) as 1 mmol in 1:2 molar ratio with above synthesized heterocyclic ligands HL1 (0.696 g)/HL2 (0.676 g)/HL3 (0.688 g)/HL4 (0.638 g) as 2 mmol in 30 mL methanol which stirred for 3–4 h at room temperature to obtain the distinct colored precipitates that filtered and purify by washing with methanol and dried by hexane.
1. 4-bromo-2-(((3,4-dihydro-2H-benzo[b][1,4]dioxepin-7-yl)imino)methyl)phenol (HL1)
Yield: 83%; Color: dark yellow; M.p.: 110–112 °C. Conductivity (ohm−1 cm2 mol−1) in DMF: 12. Anal. Calcd. (%): C, 55.19; H, 4.05; N, 4.02. Found (%): C, 55.14; H, 4.07; N, 4.05. IR (KBr, cm−1): 3436 υ(O–HPhenolic), 1057 υ(–O–CH2–), 1621 υ(HC = N), 1309 υ(C–OPhenolic). 1H NMR (400 MHz, CDCl3) δ 13.30 (s, 1H, –OH), 8.52 (s, 1H, –HC = N-), 7.50 (d, J = 2.4 Hz, 1H, Ar–H), 7.46 (d, J = 2.4 Hz, 1H, Ar–H), 7.04–7.02 (d, J = 7.6 Hz, 1H, Ar–H), 6.97 (d, J = 2.4 Hz, 1H, Ar–H), 6.94 (s, 1H, Ar–H), 6.93 (s, 1H, Ar–H), 4.30–4.26 (m, 4H, –OCH2-), 2.28–2.22 (m, 2H, –CH2-). 13C NMR (100 MHz, CDCl3) δ 160.07 (-HC = N-), 159.90 (-C–OH), 151.68, 150.70, 143.12, 135.45, 134.06, 122.19, 120.65, 119.20, 116.40, 114.06, 110.45 (Ar–C), 70.60 (-CH2O-), 31.64 (-CH2-). MS for C16H14BrNO3 m/z: 347.0157, [M + H]+: 348.0235.
2. 2,4-dichloro-6-(((3,4-dihydro-2H-benzo[b][1,4]dioxepin-7-yl)imino)methyl)phenol (HL2)
Yield: 84%; Color: red; M.p.: 105–108 °C. Conductivity (ohm−1 cm2 mol−1) in DMF: 14. Anal. Calcd. (%): C, 56.82; H, 3.87; N, 4.14, Found (%): C, 56.80; H, 3.90; N, 4.18. IR (KBr, cm−1): 3435 υ(O–HPhenolic), 1042 υ(–O–CH2–), 1610 υ(HC = N), 1304 υ(C–OPhenolic). 1H NMR (400 MHz, CDCl3) δ 14.33 (s, 1H, –OH), 8.52 (s, 1H, –HC = N-), 7.46–7.45 (d, J = 2.5 Hz, 1H, Ar–H), 7.02 (s, 2H, Ar–H), 6.99–6.98 (d, J = 2.5 Hz, 1H, Ar–H), 6.94 (s, 1H, Ar–H), 4.30–4.26 (m, 4H, –OCH2-), 2.28–2.22 (m, 2H, –OCH2-). 13C NMR (100 MHz, CDCl3) δ 158.94 (-HC = N-), 155.94 (-C–OH), 151.72, 151.11, 141.95, 132.45, 129.56, 123.29, 122.78, 122.32, 120.28, 116.53, 114.04 (Ar–C), 70.60 (-OCH2-), 70.58 (-OCH2-), 31.54 (-CH2-). MS for C16H13Cl2NO3 m/z: 337.0272, [M + H]+: 338.0351.
3. 2-(((3,4-dihydro-2H-benzo[b][1,4]dioxepin-7-yl)imino)methyl)-6-methoxy-4-nitrophenol (HL3)
Yield: 86%; Color: red; M.p.: 117–120 °C. Conductivity (ohm−1 cm2 mol−1) in DMF: 11. Anal. Calcd. (%): C, 59.30; H, 4.68; N, 8.14, Found (%): C, 59.35; H, 4.64; N, 8.17. IR (KBr, cm−1): 3435 υ(O–HPhenolic), 1051 υ(–O–CH2–), 1617 υ(HC = N), 1304 υ(C–OPhenolic). 1H NMR (400 MHz, CDCl3) δ 15.31 (s, 1H, –OH), 8.63 (s, 1H, –HC = N-), 8.07–8.06 (d, J = 2.5 Hz, 1H, Ar–H), 7.79–7.78 (d, J = 2.9 Hz, 1H, Ar–H), 7.07 (s, 1H, Ar–H), 7.05 (s, 1H, Ar–H), 7.03 (s, 1H, Ar–H), 4.32–4.28 (m, 4H, –OCH2-), 4.03 (s, 3H, –OCH3), 2.29–2.23 (m, 2H, –CH2-). 13C NMR (100 MHz, CDCl3) δ 160.17 (-HC = N-), 158.99 (-C–OH), 151.83, 151.32, 149.46, 140.09, 138.89 122.49, 120.55, 116.30, 116.09, 113.93, 108.09 (Ar–C), 70.62 (-OCH2-), 70.58 (-OCH2-), 56.49 (-OCH3), 31.44 (-CH2-). MS for C17H16N2O6 m/z: 344.1008, [M + H]+: 345.1087.
4. 1-(((3,4-dihydro-2H-benzo[b][1,4]dioxepin-7-yl)imino)methyl)naphthalen-2-ol (HL4)
Yield: 81%; Color: brown; M.p.: 112–115 °C. Conductivity (ohm−1 cm2 mol−1) in DMF: 13. Anal. Calcd. (%): C, 75.12; H, 5.37; N, 4.39, Found (%): C, 75.17; H, 5.33; N, 4.43. IR (KBr, cm−1): 3437 υ(O–HPhenolic), 1062 υ(–O–CH2–), 1620 υ(HC = N), 1311 υ(C–OPhenolic). 1H NMR (400 MHz, CDCl3) δ 15.51 (s, 1H, –OH), 9.30 (s, 1H, –HC = N-), 8.11–8.09 (d, J = 7.4 Hz, 1H, Ar–H), 7.82–7.79 (d, J = 7.1 Hz, 1H, Ar–H), 7.74–7.72 (d, J = 8.0 Hz, 1H, Ar–H), 7.55–7.51 (dd, J = 7.4, 2.2 Hz, 1H, Ar–H), 7.37–7.35 (d, J = 7.5 Hz, 1H, Ar–H), 7.12–7.10 (d, J = 7.2 Hz, 2H, Ar–H), 7.06 (s, 1H, Ar–H), 6.99–6.97 (dd, J = 7.6, 2.5 Hz, 1H, Ar–H), 4.32–4.26 (m, 4H, –OCH2-), 2.29–2.23 (m, 2H, –CH2-). 13C NMR (100 MHz, CDCl3) δ 169.48 (-HC = N-), 154.38 (-C–OH), 151.92, 150.02, 140.94, 136.33, 133.20, 129.36, 128.02, 127.33, 123.47, 122.44, 122.03, 118.90, 115.63, 113.12, 108.82 (Ar–C), 70.69 (-OCH2-), 31.73 (-CH2-). MS for C20H17NO3 m/z: 319.1208, [M + H]+: 320.1287.
5. [Co(L1)2(H2O)2]
Yield: 73%; Color: reddish brown; M.P.: 251–255 °C. Conductivity (ohm−1 cm2 mol−1) in DMF: 14. Anal. Calcd. (%): C, 48.69; H, 3.83; N, 3.55; Co, 7.47. Found (%): C, 48.65; H, 3.87; N, 3.58; Co, 7.49. IR (KBr, cm−1): 3437 υ(-OHWater), 1049 υ(–O–CH2–), 1615 υ(HC = N), 1259 υ(C–OPhenolic), 564 ʋ(M–O), 449 ʋ(M–N). MS for C32H30Br2N2O8Co m/z: 788.9680, [M + H]+: 789.9759.
6. [Ni(L1)2(H2O)2]
Yield: 79%; Color: green; M.P.: 223–225 °C. Conductivity (ohm−1 cm2 mol−1) in DMF: 17. Anal. Calcd. (%): C, 48.71; H, 3.83; N, 3.55; Ni, 7.44. Found (%): C, 48.68; H, 3.85; N, 3.59; Ni, 7.45. IR (KBr, cm−1): 3435 υ(-OHWater), 1045 υ(–O–CH2–), 1615 υ(HC = N), 1254 υ(C–OPhenolic), 565 ʋ(M–O), 495 ʋ(M–N). MS for C32H30Br2N2O8Ni m/z: 787.9702, [M + H]+: 788.9780.
7. [Cu(L1)2(H2O)2]
Yield: 71%; Color: reddish brown; M.P.: 245–248 °C. Conductivity (ohm−1 cm2 mol−1) in DMF: 15. Anal. Calcd. (%): C, 48.41; H, 3.81; N, 3.53; Cu, 8.00. Found (%): C, 48.45; H, 3.77; N, 3.56; Cu, 7.99. IR (KBr, cm−1): 3436 υ(-OHWater), 1048 υ(–O–CH2–), 1616 υ(HC = N), 1254 υ(C–OPhenolic), 533 ʋ(M–O), 466 ʋ(M–N). MS for C32H30Br2N2O8Cu m/z: 792.9644, [M + H]+: 793.9723.
