Abstract
Management of Pacific halibut (Hippoglossus stenolepis), a long-lived flatfish, is complicated by possible ontogenic and sex-specific variation in migration. Archival tags promise the ability to help uncover long-term movement patterns at the individual level, if the tags can be retained and recovered from healthy fish. We examined fifteen individuals (69–90 cm fork length) for long-term physiological response to intracoelomic implantation of three types of archival tags: fully internal, internal with right angle protruding light stalk, and internal with straight protruding light stalk. Tags represented 0.05–0.16% of initial fish weights. Fish were reared at 10.8 ± 1.1°C for 59 weeks post-surgery. One fish died after 39 weeks from thermal stress unrelated to the surgical procedure. Temporal variation in behavior of tagged fish was indistinguishable from that of controls (n = 15 tagged, 5 controls). Treatment and control-group fish grew at similar rates. No tag expulsion or physiological response was evident in the individual that died at 39 weeks, but nine of eleven individuals dissected at the end of 59 weeks had developed internal responses. These responses ranged from deposition of fibrous protein and/or calcitic material on tag surfaces to partial or full tag encapsulation in either the visceral peritoneal layer (fully-internal tags) or the intestinal mesenteries (stalk-bearing tags). The responses were within the range reported for other pleuronectids implanted with tags of similar configuration and may have implications for design and interpretation of long-term tagging studies. Encapsulation may reduce the probability of tag recoveries even in the absence of tag expulsion, especially in species eviscerated at sea.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The development of electronic data storage tags has greatly expanded the capacity for researchers to examine fish behavior on a variety of temporal scales. For example, acceleration data have been used to quantify swimming in Japanese flounder (Paralichthys olivaceus) over periods of less than 1 s, and these characteristics then scaled upwards to discriminate between periods of swimming, burial, and rest over minutes to hours (Kawabe et al. 2003). Similarly, vertical movement data demonstrate periodicity in presumed feeding on both diel and lunar scales in basking sharks (Cetorhinus maximus; Shepard et al. 2007); short-period vertical movements have been used to elucidate active spawning in individual Pacific halibut (Hippoglossus stenolepis; Seitz et al. 2005) and subsequently quantify spawning season and habitat for a component of the population (Loher and Seitz 2008a). On seasonal scales, vertical distribution of pelagic species has been used to examine seasonal habitat usage relative to oceanographic parameters (Kitagawa et al. 2007) and to quantify spawning habitat (Schaber et al. 2009), and long-term depth data for benthic species has been correlated to tidal amplitude to yield positional estimates and elucidate spawning migration routes (Hunter et al. 2004) and identify the use of tidal stream transport (Nichol and Somerton 2009). Electronic tags have the potential to generate high resolution movement data through light-based geolocation (Schaefer and Fuller 2006) and, more recently, via advances in geomagnetic techniques (Stockhausen and Guðbjörnsson 2009), and have been deployed to study physiological parameters such as core temperature (Hight and Lowe 2007; Malte et al. 2007) and heart rate (Claireaux et al. 1995; Campbell et al. 2007).
In 2002, the International Pacific Halibut Commission (IPHC) began an electronic tagging program designed to investigate migration and spawning behavior of Pacific halibut. This program has five main goals: (1) quantify migration between feeding and spawning grounds, (2) identify spawning areas, (3) examine interannual site fidelity and straying, (4) define seasonal migration periods and depth-specific habitat use, and (5) define regional spawning periods. To date, these processes have primarily been examined through the use of externally-affixed electronic pop-up archival transmitting (PAT) tags. The IPHC has deployed more than 300 PAT tags on Pacific halibut. Endpoint locations and light-based geolocation estimates during time at liberty have helped to characterize seasonal dispersal in the Gulf of Alaska (Loher and Seitz 2006; Loher and Blood 2009) and eastern Bering Sea (Seitz et al. 2007a, b), including dispersal into areas where fishery-based recaptures would have been highly unlikely (Loher and Clark 2010). In addition, broadcast depth data have been used to define seasonal redistribution between depth strata (Loher and Seitz 2008b) and archived data from physically-recovered tags have confirmed homing to feeding grounds following seasonal dispersal (Seitz et al. 2007a, b, Loher 2008), and have been used to quantify the spawning period (Loher and Seitz 2008a).
Although PAT tags have proven valuable for studying a variety of behaviors, there are numerous questions for which PAT tags are not appropriate. In particular, PAT tag deployments of more than a year are unadvisable due to tag loss and battery-life limitations and the tags’ large size has prevented extrapolation of results across population components. Most fish tagged to date have been >100 cm fork length; halibut of these sizes these have a high probability of being mature females. Given the strong possibility for differential energy allocation to reproductive products, it is reasonable to expect male halibut and small, non-reproductive individuals to behave differently than large reproductive females. Sex-biased dispersal has been suggested in salmonids (Hutchings 2003; Fraser et al. 2004) and cichlids (Knight et al. 1999), and sex-specific differences in energy storage (Faahraeus-Van Ree and Spurrell 2003) and proportional allocation to spawning (Rijnsdorp and Ibelings 1989) have been identified in flatfish. Because male halibut do not need to allocate energy reserves to egg production, they may be able to expend greater proportions of their energy budget on seasonal dispersal or transient migratory behavior. Their migration distances may be larger, migratory periods more variable, and might visit multiple spawning grounds within individual seasons or over their lifetime. In order to fully understand halibut spawning behavior and population structure, research must be expanded to include small fish and individual monitoring over multi-year time scales.
In recent years, battery life and memory capacity has advanced to such a degree that modern electronic archival tags have the capacity to record high-resolution data for periods in excess of 5 years, and their capabilities are continually increasing. This provides an opportunity to monitor behavior for periods that span the juvenile-to-adult transition in long-lived species such as halibut, and may approach the entire life-span of some commercial species. However, such extreme tag life challenges our previous definitions of “long-term” tag retention and holding studies. As opposed to being limited by tag life, modern behavioral studies may be constrained to a greater degree by the ability to secure tags to fish and recover unbiased data after periods that may well approach a decade. Modern electronic tags necessitate attachment protocols that do not impede growth or fitness during periods at large, taking into consideration the potentially large scope for growth in tagged individuals.
In designing or interpreting the results of any tagging study, potential impacts of the attachment protocols must be considered. In particular, external tags can cause drag and lift effects, potentially reducing swimming performance (Arnold and Holford 1978; Lewis and Muntz 1984) and increasing energetic costs to the tagged fish (Grusha and Patterson 2005). Biofouling may further increase drag (Thorstad et al. 2001) and fouling organisms may directly affect fish health. Dicken et al. (2006) observed high rates of biofouling on disc and dart tags deployed on south African raggedtooth sharks (Carcharias taurus), reporting that biofouling exacerbated tagging damage and abrasion around the tagging site. In our own work (T. Loher, unpublished), growth of barnacles and other fouling organisms has been observed on PVC cradles and metal tagging wires used to affix electronic tags to Pacific halibut. Where fouling organisms have come into contact with the fish, we have seen skin lesions and epidermal necrosis in excess of that observed where no fouling was present. Although long-term response to biofouled tags does not appear to have been extensively studied, it is reasonable to expect fouling organisms to inhibit attempts by the host fish’s body to “overgrow” external tags in cases where fish growth would otherwise be sufficient to internalize the tag. Even when not biofouled, overgrowth of an external tag mount might still invoke energy expenditures that could affect behavior(s) of interest, such as spawning frequency or dispersal distance. Given the difficulty in quantifying, in situ, relative differences in behavior between tagged and untagged individuals, it is imperative to seek deployment protocols that minimize tagging effects.
A growing body of evidence suggests that surgical implantation may represent a superior approach for long-term tag deployment, yielding high tag-retention rates, low physiological stress, and ultimately data that may be more representative of normal behavior than derived from externally-affixed tags (Eristhee et al. 2001; Collins et al. 2002; Cottrill et al. 2006; Righton et al. 2006). The present study was designed to refine protocols for intracoelomic (also often referred to as “intraperitoneal”) implantation of electronic tags in Pacific halibut, paying special attention to the possible occurrence of physiological responses such as tag encapsulation (sensu LaCroix et al. 2004) and expulsion (Marty and Summerfelt 1986; LaCroix et al. 2004). In the case of Pacific halibut, the potential for tag encapsulation presents an important logistical consideration: encapsulation may substantially reduce tag recovery by the commercial fishing fleet due to at-sea evisceration. Ultimately, tag recovery rates will depend upon high rates of detection during at-sea fish processing. In the present study, a total of fifteen halibut were tagged with three configurations of internal tag, one fully-internal and two possessing an externally-projecting light-sensing stalk, and fish growth and behavior monitored in a captive setting for 59 weeks. At the end of the holding period, twelve halibut were dissected to determine whether physiological responses to the tags had occurred. In order to more effectively place our findings in the context of current understanding in related species, and to broaden the potential application of our findings, we also conducted a brief review of internal tagging methods in the Pleuronectidae, with attention to observations of tag shedding and physiological responses.
