Abstract
Wastewater treatment plants are known to be important point sources for nitrous oxide (N2O) in the anthropogenic N cycle. Biofilm based treatment systems have gained increasing popularity in the treatment of wastewater, but the mechanisms and controls of N2O formation are not fully understood. Here, we review functional groups of microorganism involved in nitrogen (N) transformations during wastewater treatment, with emphasis on potential mechanism of N2O production in biofilms. Biofilms used in wastewater treatment typically harbour aerobic and anaerobic zones, mediating close interactions between different groups of N transforming organisms. Current models of mass transfer and biomass interactions in biofilms are discussed to illustrate the complex regulation of N2O production. Ammonia oxidizing bacteria (AOB) are the prime source for N2O in aerobic zones, while heterotrophic denitrifiers dominate N2O production in anoxic zones. Nitrosative stress ensuing from accumulation of NO2 − during partial nitrification or denitrification seems to be one of the most critical factors for enhanced N2O formation. In AOB, N2O production is coupled to nitrifier denitrification triggered by nitrosative stress, low O2 tension or low pH. Chemical N2O production from AOB intermediates (NH2OH, HNO, NO) released during high NH3 turnover seems to be limited to surface-near AOB clusters, since diffusive mass transport resistance for O2 slows down NH3 oxidation rates in deeper biofilm layers. The proportion of N2O among gaseous intermediates (NO, N2O, N2) in heterotrophic denitrification increases when NO or nitrous acid (HNO2) accumulates because of increasing NO2 −, or when transient oxygen intrusion impairs complete denitrification. Limited electron donor availability due to mass transport limitation of organic substrates into anoxic biofilm zones is another important factor supporting high N2O/N2 ratios in heterotrophic denitrifiers. Biofilms accommodating Anammox bacteria release less N2O, because Anammox bacteria have no known N2O producing metabolism and reduce NO2 − to N2, thereby lowering nitrosative stress to AOB and heterotrophs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
In the last decades, wastewater treatment plants have received increasing attention for their role as point sources for N2O emissions in the anthropogenic nitrogen cycle (Kampschreur et al. 2009b; Schreiber et al. 2012). N2O is a strong greenhouse gas contributing to global warming and stratospheric ozone depletion (Ravishankara et al. 2009). Even though wastewater treatment only accounts for an estimated 1.3 % of the total anthropogenic N2O emission, its contribution to the total greenhouse gas production associated with the human water supply and sanitation chain may be as high as 26 % (Kampschreur et al. 2009b). This includes production of drinking water as well as treatment of wastewater.
Numerous studies and review articles about N2O emissions from wastewater treatment systems have been published (e.g. Kampschreur et al. 2009b; Law et al. 2012b; Tallec et al. 2006; Wunderlin et al. 2012). Most of these studies focus on suspended biomass (activated sludge), while biofilm systems have received little attention. At the same time, biofilm systems enjoy increasing popularity owing to their robustness and efficiency, and their ability to combine several microbial processes within one system (Khan et al. 2013; Morgenroth 2008b). However, the understanding of N2O producing processes in biofilm systems is less developed than for suspended biomass cultures, because diffusion resistance and biomass competition across the biofilm need to be considered when studying N-transformation processes in biofilms (Morgenroth 2008a; Wanner and Gujer 1985). More knowledge is needed about the critical factors and biomass interactions controlling N2O production in biofilms (Schreiber et al. 2012).
This review links known biomass interactions in wastewater treating biofilm systems to mechanisms and rates of microbial N transformation processes and evaluates their potential role in N2O production. Chapter 2 introduces the biochemistry of N2O production and consumption found among the main functional groups of organisms relevant to wastewater treatment systems. Chapter 3 combines the known biochemistry of N2O production and consumption with current models of mass transfer and biomass interactions in biofilms and links them to environmental factors that are typically measured and controlled in wastewater treatment systems. Finally, Chapter 4 summarises N2O emissions reported for different types of wastewater treating biofilm systems.