8. [Zn(L1)2(H2O)2]
Yield: 74%; Color: light yellow; M.P.: 238–240 °C. Conductivity (ohm−1 cm2 mol−1) in DMF: 19. Anal. Calcd. (%): C, 48.30; H, 3.80; N, 3.52; Zn, 8.22. Found (%): C, 48.35; H, 3.75; N, 3.57; Zn, 8.20. IR (KBr, cm−1): 3435 υ(-OHWater), 1047 υ(–O–CH2–), 1601 υ(HC = N), 1259 υ(C–Ophenolic), 555 ʋ(M–O), 449 ʋ(M–N). 1H NMR (400 MHz, DMSO- d6) δ 9.32 (s, 1H, –HC = N-), 8.62 (s, 1H, Ar–H), 7.11 (d, J = 2.3 Hz, 2H, Ar–H), 7.09 (d, J = 2.2 Hz, 2H, Ar–H), 6.90 (s, 1H, Ar–H), 3.72–3.67 (m, 4H, –OCH2-), 2.59–2.54 (m, 2H, –CH2-). 13C NMR (100 MHz, DMSO- d6) δ 168.68 (-HC = N-), 161.45 (-C-O-), 151.74, 151.67, 150.40, 147.73, 143.48, 128.24, 123.78, 122.13, 118.45, 116.35, 113.93 (Ar–C), 70.59 (-OCH2-), 64.70 (-OCH2-), 31.71 (-CH2-). MS for C32H30Br2N2O8Zn m/z: 793.9640, [M + H]+: 794.9718.
9. [Co(L2)2(H2O)2]
Yield: 72%; Color: red; M.P.: 272–275 °C. Conductivity (ohm−1 cm2 mol−1) in DMF: 18. Anal. Calcd. (%): C, 49.96; H, 3.67; N, 3.64; Co, 7.66. Found (%): C, 49.94; H, 3.62; N, 3.65; Co, 7.68. IR (KBr, cm−1): 3437 υ(-OHWater), 1035 υ(–O–CH2–), 1595 υ(HC = N), 1266 υ(C–OPhenolic), 537 ʋ(M–O), 453 ʋ(M–N). MS for C32H28Cl4N2O8Co m/z: 768.9902, [M + H]+: 769.9981.
10. [Ni(L2)2(H2O)2]
Yield: 81%; Color: green; M.P.: 277–280 °C. Conductivity (ohm−1 cm2 mol−1) in DMF: 16. Anal. Calcd. (%): C, 49.97; H, 3.67; N, 3.64; Ni, 7.63. Found (%): C, 49.99; H, 3.70; N, 3.69; Ni, 7.62. IR (KBr, cm−1): 3434 υ(-OHWater), 1029 υ(–O–CH2–), 1602 υ(HC = N), 1274 υ(C–OPhenolic), 551 ʋ(M–O), 448 ʋ(M–N). MS for C32H28Cl4N2O8Ni m/z: 767.9924, [M + H]+: 769.0002.
11. [Cu(L2)2(H2O)2]
Yield: 75%; Color: green; M.P.: 265–267 °C. Conductivity (ohm−1 cm2 mol−1) in DMF: 15. Anal. Calcd. (%): C, 49.66; H, 3.65; N, 3.62; Cu, 8.21. Found (%): C, 49.63; H, 3.68; N, 3.67; Cu, 8.22. IR (KBr, cm−1): 3432 υ(-OHWater), 1057 υ(–O–CH2–), 1595 υ(HC = N), 1296 υ(C–OPhenolic), 539 ʋ(M–O), 459 ʋ(M–N). MS for C32H28Cl4N2O8Cu m/z: 772.9866, [M + H]+: 773.9945.
12. [Zn(L2)2(H2O)2]
Yield: 76%; Color: light yellow; M.P.: 258–260 °C. Conductivity (ohm−1 cm2 mol−1) in DMF: 16. Anal. Calcd. (%): C, 49.54; H, 3.64; N, 3.61; Zn, 8.43. Found (%): C, 49.57; H, 3.69; N, 3.65; Zn, 8.41. IR (KBr, cm−1): 3431 υ(-OHWater), 1033 υ(–O–CH2–), 1591 υ(HC = N), 1282 υ(C–OPhenolic), 544 ʋ(M–O), 463 ʋ(M–N). 1H NMR (400 MHz, CDCl3) δ 8.23 (s, 1H, –HC = N-), 7.54 (s, 1H, Ar–H), 7.13 (s, 1H, Ar–H), 6.84 (s, 1H, Ar–H), 6.67–6.66 (d, J = 7.2 Hz, 1H, Ar–H), 6.65–6.64 (d, J = 7.0 Hz, 1H, Ar–H), 4.22–4.16 (m, 4H, –OCH2-), 2.21–2.16 (m, 2H, –CH2-).13C NMR (100 MHz, CDCl3) δ 167.64 (-HC = N-), 164.06 (-C-O-), 151.77, 150.72, 143.17, 134.96, 133.37, 128.27, 122.48, 119.19, 118.51, 116.47, 114.06 (Ar–C), 70.53 (-OCH2-), 70.49 (-OCH2-), 31.40 (-CH2-). MS for C32H28Cl4N2O8Zn m/z: 773.9862, [M + H]+: 774.9940.
13. [Co(L3)2(H2O)2]
Yield: 73%; Color: light yellow; M.P.: 238–241 °C. Conductivity (ohm−1 cm2 mol−1) in DMF: 15. Anal. Calcd. (%): C, 52.25; H, 4.38; N, 7.17; Co, 7.54. Found (%): C, 52.27; H, 4.40; N, 7.20; Co, 7.57. IR (KBr, cm−1): 3401 υ(-OHWater), 1049 υ(–O–CH2–), 1594 υ(HC = N), 1262 υ(C–OPhenolic), 553 ʋ(M–O), 479 ʋ(M–N). MS for C34H34N4O14Co m/z: 781.1404, [M + H]+: 782.1482.
14. [Ni(L3)2(H2O)2]
Yield: 70%; Color: light green; M.P.: 241–245 °C. Conductivity (ohm−1 cm2 mol−1) in DMF: 17. Anal. Calcd. (%): C, 52.26; H, 4.39; N, 7.17; Ni, 7.51. Found (%): C, 52.24; H, 4.42; N, 7.19; Ni, 7.50. IR (KBr, cm−1): 3431 υ(-OHWater), 1046 υ(–O–CH2–), 1613 υ(HC = N), 1261 υ(C–OPhenolic), 552 ʋ(M–O), 452 ʋ(M–N). MS for C34H34N4O14Ni m/z: 780.1425, [M + H]+: 781.1503.
15. [Cu(L3)2(H2O)2]
Yield: 76%; Color: light green; M.P.: 222–225 °C. Conductivity (ohm−1 cm2 mol−1) in DMF: 14. Anal. Calcd. (%): C, 51.94; H, 4.36; N, 7.13; Cu, 8.08. Found (%): C, 51.97; H, 4.34; N, 7.15; Cu, 8.07. IR (KBr, cm−1): 3421 υ(-OHWater), 1044 υ(–O–CH2–), 1614 υ(HC = N), 1258 υ(C–OPhenolic), 533 ʋ(M–O), 472 ʋ(M–N). MS for C34H34N4O14Cu m/z: 785.1368, [M + H]+: 786.1446.
16. [Zn(L3)2(H2O)2]
Yield: 79%; Color: light orange; M.P.: 234–236 °C. Conductivity (ohm−1 cm2 mol−1) in DMF: 18. Anal. Calcd. (%): C, 51.82; H, 4.35; N, 7.11; Zn, 8.30. Found (%): C, 51.85; H, 4.37; N, 7.14; Zn, 8.33. IR (KBr, cm−1): 3417 υ(-OHWater), 1051 υ(–O–CH2–), 1600 υ(HC = N), 1278 υ(C–Ophenolic), 550 ʋ(M–O), 455 ʋ(M–N). 1H NMR (400 MHz, CDCl3) δ 8.38 (s, 1H, –HC = N-), 8.03–8.02 (d, J = 2.3 Hz, 1H, Ar–H), 7.74 (s, 1H, Ar–H), 6.87–6.85 (d, J = 8.6 Hz, 1H, Ar–H), 6.64 (s, 2H, Ar–H), 4.22–4.17 (m, 4H, –OCH2-), 3.97 (s, 3H, –OCH3), 2.24–2.18 (m, 2H, –CH2-). 13C NMR (100 MHz, CDCl3) δ 168.11 (-HC = N-), 166.78 (-C-O-), 152.51, 151.85, 150.94, 142.71, 136.07, 125.64, 122.57, 116.28, 115.86, 114.08, 108.14 (Ar–C), 70.54 (-OCH2-), 70.50 (-OCH2-), 56.49 (-OCH3), 31.31 (-CH2-). MS for C34H34N4O14Zn m/z: 786.1363, [M + H]+: 787.1441.
17. [Co(L4)2(H2O)2]
Yield: 76%; Color: reddish brown; M.P.: 238–240 °C. Conductivity (ohm−1 cm2 mol−1) in DMF: 16. Anal. Calcd. (%): C, 65.66; H, 4.96; N, 3.83; Co, 8.05. Found (%): C, 65.69; H, 4.94; N, 3.85; Co, 8.04. IR (KBr, cm−1): 3430 υ(-OHWater), 1054 υ(–O–CH2–), 1603 υ(HC = N), 1266 υ(C–OPhenolic), 535 ʋ(M–O), 459 ʋ(M–N). MS for C40H36N2O8Co m/z: 731.0968, [M + H]+: 732.1046.
18. [Ni(L4)2(H2O)2]
Yield: 77%; Color: green; M.P.: 246–248 °C. Conductivity (ohm−1 cm2 mol−1) in DMF: 15. Anal. Calcd. (%): C, 65.68; H, 4.96; N, 3.83; Ni, 8.02. Found (%): C, 65.70; H, 4.97; N, 3.85; Ni, 8.03. IR (KBr, cm−1): 3423 υ(-OHWater), 1044 υ(–O–CH2–), 1611 υ(HC = N), 1279 υ(C–OPhenolic), 529 ʋ(M–O), 455 ʋ(M–N). MS for C40H36N2O8Ni m/z: 730.1807, [M + H]+: 731.1855.