Materials and methods
Electronic archival tags
Three models of electronic archival tags were employed: (1) Lotek Wireless (St. John’s, NL, Canada) LTD 1110 temperature-depth recorders, (2) Lotek LTD 2310 temperature-depth-light recorders, (3) Wildlife Computers (Redmond, WA, USA) Mk9 temperature-depth-light recorders. LTD 1110 tags (Fig. 1, center) were constructed of biologically neutral resin cast in a cylindrical configuration that measured 32 mm by 11 mm, with one rounded and one flat end. At the outer margin of the tag’s flat end was mounted an ~3 mm diameter loop of stainless steel wire designed for suturing the tag to the peritoneal wall. LTD 1110 tags weighed 5 g in air, thus representing approximately 0.05–0.16% of the estimated initial body weights of the tagged halibut.
The three varieties of electronic archival tag implanted into Pacific halibut. All tags recorded temperature and depth; the LTD 2310 and Mk9 also recorded light-levels (irradiance) via a stalk protruding externally through the fish’s eyed-side peritoneal wall. An American quarter dollar is added for scale
LTD 2310 tags (Fig. 1, upper) were encased in a cylindrical silicon-coated stainless steel housing measuring 76 mm by 16 mm, with one rounded and one flat end, and a 2 mm diameter, 22 cm long Teflon light-sensing stalk protruding from the tag’s flat end, oriented parallel with the long axis of the tag body. Where the stalk entered the tag body it was sheathed in a spring designed to allow the stalk to flex without breaking. This spring protruded 6 cm from the tag body and was coated in flexible silicon, increasing the stalk’s diameter to 4 mm along the length of the spring. At the outer margin of the tag’s flat end a 5 mm stainless steel suture loop was mounted. LTD 2310 tags weighed 46 g in air, representing 0.5–1.5% of estimated initial halibut body weights.
Mk9 tags (Fig. 1, lower) were constructed of biologically neutral resin cast in a cylindrical configuration measuring 73 mm by 18 mm, with one rounded and one flat end. A 4 mm diameter, 19.5 cm long polyolefin-coated light-sensing stalk protruded from the side of the tag body at a point approximately 4 mm from the tag’s flat end, extending at a right angle to the tag’s long axis. Mk9 tags weighed 30 g in air, representing approximately 0.3–1.0% of estimated initial halibut body weights.
Fish collection and husbandry
Twenty-four Pacific halibut ranging from 65 to 90 cm fork length (FL) (estimated weigh = 3.1–9.0 kg) were collected on July 31, 2006, aboard a commercial fishing vessel capable of flooding its fish holds for live-fish transport. Capture was timed to correspond with relatively calm weather so as to minimize transport stress. The fish were captured using benthic longline gear rigged with Mustad (Auburn, NY, USA) 12/0 (#7) circle hooks at 0.98 m spacing and baited with Pacific sardine (Sardinops sagax). Gear was set immediately prior to sunrise (~0500 h, local time) and allowed to soak for 2½ h prior to retrieval. Fish were carefully released from the hooks and assessed for injuries and parasites. Retained individuals were externally tagged with a 2.5 cm vinyl Peterson disc affixed just below the dorsal fin using a 1.6 mm diameter nickel pin, and placed into the fish hold flooded with circulating raw sea water. All study specimens had been collected by 0900 h.
Retained halibut were landed at Newport, OR, following approximately 6 h of at-sea transit, and were subsequently trucked to the Oregon Coast Aquarium (Newport, OR, USA) in insulated fish totes containing sea water aerated with pressurized oxygen. The fish were transferred to a 9.1 m diameter pool filled to a depth of 0.9 m with filtered circulating seawater (total water volume ≈ 65 000 l) at 10°C. Fish were reared in this pool throughout the entire pre- and post-tagging period, as a common population without the use of any pool dividers. Three fish died shortly after transfer, apparently from hooking injuries, handling stress and failure to resume feeding; a fourth fish was euthanized in October because it had acquired a heavy load of ectoparasites and presented a possible health risk to the other fish. Food was offered to the captive fish shortly after introduction to the pool, but no fish fed within the first 2 weeks of captivity. Feeding rates steadily increased during the next 3 weeks, reaching 7.3 kg of consumption for the entire population by the end of week-5. Consumption of approximately 8 kg per feeding was typical thereafter. The fish were fed bi-weekly to satiation on a diet of whole, previously frozen capelin (Mallotus villosus), Pacific herring (Clupea pallasii), and squid (Teuthida) species.
Approximately 4 weeks post-capture, the captive halibut population began to develop an infestation of trematode ectoparasites (Entobdella hippoglossi) that are common in captive halibut culture. On two occasions, at 20 and 33 weeks post-capture, the fish were treated with Praziquantel (2-(cyclohexylcarbonyl)-1,2,3,6,7,11b-hexahydro-4H-pyrazino[2,1-a]isoquinolin-4-one) for 3 h at a concentration of 10 ppm. Although these treatments were insufficient to break the egg-hatching cycle and prevent subsequent re-infestation, each treatment eliminated all visible trematodes for approximately 1 month, and maintained relatively low parasite loads within the halibut populations throughout the post-tagging holding period.
Tag implantation
After a post-capture acclimation period of 80 days, representing a minimum of 6 weeks active feeding by all surviving individuals, the fish (n = 20) were deemed ready for tag implantation. Individuals were divided by length into two size categories (<80 cm Fl, ≥80 cm FL), and then randomly assigned within size categories to one of four treatment groups representing each of the three tag types and a control group, resulting in five individuals per treatment. The tagging/processing sequence for each fish was as follows: (1) remove fish from the water, (2) place in an anesthetic bath, (3) replace external Peterson disc, (4) implant internal tag, unless designated as a control fish, (6) measure, (7) return to water, (8) monitor until swimming ability was regained. Control fish were processed on October 18, LTD 1110 and 2310 tags implanted on October 19, and Mk9 tags implanted on October 20.
Fish were removed from the water using a 0.9 m diameter dip net fitted with 6 mm soft knotless nylon netting. Each fish was placed immediately into a bath consisting of seawater and MS-222 (tricaine methanesulfonate; Argent Chemical Laboratories, Redmond, WA, USA) at approximately 11°C. Control fish were subjected to MS-222 at 160 mg l−1 based on published information (Malmstroem et al. 1993) for anesthesia of Atlantic halibut (Hippoglossus hippoglossus). Control fish achieved full anesthesia, defined as markedly slowed and irregular opercular ventilation, complete absence of reflex reactivity to tactile stimulus upon the caudal peduncle, and no visible attempt to right themselves when placed eyed-side down, in approximately 2½ min (i.e., 2:30). This rate of anesthesia (2:26 ± 0:19) was deemed to be slightly more rapid than desired and anesthetic concentration was reduced to 130 mg l−1 for fish subsequently implanted with electronic tags. Despite the lowered concentration of MS-222, time-to-anesthesia for fish implanted with LTD 1110 tags (2:11–2:45, mean = 2:28 ± 0:05) was not significantly different than for control fish (one-factor ANOVA: F = 21.104, P < 0.001; Fisher LSD, P = 0.796). Induction times employed for implantation of LTD 2310 and Mk9 tags (3:03 ± 0:02 and 3:09 ± 0:02, respectively) were similar to one another (Fisher LSD, P = 0.320) but longer than for either controls or LTD 1110 tags (Fisher LSD, P < 0.001 for all pairwise comparisons) because light-stalk-bearing tag configurations took longer to implant and we wished the fish to be more fully sedated.
Once anesthetized, fish were removed from the bath and placed on a concave acrylic cradle lined with a clean towel moistened with seawater. No further anesthetic was administered once removed to the cradle. Following Peterson disc replacement, fish destined for electronic tag implantation were covered with a second dampened towel in which a slot was cut to allow access to the fish’s visceral region. The cradle was equipped with a restraint band made of neoprene fabric and elastic shock cord, which served to hold the posterior half of the fish against the cradle. Implantation of LTD 1110 tags was accomplished by making an incision through the body wall approximately 1 cm long, roughly parallel to the long axis of the fish, positioned about 1 cm dorsal to the anterior end of the first interhaemal spine. The tag was pushed through the entry incision and the incision sutured using three or four sutures. Each specimen was randomly assigned one of two suture types: either 3-0 (n = 9) or 4-0 (n = 5) monofilament polypropylene, mounted on either 23 mm NFS-1 or 19 mm C-13 reverse cutting needles, respectively. A single fish (implanted with an LTD 1110) was sutured using 3-0 braided silk on a 23 mm X-1 reverse cutting needle. The use of silk sutures was immediately discontinued because the silk became weak when wet and broke, requiring two sutures to be removed and re-tied, and due to concern that braided material might increase the risk of infection by wicking pathogens into the wound (T. Miller-Morgan, DVM, Hatfield Marine Science Center, Newport, OR, pers comm).
