Abstract
Effects of subcutaneous visible implanted alphanumeric (VIA) microtags, pelvic fin excision, and individual photo-identification (Pid) based on natural spot patterns were experimentally evaluated in the small stream benthic Patagonian catfish Hatcheria macraei. VIA tag retention was 90 % during the first 45 days, decreasing to 80 % at day 200, and 66 % at the end of the experiment, at day 254. Fin regeneration was not evident during the experiment. Survival, growth rate or condition factor (Fulton’s K) did not differ between tagged or pelvic fin clipped catfish and a control group. Spot patterns varied greatly among individuals and remained constant throughout the experimental period, thereby making identification of individual Pid possible in H. macraei. Tagging was not effective in identifying individual fish due to mark loss or difficulty in reading VIA tag codes. The combination of Pid with VIA tagging or fin clipping techniques in longterm experiments is advisable in order to facilitate individual recognition. The Pid analysis described in this study is a low-cost method that could potentially be applied to any fish with a variable spot pattern.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Identification of individual fish is often required in studies of life-history, age validation, stock status, behavior, migration, distribution, success of stocking programs, etc. (Phelps and Rodriguez 2011; Marshall and Pierce 2012). There are basically 2 animal identification techniques: invasive and non-invasive methods. The former implies the use of marks or tags, and is widely applied in ecology and management studies (Lucas and Baras 2000). These experiments must meet some basic requirements in order to obtain reliable population parameters (Williams et al. 2002; Amstrup et al. 2005); for example, marks must not alter fish behaviour, growth, or survival (Jepsen et al. 2008), and tag or mark loss must be minimal or known (Cowen et al. 2009). On the other hand, non-invasive methods such as photo-identification (Pid) have no deleterious effects on individuals, but can only be performed in species with natural features or marking (Martin-Smith 2011; Kitchen-Wheeler et al. 2012).
Skin pigmentation patterns have frequently been used to track individuals of diverse fish species, e.g. barracudas (Wilson et al. 2006), seadragons (Martin-Smith 2011), salmon (Merz et al. 2012) and sharks (Barker and Williams 2010). The arrangement of melanophores into macroscopic configurations, such as stripes or spots, may remain only during early life periods (e.g. Donaghy et al. 2005) or for many years, probably until death, in long-living species (Bansemer and Bennett 2008).
The small benthic catfish Hatcheria macraei (Girard, 1855) is a rheophilic and negatively phototactic (Menni 2004) species of the family Trichomycteridae that lives in cold, well-oxygenated waters (Ringuelet et al. 1967). The diurnal microhabitat use of H. macraei is associated with large substrate sizes with conspicuous interstitial space (Barriga et al. 2013). Arratia and Menu-Marque (1981) described a size-related habitat preference, mostly associated with the type of substrate and water depth. In addition, Barriga and Battini (2009) determined the indirect ontogeny (sensu Balon 1990) of this species and related its morphological constraints to habitat and feeding preferences. Furthermore, H. macraei presents an extremely wide variation in its spotted pattern (Arratia and Menu-Marque 1981); therefore, this species is an excellent candidate for the evaluation of Pid techniques based on spot pattern configuration.
Hatcheria macraei is widely distributed in low-order rivers of Patagonia (Unmack et al. 2009, 2012), in contrast with other native fishes [e.g. creole perch, Percichthys trucha (Valenciennes, 1833), Patagonian silverside, Odontesthes hatcheri (Eigenmann, 1909), or the small puyen, Galaxias maculatus (Jenyns, 1842)], which inhabit mostly lentic (lakes and reservoirs) or high-order lotic environments. The main goal of this study was to develop an individual identification technique to be applied in H. macraei population studies. The specific aims were to i) estimate tag retention rates and the effect of implanted tags on growth and survival, ii) evaluate the effect of pelvic fin excision on growth and survival, as well as time of fin regeneration in this species, and iii) evaluate temporal variation in melanophore spot patterns on the flanks of H. macraei as a potential Pid tool.
Materials and methods
Fish collection
Hatcheria macraei individuals were captured in the Pichileufu River, Río Negro province, Argentina (41°05’24” S, 70°49’42” W, 926 m a.s.l.) using a 24 V DC backpack electrofishing unit, model 12-B (Smith-Root Inc., Vancouver, WA, USA) and hand nets. Fishing was performed mainly in riffles in an upstream direction on March 18th 2011. Fish captured (n = 131), ranged from 45 mm to 120 mm total length (TL) including juveniles and adults, were transported to the Centro de Salmonicultura Bariloche of the Universidad Nacional del Comahue in San Carlos de Bariloche city, Río Negro province, Argentina.