19. [Cu(L4)2(H2O)2]
Yield: 73%; Color: green; M.P.: 227–230 °C. Conductivity (ohm−1 cm2 mol−1) in DMF: 17. Anal. Calcd. (%): C, 65.25; H, 4.93; N, 3.80; Cu, 8.63. Found (%): C, 65.28; H, 4.95; N, 3.83, Cu, 8.62. IR (KBr, cm−1): 3431 υ(-OHWater), 1044 υ(–O–CH2–), 1613 υ(HC = N), 1260 υ(C–OPhenolic), 522 ʋ(M–O), 472 ʋ(M–N). MS for C40H36N2O8Cu m/z: 735.4915, [M + H]+: 736.4993.
20. [Zn(L4)2(H2O)2]
Yield: 80%; Color: yellow; M.P.: 234–237 °C. Conductivity (ohm−1 cm2 mol−1) in DMF: 19. Anal. Calcd. (%): C, 65.09; H, 4.92; N, 3.80; Zn, 8.86. Found (%): C, 65.11; H, 4.95; N, 3.87; Zn, 8.88. IR (KBr, cm−1): 3411 υ(-OHWater), 1050 υ(–O–CH2–), 1605 υ(HC = N), 1289 υ(C–OPhenolic), 538 ʋ(M–O), 468 ʋ(M–N). 1H NMR (400 MHz, CDCl3) δ 9.29 (s, 1H, –HC = N-), 8.03–8.00 (d, J = 8.5 Hz, 1H, Ar–H), 7.82–7.79 (d, J = 7.2 Hz, 1H, Ar–H), 7.49 (d, J = 1.6 Hz, 1H, Ar–H), 7.30 (d, J = 2.1 Hz, 1H, Ar–H), 7.13–7.08 (m, 2H, Ar–H), 6.83 (s, 1H, Ar–H), 6.78–6.77 (d, J = 2.7 Hz, 1H, Ar–H), 6.74 (d, J = 2.8 Hz, 1H) 4.18–4.12 (m, 4H, –OCH2-), 2.19–2.13 (m, 2H, –CH2-). 13C NMR (100 MHz, CDCl3) δ 173.58 (-HC = N-), 162.65 (-C-O-), 154.44, 151.76, 145.36, 137.44, 135.54, 129.29, 127.87, 126.63, 122.32, 118.85, 116.68, 115.65, 114.09, 113.13, 109.04 (Ar–C), 70.68 (-OCH2-), 70.52 (-OCH2-), 31.62 (-CH2-). MS for C40H36N2O8Zn m/z: 736.4039, [M + H]+: 737.4117.
Biophysical-experiments
To find out a significant medicinal agent for pathogen causing illness, we analyzed all the synthesized compounds (1–20) against antioxidant, antimicrobial and anti-inflammatory activities; and the highly potent antimicrobial and anti-inflammatory heterocyclic Schiff base ligands HL2 (2), HL3 (3) and their complexes are also evaluated for cytotoxicity analysis on Vero cells to check their toxicity level.
Antioxidant activity
In vitro 2,2-dipheny-1-picrylhydrazyl (DPPH) and 2,2'-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid (ABTS) assays were evaluated for a set of compounds (1–20) for observing the % radical scavenging and IC50 values using ascorbic acid (standard drug).
DPPH assay
Compound concentrations
The synthesized compounds were prepared in DMSO for 200, 100, 50, 25, 12.5 μg/mL concentrations. The above prepared solutions (1 mL) were added with another 1 mL DPPH solution (1 mg DPPH in 20 mL DMSO) and dissolve the resulting solution into 1 mL DMSO [27]. Then obtained solutions were put in dark place for 30 min. after vigorous shaking.
Determination of radical scavenging activity
The diversion in absorbance of all the prepared solutions was assessed at 517 nm in triplicates by using DPPH solution in DMSO (reference). The % radical scavenging values of the compounds were assessed by using the below equation-
Xreference - Absorbance of the reference; Ycompound - Absorbance of the compounds.
The graphs were plotted in between concentration (μg/mL) on X-axis and scavenging activity on Y-axis for calculating % radical scavenging; further their IC50 values were also calculated and compared with standard drug.
ABTS radical assay
The ABTS radical assay was performed in triplicates by producing the blue green ABTS radical cation by reacting 70 mM potassium persulfate with 7 mM ABTS in water which stored for 16 h at room temperature before use. Further, the ABTS solution was diluted with 80% methanol to obtain 0.700 ± 0.005 absorbance at 734 nm. Then mix the 2 mL ABTS solution in 100 μL solution of different concentrations (200, 100, 50, 25, 12.5 μg/mL) after that incubate the solution for 1 min at room temperature and record the absorbance of the solutions at 734 nm by taking blank solution (2 mL ABTS solution in 200 μL methanol). A standard curve was acquired by using ascorbic acid reference solution at different concentrations. The radical scavenging ability of the compounds (1–20) was measured to calculate the IC50 values [30].
Xcontrol - Absorbance of the control (control did not have any compound or standard); Ycompound - Absorbance of the compounds.
Antimicrobial activity
In vitro anti-fungal and anti-bacterial potential of the derived compounds were screened against two fungal and four bacterial strains (Rhizopus oryzae (MTCC 262), Candida albicans (MTCC 227), Escherichia coli (MTCC 732), Pseudomonas aeruginosa (MTCC 424), Staphylococcus aureus (MTCC 2901) and Bacillus subtilis (NCIM 2063)) via serial dilution methodology [28, 29]. Fluconazole and ciprofloxacin were taken as standard drugs for fungi and bacteria, respectively. DMSO used as negative control and the MIC (minimum inhibitory concentrations) values were recorded in the unit of μmol/mL.
Compounds concentrations
MIC values were calculated by two-fold serial dilution methodology using 1000 μg/mL stock solutions of the compounds which was prepared by dissolving 5 mg of the synthesized ligands and their complexes in 5 mL DMSO. For preparing 100 μg/mL stock solution, took 1 mL of 1000 μg/mL solution in a test tube and made up it up to 10 mL by DMSO.
Subculture of pathogens
The bacterial strains were sub-cultured in nutrient broth (NB) which was obtained by adding 1.3 g of NB in distilled water (100 mL). The fungal strains were sub-cultured in potato dextrose broth (PDB) which was obtained by adding 2.4 g of PDB in distilled water (100 mL). Both the mixtures were autoclaved for 30 min at 15 psi and the subcultures were incubated at 25 °C for seven days and at 37 °C for one day for fungal and bacterial strains, respectively.
Determination of minimum inhibitory concentration (MIC) values
The two-fold serial dilution assay was employed in triplicate manner to detect the antimicrobial properties of the compounds. In this assay, 1 mL of 100 μg/mL stock solution was added with 1 mL of broth in a test which was serially diluted for 50, 25, 12.50, 6.25 and 3.12 μg/mL concentration solutions. After that, bacteria and fungi were added in every test tube and incubated for a particular time for growth of the culture. Growth of the pathogens was checked spectrophotometrically and visually; and record the minimum inhibitory concentrations of the compounds which were compared with reference drugs.
Anti-inflammatory activity
The in vitro anti-inflammatory evaluation of the compounds was observed in triplicate by using egg albumin method and taking diclofenac sodium as a standard drug. The solution of various concentrations was prepared in DMSO and evaluated their % inhibition and IC50 values.
Compound concentrations
The percentage inhibition of the compounds was evaluated by using 200, 100, 50, 25, 12.5 µg/mL concentration solutions which were obtained by mixing the compounds in the DMSO.
Determination of percentage inhibition
The above prepared solutions (2 mL) added in a test tube having egg albumin (0.2 mL of fresh hen’s egg) and phosphate buffer saline (2.8 mL) of 6.4 pH. The resulting solutions of the compounds were incubated for 15–20 min at 37 °C in BOD and warmed at 70 °C for 5 min. On cooling, the alteration in absorbance of the solutions was observed at 660 nm on cooling using double distilled water as a reference/control [30, 31]. The % inhibition of the denatured protein was evaluated by the given formula-
Xcompound - Absorbance of the compounds; Ycontrol - Absorbance of the control.
Further, the obtained IC50 values of the solutions were compared with the value of diclofenac sodium (standard drug).
Cytotoxicity on Vero cell lines
In vitro cytotoxic studies [32] of highly active antimicrobial and anti-inflammatory Schiff base ligands HL2 (2), HL3 (3) and their (9–16) complexes were performed on Vero cell lines by colorimetric assay using resazurin dye (7-hydroxy-3H-phenoxazin-3- one 10-oxide) and DMSO (reference). The resazurin dye is nonfluorescent and blue in color until reduction to highly fluorescent pink color resorufin.
Compound concentrations
Percentage cytotoxicity was calculated by using 1000 µg/mL stock solution (1 mg of compound in 10 µL DMSO and 900 µL media) which was utilized to prepare the solution of 250, 62.50 µg/mL concentration.
Subculture of cell lines
Vero cell lines were cultured in 100 µL of cell culture medium (EMEM supplemented with 10% fatal bovine serum, 25 mM sodium bicarbonate, 2 mM L-glutamine, 10 mM HEPES, amphotericin B 25 mg/mL, penicillin 10,000 units/mL, and streptomycin 10 mg/mL) at density of 1 × 104 cells per well, in 96-well cells culture plate.
Determination of percentage cytotoxicity
The above culture cells were treated with different concentrations (1000, 250 and 62.5 µg/mL) of compounds in triplicate which was incubated for 24 h at 37 °C in 5% CO2 and incubated again for 24 h after treatment. After incubation, the compounds were treated with 10 µL of resazurin that was prepared in distilled water (1 mg/mL) and incubated again for under conditions as mentioned above which show the formation of pink color resorufin after 4 h incubation due to cellular metabolic activity. Then the absorbance of the compounds was recorded by spectrophotometer (ELISA plate reader, PowerWave™ XS2; Bio-tek, VT, USA) at 590 nm. Percentage cytotoxicity of the compounds was calculated after normalizing the background absorbance of media with reference to untreated cells [33].