LTD 2310 and Mk9 tags required a more complex implantation process than LTD 1110 tags because the light stalks needed to protrude externally from the fish’s eyed side. Allowing the light stalk to protrude from the entry incision was undesirable because the protruding light stalk could serve to guide the tag back out the entry incision if the sutures failed, and because the light stalk should ideally project posteriorly and somewhat dorsal so that it lies above the body of the fish as it swims. A dorso-posterior incision would have been required to result in the desired stalk position; such an incision was undesirable due to the thickness of the body wall. Thus, for LTD 2310 and Mk9 tags a 2 cm incision was made approximately half way between the anus and anterior end of the first interhaemal spine (Fig. 2), about 1.5 cm from the fish’s ventral margin. The incision was made at an angle of approximately 25–30° relative to the fish’s midline, with the anterior end of the incision more dorsal than the posterior end. A blunt-ended stainless steel rod was inserted into the incision in order to lift the body wall away from the internal organs. A 3.2 mm diameter solid stainless steel needle was then fed along the length of the rod to a point located approximately 4 cm dorsal to the sixth anal fin ray and used to pierce a hole in the body wall. A hollow aluminum stalk-guiding fid was fitted to the end of the needle that still protruded from the entry incision, the light stalk inserted into the fid, and the fid and needle assembly used to guide the tag’s light stalk into the entry incision, through the peritoneum, and out the pierced hole (Fig. 2). Fids were either 6.4 mm or 7.9 mm diameter, for LTD 2310 and Mk9 tags, respectively. The leading end of the fid was tapered so as to present a smooth transition in diameter between the junction of the piercing needle and the maximum diameter of the fid. Once the tag’s light stalk was fed through the exit hole, the tag’s body was inserted into the peritoneum and the entry incision sutured using five or six sutures of either 3-0 or 4-0 monofilament polypropylene.
Sterile technique was maintained throughout the surgical process. A sterile surgical kit was prepared for each fish, containing Peterson discs and nickel pin, electronic tag, blunt-ended rod, piercing needle, stalk-guiding fid, and suture pack, shrink-wrapped into microwave-sterilized plastic packaging. Metallic implements were heat-sterilized by boiling in fresh water for 15 min followed by baking at 200°C for 30 min. Non-metallic items were immersed for 16 h in Banicide Plus (3.4% glutaraldehyde; Pascal Dental, Bellevue, WA, USA). Suture packs and scalpel blades were purchased as pre-sterilized packages and not reused. The suture-needle holder, scalpel handle and operating cradle were cleaned between fish by scrubbing and soaking in Hibiclens (4.0% chlorohexadine gluconate; Mölnycke Health Care, Norcross, GA, USA) for at least 3 min. Nitrile gloves were worn when operating on fish and not reused; surgical towels were replaced after each fish.
For each fish, total time out of water (TOW) and total time required to recover from anesthesia was recorded. Anesthesia recovery time (ART) was defined as time required for the fish to recover swimming ability; this was typically spontaneous and did not require prompting. Differences among treatments in TOW and ART were statistically compared via one-factor ANOVA. Statistically-significant factors were further explored via Fisher’s LSD post hoc multiple comparisons. The relationships between ART and time-to-anesthesia, TOW, and estimated body weight were investigated via linear regression, where ART was treated as the dependent variable and individual fish weights were estimated using the following relationship (Clark 1992): weight (kg) = (9.227 × 10−6 × L 3.24) × 0.4536, where L = fork length in centimeters. All tests were conducted using the Statistica 7 software package (StatSoft Pacific Pty. Ltd, North Melbourne, Australia); all errors in this manuscript will be reported as one standard deviation about the mean.
Post-tagging observations
Fish recovery and behavior was observed for 410 days following the completion of all tag implantations (i.e., from October 19, 2006, through December 5, 2007). Observations occurred on October 20, 2006, and at 2, 4, 7, 17, 22, 26, 36, 42 and 59 weeks thereafter. Each observation period consisted of in-water examinations in which the physical condition of each fish was assessed, followed by a behavioral observation session that lasted approximately 3.5 h for all fish, combined.
During physical examinations, entry incisions were inspected for degree of closure, evidence of inflammation or infection, and number of sutures present; stalk exit piercings were inspected for inflammation or infection, general morphology, and diameter relative to that of the tag stalk. General fish health was noted with particular reference to clarity and responsiveness of the eyes, abrasions on the chin and rostrum, condition of the fins, presence of skin irritations or lesions, and ectoparasite load. Fish were not removed from the pool at any time during the 59-week post-operative period in order to avoid inducing handling stress (sensu Waring et al. 1992) and possible injury to incompletely-closed incisions, potentially biasing recovery and growth rates. In particular, the likelihood of re-injury would presumably have been greatest for tags with external stalks due to their potential to snag in the capture net, potential yielding spurious treatment effects unassociated with actual tag responses. Instead, incision, light-stalk puncture, and general condition of each fish was determined during regular in-water examination, via snorkeling. Study specimens tended to be docile and cooperative during examinations. The fish would commonly approach the examiner and initiate physical contact, often allowing the examiner to lift the fish to the water’s surface and hold it stationary for a sufficient period to allow thorough inspection of the tagging incision. At the termination of the experiment, all halibut were removed from the water, anesthetized in MS-222, measured, and externally inspected. Four fish from each tag treatment group were sacrificed and dissected to document physiological responses to the electronic tags. Growth differences (in length) among treatment groups were statistically compared via one-factor ANOVA using Statistica 7.
Behavioral sessions consisted of 10 min of continuous observation of each fish. Time of occurrence was recorded each time the fish under observation began or terminated swimming or executed a “spyhop”, a behavior in which the fish would come to the surface in a vertical orientation and extend its head out of the water at least as far as the eyes. Spyhopping is often observed in captive-reared halibut and has been suggested as an indicator of stress or suboptimal rearing conditions (Kristiansen et al. 2004). Observations were conducted sequentially in a nested design comprised of five observation blocks during which one fish from each of the four treatment groups was observed. In order to better control for observation position than could likely have been achieved through strict randomization within such a small sample, observation order was determined randomly for the first block and then structured orthogonally in subsequent blocks by “leapfrogging” the first treatment to the end of the subsequent observation block. For example: if block 1 = A-B-C-D; then block 2 = B-C-D-A, block 3 = C-D-A-B, block 4 = D-A-B-C, block 5 = A-B-C-D. Differences in proportion of time spent swimming, “agitation level”, defined as number of switches between swimming and resting behavior per observation period, and number of spyhops per observation period were statistically compared via two-factor ANOVA with tag treatment and observation date as independent variables using Statistica 7.
Results
Surgical implantation
Individuals ranged from 65 to 84 cm FL at time of tagging. Average fish lengths, by treatment group, were as follows: control = 77.4 ± 7.0 cm, LTD 1110 = 72.4 ± 6.1 cm, LTD 2310 = 77.4 ± 7.4 cm, Mk9 = 77.0 ± 4.7 cm. No significant differences existed among treatments (one-factor ANOVA: F = 0.661, P = 0.589).
Significant differences in TOW were experienced during tagging by treatment group (one-factor ANOVA, F = 39.700, P < 0.001). Time required to implant stalk-bearing tags was similar regardless of tag model (LTD 2310 = 6:46 ± 0:36; Mk9 = 7:05 ± 1:35; Fisher LSD, P = 0.533) and significantly longer than required to implant LTD 1110 tags (4:42 ± 0:45; Fisher LSD, P < 0.001 for both comparisons). Processing of control fish (2:03 ± 0:26) was significantly shorter than for all other treatments (Fisher LSD, P < 0.001 for all pairwise comparisons).
ART was highly variable, ranging from 03:15 to 11:35 for fish that swam spontaneously upon recovery. One fish failed to spontaneously resume swimming after 18 min and was prodded gently on the caudal peduncle to assess its reactivity; it swam almost immediately, and had likely regained its swimming ability somewhat earlier. Excluding the fish that was prompted, no significant differences in ART were observed among treatments (one-factor ANOVA; F = 0.170, P = 0.915). No significant relationship was detected between ART and estimated fish weights (linear regression, F = 0.228, P = 0.639; Fig. 3) nor between ART and any combination of time-to-anesthesia, TOW and estimated weight (multiple regression, multiple R 2 = 0.00682, F = 0.110, P = 0.744).