Experimental design of trials
Fish were placed in 4 circular tanks of 100 l with a continuous water supply. As H. macraei is a negatively phototactic fish and remains hidden during daytime, the circular tanks were prepared with cobble substrate as shelter. After individuals allowed to acclimate for one week to the experimental conditions, all catfish were anaesthetized using benzocaine (0.02 g l−1) and randomly assigned to one of four groups. Individuals in the first group (n = 37) were marked with small (1.2 mm × 2.7 mm) alphanumeric code tags (Northwest Marine Technology Inc., Shaw Island, WA, USA). These visible implant alphanumeric (VIA) tags were placed under the skin with a handheld injector. Owing to the small size of this species, up to 120 mm TL in the Pichileufu population, the chosen implantation area of tags was in the caudal peduncle flanks. Individuals in the second group consisted in animals previously marked with VIA tags (n = 13) on different dates, released in the Pichileufu River, and recaptured on 18 March 2011 (Table 1). This group was used to test whether marked fish presented the same probability of survival as recently marked fish (i.e. group one). Fish belonging to the third group (n = 40) were marked by clipping on the left pelvic fin. The final group (n = 41), or control group, was made up of unmarked fish. After the group designation of individuals, each fish was photographed laterally, on its left side, with a digital camera. The focal distance was kept at a constant 20 cm during the experiment using a stative. A ruler was placed near the fish as a reference for the digital measurement of fish size (nearest 1 mm) in each picture. Following this, catfish were weighed to the nearest 0.01 g using an electronic scale (Scout Pro 400, Ohaus Corp., Florham Park, NY, USA) and were finally returned to their respective circular tank to recuperate. This procedure of anesthetization, photographing and weighing was repeated every two weeks for the first 4 months and monthly for the following four months. A total of 14 sessions were performed during the 254-day experiment.
Fish were live prey (Tubifex sp.) fed ad libitum every 2 or 3 days throughout the experiment. Even though all circular tanks were maintained in a similar way, fish were randomly distributed in the four circular tanks to avoid bias owing to eventual differences between tanks. Water temperature was registered each 30 min during the experiment using a data logger HOBO® Pendant (Onset, Cape cod, MA, USA) with an accuracy of 0.54 °C.
Tag retention and fin regeneration
VIA tag retention and pelvic fin regeneration were visually evaluated after fish sedation, from the second to the final session. Only fish without a tag were carefully examined. First, pelvic fins were inspected to identify individuals with clipped fins. Following this, individuals with intact fins were checked, looking for possible scars caused by losing the tags. Individual identification of all fish, including those with legible tag codes, was performed by Pid based on spot patterns (see Photo-identification analysis).
Growth, condition and survival
Growth was evaluated by measuring changes in both TL and total wet body mass (M) throughout the duration of the experiment. Somatic condition was also assessed using Fulton’s condition factor, K = M TL−3 105, where M is the total wet body mass (in g) and TL is the total length (in mm). Differences between groups throughout the experiment regarding TL, M and K were tested by using a repeated measures analysis of variance (ANOVA) (McCullagh and Nelder 1989). Each repeated measure corresponded to a session of data recording (i.e. fourteen dates).
Life tables were constructed from survival data for each experimental group. Survival analysis was performed using the Kaplan-Meier method (Kaplan and Meier 1958). A Wilcoxon chi-square (χ 2) test was used to analyze cumulative survivorship in experimental groups. To test the influence of TL or M on the survival model, a Cox regression was performed using these variables as covariates.
Photo-identification analysis
Spot varies widely in shape, size, density and position on the body of H. macraei (Fig. 1). Spot range from large black spots of different sizes and shapes to tiny dots uniformly distributed on the body, except for the ventral zone. The first and second groups (i.e. only marked individuals) were used to test whether the spot pattern could be used as an identification fingerprint. The image database generated over the 14 dates allowed the evaluation of temporal variation of the pigmentation pattern. The software I3S Manta v. 2.1 (Speed et al. 2007), freely available at http://www.reijns.com, was used to compare spot patterns from 4-megapixel pictures of each individual at t0 (time at the beginning of the experiment) with subsequent photographic sessions. The software, created to identify individual manta rays [Manta birostris (Walbaum, 1792) and Manta alfredi (Krefft, 1868)], is based on the differences in spot patterns between individuals. This software takes into account the number of spots, their shapes, and their position relative to three reference points. Spots and reference points are marked on each photograph manually. The structures taken as reference points in H. macraei were the origin of the dorsal fin, the origin of pelvic fins and the beginning of the caudal fin. Only spots delimited within these points were used as the fingerprint pattern (Fig. 1e). The program compares the unidentified fish image against the complete database of known individuals and generates a ranking of probability using an information criterion algorithm (Speed et al. 2007).