X - Absorbance of the untreated control; Y - Absorbance of the compounds.
Computational study
In the recent time, the computational studies are highly supports the biological evaluation, therefore, the less cytotoxic and most antimicrobial active zinc(II) complex (12) and its ligand (2) were evaluated by molecular docking study.
Molecular docking
Molecular docking study was used to determine the mode of interaction and binding energy via ligand-enzyme interaction which support the biological activity of the compounds. The structure of highly microbial active and less cytotoxic compounds (2) and (12) were drawn by chemDraw3d pro and energy minimization was done by UCSF chimera. The crystal structures of the S. aureus S1: DHFR (PDB code-2W9S), E. coli DNA gyrase B (PDB code–4DUH) and C. albicans sterol-14-alpha-demethylase (PDB code–5TZ1) enzymes were retrieved from protein data bank (https://www.rcsb.org/); further the compounds and enzymes were docked by the Autodock Vina software. Swiss PDB application was used for structure optimization of enzymes. The comparison and observations of the docking sites of compounds and targeted enzymes were done by BIOVIA discovery studio [34].
Results and discussion
Chemistry
In the present work, the synthesis of four heterocyclic ligands and their sixteen transition metal complexes were reported. The ligands (1–4) were acquired in significant amount by condensing the 3,4-dihydro-2H-benzo[b][1,4]dioxepin-7-amine (3,4-dihydro-2H-1,5-benzodioxepin-7-amine) with derivatives of salicylaldehyde/2-hydroxy-1-naphthaldehyde using methanol as solvent which further treated with methanolic solution of Co(II), Ni(II), Cu(II) and Zn(II) acetates in 1:2 molar ratio to obtain the metal(II) complexes. All the obtained compounds were solids, insoluble in the organic solvents except DMF, THF, CDCl3, DMSO and stable at room temperature. The crystalline and non-electrolytic nature of the compounds were confirmed by powder XRD and molar conductance measurements, respectively. The single crystals of the compounds were not grown in numerous of attempts which carried out in different ratio of solvents. The octahedral geometry and binding sites in the complexes were confirmed by a number of spectroscopic and physical techniques which suggested that the ligands bonded to the metal centre in bidentate way by the oxygen and nitrogen atoms of the deprotonated phenolic and azomethine group, respectively.
Mass spectra
The mass spectral analysis in the positive ion mode was recorded in acetonitrile solvent that proposed the stoichiometry of the complexes [35, 36] and the obtained results are mentioned in Table S1 and Fig. S1–S8 of the supplementary. The heterocyclic Schiff ligands HL1, HL2, HL3 and HL4 shows significant ion peaks at m/z 348.0235, 338.0351, 345.1087 and 320.1287, respectively because of [M + H]+ ion. The Co(II), Ni(II), Cu(II) and Zn(II) complexes of ligand (4) exhibited [M + H]+ ion peak at 732.1046, 731.1855, 736.4993 and 737.4117, respectively which is same as their molecular mass ion values and confirmed octahedral geometry of the complexes. The mass spectral results confirm the complex formation of ligands with metal ion in the molar ratio of 2:1 as represented in Scheme 1.
Molar conductance measurements
The molar conductance of the compounds was recorded at ambient temperature using digital conductivity meter in 1 × 10−3 M DMF solution. The obtained conductance value exhibited in the region of 11–19 Ω−1 cm2 mol−1 (Table S1 of the supplementary) which confirmed the non-electrolytic behavior of the compounds [37].
IR spectra
The mode of coordinating sites and presence of functional groups in the compounds is examined by infrared spectroscopy at 4000 cm−1–400 cm−1. The obtained spectra of the ligands were compared with their respective complexes and confirmed complex formation due to appearance or disappearance of bands and shifting in frequencies because of the change in electronic environment [38, 39] (Table S2 and Fig. S9–S16 of the supplementary). In the spectra of ligands (HL1–HL4), a strong absorption band appears near 3437–3435 cm−1 because of ʋ(O–HPhenolic) stretching vibration frequency that disappear on complexation which confirmed the bonding of ligands with metal ions. Another band appears at 1621–1610 cm−1 as result of ʋ(HC = N) frequency which shifts at lesser frequency after complexation shows ligation of azomethine group from N- atom to the central metal atom [40]. During complexation, there is a shifting of ʋ(C-OPhenolic) band frequency from 1311–1304 cm−1 to 1296–1254 cm−1 indicates bonding through deprotonated oxygen atom [41]. The IR spectra also shows a band at 1062–1029 cm−1 in the synthesized compounds as a result of (-O-CH2-) group and the appearance of a broad absorption band in the complexes at 3437–3401 cm−1 confirmed the coordination of water molecules with central metal atom. Further, the infrared spectra of the complexes show some new bands around 565–522 cm−1 and 495–448 cm−1 which ascribed for stretching vibrations frequency of metal–oxygen (M–O) and metal-nitrogen (M–N) bonds, respectively [42]. The presence of new absorption bands in the spectra of the complexes confirms that the heteroatoms such as deprotonated oxygen atom, nitrogen atom of –HC = N- group and oxygen atom of water molecule are bonded to the metal centre [43] which validate the octahedral nature of the complexes as shown in Scheme 1.
1H NMR spectra
1H NMR spectra of the heterocyclic ligands and zinc(II) complexes were carried out in DMSO- d6 and CDCl3 using trimethylsilane as a reference which are shown in Table S3 and Fig. S17–S24 of the supplementary. The (HL1–HL4) ligands shows a singlet in the region of δ 15.51–13.30 ppm due to hydroxyl proton which disappeared on complexation due to the proton abstraction of –OH group on bonding of ligands with metal centre confirm the complexation. The Schiff base ligands (HL1–HL4) have a singlet around δ 9.30–8.52 ppm because of the proton of azomethine group which was shifted at δ 9.32 ppm in case of complex (8) and in region of δ 9.29–8.23 ppm in the case of other complexes of zinc(II) metal, indicates chelation of azomethine nitrogen with metal. The upward shifting of peaks indicates releasing of electrons of nitrogen atom of the moiety to the zinc(II) metal centre and downward shifting of peaks might be due to the steric effects and geometry of the zinc(II) complexes [44, 45]. Some singlet, doublet and multiplet displayed around δ 8.11–6.93 ppm corresponds to the protons of phenyl ring which shifted on complexation and supports the Zn(II) complexes formation. The signal for the protons of the (-OCH2-) groups appears near δ 4.32–4.26 ppm in ligands which gets shifted downward on complexation. A multiplet is exhibited at δ 2.29–2.22 ppm due to methylene protons, coupled with the (–OCH2–) protons that showed shifting on complexation. Therefore, the obtained 1H NMR data is supports the complex formation with ligands in 1:2 molar ratio as represented in Scheme 1.
13C NMR spectra
The 13C NMR spectral data of the heterocyclic ligands and Zn(II) complexes [46, 47] were observed in DMSO-d6 and CDCl3 which also supports the structures of the complexes. The obtained data displayed in Table S3 and Fig. S25–S32 of the supplementary. The (HL1–HL4) ligands shows a signal at δ 169.48–158.94 ppm because of carbon atom of of –HC = N- group which was shifted upward in the complexes, indicates bonding of nitrogen atom of the –HC = N- group to the metal centre, revealing that the electron density of N- atom of –HC = N- group shifts towards the zinc(II) metal ions. A sharp peak around δ 159.90–154.38 ppm designated for carbon atom having hydroxyl group in Schiff base ligands which was shifted around δ 166.78–161.45 ppm in the spectra of Zn(II) complexes which indicate the deprotonation of hydroxyl group by coordinating via metal ion. The signals of aromatic carbon were assigned in the region of δ 151.92–108.09 ppm for ligands and shifting of these signals clearly indicates the complexation. The (HL1–HL4) ligands shows some peaks at δ 70.69–70.58 ppm due to –OCH2- group and at δ 31.73–31.44 ppm due to methylene group which also get shifted on complexation. The 13C and 1H NMR spectral data shows that the obtained signals of ligands shifted on complexation and confirm the coordination behavior of the ligands with central metal atoms in a bidentate pattern as proposed by other techniques.
Magnetic susceptibility and electronic spectra
The UV–Vis spectral analysis of the compounds were examined in THF solvent at room temperature (Table S4 of the supplementary) which is very significant technique to confirm the stereochemistry of metal complexes. The electronic absorption spectra of ligands show strong absorption band at 25,895–27,580 cm−1 because of n → π* transition of –HC = N- group and at 38,765–39,905 cm−1 as a result of π → π* transition of aromatic ring [48, 49].