2 Key organisms and enzymatic processes involved in biological Nitrogen conversion
2.1 Ammonia oxidizing bacteria (AOB)
Ammonia oxidizing bacteria (AOB) are obligate chemolitoautotrophic, sometimes mixotrophic bacteria of the phylum Proteobacteria, which generate energy for growth and maintenance from the oxidation of ammonia (NH3) to nitrite (NO2 −). This group comprises several dozen species, which are adapted to different environments (e.g. Bothe et al. 2000). AOB are the key species mediating nitrification, i.e. the microbial conversion of NH4 + to NO2 − in wastewater treatment systems and have therefore been at the core of interest as potential sources for N2O emissions (Kampschreur et al. 2009b). Here, we focus on Nitrosomonas sp., which is the most studied and most important AOB genus in wastewater treatment systems (Law et al. 2012a; Schreiber et al. 2009) and review metabolic pathways involved in N2O production.
2.1.1 AOB metabolism
AOB oxidize ammonia (NH3) to hydroxylamine (NH2OH) and further to nitrite (NO2 −). This two-step reaction is performed by the membrane bound enzyme ammonia monooxygenase (AMO) and the periplasmic enzyme hydroxylamine dehydrogenase (formerly hydroxylamine oxidoreductase) (HAO), respectively (Fig. 1). The HAO reaction releases one mole of NO2 −, four moles of electrons and five moles of H+ per mole NH2OH oxidized (Wood 1986). Two of the electrons are shuttled back to AMO where they aid the oxidation of NH3 to NH2OH. The electron transport chain is mediated by the hemeproteins cytrochrome C554, cytochrome C552 and a quinone pool (Arp et al. 2002; Whittaker et al. 2000). The two other electrons are used to generate a proton motive force through reverse electron transport (Schmidt and Bock 1998; Whittaker et al. 2000; Wood 1986). Together, the nitrification of one mole of NH3 releases three moles of protons resulting in the overall reaction:
Electron flow during oxidation of ammonia (NH3) to nitrite (NO2 −) in Nitrosomonas sp. driven by a two-step reaction involving the enzymes ammonia monooxygenase (AMO) and hydroxylamine oxidoreductase (HAO). Hydroxylamine oxidation generates 4 electrons which are channelled via the hemeproteins cytochrome C554 and cytochrome Cm552 to a quinone pool (QH2) where two of the four electrons are transferred to an electron chain for energy conservation (adapted from Arp et al. 2002)
The design of wastewater treatment processes is often based on total ammonia nitrogen (TAN) (e.g. Ekama and Wentzel 2008; Gujer et al. 1999; Kaelin et al. 2009), while the true substrate for AMO is free NH3 which is in equilibrium with undissociated NH4 + (Hyman and Arp 1995; Suzuki et al. 1974). Owing to the low acid dissociation of NH4 + in water (pKa NH3/NH4 + 9.24) and its dependency on temperature and pH, the acid-base equilibrium between ammonium (NH4 +) and the AOB substrate NH3 is an important factor, which needs to be taken into account when modelling nitrification based on TAN, especially in systems operating at low pH (Kaelin et al. 2009; Quinlan 1984).
The oxidation of ammonia to hydroxylamine by AMO is mediated by a complex reaction chain, which is not yet fully unravelled. A hypothesis put forward by Schmidt et al. (2001a) proposes a two-step reaction based on an internal recirculation of the oxidant dinitrogen tetroxide (N2O4) (Fig. 2). In this so-called NOx cycle, NH3 is oxidized with N2O4 releasing NH2OH and nitric oxide (NO). The latter is reoxidized with O2 at another active site of the enzyme to nitrogen dioxide (NO2) which is in equilibrium with its dimeric form N2O4. Under aerobic conditions, NO or NO2 were shown to significantly enhance the NH3 oxidation rate (Beyer et al. 2009; Schmidt et al. 2001a; Zhang et al. 2010). However, in another study, the NO scavenger PTIO had little effect on the ammonia oxidation rate of Nitrosospira multiformis (Shen et al. 2013). This finding questions the significance of NO and of the proposed NOx cycle for NH3 oxidation in N. multiformis. An alternative hypothesis proposes that NO2 or N2O4 act as direct oxidants in addition to O2 (Zhang et al. 2010). The conversion of NH2OH to NO2 − by HAO is a complex reaction involving several intermediates (Fig. 2). NH2OH binds to the active site of P460, an iron containing multi-heme, where it is oxidized via the intermediates nitroxyl (HNO) and nitric oxide (NO) to nitrite (Kostera et al. 2008).