Results
Tag retention and fin regeneration
Inflammation was sometimes observed in the area where tags were inserted. In the cases where the tag had been lost, the insertion site had healed, leaving a scar indicating where the tag had been located. As it was difficult to implant the tags in zones without pigmentation, independently of the particular individual spot pattern, the reading of VIA tag codes was almost impossible.
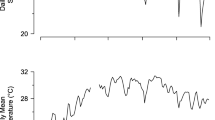
VIA tag retention was 90 % up to day 45, but then decreased to 80 % at day 200, and was 66 % at day 254 (Fig. 2a). There was no relationship between fish size (ranges every 5 mm TL) and percentage of tag retention (Spearman, ρ = −0.149, n = 10, P = 0.66).
Fin regeneration was not evident even at the end of the experiment; consequently, there was no misidentification between fin-clipped individuals and the control group.
Growth, condition and survival
The monthly growth rate was very slow in H. macraei: about 0.1 mm month−1 in length and 0.05 g month−1 in weight for each experimental group (Table 2). The K values increased up to day 100, then decreased until day 163, and finally stabilized towards the end of the experiment (Fig. 2b). There were no differences between groups in the relative growth rate in TL (ANOVA, F 3, 113 = 2.08, P = 0.11), M (ANOVA, F 3, 113 = 2.09, P = 0.11), or in K (ANOVA, F 3, 113 = 0.72, P = 0.54).
Overall survival decreased to 90 % at day 86, thereafter remaining constant till the end of the experiment. No differences in survival rate between groups (Wilcoxon, χ 2 = 0.92, d.f. = 3, P = 0.82) were found (Fig. 2c). There was no relationship between TL or M of individuals and their survival (Cox regression, P = 0.082 and P = 0.098, respectively).
Photo identification analysis
Spot patterns varied greatly between individuals and differences remained constant throughout the experimental period in each individual (Fig. 3). A total of 43 out of 45 individuals (96 %) were correctly classified within the first position of the ranking generated by the software I3S Manta. Only two individuals were misclassified owing to their peculiar pigmentation pattern. These catfish had very small, uniformly distributed dots on their flanks (Fig. 1a), which made delimitation of these dots extremely difficult. Despite this, all catfish were accurately indentified within the fifth positions of the ranking generated by the programme. When only the largest spots within the delimited area were taken into account all individuals were correctly identified using I3S Manta.
Discussion
This is the first study to evaluate the use of fish tags and a Pid procedure to perform mark-recapture studies in the Patagonian catfish, H. macraei. The importance of population studies on this species lies not only in the knowledge of its life history and ecology, but also in the understanding of the invasive salmonid effect on native fish populations. The detrimental effect of salmonids on native species in rivers of low order in Patagonia (e.g. on galaxiid species; Habit et al. 2010) could have been better tolerated by H. macraei, judging by their current distribution (Unmack et al. 2009, 2012) and density (Barriga et al. 2013).
In some individuals of H. macraei inflammation was observed in the area where tags were inserted, or the insertion site could be recognized by the lesion or scar it left. VIA tag retention depends on several factors, such as fish species, size, and tag location. In general, tag retention is lower in smaller individuals than in larger ones (Table 3). However we did not find any association between retention time and H. macraei size. Thus, this method is not biased by size in this species, at least in the size range analyzed.
The election of VIA tag location is mostly associated with rigid motionless structures covered by transparent skin, such as the sector between eyes and the opercular zone (Buckley et al. 1994; Crook and White1995; Shepard et al. 1996; Hughes et al. 2000; Rikardsen et al. 2002; Summers et al. 2006), jaws (Olsen et al. 2004; Meerbeek et al. 2013) or nape (Griffiths 2002). These zones in H. macraei were not used for tag insertion owing to their small size and the unsuccessful results found in pilot experiments (J. P. Barriga, pers. comm.). In contrast, the caudal peduncle presented a large surface for the insertion of the VIA tag. However, the main inconvenience of this zone is that movement during swimming activity increases the chance of tag loss, and this could be the reason for low long-term retention in H. macraei compared to other species (Table 3).