The three bands appears in Co(II) complexes in the region of 10,553–10,835 cm−1 for 4T1g(F) → 4T2g(F) (υ1), 17,331–18,283 cm−1 for 4T1g(F) → 4A2g(F) (υ2) and 22,583–23,154 cm−1 for 4T1g(F) → 4T1g(P) (υ3) transitions that confirm octahedral environment around cobalt(II) atom which was also supported by the ʋ3/ʋ1, B and β values that lies at 2.11–2.19, 881.8–930.4 cm−1 and 0.908–0.958, respectively. The number of unpaired electron (three) in Co(II) complexes were confirmed by the magnetic moment value at 4.45–4.58 BM which also suggests octahedral behavior of cobalt(II) complexes. Ni(II) compounds also reported three absorption bands in the range of 10,251–10,982 cm−1 for 3A2g(F) → 3T2g(F) (ʋ1), 17,018–18,867 cm−1 for 3A2g(F) → 3T1g(F) (ʋ2) and 24,887–23,987 cm−1 for 3A2g(F) → 3T1g(P) (ʋ3) that confirmed the octahedral behavior of the complexes. The ratio of ʋ2/ʋ1 nearby 1.66–1.74, the values of magnetic moment in the region of 3.35–3.52 BM, the values of ligand field parameters B nearby 683.4–743.8 cm−1 and β around 0.722–0.663 also support the octahedral geometry of nickel(II) complexes. The octahedral bonding around the Cu(II) metal ions was supported by 1.73–1.84 BM magnetic moment and two absorption band at 15,119–15,983 cm−1 for 2B1g → 2A1g (υ1) and 23,529–24,767 cm−1 for 2B1g → 2E2g (υ2) transitions. Only one absorption band shown by the Zn(II) metal complexes in the region of 22,987–23,925 cm−1 due to the LMCT and these complexes also have diamagnetic in nature and d10 configuration that confirm octahedral geometry around Zn(II) metal. The above spectral data suggests the octahedral environment for all the synthesized metal complexes as suggested by other spectral evaluations.
Powder XRD analysis
Powder XRD analysis was carried out for the synthesized compounds in the region of 2θ = 10–80 ̊ at the ambient temperature and 1.5406 Å wavelength to verify the nature of the compounds [50]. The XRD pattern of a compound is like fingerprint of the compound which analyzes amorphous and crystalline nature of the compounds, dislocation density, peak intensities and average crystallite size (D) of the compounds [51]. The crystalline nature of the compounds was confirmed by the crystalline peaks in the XRD pattern. The X-ray diffraction pattern of the ligand (1) and complex (5) are shown in Fig. 1. The Debye- Scherrer equation was used to calculate the average crystallite size (D) by the given equation
λ - wavelength (1.5406 Å); β - full width at half maximum of the reference diffraction peak; \(\uptheta\) - diffraction angle; K - shape factor (0.95)
The ligand HL1 (1) and its Co(II) complex shows the average crystallite size (D) at 48.75 nm and 44.67 nm, respectively [52]. The complex has smaller crystallite size than its ligand due to ligation with metal ion which shows the nano-crystallite size of the complexes. The dislocation density (δ) of the ligand (1) and cobalt(II) complex (5) was evaluated by the below equation which is lie in the region of 0.00042–0.00050 nm−2 [53].
Thermal analysis
The thermogravimetric analysis was evaluated to examine the thermal behavior of the compounds and to determine whether the H2O molecules are outside or inside from the coordination sphere in the complexes [54,55,56]. The TG curve of present complexes do not indicate sharp weight loss but shows the percentage mass loss in the three steps and leaves metal oxide as residue. The results indicate the fine agreement between the proposed formulae and calculated values of the weight loss.
The thermal stabilization of the complexes was evaluated from the TG curve having function of temperature in the range of 30–900 °C. The complex [Cu(L1)2(H2O)2] (7) do not shows any type of weight loss and stable up to 170 °C but have three decomposition steps beyond this temperature (Fig. 2). The 1st step decomposition represents the loss of the bonded water molecules from 170 to 230 °C having weight loss of 4.83% (Calcd. 4.53%). The 2nd decomposition step shows the weight loss of 46.01% (Calcd. 45.84%) around the temperature of 230–420 °C due to the loss of one ligand moiety. Further, in the 3rd step, complex decomposes in the region of 420–780 °C indicating weight loss of 34.57% (Calcd. 34.27%) due to another ligand moiety and leave copper oxide as the end product.
The thermal analysis curve of [Ni(L2)2(H2O)2] (10) shows that the coordinated water molecules were loss in the first decomposition step at the temperature nearby 190–260 °C exhibiting weight loss of 4.58% (Calcd. 4.68%). Further, the complex goes for second and third steps weight loss of 46.17% (Calcd. 46.04%) and 34.71% (Calcd. 34.61%), respectively in the temperature region of 260–800 °C due to the presence of two bidentate coordinated ligands leaving behind nickel oxide as end product (Fig. 2).
The thermogravimetric evaluation of other complexes follows the same three steps for decomposition leaving metal oxide in the last which exhibit thermal stability of metal complexes up to 170 °C temperature and no weight loss was reported up to this temperature range, indicating that there is no probability of presence of H2O molecule outer side of the coordination sphere. From thermal data, we conclude that the compounds were highly stable and non-volatile in behavior at room temperature and decomposition of synthesized compounds ends with appearance of metal oxide.
The decomposition steps of the metal compounds are as follows-
SEM and EDAX analysis
SEM (scanning electron microscopy) was implemented to analyze the changes in surface morphology of Schiff base ligands on complexation [57]. The SEM micrographs of ligand HL2 (2) and its [Co(L2)2(H2O)2] complex (9) was carried at different magnifications (Fig. 3). The micrograph of the Schiff base ligand HL2 (2) have rough surface with rectangular bars having many territorial patches and its Co(II) complex (9) exhibits needle shaped surface which is quite different from its Schiff base ligand. The differences in the size and shape of the particles of ligand on complexation is because of the crystal aggregation which accumulate on the thin films due to its dependency on the transition metal ions which is another evidence of complexation.
The synthesized complexes were examined by energy dispersive X-ray analysis (EDAX) to know the elemental configuration [58]. The EDAX graphs of ligand (2) and complex (9) were shown in Fig. 3. The EDAX indicates the presence of various major constitutes (C, O, N, Cl) in Schiff base ligand (2) and complexation was confirmed by appearance of cobalt(II) metal in the graph of complex which supports the proposed structure as shown in Scheme 1 and validate the other spectral and physical data.
Electron spin resonance spectroscopy
The ESR spectrum (solid state X-band) of copper(II) complexes were examined at liquid nitrogen temperature (77 K) and at room temperature (300 K) as shown in Fig. 4 [59, 60]. The g∥ and g⊥ values of copper(II) complexes were evaluated and reported in Table 1. The values of g∥ and g⊥ at 2.23 and 2.07 for Cu(II) complex (15) is according to g∥ > g⊥ > 2.0023, that supports the octahedral geometry of the complex by having unpaired electron in the dx2-dy2 orbital. The g tensor values predicted the ionic (g∥ > 2.3) and covalent (g∥ < 2.3) nature of the complexes. Therefore, the covalent character of the complex (15) was supported by the g∥ value which is less than 2.3. The lesser value of orbital coupling constant in comparison of free copper metal ion (832 cm−1) supported the covalent nature of M-L bonds; it was determined with the help of gav value (2.12) which calculated by the given equation-
The G (anisotropic geometric parameter) value was observed by the given formula which indicates some exchange interactions in copper(II) complexes.
Hathway and Billing says that there is negligible interaction in copper metal ions if G > 4 and significant exchange interaction between Cu(II) centres if the value of G < 4 in the solid state. Here, the value of G was 3.36 which give an idea about exchange interaction in copper(II) metal complex.
Biological investigations
In the present time, we are continuously facing the onslaught of many microorganisms causing illness which is very serious matter for the scientific community, therefore, to find a significant agent for these diseases, we inspected the synthesized compounds for antioxidant, antimicrobial and anti-inflammatory activities.
Antioxidant activity
The extremely reactive oxygen species (ROS) like peroxide, superoxide and hydroxyl radical were arise in human body because of various biochemical process that damage DNA, proteins, lipids, nucleic acid and another biological molecule which is major cause of numerous serious diseases like diabetes, cancer, Parkinson, Alzheimer, aging, coronary heart disease etc. Therefore, it is important to avert our body from free radical scavenging by taking the enriched antioxidants drugs which can repair and stop the oxidative contamination. Therefore, the derived compounds were analyzed for in vitro antioxidant efficacy [61] in triplicates at the 200, 100, 50, 25, 12.5 μg/mL concentrations by DPPH and ABTS assays taking ascorbic acid as standard drug to find pot a compelling antioxidant agent. The dose dependence data of the compounds were mentioned in Table S5 of the supplementary and the graphical representations was shown in Fig. 5 and the IC50 values of the compounds were mentioned in Table 2 and supplementary Fig. S37.
The following conclusions are drawn from the antioxidant activity-
-
1.
The antioxidant evaluation indicates that the activity of all the synthesized compounds and standard drug behave in dose dependence manner which clearly exhibit that the activity of the compounds was enhanced with increasing concentration of the compounds and with decreasing IC50 value.
-
2.
The ligands (1–4) exhibit the % radical scavenging ability in the region of 17.90 ± 0.11–28.98 ± 0.03 and 29.57 ± 0.09–46.44 ± 0.01 at lower and higher concentrations, respectively and IC50 value at 7.35 ± 0.01–9.33 ± 0.06 μM and 7.56 ± 0.09–9.63 ± 0.06 μM in DPPH and ABTS assays, respectively which follows the activity order as HL4 > HL1 > HL3 > HL2. The most active Schiff base ligand (HL4) indicate 28.98 ± 0.03 and 46.44 ± 0.01 percentage radical scavenging at lower and higher concentrations, respectively; and IC50 value at 7.35 ± 0.01 μM and 7.56 ± 0.09 μM in DPPH and ABTS assays, respectively because of the presence of electron donating groups in the moiety [62].
-
3.
The antioxidant potency of the ligands was increased on complex formation in the region of 24.53 ± 0.08–38.11 ± 0.03 and 39.79 ± 0.06–50.25 ± 0.10 at lower and higher concentrations, respectively with IC50 value at 4.70 ± 0.07–7.99 ± 0.02 μM and 4.65 ± 0.01–8.46 ± 0.02 μM in the DPPH and ABTS assays, respectively because of the polarity, metallic effect, hydrophobicity, lipophilicity and spreading of negative charge of –OH group on the aromatic ring.
-
4.