Post-surgical recovery
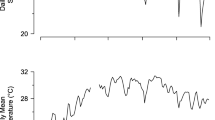
In general, fish health was good throughout the holding period; no systemic problems were observed in the population, aside from the trematode infestation noted earlier. One fish (implanted with an LTD 1110) developed an infection in its right eye shortly after surgery (first noted at week-2), likely due to a netting injury, and appeared to lose vision in that eye. However, its behavior did not change in any discernible manner and its total post-operative growth was close to the mean for the other members of its treatment group (12 cm FL, vs. 13.0 ± 3.9 cm FL for the others). A single mortality occurred 39 weeks after tag implantation. This mortality did not appear to be related to either surgical complications nor to a tag interaction. Rather, immediately prior to the mortality there occurred a rapid increase in water temperature. A strong freshwater intrusion event occurred within Newport Bay that inundated the facility’s seawater intake line and forced reliance upon stored sea water and recirculation. From July 13 to July 20, rearing temperature increased from its long-term mean of 10.8 ± 1.1°C to a peak of 16.3°C, returning to <11°C on July 28 (Fig. 4). The elevated temperature caused all fish to cease feeding for a period of approximately 1 week; the mortality occurred before the water temperature could be lowered to target rearing temperature. A second temperature elevation was observed from August 19–28 (Fig. 4), but this was of lesser magnitude (peak temperature = 14.3°C) and no marked changes in feeding or group activity was observed. Aside from these events, water temperatures were generally maintained at 9–13°C.
Mean daily rearing temperatures throughout the 59-week post-surgical holding period. A large increase in temperature occurring from July 13–28 (A) resulted in disruption of feeding and the only post-surgical mortality during the experiment. A second temperature increase of lesser magnitude, occurring from August 19–28 (B), did not markedly interrupt feeding
All fish displayed growth during the post-surgical holding period. Individual growth ranged from 6 to 25 cm FL (7–38% of initial body length (IBL)). Mean growth by treatment was as follows: control = 13.6 ± 5.77 cm (17 ± 7.4% IBL); LTD 1110 = 12.8 ± 3.42 cm (18 ± 4.7% IBL); LTD 2310 = 13.0 ± 8.49 cm (17 ± 14.0% IBL); Mk9 = 11.2 ± 3.03 cm (15 ± 3.7% IBL). No significant differences among treatments or between tagged and control fish were detected in either mean growth (one-factor ANOVA: F = 0.180, P = 0.908) or final length (one-factor ANOVA: F = 0.633, P = 0.605).
Incision closure began within 2 weeks of surgery. Complete closure was first observed after 5 weeks and had occurred in all individuals within 16; changes characterized by apparent thickening of overlying scar tissue and return of dark skin pigmentation continued throughout the holding period. Upon dissection, it was confirmed that the inner peritoneal wall was fully healed in all individuals, including those that had not shed all sutures and were experiencing epidermal tissue sloughing at the points of suture insertion. Suture shedding was first observed after 7 weeks and reached 70% by the end of the holding period (Fig. 5); shedding rates appeared to be similar for 3-0 and 4-0 gauge polypropylene. By week-36, all individuals had shed at least one suture. At the end of the holding period, two individuals had shed only a single suture; five fish shed all but one suture, and four individuals had shed all sutures. The rate of suture shedding had slowed considerably by week-59, but continued shedding throughout the holding period suggested that additional suture shedding likely would have occurred had the holding period been extended.
Behavior
General observation indicated no obviously aberrant behavior by any individual, either tagged or control, and no patterns of behavior that could be attributed to any particular treatment group. Total activity within the population had a tendency to vary cyclically, over periods of tens of minutes to a few hours. For extended periods most of the fish would rest on bottom, typically congregating in a large group within which individuals would be in physical contact with one another despite the availability of a preponderance of unoccupied bottom area. Without any obvious cue, some individuals would initiate swimming and move in a circular motion around the periphery of the tank; this would eventually lead to departure from bottom by an increasing number of individuals, leading to a period when most fish would be in motion.
Quantitative behavioral observations indicated no significant difference between treatment groups with respect to proportion of time spent swimming (two-factor ANOVA, factors = tag treatment and observation date, F = 1.188, P = 0.316), agitation level (two-factor ANOVA, F = 1.549, P = 0.205), or spyhop frequency (two-factor ANOVA, factors = tag treatment and observation date, F = 1.424, P = 0.238) (Table 1; Fig. 6). No significant trends in agitation level were observed (two-factor ANOVA, factors = tag treatment and observation date, F = 1.370, P = 0.206), but both proportion of time spent swimming (two-factor ANOVA, factors = tag treatment and observation date, F = 3.105, P = 0.002) and spyhop frequency (two-factor ANOVA, factors = tag treatment and observation date, F = 4.518, P < 0.001) varied significantly among observation periods (Table 1; Fig. 6). Pairwise post hoc analyses (Fisher LSD) indicated that, in general, swimming activity was lowest at weeks 2, 7, 42 and 59 (Table 2; Fig. 6) and spyhop frequency elevated at weeks 26 and 36 (Table 1; Fig. 6). No interaction effects (Table 1) were detected between tag treatment and observation period for any behavioral category (two-factor ANOVAs: swimming proportion, tag treatment × week, F = 0.878, P = 0.641; spyhops, tag treatment × week, F = 0.734, P = 0.820; agitation level, tag treatment × week, F = 0.873, P = 0.647).
Mean time spent swimming, number of spyhops executed, and agitation level (defined as number of changes in behavior between swimming and resting) observed during 10 min of observation for individuals within each treatment group (control, LTD 110, LTD 2310, Mk9), by week. Shading of bars indicates week of observation, as labeled on the abscissa; observations occurred at 0, 2, 4, 7, 17, 22, 26, 36, 42 and 59 weeks post-surgery. Error bars represent one standard deviation about the mean
Physiological tag responses
Dissected fish displayed no notable internal anomalies or tissue necrosis. Those dissected after 59 weeks (n = 11) displayed considerable gonad development. These fish were dissected during the normal spawning season for wild halibut and two fish were in spawning condition: one female implanted with an LTD 1110 and one male implanted with an LTD 2310. The female’s ovaries contained a considerable amount of fluid-filled space, indicating that she had recently spawned at least one batch of eggs, and fully-hydrated eggs were also present. Archival tagging data suggest that female halibut release batches of eggs roughly every 4 days in the wild (Loher and Seitz 2008a). The male was not actively running, but its testes were large, swollen, and milky in appearance.
No internal tags were expelled during the course of the experiment, but some degree of tissue response was observed in 75% of the dissected individuals. In each treatment group, three individuals displayed tissue responses and one did not. Responses ranged from deposition of a thin film of apparently proteinaceous material upon the surface of the tags (one LTD 1110 and one MK9) to deposition of calcitic material on tag surfaces (two LTD 1110 and one LTD 2310; Fig. 7), partial or complete encapsulation of tags in the body wall (two LTD 1110; Fig. 7), or some degree of encapsulation generated by the intestinal mesenteries (two LTD 2310 and two Mk 9; Fig. 7). Complete encapsulation in the peritoneal wall was observed for two LTD 1110 tags; one stalk-bearing electronic tag (LTD 2310) was completely isolated from the peritoneal cavity via mesenterial encapsulation. For tags bearing an external stalk, mesenterial encapsulation appeared to have progressed from the stalk’s exit-hole inward. All seven fish implanted with stalk-bearing electronic tags and dissected after 59 weeks had intestinal mesentery attached to the inner peritoneal lining at the margins of the stalk’s exit-hole. In three individuals, this was the only mesenterial response and no portion of the stalk or tag was covered to any degree by mesentery. In one fish, mesentery was wrapped around the stalk to form a sheath of tissue (Fig. 7) that extended nearly as far as the tag body. In two others, the stalks were entirely sheathed and the mesenteries had begun to encapsulate the tag body. In the final specimen, the entire tag was encapsulated such that the tag and stalk were resting in a unique chamber that was presumably exposed to the outside environment via the stalk exit, but fully isolated from the peritoneal cavity. The fish that died at week-39 was the only fish implanted with a stalk-bearing electronic tag in which no mesenterial response was observed; however, there was deposition of calcitic material on the tag body.