Implanted tags could be used in short-term studies (i.e. less than 45 days). Although in this study individuals of H. macraei were marked, released and recaptured in their own habitat during periods longer than 45 days, this technique is not recommended for long-term population studies. The other disadvantage of these tags was associated with the difficulty of reading the tag codes because of the high degree of pigmentation found in this species. On the other hand, pelvic fin excision was detectable till the end of the experiment, showing no evidence of fin regeneration, allowing easy discrimination between marked and non-marked individuals. The major drawback of this method is obviously the impossibility of individual identification.
Given that implanted tags or pelvic fin excision did not affect survival, growth or body condition, would meet the requirements of mark-recapture studies, i.e. marks must not alter growth or survival (Jepsen et al. 2008). However, fish behaviour should be studied in order to evaluate possible effects in terms of vulnerability to predation and decrease in fitness in terms of resource competition.
Although H. macraei growth rate was extremely low (i.e. less than 1 mm in length during the whole experiment), its K increased up to day 100 of the experiment (in July) from ~0.46 to ~0.56. This change in body condition could be the result of the post-spawning recovery period, as the spawning period of this species ranges from December to February during the austral summer season (Barriga and Battini 2009). In addition, low water temperatures during most of the experiment probably contributed to the low growth rate measured in H. macraei.
Injectable fluorescent marks, such as visible implant elastomer (VIE) or injectable photonic dye (IPD), are other marking techniques often used for individual identification of small stream fishes. These methods have been applied to estimate population size (Trajano 2001), fish dispersion (Belica and Rahel 2008; Ficke and Myrick 2009; Mitsuo et al. 2013), growth rate (Trajano and Bichuette 2007), survivor rates (Reznick and Bryant 2007) and natural selection (Weese et al. 2010). High retention rates and low mortalities have been reported applying VIE or IPD (Roberts and Angermeier 2004). However, VIA tags are less invasive than VIE or IPD because just one injection is enough to mark a fish. In contrast, when VIE or IPD are used a combination of marks is necessary to individualize fish.
The enormous population variation in spot patterning can be a useful tool for individual Pid. The small percentage of individuals (less than 5 %) were misclassified owing to their homogeneous dot patterning, which is present only in a small fraction of wild populations. For example, in Pichileufu River only 3.4 % of 1334 individuals captured and released for a mark-recapture study had this pigmentation pattern (J. P. Barriga, unp. data). However, this small percentage can also be identified when only the largest dots in the delimited area are considered. Despite this misclassification when applying the software, these individuals may also be successfully identified by using particular characteristics such as scars or identifying specific dots on the whole fish.
Photo-identification is low-cost method, but it is time-consuming; therefore, this procedure is best suited for use in field or laboratory trials when the number of experimental individuals is low. This technique could potentially be applied to other species of the family Trichomycteridae (e.g. Trichomycterus areolatus Valenciennes, 1846) and to non-related species whose spot pattern is highly variable, like some Galaxiidae or Rivulidae in the Southern hemisphere and Salmonidae or Esocidae in the North.
In conclusion, selection of the identification method or combination of methods to be used must be based on clearly identified goals, the type of information needed, the degree of loss that is acceptable, and how long the study will last. None of the methods used here was a significant source of mortality or hindered fish growth. However, VIA tags had a low retention time and information was not always legible when fish were recaptured. Fin clipping was easily recognized with no evident regeneration during the study period. Pid, using spot pattern analyses as a tool for individual identification, is strongly recommended in H. macraei. However, as this method is time-consuming, and as a minor fraction of the population could present a homogeneous pattern of small dots, the simultaneous use of complementary methods, such as pelvic fin excision or VIA tagging is also advisable in order to facilitate identification.