The potency of metal compounds was assessed in the following manner Cu(II) > Zn(II) > Ni(II) > Co(II) due to the ability of the compounds to donate hydrogen atom for disturbance of free radical sequence. The 15, 17, 18, 19, 20 compounds have significant IC50 value at 4.70 ± 0.07–5.83 ± 0.10 μM and 4.65 ± 0.01–5.93 ± 0.02 μM in the DPPH and ABTS assays, respectively which are comparable with standard drug.
-
5.
Hence, the result concluded that the synthesized metal complexes have good antioxidant behavior but the copper(II) complex (19) (IC50 value at 4.70 ± 0.07 μM and 4.65 ± 0.01 μM in the DPPH and ABTS assays, respectively) is highly active species among them which give hopeful therapy for treatment of oxidative stress and pathogenic illness.
-
6.
Further, the comparison of synthesized compounds with previously reported compounds [63,64,65] revealed that the synthesized compounds (1–20) have more antioxidant activity, so, these may be act as antioxidant agents in health cares.
Antimicrobial activity
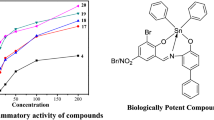
The synthesized compounds were examined by serial dilution method using S. aureus, B. subtilis, Escherichia coli, P. aeruginosa, R. oryzae, C. albicans as microbial (bacterial and fungal) strains in triplicates to know their in vitro antimicrobial potency. The fluconazole and ciprofloxacin were utilized as standard drugs for fungi and bacteria, respectively and the obtained results are mentioned as MIC values in Table 3 and Fig. 6.
The results concluded from antimicrobial data are given below-
-
1.
The antimicrobial activity shown by the synthesized compounds is because of the presence of –HC = N- (azomethine) group in the moiety which have a significant role in resamination and transamination of the biological systems. The presence of nitrogen and oxygen donor atoms on the ligands may be prohibit the production of enzyme. According to chelation theory, the polar behavior of the metals decreases because of the partial distribution of its positive charge with ligand upon chelation that increase the lipophilicity of the metal atom and favors their permeability from the lipid layer of cell membrane which enhance antimicrobial potency of the metal complexes [66,67,68]. Therefore, the chelating behavior of the ligand increase the biological activity on complexation with some another factors like bond length, dipole moment, size of the moiety, hydrophobicity, concentration etc.
-
2.
The synthesized ligands show the antimicrobial results in the following manner HL2 > HL3 > HL1 > HL4 for all the tested strains of microbes which is due to the electron withdrawing groups attached in the moieties [69, 70].
-
3.
For gram-positive bacteria, the compounds 9, 10, 11, 12, 15, 16 are more potent against S. aureus then all the other compounds with MIC value 0.0080–0.0162 \(\mathrm{\mu mol}/\mathrm{mL}\) and the metal compounds 5, 8, 9, 12 are more potent against B. subtilis with MIC value 0.0157–0.162 \(\mathrm{\mu mol}/\mathrm{mL}\) in comparison of another compounds.
-
4.
For the gram-negative bacteria, the compounds 6, 11, 12, 14, 16 are more active against E. coli with MIC value 0.0080–0.0161 \(\mathrm{\mu mol}/\mathrm{mL}\) and the compounds 7, 9, 12, 15 are more efficient for P. aeruginosa with MIC value 0.0157–0.0162 \(\mathrm{\mu mol}/\mathrm{mL}\) then another compounds.
-
5.
Against fungal strains, 10, 11, 12, 15 complexes are more active for R. oryzae with minimum inhibitory concentration value 0.0079–0.0081 \(\mathrm{\mu mol}/\mathrm{mL}\) and the 8, 9, 12, 15, 16 compounds are active for C. albicans with MIC value 0.0040–0.0081 \(\mathrm{\mu mol}/\mathrm{mL}\).
-
6.
Among all the synthesized metal compounds, the general trend for reactivity and growth of inhibition against all the tested microbes is Zn(II) > Cu(II) > Co(II) > Ni(II) and compound 12 have more activity and least MIC value for all the tested microbes.
-
7.
The more antimicrobial efficacy of the synthesized compounds (1–20) was revealed by comparing the previously reported compounds [71, 72] with synthesized compounds (1–20) which states that the compounds (1–20) have significant MIC values with more antimicrobial activity and may be used to control the microbial dysfunctions in the medicinal industries.
Anti-inflammatory activity
The antimicrobial and anti-inflammatory activities were depended on the electron withdrawing groups of the moieties [73, 74] and the compounds have significant potency against microbial strains, therefore to find out the anti-inflammatory potency of the compounds, the percentage inhibition of the standard drug, ligands and their transition metal compounds were measured on denatured protein by egg albumin assay in triplicates.
Inflammation is the main reason of numerous diseases like cancer, asthma, rheumatoid arthritis etc. which shows the genomic changes and tissue injuries around the infected organs. Therefore, the anti-inflammatory drugs are responsible to destruct the organisms to abolish the irritant from the body and repair the damaged tissues. So, the obtained results are mentioned in Table S6 and Fig. S38 of the supplementary and Fig. 7 and Table 4.
The results revealed the following conclusions-
-
1.
The data of inflammatory evaluation depends on the concentration of the compounds. The percentage inhibition was enhanced with the concentration of the compounds. The results also shows that the IC50 values decreases with increasing percentage inhibition.
-
2.
The ligands (HL1–HL4) indicate the percentage inhibition in the range of 17.44 ± 0.02 to 31.44 ± 0.02% at higher concentration (200 µg/mL) and IC50 values from 10.06 ± 0.02 to 19.07 ± 0.08 µM which follows the following activity order HL2 > HL3 > HL1 > HL4 due to the electron withdrawing groups attached to the moiety.
-
3.
On complexation, the percentage inhibition increased in the range of 19.23 ± 0.12 to 40.21 ± 0.02% at 200 µg/mL concentration which shows IC50 values from 7.42 ± 0.02 to 18.28 ± 0.05 µM, suggesting more activity of the compounds in comparison of the ligands for inflammation due to the lipophilicity, metallic effect, ligation behavior, chelation and dispersal of charge.
-
4.
The synthesized complexes show the following order for anti-inflammation Zn(II) > Cu(II) > Co(II) > Ni(II) and the 9, 10, 11, 12 compounds have the IC50 values very close to standard drug (diclofenac sodium). Compound 12 with IC50 value 7.42 ± 0.02 µM is more potent among all the synthesized compounds and might be used for the inflammation ailments.
-
5.
The highest activity of the ligand HL2 (2) and its complexes are explained on the basis of chelation and two electronegative chloro groups of the moiety.
-
6.
Further, we compared the previously reported compounds [75] with the synthesized compounds (1–20) which advocates the more inflammation inhibition ability of the synthesized compounds and revealed that these compounds are many new advancements in the pharmaceutical industry for treating the inflammation causing diseases.
Structure activity relationship
The structure activity relationship was utilized to depict the effect of attached groups on the biological activities of the compounds (Scheme 2). The (HL4) ligand is highly active for antioxidant activity because there is no electron withdrawing group present on the aromatic ring while the presence of bromo group in ligand (HL1) and methoxy, nitro groups in (HL3) ligand justify the moderate efficiencies of the (HL1) and (HL3) ligands, respectively. The lowest antioxidant effect of the (HL2) ligand is justify by the attached electron withdrawing chloro groups at the aromatic ring. Therefore, the antioxidant activity is depends on the electron donating ability of the attached group at the moiety.
The antimicrobial and anti-inflammatory activities are depended on the electron withdrawing group effect of the attached group at the moiety, therefore, the highest antimicrobial and anti-inflammatory potency of the (HL2) ligand is advocated by the presence of two chloro groups at the phenyl ring while the moderate activity are shown by (HL3) and (HL1) ligands as a consequences of methoxy and nitro groups in (HL3) ligand and bromo group in (HL1) ligand. The unsubstituted aromatic ring in (HL4) ligand attributes the least activity of (HL4) ligand.
The performed pharmacological activities show that the biological efficiency of the ligands was enhanced on complexation with transition metal atoms as a consequence of polarity, chelation, hydrophobicity, DNA binding ability, lipophilicity and metallic effect.
Cytotoxicity on Vero cell lines
The in vitro cytotoxicity was performed for highly potent antimicrobial and anti-inflammatory heterocyclic Schiff base ligands HL2 (2) and HL3 (3) and their respective transition metal complexes using DMSO as reference on Vero cell lines (Mammalian cells from African Green Monkey Kidney) by calorimetric method in triplicate manner [76]. The cytotoxic data revealed that the toxicity of the compounds behave in the dose dependence manner which decreased with decrease in concentration of the compounds as shown in Fig. 8 and Table 5. The assay shows that metabolic active cells reduced the blue color non-fluorescent resazurin dye into pink color fluorescent resorufin after 4 h of incubation, hence, the optical density measured at 590 nm which was directly depends on the number of viable cells. The obtained data indicates that the Schiff base ligand HL2 was less toxic than HL3 at all the treated concentrations due to the different groups present on the phenyl ring, which is also more potent for microbial strains and anti-inflammation. On chelation, the toxicity of the compounds was decreased due to the delocalization of negative charge of –OH group on the phenyl ring, metallic effect and lipophilicity. The cytotoxicity order of the compounds was observed as Zn(II) < Cu(II) < Ni(II) < Co(II). The result of the present study indicates that all the compounds are less toxic and have significant properties for medicinal industries, but Zn(II) complex (12) is more potent for antimicrobial and anti-inflammatory with very low toxicity at all the treated concentration in comparison of other tested compounds which attracted the attention of the researcher for new drug discovery for some serious diseases.
Molecular docking
Molecular docking is a computational study and very much demanding in medicinal chemistry. It plays a significant role in drug designing and stimulate the molecular recognition process. Molecular docking is used to evaluate the binding energy that indicates the interaction between the enzyme and ligand molecules. The lower binding energy value indicates the higher binding affinity and more potency of the compounds.