Physiological responses to intraperitoeally-implanted tags observed in Pacific halibut after 59 weeks. a Encapsulation in the peritoneal wall. The arrow indicates the tag, which is partially visible and extends to the right; the dashed line indicates the outline of the tag. b Deposition of apparently calcitic material on the tag surface. An LTD 1110 is shown: the upper arrow indicates material flaked from the tag, the lower arrow indicates material still attached. c Encapsulation of an Mk9 by intestinal mesenteries. The light-stalk (arrow 1) exits the body wall at the point indicated by arrow 2; the mesenteries have attached themselves to the peritoneal lining at the exit-point. Anteriorly, the light-stalk has been covered by intestinal mesenteries, as has been the tag body, which is embedded in the intestinal mass; the tag’s orientation is indicated by the dashed lines. d The mesenterial sheath that covered the light stalk depicted in c, with the tag removed
The apparent progression of tissue response from initial attachment of mesentaries to the margins of the exit hole to complete tag encapsulation appeared to be consistent with changes in external morphology noted during in-water exams. Through week-17, little was observed with respect to the stalk exit holes. During this period, the flesh around the stalks tended to be pink and occasionally associated with sloughing, necrotic tissue, but this tissue essentially became flush with the fish’s undisturbed skin. At week-22, the tissue surrounding the stalks of two individuals (one from each treatment group) was observed to be slightly “extruded”, or expanded above the plane of the fish’s skin. By week-26, tissue extrusion was observed in nine individuals, and in one fish the disturbed tissue had begun to obtain dark pigmentation. After 36 weeks, the extruded tissue in most individuals (n = 6) had developed into a lumpy, dark-pigmented toroid encircling the base of the light stalk, with a structural appearance not unlike mild colorectal prolapse. By week-42, all surviving individuals had developed this “pseudo-prolapse”, and at the end of the holding period all such tissue had darkened to achieve coloration equivalent to eyed-side pigmentation. The individual that died during week-39 was the only individual that did not develop pseudo-prolapse; dissection confirmed that intestinal mesenteries had not become attached to the peritoneal wall. Hence, the observations suggest that evolution of pseudo-prolapse was most likely associated with growth of intestinal meseneterial tissue through the exit hole to extend the mesentearial tag-sheath completely through the body wall.
Discussion
A review of internal tagging in flatfishes
Tagging of fishes can be viewed as occurring along a continuum that begins with conventional external tagging, advances to “growth-through” intramuscular implants used to anchor an externally-visible tag (sensu Pletcher 1968) and fully subcutaneous tag insertion, followed by insertion of components of an external tag into the peritoneum (sensu Sureau and Lagardere 1991), and culminates in complete intracoelomic implantation. The present review will focus upon methods in which the tag body is internalized, beginning with subcutaneous and intramuscular (SC-IM) implants, followed by intracoelomic (IC) methods. We will pay particular attention to accounts of physiological responses to implanted tags and to tag expulsion.
For the most part, complete SC-IM tag implantation in flatfish has been used for relatively small tag configurations: coded wire (CW), passive integrated transponder (PIT) and visible implant elastomer (VIE) tags (Table 3). Practical applications have included identification of individuals during physiological research in aquaculture (Imsland et al. 2000; Kristiansen et al. 2004) and field (Meng et al. 2001) studies, batch identification in stock enhancement programs (Fairchild et al. 2005), and conventional mark-recapture analysis (Webster 2010). These tags are typically implanted using a hypodermic needle. In some cases it has been shown that choice of implantation site can affect fish health or growth (Lee et al. 2009), but in many cases the choice appears to be logistical. For example, recovery of PIT tags in the IPHC’s Pacific halibut mark-recapture study was accomplished through systematic scanning of fish heads at processing plants and therefore required a cranial insertion site. VIE tags are often inserted so as to facilitate visual tag detection, typically by using a shallow subcutaneous insertion on the uneyed-side of flatfish where the skin is unpigmented and translucent (sensu Fairchild et al. 2005). Adverse physiological reactions have generally not been observed for small SC-IM tag implants, although very close inspection would be required to identify tissue responses such as increased scar tissue deposition around the implantation site. Holding studies conducted with Pacific halibut have confirmed relocation and expulsion of subcutaneous PIT tags (Kaimmer and Geerneart 2003), but retention periods of over 6 years have also been confirmed (Webster 2010).
While studies employing externally-affixed data storage tags (DSTs) on flatfish are numerous, addressing topics that range in temporal scope from characterization of short-period swimming behavior (sensu Kawabe et al. 2003) to seasonal redistribution (sensu Loher and Blood 2009) and interannual migration patterns (sensu Hunter et al. 2004), SC-IM implantation of DSTs appears absent from the literature. We are aware of only one research program that has investigated the technique. Researchers in the UK (E. Hunter and J. D. Metcalfe, CEFAS, Lowestoft, UK, pers comm) have tested subcutaneous implantation of DSTs weighing approximately 1.6 g in sole (Solea solea) weighing roughly 240 g (i.e., tag-to-fish mass proportion = 0.007). However, high rates of tag expulsion were observed within 3–4 weeks and the experiment was terminated. Thus, the researchers have continued deployments using external tag attachment while simultaneously investigating the feasibility of intracoelomic implantation (treated subsequently).
Intracoelomic tag implantation has been successfully conducted on at least eight pleuronectid species (Table 3) native to the north Pacific, north Atlantic and Mediterranean Sea. The size of implanted tags have ranged from PIT tags (2 mm × 12 mm; Foss et al. 2009) to heart monitors (30 × 69 cm; Rabben and Furevik 1993), resulting in tag-to-fish mass proportions ranging from roughly 2 × 10−5 [~75 cm Atlantic halibut (Hippoglossus hippoglossus) implanted with PIT tags; Foss et al. 2009] to 0.025 [~200 g English sole (Parophrys vetulus) and summer flounder (Paralichthys dentatus) implanted with acoustic tags; Moser et al. 2005; Fabrizio and Pessutti 2007]. Perhaps the earliest foray into intracoelomic tagging in flatfish was conducted on Pacific halibut: Kask (1936) employed a method devised for tagging gray weakfish (Cynoscion regalis; Nesbit 1933) in which thin strips of labeled celluloid were inserted into the visceral cavity. The tags measured approximately 64 mm × 22 mm and were inserted through the abdominal wall on the fish’s eyed-side. Despite no mention of having employed sterile procedures nor of having sutured the incisions, the method yielded tag recoveries for roughly 7 years thereafter (Myhre 1966). A later analysis of the recovery data (Myhre 1966) suggested ~3% tag shedding, but there is no evidence that expulsion could be resolved from non-detection and non-reporting of the internal tags. The halibut were double-tagged using a secondary opercular tag and putative shedding rates estimated simply from the proportion of external tags returned by fishermen in the absence of a celluloid tag. The tagging method was intended as an alternative to conventional external tagging and was never used in applied research. Nonetheless, the experiment confirmed that a large pleuronectid could retain intracoelomically-implanted foreign material at high rates for periods that approach the maximum operational span of modern electronic archival tags.
Approximately six decades later, intracoelomic tag implantation was employed again, this time for physiological monitoring of Atlantic halibut. Interested in optimizing growing conditions for commercial aquaculture, researchers sought to monitor heart rate as a proxy for stress. Initial work with flatfish had employed fully-external heart rate transmitters (West et al. 1978) and eventually progressed to surgical insertion of the electrodes in order to better articulate with the pericardium (Sureau and Lagardere 1991). Rabben and Furevik (1993) implanted the entire transmitter into fish ranging from 73 to 110 cm (5–26 kg), placing the body of the instrument in the coelomic cavity with the electrodes fed between the pelvic bones and attached to the pericardium via gold-plated hooks. No mortalities were induced by the process and one fish was monitored for 4 months before being sacrificed and dissected. The authors make no mention of physiological tag responses, but the incisions had fully healed by the end of the experiment and the fish achieved spawning condition (running milt) while implanted. This was an important finding, demonstrating that the flattened visceral cavity typical of flatfish could accommodate a large tag for at least a short period with little discernible effect on health or behavior.
Accounts of intracoelomic implantation of PIT tags begin to appear in the literature in the late 1990s in aquaculture research involving Atlantic halibut and turbot (Scopthalmus maximus). At least four published accounts of intracoelomic PIT tag implantation exist for these species (Table 3) and usage is likely more widespread. Neither tag interactions nor tag loss have been reported after verified retention periods ranging from 13 to 160 weeks, although the cited studies were not specifically designed to monitor these phenomena. The research has generally focused on tracking individual growth trajectories under varied rearing conditions. More recently, Lee et al. (2009) conducted a study explicitly designed to compare intramuscular versus intracoelomic implantation of PIT tags in Japanese flounder, with specific attention to growth impacts. Tags were injected using a hypodermic needle and fish held for 8 months post-tagging. Neither mortality nor tag shedding resulted and growth of implanted individuals was indistinguishable from untagged controls; dissection was not conducted and tissue responses not assessed.