References
Amstrup SC, McDonald TL, Manly BFJ (2005) Handbook of Capture-Recapture Analysis. Princeton University Press, New Jersey
Arratia G, Menu-Marque S (1981) Revision of the freshwater catfishes of the genus Hatcheria (Siluriformes, Trichomycteridae) with commentaries on ecology and biogeography. Zool Anz 207:88–111
Balon EK (1990) Epigenesis of an epigeneticist: the development of some alternative concepts on the early ontogeny and evolution of fishes. Guelph Ichthyol Rev 1:1–48
Bansemer CS, Bennett MB (2008) Multi-year validation of photographic identification of grey nurse sharks, Carcharias taurus, and applications for non-invasive conservation research. Mar Freshw Res 59:322–331
Barker SM, Williams JE (2010) Collaborative photo-identification and monitoring of grey nurse sharks (Carcharias taurus) at key aggregation sites along the eastern coast of Australia. Mar Freshw Res 61:971–979
Barriga JP, Battini MA (2009) Ecological significances of ontogenetic shifts in the stream-dwelling catfish, Hatcheria macraei (Siluriformes, Trichomycteridae), in a Patagonian river. Ecol Freshw Fish 18:395–405
Barriga JP, Espinós NA, Chiarello-Sosa JM, Battini MA (2013) The importance of substrate size and interstitial space in the microhabitat selection by the stream-dwelling catfish Hatcheria macraei (Actinopterygii, Trichomycteridae). Hydrobiologia 705:191–206
Belica LAT, Rahel FJ (2008) Movements of creek chubs, Semotilus atromaculatus, among habitat patches in a plains stream. Ecol Freshw Fish 17:258–272
Buckley RM, West JE, Doty DC (1994) Internal micro-tag systems for marking juvenile reef fishes. B Mar Sci 55:848–857
Buckmeier DL, Irwin ER (2000) An evaluation of soft visual implant tag retention compared with anchor tag retention in channel catfish. N Am J Fish Manag 20:296–298
Cowen L, Walsh SJ, Schwarz CJ, Cadigan N, Morgan J (2009) Estimating exploitation rates of migrating yellowtail flounder (Limanda ferruginea) using multistate mark–recapture methods incorporating tag loss and variable reporting rates. Can J Fish Aquat Sci 66:1245–1255
Crook DA, White RWG (1995) Evaluation of subcutaneously implanted visual implant tags and coded wire tags for marking and benign recovery in small scaleless fish, Galaxias truttaceus (Pisces: Galaxiidae). Mar Freshw Res 46:943–946
Donaghy MJ, Youngson AF, Bacon PJ (2005) Melanophore constellations allow robust individual identification of wild 0+ year Atlantic salmon. J Fish Biol 67:213–222
Ficke AD, Myrick CA (2009) A method for monitoring movements of small fishes in urban streams. N Am J Fish Manag 29:1444–1453
Griffiths SP (2002) Retention of visible implant tags in small rockpool fishes. Mar Ecol-Prog Ser 236:307–309
Habit E, Piedra P, Ruzzante DE, Walde SJ, Belk MC, Cussac VE, Gonzalez J, Colin N (2010) Changes in the distribution of native fishes in response to introduced species and other anthropogenic effects. Glob Ecol Biogeogr 19:697–710
Hughes TC, Josephson DC, Krueger CC, Sullivan PJ (2000) Comparison of large and small visible implant tags: retention and readability in hatchery Brook Trout. N Am J Aquac 62:273–278
Jepsen N, Mikkelsen JS, Koed A (2008) Effects of tag and suture type on survival and growth of brown trout with surgically implanted telemetry tags in the wild. J Fish Biol 72:594–602
Kaplan EL, Meier P (1958) Nonparametric estimation from incomplete observations. J Am Stat Assoc 53:457–481
Kitchen-Wheeler AM, Ari C, Edwards AJ (2012) Population estimates of Alfred mantas (Manta alfredi) in central Maldives atolls: North Male, Ari and Baa. Environ Biol Fish 93:557–575
Lucas MC, Baras E (2000) Methods for studying spatial behaviour of freshwater fishes in the natural environment. Fish Fish 1:283–316
Marshall AD, Pierce SJ (2012) The use and abuse of photographic identification in sharks and rays. J Fish Biol 80:1361–1379
Martin-Smith KM (2011) Photo-identification of individual weedy seadragons Phyllopteryx taeniolatus and its application in estimating population dynamics. J Fish Biol 78:1757–1768
McCullagh P, Nelder JE (1989) Generalized Linear Models. Chapman and Hall, London
Meerbeek JR, Larscheid JG, Hawkins MJ, Scholten GD (2013) Retention of large-format, soft visible implant alphanumeric tags in Walleye. N Am J Fish Manag 33:26–31
Menni RC (2004) Peces y ambientes en la Argentina continental. Monografías del Museo Argentino de Ciencias. Naturales, Buenos Aires