The zinc(II) complex (12) [Zn(L2)2(H2O)2] of heterocyclic Schiff base ligand (2) (HL2) have very low cytotoxicity and more potency for S. aureus, E. coli and C. albicans strains among tested gram ( +) bacteria, gram (-) bacteria and fungus, respectively. Therefore, the evaluation of probable binding score and binding affinity of these compounds is point of interest. To analyze the binding energy of these compounds, three enzymes such as 2W9S, 4DUH, 5TZ1 are chosen for molecular docking studies with respect to S. aureus, E. coli and C. albicans microbial strains [14, 77, 78]. The molecular docking studies show the various interactions between the compound and amino acid of the enzyme (Table 6 and Figs. 9, 10, 11) which supports the antimicrobial activity and its mechanism of action.
The results of molecular docking are mentioned below-
-
1.
For S. aureus S1: DHFR, the ligand (2) indicates the seven good interaction with the Ile50, Asn18, Leu20, Ile31, Phe92, Gly94, Gln95 residues while on complexation with zinc(II) metal, the complex (12) exhibits the Leu20, Ile14, Gln19 interaction. The binding energy of the ligand (2) and complex (12) was reported at − 8.9 and − 10.2 kcal/mol, respectively.
-
2.
The ligand (2) and its complex (12) show the binding energy at − 7.7 and − 10.1 kcal/mol, respectively for E. coli DNA gyrase B. The ligand (2) indicates the four significant interactions with Asn46, Ile78, Lys103, Phe104 amino acid residues and the complex (12) have Glu58, Ile60, Asp74, Arg76, Thr163, Lys162, Arg136 interaction with the residues of enzyme.
-
3.
Against C. albicans sterol-14-alpha-demethylase enzyme, the ligand (2) shows the − 8.4 kcal/mol binding energy and have the nine interactions (Tyr64, Tyr505, His377, Leu376, Leu87, Leu88, Pro230, Leu121, Tyr118) with the active sites of the enzyme. The complex (12) indicates the binding energy at − 12.6 kcal/mol and shows the following significant interactions with Cys470, Gly472, Phe475, Leu150, Gly308, Ile304, Gly307, Gly303, Ile131, Leu376, His377, Met508, Phe233, Pro233 residues.
-
4.
From the molecular docking study, we conclude that the binding affinity of the complex is less than the ligand and supports the enhancement of antimicrobial activity on complexation. Among all the three chosen enzymes for docking, complex (12) has lowest binding energy (-12.6 kcal/mol) for C. albicans sterol-14-alpha-demethylase enzyme which significantly supports the higher antifungal activity against C. albicans and attracted the attention of chemist for synthesis of new antifungal drug.
Conclusion
In the current work, we have synthesized four heterocyclic ligands and their sixteen Co(II), Ni(II), Cu(II) and Zn(II) metal complexes which further characterized by numerous analytical techniques i.e. NMR, FT-IR, mass spectrometry, UV–Vis, TGA, ESR, powder XRD, molar conductivity, elemental analysis and magnetic moment for structure elucidation. The characterization data implies that the complex formation of metal ions occurs with ligands in 1:2 molar ratio forming octahedral geometry which indicates that the ligands coordinated with metal centre in bidentate way from the nitrogen atom of imine linkage and deprotonated phenolic oxygen atom. The biological evaluations strongly advocate that the activity of the ligands was increased on complexation with transition metals. The antioxidant results show that the compounds 15, 17, 18, 19 and 20 are highly antioxidant than the other compounds and the IC50 values of these compounds are comparable with standard drug. The antimicrobial evaluation indicates that the compounds 9, 10, 11, 12, 15, 16 are more potent against S. aureus; compounds 5, 8, 9, 12 are more active against B. subtilis; compounds 6, 11, 12, 14, 16 are highly active against E. coli; compounds 7, 9, 12, 15 are more efficient for P. aeruginosa; compounds 10, 11, 12, 15 are more active for R. oryzae; compounds 8, 9, 12, 15, 16 are highly potent for C. albicans then all the other compounds. The anti-inflammatory activity revealed that the compounds 9, 10, 11, 12 are more potent and IC50 values of these compounds very close to standard drug (diclofenac sodium). Further, in vitro cytotoxicity was analyzed for highly active antimicrobial and anti-inflammatory ligands HL2 (2), HL3 (3) and their respective metal complexes on Vero cell lines; and found that the complex (12) is less cytotoxic than other compounds. The molecular docking study was evaluated for the less toxic and highly antimicrobial active (against S. aureus among tested gram ( +) bacteria, E. coli among tested gram (-) bacteria and C. albicans among tested fungal strains) zinc(II) metal complex (12) and its ligand (2) against the active sites of S. aureus S1: DHFR, E. coli DNA gyrase B and C. albicans sterol-14-alpha-demethylase enzymes which indicate that the compounds have significant binding interactions and validate their biological efficiency. The docking results shows that the ligand (2) and complex (12) have binding energy at − 8.9 and − 10.2 kcal/mol for S. aureus S1: DHFR, − 7.7 and − 10.1 kcal/mol for E. coli DNA gyrase B, − 8.4 and − 12.6 kcal/mol for C. albicans sterol-14-alpha-demethylase, respectively which are significantly supported the MIC values of antimicrobial activity and confirm that the activity of ligands increases on complexation. The lowest binding energy of the complex (12) against C. albicans sterol-14-alpha-demethylase indicate that it is more potent for antifungal activity against C. albicans and may be used for fungal deformities in health cares. So, the present research emphasized the biological importance of transition metal complexes of Schiff base ligands in pharmaceutical industries which also advocates by the comparison of previously reported compounds. Hence, this research has an exclusive perception in the constructing of cost-effective, less toxic and more potent medicinal drug for health cares.
Availability of data and materials
All the spectra are provided in the supplementary information.
References
M. Seki, Org. Process. Res. Dev. 20, 867 (2016)
T. Jurca, E. Marian, L.G. Vicaş, M.E. Mureşan, L. Fritea, Spectrosc. Anal. Dev. Appl. 123 (2017)
A. Erxlebe, Inorganica Chim. Acta. 472, 40 (2018)
W.H. Mahmoud, R.G. Deghadi, G.G. Mohamed, Appl. Organomet. Chem. 30, 221 (2016)
I.P. Ejidike, P.A. Ajibade, Rev. Inorg. Chem. 35, 191 (2015)
G. Kumar, S. Devi, D. Kumar, J. Mol. Struct. 1108, 680 (2016)
O. Danilescu, I. Bulhac, S. Shova, G. Novitchi, P. Bourosh, Russ. J. Coord. Chem. 46, 838 (2020)
W.H. Mahmoud, G.G. Mohamed, O.Y. El-Sayed, Appl. Organomet. Chem. 32, e4051 (2018)
M. Lazou, A. Tarushi, P. Gritzapis, G. Psomas, J. Inorg. Biochem. 206, 111019 (2020)
D. Kumar, N. Sharma, M. Nair, J. Biol. Inorg. Chem. 22, 535 (2017)
B. Čobeljić, M. Milenković, A. Pevec, I. Turel, M. Vujčić, B. Janović, K. Anđelković, J. Biol. Inorg. Chem. 21, 145 (2016)
J. Devi, B. Kumar, B. Taxak, Inorg. Chem. Commun. 109208 (2022)
A.N. Srivastva, N.P. Singh, C.K. Shriwastaw, Arab. J. Chem. 9, 48 (2016)
J. Devi, S. Kumar, B. Kumar, S. Asija, A. Kumar, Res. Chem. Intermed. 48, 1541 (2022)
S. Bu, G. Jiang, G. Jiang, J. Liu, X. Lin, X. Shen, X. Liao, J. Biol. Inorg. Chem. 25, 747 (2020)
R. Fekri, M. Salehi, A. Asadi, M. Kubicki, Inorganica Chim. Acta. 484, 245 (2019)
Z. Meng, H. Yu, L. Li, W. Tao, H. Chen, M. Wan, P. Yang, D.J. Edmonds, J. Zhong, A. Li, Nat. Commun. 6, 1 (2015)
F.E. Held, A.A. Guryev, T. Fröhlich, F. Hampel, A. Kahnt, C. Hutterer, M. Steingruber, H. Bahsi, C.V. Bojnici-Kninski, D.S. Mattes, T.C. Foertsch, A. Nesterov-Mueller, M. Marschall, S.B. Tsogoeva, Nat. commun. 8, 1 (2017)