To date, intracoelomic implantation of electronic tags has primarily been conducted for acoustic tagging experiments investigating migration and dispersal. The tags used in these studies have been smaller than the stalk-bearing electronic tags tested in the present study, and of configurations lacking an external antenna. Implantation of acoustic tags evolved from routine use of external mounts (sensu Lagardère and Sureau 1989), and was preceded in two species by laboratory studies to develop surgical techniques and protocols; field deployments have occurred in at least four species (Table 3). Laboratory studies have demonstrated tag retention of over 1 year in summer flounder, and field studies have confirmed retention periods of more than 3 years in Pacific halibut. To date, only two published accounts exist in which specimens were dissected to assess tissue response, and the results of those experiments were largely divergent. Fabrizio and Pessutti (2007) found no detectable tag interactions for summer flounder 53 weeks after tag implantation, whereas Moser et al. (2005) observed partial encapsulation in English sole after only 4 weeks. Resolving the reasons for such marked differences is difficult. While both studies employed fish and tags of approximately equal size (200 g fish and 5 g tags), the tag configurations varied in potentially important respects: Moser et al. (2005) used cylindrical epoxy resin whereas Fabrizio and Pessutti (2007) tested a flattened configuration coated in beeswax. It is possible that a flattened configuration will elicit less response in compressiform fishes or that wax coating either suppresses the foreign-body response or resists proteinacious deposition more so than bare epoxy. Alternatively, responses may be species-specific. Acoustic tag implantation is presently being investigated in a third species, the spotted halibut (Verasper variegatus) (T. Wada, Fukushima Prefectural Fisheries Experimental Station, Iwaki, Japan, and H. Mitamura, Graduate School of Informatics, Kyoto University, Japan, pers comm). The work employs Vemco (Halifax, NS, Canada) V9 transmitters (24 mm × 9 mm) implanted into hatchery-reared fish approximately 16–20 cm in length. In late November, 2009, fifteen individuals were implanted with tags following methods described in Mitamura et al. (2009). Ten have been released into Matsukawa-ura Inlet (northeastern Honshu) in order to acoustically monitor first wintering movements of young-of-the-year spotted halibut, and another five fish remain in captivity to assess handling and tagging stress. After 4 months, no negative impacts had been observed in the captive fish and no tag expulsion has occurred. Final results of both components of the study are expected in April, 2010.
To our knowledge, the present study represents the first published report of the impacts of intracoelomically-implanted data storage tags on a flatfish, but should soon be complemented by results of joint research by Ifremer and Cefas that is currently examining tag impacts upon sole, Solea solea (M. L. Bégout and E. Hunter, Ifremer, La Rochelle, France, pers comm). The sole research is ongoing and results are not anticipated until late in 2010, but the study is comprised of fish- and tag-sizes similar to those used in a previous study of the effects of externally attached acoustic tags (Bégout Anras et al. 2003). This latter study should ultimately provide additional context with regards the effects of intracoelomic tagging on pleuronectid growth and behaviour.
Implications of the present results
The time required for Pacific halibut to fully close their entry incisions (5–16 weeks) was longer than reported for English sole (<4 weeks; Moser et al. 2005) and summer flounder (~4 weeks; Fabrizio and Pessutti 2007). This may represent species-specific variance or different experimental conditions among studies, such as temperature or feeding regimes. In the present study, halibut that fed voraciously would distend considerably, resulting in slight gaping of their implantation incision. Such gaping likely impeded closure and lower feeding rates may have resulted in accelerated healing rates. Although we reduced feed for 1 week post-tagging to mitigate such effects, we wished to mimic natural post-tagging behavior to the greatest degree possible. It may be unrealistic to expect wild fish to temper their feeding after surgery and similar stress to incisions should probably be expected for field deployments. The only reasonable manner of minimizing the effect of overfeeding in a wild setting might be to ensure tight suturing using closely-spaced stitches. Given the considerable resistance of Pacific halibut to air exposure (Davis and Schreck 2005), there is little reason in this species to be overly concerned with processing time and we suggest that it would be wise to maintain stitch spacing of no more than a few millimeters to produce the tightest closure practicable.
The tissue responses we observed were within the range described for other flatfish species using similar tag-to-fish mass ratios. Moser et al. (2005) reported peritoneal adhesions and partial tag encapsulation in English sole whereas Fabrizio and Pessutti (2007) reported no responses in summer flounder. Although we have only one mid-period observation (at week-39), the observed responses suggest a process in Pacific halibut that requires tens of weeks to visibly develop and a year or more to progress to full encapsulation. Stronger responses to stalk-bearing tags than to fully-internal configuration should probably be expected because a stalk’s exit represents a persistent breach of the peritoneum wall that resists closure. Mesenterial attachment and subsequent encapsulation of the tag may be the most rapid, if not the only, manner in which the animal can seal the hole and eliminate intrusion of foreign material. In the current study, all external stalks represented light-sensing elements of the tag, and so their composition was essentially predetermined. Alternatively, an external stalk may be employed simply as a means of providing an external marker to increase tag recovery rates. In such cases, tissue reactions might be ameliorated, or enhanced, by using alternative materials. Despite the expectation that externally-protruding elements should elicit a more rapid physiological response, we observed similar encapsulation of fully internal tags, for which breach of animals’ integuments was not an issue. This suggests species-specific response to varying tag configurations. We initially hypothesized that encapsulation of these tags was associated with healing the incised visceral peritoneal layer, perhaps by incorporating the tag within newly formed tissue. This may have been the case in one individual. However, inspection of the second fish suggested that the encapsulation site was at least 3 cm ventral of the fully-healed incision scar. Thus, it appears that Pacific halibut have the capability to encapsulate foreign bodies into undisturbed tissue.
While our results suggest very low probability of tag expulsion for Pacific halibut within 1 year, the likelihood of expulsion over longer periods, especially those consistent with the operational life of new-generation electronic archival tags (i.e., 5–10 years), remains ambiguous. We are unable to speculate whether encapsulation represents the endpoint in stabilizing a foreign body within the peritoneum, or a midpoint in the progression toward expulsion. Encapsulation is a typical response to foreign objects introduced into vertebrates (Coleman et al. 1974) and is observed across taxa, from fishes (Moore et al. 1990; Penne et al. 2007) to reptiles (Pearson and Shine 2002), birds (Korschgen et al. 1996; Shulz et al. 1998) and mammals (Guynn et al. 1987; Van Vuren 1989). Studies have clearly demonstrated the ability of Pacific halibut to retain celluloid tags for up to 7 years (Myhre 1966; Foss et al. 2009) and acoustic tags for more than 3 years (P. N. Hooge, US Park Service, Denali, USA, unpublished data) and anecdotal evidence suggests that acoustic tag retention was likely accompanied by encapsulation: numerous halibut were recovered while bearing an active acoustic tag, but only the fish’s external wire tag was returned despite the much higher monetary reward associated with return of the acoustic tags. The simplest explanation is tag encapsulation resulting in non-detection of the internal tag. Still, no study has been designed to explicitly detect expulsion after periods of more than 1 year. Longer-term holding is warranted, especially given that different tag configurations may be encapsulated in different manners; i.e., encapsulation entirely contained within the peritoneal lining versus mesenterial encapsulation with an associated external orifice. Different ejection mechanisms might be invoked depending upon the form of the encapsulation, such as transmission through the body wall, as documented in salmonids (Moore et al. 1990), versus expulsion through the intestinal tract, as observed in ictalurids (Summerfelt and Mosier 1984), cyprinids (Penne et al. 2007) and carangids (Meyer and Honebrink 2005). One form of encapsulation may be more likely to result in expulsion, and the time course of processes may be markedly different. Even in the absence of tag expulsion, encapsulation represents a logistical consideration for studies requiring physical tag recovery. If encapsulation is common, it may be necessary to routinely use an external stalk or streamer to maximize recovery rates. A stalk-bearing tag would presumably allow the same scope for growth as a fully-internal tag, but some of the issues associated with external tagging would remain. In particular, an external stalk could be prone biofouling, requiring that it be constructed of material resistant to settlement of sessile benthic species.
In conclusion, intracoelomically-implanted electronic tags were well-tolerated in Pacific halibut, with no evidence of effects on growth or behavior. The results support a growing body of evidence suggesting that intracoelomic implantation may be superior in many respects to external tag attachment for species with sufficient intracoelomic space to accommodate them. Still, concerns associated with non-detection due to encapsulation and potential long-term expulsion must be addressed. Given the broad range of physiological responses observed in the relatively few pleuronectid species studied to date, researchers should be careful in extrapolating results across species. Given the ubiquity of the foreign-body response across vertebrate taxa, researchers would do well to conduct holding experiments of appropriate length and scope to quantify the potential for tissue encapsulation and establish rates at which it might occur in their particular study species. The need for extended studies is exacerbated by the very long (nearly decadal) operating lives of modern electronic archival tags, which challenge our current definitions of “long-term” experimentation, both with respect to holding experiments and the duration of the ecological processes that can now be studied.