Oxidation of NH2OH to NO2 − catalysed by hydroxylamine dehydrogenase involving the reduction and re-oxidation of iron in the multi-heme P460 (adapted from Kostera et al. 2008)
Nitrosomonas europaea and other AOB species have been shown to be equipped with respiratory enzymes which reduce NO2 − to NO, N2O or N2. This process, denoted as “nitrifier denitrification” is considered to constitute a chemoorganotrophic survival metabolism under O2 limiting conditions (Hooper 1968; Poth and Focht 1985; Schmidt and Bock 1997). Alternatively, nitrifier denitrification might be involved in protecting the organisms from high NO2 − concentrations (Beaumont et al. 2002, 2005; Cua and Stein 2011). The latter has been questioned however, because nitrifier denitrification has relatively low reaction rates (Schmidt 2008). Nitrite reductase (NIR), the enzyme reducing NO2 − to NO, was for a long time considered to be the central enzyme of nitrifier denitrification (Casciotti and Ward 2001; Hooper 1968). However, N. europaea expresses NirK (one of the known NIR enzymes) under fully aerobic conditions (Beaumont et al. 2004b) and mutants lacking NirK were shown to reduce NO2 − under anoxic conditions at rates comparable to the wild type (Beaumont et al. 2002). Hence, the first step in nitrifier denitrification is performed by another, so far unidentified nitrite reductase, while NirK seems to play an important role in the energy conservation during NH2OH oxidation under oxic conditions (Kozlowski et al. 2014). The enzyme nitric oxide reductase (NOR) (Beaumont et al. 2004b) reduces NO to N2O (Kostera et al. 2008; Schmidt et al. 2004a). The gene coding for NOR, norB, was shown to be essential for AOBs’ ability to reduce NO2 −, suggesting that NOR is the sole NO reductase in the nitrifier denitrification pathway (Kozlowski et al. 2014). Notwithstanding, norB-deficient N. europaea strains have been shown to reduce added NO to N2O (Beaumont et al. 2004b). These findings point to two alternative NO reduction pathways: NO produced via reduction of NO2 − by NOR coupled to energy conversation (Kozlowski et al. 2014; Schreiber et al. 2009) and NO released during ammonia oxidation (NOx cycle, AMO intermediates, Fig. 2) being reduced by an alternative NO-reductase in the course of detoxification, likely connected to Cytochrome C554 (Upadhyay et al. 2006). N. europaea (Schmidt et al. 2004b), Nitrosomonas eutropha (Schmidt and Bock 1997, 1998) and likely also other AOB species (Beyer et al. 2009) are able to perform a complete reduction of NO2 − to N2 (via NO and N2O), even though no homologue of N2O reductase (N2OR), the enzyme reducing N2O to N2 in canonical denitrification has been found in AOB (Chain et al. 2003). However, Nitrosocyanin (nsc), a protein similar to N2OR in structure, could mediate this function, thus rendering AOB fully fledged denitrifiers (Arciero et al. 2002; Beyer et al. 2009; Schmidt 2009; Whittaker et al. 2000).
2.1.2 Potential N2O producing pathways in AOB
Several studies suggest that the highly reactive intermediates NH2OH, NOH or NO can be released from AOB during imbalanced NH3 oxidation and that these intermediates serve as substrates for chemical N2O formation under aerobic conditions (Law et al. 2012a; Wunderlin et al. 2013). This would explain the often times observed positive correlation between apparent NH3 oxidation rates and N2O emission rates (Law et al. 2011, 2012a; Yu et al. 2010). Proposed pathways for N2O formation connected to the release of HAO intermediates are release of NOH and subsequent chemical decomposition to N2O (Law et al. 2012a; Poughon et al. 2001) or release of NO and subsequent enzymatic reduction by cytochrome C554 to N2O (Hooper and Terry 1979; Kostera et al. 2010, 2008; Upadhyay et al. 2006). Nitroxyl (NOH) is an early intermediate in the HAO pathway (Fig. 2), that can decompose abiotically to N2O (Law et al. 2012a; Poughon et al. 2001; Stuven and Bock 2001). NH2OH uptake by HAO is believed to be greater than electron transfer to cytochrome C554 (Kostera et al. 2010), which, at high NH2OH oxidation rates, may result in build-up of excess reduction power. Stuven and Bock (2001) proposed that HAO transfers only two instead of four electrons to cytochrome C554 under high NH2OH oxidation rates while releasing HNO. Yet, even under those conditions, the electron supply by AMO may exceed the reducing potential available for energy conservation, so that HNO is released from HAO and leads to chemical N2O formation (Law et al. 2011, 2012a; Wunderlin et al. 2013). The latter may become as a self-accelerating process, which could be an explanation for the observed exponential relationship between NH3 oxidation rates and N2O emissions (Law et al. 2012a).