E.M. Carvalho, T. de Freitas Paulo, A.S. Saquet, B.L. Abbadi, F.S. Macchi, C.V. Bizarro, V. Bernardes-Génisson, J. Biol. Inorg. Chem. 25, 887 (2020)
U.H. Ramadhan, H.M. Haddad, Z.G. Ezaria, World J. Pharm. Pharm. Sci. 5, 98 (2006)
M. Nath, P.K. Saini, A. Kumar, J. Organomet. Chem. 695, 1353 (2010)
F. Nareetsile, J.T. Matshwele, S. Ndlovu, M. Ngaski, Chem. Rev. lett. 3, 140 (2020)
D.M. Yufanyi, H.S. Abbo, S.J. Titinchi, T. Neville, Coord. Chem. Rev. 414, 213285 (2020)
S. Savir, Z.J. Wei, J.W.K. Liew, I. Vythilingam, Y.A.L. Lim, H.M. Saad, S.S. Kae, K.W. Tan, J. Mol. Struct. 1211, 128090 (2020)
J.M. Méndez-Arriaga, G.M. Esteban-Parra, M.J. Juárez, A. Rodríguez-Diéguez, M. Sánchez-Moreno, J. Isac-García, J. Inorg. Biochem. 175, 217 (2017)
A.A. Garcia-Valdivia, A. García-García, F. Jannus, A. Zabala-Lekuona, J.M. Mendez-Arriaga, B. Fernández, A. Rodriguez-Dieguez, J. Inorg. Biochem. 208, 111098 (2020)
J. Devi, S. Pachwania, J. Yadav, A. Kumar, Phosphorus Sulfur Silicon Relat. Elem. 196, 119 (2020)
L. Deswal, V. Verma, D. Kumar, Y. Deswal, A. Kumar, R. Kumar, M. Bhatia, Chem. Pap. 76, 7607 (2022)
I. Potočňák, P. Vranec, V. Farkasová, D. Sabolová, M. Vataščinová, J. Kudláčová, S.R. Trifunović, J. Inorg. Biochem. 154, 67 (2016)
A. Mumtaz, T. Mahmud, M. Khalid, H. Khan, A. Sadia, M.M. Samra, M.A.R Basra, J. Pharm. Innov. 1 (2020)
S. Chandra, P. Chatterjee, P. Dey, S. Bhattacharya, Asian Pac. J. Trop. Biomed. 2, 178 (2012)
P. Kaur, R. Thakur, M. Barnela, M. Chopra, A. Manuja, A. Chaudhury, Chem. Technol. Biotechnol. 90, 867 (2015)
A. Manuja, B. Kumar, T. Riyesh, T.R. Talluri, B.N. Tripathi, Int. J. Biol. Macromol. 165, 71 (2020)
E.F. Pettersen, T.D. Goddard, C.C. Huang, E.C. Meng, G.S. Couch, T.I. Croll, T.E. Ferrin UCSF ChimeraX: Protein Sci. 30, 70 (2021)
I.K. Kareem, M.A. Hadi, Egypt J. Chem. 63, 301 (2020)
M. Munjal, J. Pharmacogn. Phytochem. 7, 864 (2018)
C.T. Prabhakara, S.A. Patil, S.S. Toragalmath, S.M. Kinnal, P.S. Badami, J. Photochem. Photobiol. B Biol. 157, 1 (2016)
J. Devi, S. Sharma, S. Kumar, B. Kumar, D. Kumar, D.K. Jindal, S. Das, Appl. Organomet. Chem. 36, e6760 (2022)
Z. Zhao, J. Zhang, S. Zhi, W. Song, J. Inorg. Biochem. 196, 110696 (2019)
Y. Deswal, S. Asija, A. Dubey, L. Deswal, D. Kumar, D.K. Jindal, J. Devi, J. Mol. Struct. 1253, 132266 (2022)
M. Yadav, S. Sharma, J. Devi, J. Chem. Sci. 133, 1 (2021)
S.E. Abd El-Razek, S.M. El-Gamasy, M. Hassan, M.S. Abdel-Aziz, S.M. Nasr, J. Mol. Struct. 1203, 127381 (2020)
H. Kargar, F. Aghaei-Meybodi, R. Behjatmanesh-Ardakani, M.R. Elahifard, V. Torabi, M. Fallah-Mehrjardi, K.S. Munawar, J. Mol. Struct. 1230, 129908 (2021)
J. Devi, M. Yadav, D. Kumar, L.S. Naik, D.K. Jindal, Appl. Organomet. Chem. 33, e4693 (2019)
K. Singh, M.S. Barwa, P. Tyagi, Eur. J. Med. Chem. 41, 147 (2006)
V.P. Radha, S. Chitra, S. Jonekirubavathi, I.M. Chung, S.H. Kim, M. Prabakaran, J. Coord. Chem. 73, 1009 (2020)
S.G. Nozha, S.M. Morgan, S.A. Ahmed, M.A. El-Mogazy, M.A. Diab, A.Z. El-Sonbati, M.I. Abou-Dobara, J. Mol. Struct. 1227, 129525 (2021)
D. Aggoun, M. Fernández-García, D. López, B. Bouzerafa, Y. Ouennoughi, F. Setifi, A. Ourari, Polyhedron 187, 114640 (2020)
G. Lupașcu, E. Pahonțu, S. Shova, Bărbuceanu, Ș.F., Badea, M., Paraschivescu, C.E. Dinu‐Pîrvu, Appl. Organomet. Chem. 35, e6149 (2021)
J. Devi, N. Batra, Spectrochim. Acta A Mol. Biomol. Spectrosc. 135, 710 (2015)
I. Gonul, M. Kose, G. Ceyhan, S. Serin, Inorg. Chim. Acta. 453, 522 (2016)
C. Vidya Rani, M.P. Kesavan, S. Haseena, R. Varatharaj, J. Rajesh, G. Rajagopal, Appl. Biochem. Biotechnol. 191, 1515 (2020)
J.R. Anacona, J.L. Rodriguez, J. Camus, Spectrochim. Acta A Mol. Biomol. Spectrosc. 129, 96 (2014)
S. Velumani, X. Mathew, P.J. Sebastian, S.K. Narayandass, D. Mangalaraj, Sol. Energy Mater Sol. Cells. 76, 347 (2003)
N. Kavitha, P.A. Lakshmi, J. Saudi Chem. Soc. 21, S457 (2017)
M.M. Abo-Aly, A.M. Salem, M.A. Sayed, A.A. Aziz, Spectrochim. Acta A Mol. Biomol. Spectrosc. 136, 993 (2015)
M.S. Refat, H.A. Saad, A.A. Gobouri, M. Alsawat, A.M.A. Adam, S.M. El-Megharbel, J. Mol. Liq. 345, 117140 (2022)
A.A.M. Belal, I.M. El-Deen, N.Y. Farid, R. Zakaria, M.S. Refat, Spectrochim. Acta A Mol. Biomol. Spectrosc. 149, 771 (2015)
N.N. Rao, K. Gopichand, R. Nagaraju, A.M. Ganai, P.V. Rao, Chem. Data Collect. 27, 100368 (2020)
A. Palanimurugan, A. Dhanalakshmi, P. Selvapandian, A. Kulandaisamy, Heliyon 5, e02039 (2019)
V.P. Singh, S. Singh, D.P. Singh, K. Tiwari, M. Mishra, J. Mol. Struct. 1058, 71 (2014)
H.M. Vinusha, S.P. Kollur, H.D. Revanasiddappa, R. Ramu, P.S. Shirahatti, M.N. Prasad, M. Begum, Results Chem. 1, 100012 (2019)
Q.M. Hasi, Y. Fan, X.Q. Yao, D.C. Hu, J.C. Liu, Polyhedron 109, 75–80 (2016)
L.H. Abdel-Rahman, M.S.S. Adam, N. Al-Zaqri, M.R. Shehata, H.E.S. Ahmed, S.K. Mohamed, Arab. J. Chem. 15, 103737 (2022)
G. Kalaiarasi, C. Umadevi, A. Shanmugapriya, P. Kalaivani, F. Dallemer, R. Prabhakaran, Inorganica Chim. Acta 453, 547 (2016)
N. Ganji, S. Daravath, A. Rambabu, K. Venkateswarlu, D.S. Shankar, Inorg. Chem. Commun. 121, 108247 (2020)
L. Wang, C. Hu, L. Shao, Int. J. Nanomed. 12, 1227 (2017)
N. Kumar, S. Asija, Y. Deswal, S. Saroya, A. Kumar, J. Devi, Phosphorus Sulfur Silicon Relat. Elem. 1 (2022)
H.F. Abd El‐Halim, G.G. Mohamed, M.N. Anwar, Appl. Organomet. Chem. 32, e3899 (2018)
Y. Deswal, S. Asija, D. Kumar, D.K. Jindal, G. Chandan, V. Panwar, S. Saroya, N. Kumar, Res. Chem. Intermed. 48, 703 (2022)
S. Slassi, A. El-Ghayoury, M. Aarjane, K. Yamni, A. Amine, Appl. Organomet. Chem. 34, e5503 (2020)
A.N. Kursunlu, E. Guler, F. Sevgi, B. Ozkalp, J. Mol. Struct. 1048, 476 (2013)
S.S. Hassan, P.A. Khalf-Alla, Appl. Organomet. Chem. 34, e5432 (2020)
J. Devi, J. Yadav, N. Singh, Res. Chem. Intermed. 45, 3943 (2019)
M.S. Kasare, P.P. Dhavan, B.L. Jadhav, S.D. Pawar, Synth. Commun. 49, 3311 (2019)
A. Manuja, N. Rathore, S. Choudhary, B. Kumar, Med. Chem. 17, 576 (2021)
P. Jain, S. Sharma, N. Kumar, N. Misra, Appl. Organomet. Chem. 34, e5736 (2020)
M.J. Ahmad, S.F. Hassan, R.U. Nisa, K. Ayub, M.S. Nadeem, S. Nazir, U. Rashid, Med. Chem. Res. 25, 1877 (2016)
Acknowledgements
The author, Mr. Binesh Kumar (File No. 09/752(0105)/2020-EMR-I) is highly thankful to CSIR-HRDG, New Delhi for financial support and also the authors acknowledge to Dr. APJ Abdul Kalam Central Instrumentation Laboratory and Department of Chemistry, Guru Jambheshwar University of Science and Technology, Hisar for providing facilities to carry out this research work.
Funding
The author, Mr. Binesh Kumar (File no. 09/752(0105)/2020-EMR-I) is highly thankful to CSIR-HRDG, New Delhi for financial support in the form of senior research fellowship.
Author information
Authors and Affiliations
Contributions
BK: Data curation, Formal analysis, Investigation, Methodology, Writing-original draft, Software. JD: Supervision, Validation, Writing—review & editing, Data curation. AM: Formal analysis, Investigation.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
No animal/human studies were carried out in the present work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kumar, B., Devi, J. & Manuja, A. Synthesis, structure elucidation, antioxidant, antimicrobial, anti-inflammatory and molecular docking studies of transition metal(II) complexes derived from heterocyclic Schiff base ligands. Res Chem Intermed 49, 2455–2493 (2023). https://doi.org/10.1007/s11164-023-04991-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-023-04991-y