References
Arnold GP, Holford BH (1978) The physical effects of an acoustic tag on the swimming performance of plaice and cod. J Cons CIEM 38:189–200
Aune A, Imsland AK, Pittman K (1997) Growth of juvenile halibut, Hippoglossus hippoglossus (L.), under a constant and switched temperature regime. Aquacult Res 28:931–939
Bégout Anras ML, Covès D, Dutto G, Laffargue P, Lagardère F (2003) Tagging juvenile sea bass and sole with telemetry transmitters: medium-term effects on growth. ICES J Mar Sci 60:1328–1334
Campbell HA, Fraser KPP, Peck LS, Bishop CM, Egginton S (2007) Life in the fast lane: the free-ranging activity, heart rate and metabolism of an Antarctic fish tracked in temperate waters. J Exp Mar Biol Ecol 349:142–151
Claireaux G, Webber DM, Kerr SR, Boutilier RG (1995) Physiology and behaviour of free-swimming Atlantic cod (Gadus morhua) facing fluctuating temperature conditions. J Exp Biol 198:49–60
Clark WG (1992) Estimation of halibut body size from otolith size. IPHC scientific report 75. International Pacific Halibut Commission, Seattle
Coleman DL, King RN, Andrade JD (1974) The foreign body reaction: a chronic inflammatory response. J Biomed Mater Res 8:199–211
Collins MR, Cooke DW, Smith TIJ, Post WC, Russ DC, Walling DC (2002) Evaluation of four methods of transmitter attachment on shortnose sturgeon, Acipenser brevirostrum. J Appl Ichthyol 18:491–494
Cottrill RA, Oekland F, Aarestrup K, Jepsen N, Koed A, Hunter KJ, Butterworth KG, McKinley RS (2006) Evaluation of three telemetry transmitter attachment methods for female silver-phase American eels (Angiulla rostrata Lesueur). J Great Lakes Res 32:502–511
Davis MW, Schreck CB (2005) Responses by Pacific halibut to air exposure: lack of correspondence among plasma constituents and mortality. Trans Am Fish Soc 134:991–998
Dicken ML, Booth AJ, Smale MJ (2006) Preliminary observations of tag shedding, tag reporting, tag wounds, and tag biofouling for raggedtooth sharks (Carcharias taurus) tagged off the east coast of South Africa. ICES J Mar Sci 63:1640–1648
Eristhee N, Popple I, Oxenford H, Hunte W (2001) Methods and lessons learned in the application of ultrasonic telemetry to coral reef fish movement studies. Proc Gulf Caribb Fish Inst 52:145–160
Faahraeus-Van Ree GE, Spurrell DR (2003) Structure of and energy reserves in the liver of wild and cultured yellowtail flounder, Limanda ferruginia. Mar Biol 143:257–265
Fabrizio MC, Pessutti JP (2007) Long-term effects and recovery from surgical implantation of dummy transmitters in two marine fishes. J Exp Mar Biol Ecol 351:243–254
Fairchild EA, Fleck J, Howell WH (2005) Determining an optimal release site for juvenile winter flounder Pseudopleuronectes americanus (Walbaum) in the Greta Bay Estuary, NH, USA. Aquacult Res 36:1374–1383
Forsberg JE (2010) Portside and survey vessel sampling for recovered PIT tags in Pacific halbut. In: Sadorus L (ed) Report of assessment and research activities 2009. International Pacific Halibut Commission, Seattle, pp 487–512
Foss A, Imsland AK, Vikingstad E, Stefansson SO, Norberg B, Pederson S, Sandvik T, Roth B (2009) Compensatory growth in Atlantic halibut: effect of starvation and subsequent feeding on growth, maturation, feed utilization and flesh quality. Aquaculture 290:304–310
Fraser DJ, Lippe C, Bernatchez L (2004) Consequences of unequal population size, asymmetric gene flow and sex-biased dispersal on population structure in brook char (Salvelinus fontinalis). Mol Ecol 13:67–80
Grusha DS, Patterson MR (2005) Quantification of drag and lift imposed by pop-up satellite archival tags and estimation of the metabolic cost to cownose rays (Rhinoptera bonasus). Fish Bull 103:63–70
Guynn DC, Davis JR, Von Recum AF (1987) Pathological potential of intraperitoneal transmitter implants in beavers. J Wildl Manage 51:605–606
Hight BV, Lowe CG (2007) Elevated body temperatures of adult female leopard sharks, Triakis semifasciata, while aggregating in shallow nearshore embayments: evidence for behavioral thermoregulation? J Exp Mar Biol Ecol 352:114–128
Hunter E, Metcalfe JD, Holford BH, Arnold GP (2004) Geolocation of free-ranging fish on the European continental shelf as determined from environmental variables. 2. Reconstruction of plaice ground tracks. Mar Biol 144:787–798
Hutchings JA (2003) Sex-biased dispersal in a salmonid fish: implications for growth rate and survival. J Fish Biol 63(suppl 1):233
Imsland AK, Jonassen TM, Stefansson SO, Kadowaki S, Berntssen MHG (2000) Intraspecific differences in physiological efficiency of juvenile Atlantic halibut Hippoglossus hippoglossus L. J World Aquacult Soc 31:285–296
Imsland AK, Gunnarsson S, Ásgeirsson Á, Kristjánsson ÁrnasonJ, Jónsson AF, Smáradóttir H, Thorarensen H (2010) Long-term rearing of Atlantic halibut at intermediate salinity: effect on growth, feed conversion efficiency, and blood physiology. J World Aquacult Soc 41:115–123
Kaimmer SM, Geerneart TO (2003) 2003 PIT tagging: tagging equipment and prostocol, and shedding studies. In: Sadorus L (ed) Report of assessment and research activities 2002. International Pacific Halibut Commission, Seattle, pp 351–360
Kask JL (1936) The experimental marking of halibut. Science 83(2158):435–436
Kawabe R, Nashimoto K, Hiraishi T, Naito Y, Sato K (2003) A new device for monitoring the activity of free-swimming flatfish, Japanese founder Paralichthys olivaceus. Fish Sci 69:3–10
Kitagawa T, Boustany AM, Farwell CJ, Williams TD, Castleton MR, Block BA (2007) Horizontal and vertical movements of bluefin tuna (Thunnus orientalis) in relation to seasons and oceanographic conditions in the eastern Pacific Ocean. Fish Oceanogr 16:409–421
Knight ME, Van Oppen MJH, Smith HL, Rico C, Hewitt GM, Turner GF (1999) Evidence for male-biased dispersal in Lake Malawi cichlids from microsatellites. Mol Ecol 8:1521–1527
Korschgen CE, Kenow KP, Gendron-Fitzpatrick A, Green WL, Dein FJ (1996) Implanting intra-abdominal radiotransmitters with external whip antennas in ducks. J Wildl Manage 60:132–137
Kristiansen TS, Fernö A, Holm JC, Privitera L, Bakke S, Fosseidengen JE (2004) Swimming behaviour as an indicator of low growth rate and impaired welfare in Atlantic halibut (Hippoglossus hippoglossus L.) reared at three stocking densities. Aquaculture 230:137–151
LaCroix GL, Knox D, McCurdy P (2004) Effects of implanted dummy acoustic transmitters on juvenile Atlantic salmon. Trans Am Fish Soc 133:211–220
Lagardère JP, Sureau D (1989) Changes in the swimming activity of the sole (Solea vulgaris Quensel, 1806) in relation to winter temperatures in a saltmarsh: observations using ultrasonic telemetry. Fish Res 7:233–239
Lee J, Park I-S, Cho SH (2009) Long-term effects of passive integrated transponder tagging on growth of olive flounder, Paralichthys olivaceus. J World Aquacult Soc 40:134–139
Lewis AE, Muntz WRA (1984) The effects of external ultrasonic tagging on the swimming performance of rainbow trout, Salmo gairdneri Richardson. J Fish Biol 25:577–585
Loher T (2008) Homing and summer feeding site fidelity of Pacific halibut (Hippoglossus stenolepis) in the Gulf of Alaska, established using satellite-transmitting archival tags. Fish Res 92:63–69
Loher T, Blood CL (2009) Dispersion of Pacific halibut (Hippoglossus stenolepis) summering off British Columbia and the US Pacific Northwest, evaluated via satellite archival tagging. Can J Fish Aquat Sci 66:1409–1422
Loher T, Clark WG (2010) Deployment, recovery, and reporting of pop-up archival transmitting (PAT) tags to study interannual dispersal and seasonal migration timing in IPHC Regulatory Area 4. In: Sadorus L (ed) Report of assessment and research activities 2009. International Pacific Halibut Commission, Seattle, pp 537–551
Loher T, Rensmeyer R (2009) Archival tagging to study halibut migration and behavior: surgical techniques for internal implantation and deployment of externally-mounted tags in Area 2B. In: Sadorus L (ed) Report of assessment and research activities 2008. International Pacific Halibut Commission, Seattle, pp 439–460