NO can be released from the P460FeIII-NO complex, during the final step of HAO metabolism (Kostera et al. 2008, see Fig. 3). Its further reduction to N2O is likely performed by the protein cytochrome C554 (Upadhyay et al. 2006), which is believed to act as an immediate acceptor for electrons transferred by the HAO enzyme (Upadhyay et al. 2006; Yamanaka and Shinra 1974). Therefore, the putative NO reducing activity of cytochrome C554 is likely driven by electrons supplied by the HAO process. In presence of O2, NO is oxidized abiotically to NO2, which again may fuel the NOx cycle and AMO activity (Schmidt et al. 2001b). However, this chemical reaction does probably not reach significant rates under normal growth conditions (Schmidt et al. 2001b; Udert et al. 2005). Upadhyay et al. (2006) proposed that the release of NO is a consequence of a decreased electron transfer rate from HAO to the cytochrome complex, which may be provoked when O2 depletes to a level exceeding the capacity of AMO for receiving electrons from NH2OH oxidation (Kostera et al. 2008; Upadhyay et al. 2006). Acidity was shown to be another potential factor provoking incomplete HAO reaction (Jiang and Bakken 1999). Under strongly acidic conditions, abiotic N2O formation by N-nitrosation of NH2OH and NO2 − has been proposed (Freeman 1973; Spott et al. 2011).
Chemical decomposition of the intermediate NH2OH as a source of N2O from NH3 oxidation was proposed in the early literature (Bremner et al. 1980; Yoshinari 1990). NH2OH may accumulate when the NH2OH oxidation becomes slower than the NH3 oxidation, for example in the case of limited availability of reducing equivalents (Wunderlin et al. 2013). Recent in vitro experiments showed that abiotic NH2OH decomposition does not reach significant rates under normal growth conditions (Harper et al. 2009; Jason et al. 2007; Wunderlin et al. 2012). However, the latter abiotic reaction likely involves mandatory oxidation of NH2OH to NOH with NO2 − as electron acceptor. Hence, chemical decomposition of NH2OH to N2O may be promoted when NO2 − is present at high concentrations (Tallec et al. 2006).
The exact biochemistry of NO2 − reduction in nitrifier denitrification is not fully understood. In particular, the role of the copper-containing nitrite reductase NirK, a homologue of the dissimilatory nitrate reductase in canonical denitrification, remains unclear. Some studies found positive (Cua and Stein 2011; Kim et al. 2010), no (Beaumont et al. 2002) or even negative correlation (Jason et al. 2007) between the expression of NirK and N2O formation in N. europaea. In vitro studies have shown that NirK-deficient strains of N. europaea released four times more N2O than the wild type (Beaumont et al. 2002), while NOR-deficiency did not significantly affect N2O production (Beaumont et al. 2004b). This may imply that nitrifier denitrification is not a major source of N2O under normal growth conditions (Beaumont et al. 2002), which however is questioned by the recent hypothesis that NirK has a supportive function for NH2OH oxidation by providing an additional electron sink for the HAO enzyme while another, so far unknown NIR homologue performs the NO2 − reduction in nitrifier denitrification (Kozlowski et al. 2014).