Merz JE, Skvorc P, Sogard SM, Watry C, Blankenship SM, Van Nieuwenhuyse EE (2012) Onset of melanophore patterns in the head region of Chinook Salmon: a natural marker for the reidentification of individual fish. N Am J Fish Manag 32:806–816
Mitsuo Y, Ohira M, Tsunoda H, Yuma M (2013) Movement patterns of small benthic fish in lowland headwater streams. Freshw Biol 58:2345–2354
Olsen EM, Gjøsæter J, Stenseth NC (2004) Evaluation of the use of visible implant tags in age-0 Atlantic Cod. N Am J Fish Manag 24:282–286
Phelps RP, Rodriguez D (2011) Effects of tag type on red snapper Lutjanus campechanus tag retention, growth and survival under hatchery conditions. J Appl Ichthyol 27:1169–1172
Reznick D, Bryant M (2007) Comparative long-term mark-recapture studies of guppies (Poecilia reticulata): differences among high and low predation localities in growth and survival. Ann Zool Fenn 44:152–160
Rikardsen AH, Woodgate M, Thompson DA (2002) A comparison of Floy and soft VIalpha tags on hatchery Arctic charr, with emphasis on tag retention, growth and survival. Environ Biol Fish 64:269–273
Ringuelet RA, Aramburu RH, Alonso A (1967) Los peces argentinos de agua dulce. Provincia de Buenos Aires, Comisión de Investigación Científicas, Buenos Aires
Roberts JH, Angermeier PL (2004) A comparison of injectable fluorescent marks in two genera of darters: effects on survival and retention rates. N Am J Fish Manag 24:1017–1024
Shepard BB, Robison-Cox J, Ireland SC, White RG (1996) Factors influencing retention of visible implant tags by Westslope Cutthroat Trout inhabiting headwater streams of Montana. N Am J Fish Manag 16:913–920
Speed CW, Meekan M, Bradshaw CJA (2007) Spot the match – wildlife photo-identification using information theory. Front Zool 4:2
Summers DW, Roberts DE, Giles N, Stubbing DN (2006) Retention of visible implant and visible implant elastomer tags in brown trout in an English chalk stream. J Fish Biol 68:622–627
Trajano E (2001) Habitat and population data of troglobitic armored cave catfish, Ancistrus cryptophthalmus Reis, 1987, from central Brazil (Siluriformes: Loricariidae). Environ Biol Fish 62:195–200
Trajano E, Bichuette ME (2007) Population ecology of cave armoured catfish, Ancistrus cryptophthalmus Reis 1987, from central Brazil (Siluriformes: Loricariidae). Ecol Freshw Fish 16:105–115
Unmack PJ, Habit EM, Johnson JB (2009) New records of Hatcheria macraei (Siluriformes, Trichomycteridae) from chilean province. Gayana 73:102–110
Unmack PJ, Barriga JP, Battini MA, Habit EM, Johnson JB (2012) Phylogeography of the catfish Hatcheria macraei reveals a negligible role of drainage divides in structuring populations. Mol Ecol 21:942–959
Weese DJ, Gordon SP, Hendry AP, Kinnison MT (2010) Spatiotemporal variation in linear natural selection on body color in wild guppies (Poecilia reticulata). Evolution 64:1802–1815
Williams BK, Nichols JD, Conroy MJ (2002) Analysis and Management of Animal Populations. Academic, San Diego
Wilson SK, Wilson DT, Lamont C, Evans M (2006) Identifying individual great barracuda Sphyraena barracuda using natural body marks. J Fish Biol 69:928–932
Woods CMC (2005) Evaluation of VI-alpha and PIT-tagging of the seahorse Hippocampus abdominalis. Aquac Int 13:175–186
Acknowledgments
We thank two anonymous reviewers whose helpful comments increased the clarity of the manuscript. We thank Dirección de Pesca Continental of the Río Negro Province for permission to collect native fish. This study was partially funded by Agencia Nacional de Promoción Científica y Tecnológica, Argentina (ANPCyT, PICT 2010, No. 0262) and Consejo Nacional de Investigaciones Científicas y Técnicas, Argentina (CONICET, PIP No. 11220080100282). All handling, care and experimental procedures used in this research complied with the animal welfare laws stated by the Government of Argentina (Law n° 14346).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Barriga, J.P., Chiarello-Sosa, J.M., Juncos, R. et al. Photo-identification and the effects of tagging on the Patagonian catfish Hatcheria macraei . Environ Biol Fish 98, 1163–1171 (2015). https://doi.org/10.1007/s10641-014-0349-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10641-014-0349-1






 ), recaptured (
), recaptured ( ), fin excised (■), and control (◯)
), fin excised (■), and control (◯)