Loher T, Seitz AC (2006) Seasonal migration and environmental conditions of Pacific halibut Hippoglossus stenolepis, elucidated from pop-up archival transmitting (PAT) tags. Mar Ecol Prog Ser 317:259–271
Loher T, Seitz AC (2008a) Characterization of active spawning season and depth for eastern Pacific halibut (Hippoglossus stenolepis), and evidence of probable skipped spawning. J Northwest Atl Fish Sci 41:23–36
Loher T, Seitz AC (2008b) Characterization of seasonal onshore-offshore migration timing, and active spawning depth and period of Gulf of Alaska halibut, with evidence of possible skipped spawning. In: Sadorus L (ed) Report of assessment and research activities 2007. International Pacific Halibut Commission, Seattle, pp 433–450
Malmstroem T, Salte R, Gjoeen HM, Linseth A (1993) A practical evaluation of metomidate and MS-222 as anaesthetics for Atlantic halibut (Hippoglossus hippoglossus L.). Aquaculture 113:331–338
Malte H, Larsen C, Musyl M, Brill R (2007) Differential heating and cooling rates in bigeye tuna (Thunnus obesus Lowe): a model of non-steady state heat exchange. J Exp Biol 210:2618–2626
Marty GD, Summerfelt RC (1986) Pathways and mechanisms for expulsion of surgically implanted dummy transmitters from channel catfish. Trans Am Fish Soc 115:577–589
Meng L, Powell JC, Taplin B (2001) Using winter flounder growth rates to assess habitat quality across an anthropogenic gradient in Narragansett Bay, Rhode Island. Estuaries 24:576–584
Meyer CG, Honebrink RR (2005) Transintestinal expulsion of surgically implanted dummy transmitters by bluefin trevally—implications for long-term movement studies. Trans Am Fish Soc 134:602–606
Mitamura H, Uchida K, Miyamoto Y, Arai N, Kakihara T, Yokota T, Ojuyama J, Kawabata Y, Yasuda T (2009) Preliminary study on homing, site fidelity, and diel movement of black rockfish Sebastes inermis measured by acoustic telemetry. Fish Sci 75:1133–1140
Moore A, Russell IC, Potter ECE (1990) The effects of intraperitoneally implanted dummy acoustic transmitters on the behavior and physiology of juvenile Atlantic salmon. J Fish Biol 37:713–721
Moser ML, Myers MS, Burke BJ, O’Neill SM (2005) Effects of surgically-implanted transmitters on survival and feeding behavior of adult English sole. In: Lembo G, Marmulla G (eds) Aquatic telemetry: advances and applications. FAO/COISPA, Rome, pp 269–274
Myhre RJ (1966) Loss of tags from Pacific halibut as determined by double-tag experiments. IPHC scientific report 41. International Pacific Halibut Commission, Seattle
Nesbit RA (1933) A new method of marking fish by means of internal tags. Trans Am Fish Soc 63:306–307
Nichol DG, Somerton DA (2009) Evidence of tidal streams by northern rock sole (Lepidopsetta polyxystra) for transport in the eastern Bering Sea. Fish Bull 107:221–234
Pearson DJ, Shine R (2002) Expulsion of intraperitoneally-implanted radiotransmitters by Australian pythons. Herp Rev 33:261–263
Penne CR, Ahrens NL, Summerfelt RC, Pierce CL (2007) Effect of relative volume on radio transmitter expulsion in subadult common carp. N Am J Fish Manage 27:986–991
Pletcher FT (1968) A subcutaneous dart tag for fish. J Fish Res Bd Canada 25:2237–2240
Rabben H, Furevik DM (1993) Application of heart rate transmitters in behavior studies on Atlantic halibut (Hippoglossus hippoglossus). Aquacult Eng 12:129–140
Righton D, Kjesbu OS, Metcalfe J (2006) A field and experimental evaluation of the effect of data storage tags on the growth of cod. J Fish Biol 68:385–400
Rijnsdorp AD, Ibelings B (1989) Sexual dimorphism in the energetic of reproduction and growth of North Sea plaice, Pleuronectes platessa L. J Fish Biol 35:401–415
Schaber M, Hinrichsen H, Neuenfeldt S, Ruediger V (2009) Hydroacoustic resolution of small-scale vertical distribution in Baltic cod Gadus morhua—habitat choices and limits during spawning. Mar Ecol Prog Ser 377:239–253
Schaefer KM, Fuller DW (2006) Comparative performance of current-generation geolocating archival tags. Mar Technol Soc J 40:15–28
Seitz AC, Norcross BL, Wilson D, Nielsen JL (2005) Identifying spawning behavior in Pacific halibut, Hippoglossus stenolepis, using electronic tags. Environ Biol Fish 73:445–451
Seitz AC, Loher T, Nielsen JL (2007a) Seasonal movements and environmental conditions experienced by Pacific halibut in the Bering Sea, examined by pop-up satellite tags. IPHC scientific report 84. International Pacific Halibut Commission, Seattle
Seitz AC, Loher T, Nielsen JL (2007b) Seasonal movements and environmental conditions experienced by Pacific halibut along the Aleutian Islands, examined by pop-up satellite tags. IPHC scientific report 85. International Pacific Halibut Commission, Seattle
Shepard ELC, Ahmed MZ, Southall EJ, Witt MJ, Metcalfe JD, Sims DW (2007) Diel and tidal rhythms in diving behavior of pelagic sharks identified by signal processing of archival tagging data. Mar Ecol Prog Ser 328:205–2130
Shulz JH, Bermudez AJ, Tomlinson JL, Firman JD, He Z (1998) Effects of implanted radiotransmitters on captive mourning doves. J Wildl Manage 62:1451–1460
Stockhausen H, Guðbjörnsson S (2009) The earth’s geomagnetic field and geolocation of fish: first results of a new approach. ICES CM 2009/B:19
Sulikowski JA, Fairchild EA, Rennels N, Howell H, Tsang PC (2005) The effects of tagging and transport on stress in juvenile winter founder, Pseudopleuronectes americanus: implications for successful stock enhancement. J World Aquacult Soc 36:148–156
Sulikowski JA, Fairchild EA, Rennels N, Howell H, Tsang PC (2006) The effects of transport density on cortisol levels in juvenile winter founder, Pseudopleuronectes americanus. J World Aquacult Soc 37:107–112
Summerfelt RC, Mosier D (1984) Transintestinal expulsion of surgically implanted dummy transmitters by channel catfish. Trans Am Fish Soc 113:760–766
Sunde LM, Imsland AK, Folkvord A, Stefansson SO (1998) Effects of size grading on growth and survival of juvenile turbot at two temperatures. Aquacult Int 6:19–32
Sureau D, Lagardere JP (1991) Coupling of heart rate and locomotor activity in sole, Solea solea (L.) and bass, Dicentrarchus labrax (L.), in their natural environment by using ultrasonic telemetry. J Fish Biol 38:399–405
Thorstad EB, Økland F, Heggberget TG (2001) Are long-term negative effects from external tags underestimated? Fouling of an externally attached telemetry transmitter. J Fish Biol 59:1092–1094
Van Vuren D (1989) Effects of intraperitoneal transmitter implants on yellow-bellied marmots. J Wildl Manage 53:320–323
Waring CP, Stagg RM, Poxton MG (1992) The effects of handling on flounder (Platichthys flesus L.) and Atlantic salmon (Salmo salar L.). J Fish Biol 41:131–144
Webster RA (2010) Analysis of PIT tag recoveries through 2009. In: Sadorus L (ed) Report of assessment and research activities 2009. International Pacific Halibut Commission, Seattle, pp 177–184
West TJS, Mitson RB, Walker MG (1978) Fish heart rate telemetry in the open sea using sector scanning sonar. Biotelem Patient Monit 5:149–153
Acknowledgments
The authors wish to thank Captain M. Pettis and the crew of the F/V Heidi Sue for their skill in capturing and delivering the fish used in this experiment, J. Burke for assistance in rearing, and T. Miller-Morgan, DVM, for ensuring the continued health of the fish throughout the study period.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Loher, T., Rensmeyer, R. Physiological responses of Pacific halibut, Hippoglossus stenolepis, to intracoelomic implantation of electronic archival tags, with a review of tag implantation techniques employed in flatfishes. Rev Fish Biol Fisheries 21, 97–115 (2011). https://doi.org/10.1007/s11160-010-9192-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11160-010-9192-4