2.1.3 Regulation of N2O production in nitrification
2.1.3.1 Nitrite
Nitrite accumulation is often pointed out to be a major factor leading to N2O production by AOB, likely via incomplete nitrifier denitrification (Stein and Arp 1998; Beaumont et al. 2005; Yu and Chandran 2010; Cua and Stein 2011). Table 1 gives an overview over findings from AOB pure culture studies in which the proportion of N2O originating from NO2 − was determined with help of 15N labelling. The study by Shaw et al. (2006) indicates that this proportion may vary among different AOB strains. Comparing the figures of Poth and Focht (1985) with those of Schmidt et al. (2004b) suggests that high NO2 − levels increase the amount of N2O produced relative to the amount of NH3 oxidized, the proportion of N2O having NO2 − as N source and the N2O/N2 ratio. The latter might be due to incomplete nitrifier denitrification at high NO2 − concentrations (Cua and Stein 2011; Poth and Focht 1985). This is supported by a pure culture study with N. europaea in which N2O production increased substantially when NO2 − concentrations exceeded 5 mM (Yu and Chandran 2010). NO2 − concentrations >5 mM were also shown to inhibit AMO activity, especially at low pH, when HNO2 is formed (Stein and Arp 1998; Yu and Chandran 2010). However, the effect of NO2 − seems to be O2 depended. In a recent mixed culture study, the trend of increasing N2O production with increasing NO2 − concentration was shown to be less obvious at high DO than at low DO levels (<1.5 mg L−1) (Peng et al. 2015a). Increased N2O production by nitrifier denitrification in response to high NO2 − concentrations may reflect a detoxification reaction countervailing nitrosative stress (Stein and Arp 1998; Yu and Chandran 2010). However, detoxifying N2O production has been challenged by a recent study with a mixed culture, in which increasing NO2 − concentrations up to a level of 1 mM increased N2O production while a further increase to 10 mM decreased N2O production (Law et al. 2013). The authors attributed this to substrate inhibition of NIR above a certain NO2 − threshold. However, since the mixed AOB culture was studied in a highly loaded partial nitrification reactor, continued high concentrations of NO2 − may have altered the AOB community towards species that are less sensitive to NO2 − and hence produce less N2O (Castro-Barros et al. 2016; Chandran et al. 2011). A similar observation was made in a highly loaded wastewater reactor when introducing partial nitrification conditions by lowering DO and biomass retention time; biomass dominance shifted from N. europaea to N. eutropha and the transient N2O peak faded out (Ahn et al. 2011). These findings point to a greater tolerance of N. eutropha to NO2 − as compared with N. europaea. Another study with a mixed AOB culture in a wastewater reactor with low NH3 load observed a shift from N2O to NO emission when NO2 − concentrations exceeded 3.6 mM (Castro-Barros et al. 2016). In contrast, a recent pure culture study with N. europaea found only temporary increase of NO production upon increasing NO2 − (Yu et al. 2010). Clearly, more research is needed to elucidate the role of NO2 −, NO and NO2 −/HNO2 partitioning for N2O emissions from AOB activity associated with wastewater treatment.
2.1.3.2 Dissolved oxygen (DO)
Comparing DO in the studies with N. europaea by Poth and Focht (1985) and Shaw et al. (2006) in Table 1 suggests that DO has little impact on N2O production under aerobic conditions (DO > 1 mg O2 L−1). This was also found in another pure culture study with N. europaea (Kester et al. 1997) and in a recent study with a mixed AOB culture (Peng et al. 2014), both of which did not find significant differences in the N2O emission factor (relative to oxidised NH3) at steady state when DO was above 1 mg O2 L−1. Isotope labelling indicated that N2O originates from NH2OH, likely produced via HAO intermediates under fully aerobic conditions (Peng et al. 2014). N2O emissions by AOB increase dramatically when DO in the bulk liquid falls below 1 mg O2 L−1 or fluctuates between aerobic and anaerobic conditions (Jia et al. 2013; Kester et al. 1997; Peng et al. 2014). Production of N2O (and NO) during nitrification under limited O2 availability is commonly attributed to nitrifier denitrification (Jia et al. 2013).
2.1.3.3 Inorganic carbon
Another factor which influences N2O production by AOB is the availability of inorganic carbon. In a recent study with a mixed biomass that was enriched in AOB and NOB (Peng et al. 2015b), N2O emissions showed a positive relationship with inorganic carbon concentration. In intensively nitrifying reactor systems, autotrophic growth may result in a shortage of inorganic carbon, which may limit NH3 oxidation activity by AOB. Increased availability of inorganic carbon will therefore lead to higher reaction rates of AOB and associated N2O emissions (Peng et al. 2015b).
2.1.3.4 Acidity
Acidic conditions (pH < 5) may induce partial inhibition of the HAO with subsequent release of NO that might be further reduced to N2O (Jiang and Bakken 1999). In addition, pH may indirectly affect N2O production by AOB through controlling the partitioning of NO2 − and HNO2. The latter may induce the above discussed detoxification reactions and is suspected to have an inhibitory effect on AMO (Stein and Arp 1998; Yu and Chandran 2010).
2.2 Nitrite oxidizing bacteria (NOB)
Nitrite oxidizing bacteria, NOB, refers to a group of gram-negative bacteria gaining their energy from oxidizing NO2 − to NO3 − (Spieck and Lipski 2011). Wastewater treatment systems are usually dominated by the two genera, Nitrobacter sp. and Nitrospira sp. (Blackburne et al. 2007). Which of these species dominates depends on the total ammonia (NH3; NH4 +) load, but also on O2 availability and other environmental conditions (Okabe et al. 1999). The oxidation is catalysed by a complex multi-enzyme reaction chain (Spieck and Lipski 2011; Sundermeyer-Klinger et al. 1984). In brief, the membrane bound nitrite oxidoreductase (NXR) oxidizes NO2 − to NO3 −. This enzymatic reaction derives the oxygen needed from dissociation of H2O and releases two protons and two electrons. The electrons are transferred to the heme-protein cytochrome-C oxidase, which reduces the final electron acceptor O2 to O2−, which in turn reacts with the protons released by NXR to H2O (Sundermeyer-Klinger et al. 1984). This results in overall Eq. 2, which is also used for parameterizing O2 consumption during NO2 − oxidation (Kaelin et al. 2009) as well as for studying NOB kinetics (Ciudad et al. 2006).
NOB are little studied owing to their poor growth in pure cultures (Spieck and Lipski 2011). Recent studies support the notion that NO2 − rather than its protonated form nitrous acid (HNO2) (pKa NO2 −/HNO2 3.39 in water) is the true substrate for NXR (Park and Bae 2009; Poughon et al. 2001; Udert and Jenni 2013). However, NOB seem to be able to deprotonate HNO2 prior to its oxidation (Park and Bae 2009). NOB have received little attention in the literature so far with respect to N2O production. Nitrobacter sp. and Nitrospira sp. were shown to have an anoxic growth mode reducing NO3 − with pyruvate or H2 as electron donor (Colliver and Stephenson 2000; Maia and Moura 2014; Sundermeyer-Klinger et al. 1984). This metabolism is little studied but some authors assume that the terminal products are NO or N2O (Starkenburg et al. 2008). In contrast to nitrifier denitrification by AOB, which was observed at variable O2 concentrations (Beaumont et al. 2004a; Schmidt et al. 2004b), NO3 − respiration by NOB is only active under anoxic conditions (Colliver and Stephenson 2000). A potential direct contribution of NOB to N2O emissions seems therefore only relevant under oxygen limiting or anoxic conditions. However, NOB play a crucial role in controlling NO2 − concentrations in waste water treatment systems, and may thus indirectly controlling N2O emissions (Ch. 3).
It has been frequently stated that high NH3 concentrations inhibit NOB (Anthonisen et al. 1976; Park and Bae 2009). However, NOB can adapt to high NH3 and hence inhibition is species dependent (Yoo et al. 1999). Reported threshold concentrations vary from 0.04 to 0.08 mg NH3–N L−1 for Nitrospira sp. to up to 30–50 mg NH3–N L−1 for Nitrobacter sp. (Blackburne et al. 2007). HNO2, the protonated form of NO2 − is another frequently cited inhibitor of NOB (Hellinga et al. 1999; Park and Bae 2009; Zhou et al. 2011). In general, critical HNO2 levels can be expected when NO2 − accumulates at low pH. This typically occurs in weakly buffered systems with high nitrification rates owing to the proton release during AOB metabolism (Eq. 1) (Udert et al. 2005).
2.3 Heterotrophic denitrifiers
In wastewater treatment, heterotrophic denitrifiers refer to a large number of taxonomically unrelated chemoorganotrophic bacteria, which use the anoxyions NO3 − and NO2 − as terminal electron acceptors in the absence of oxygen and reduce them to gaseous NO, N2O and N2. Canonical denitrification of NO3 − comprises four consecutive reduction steps in which the intermediates NO2 −, NO and N2O serve as substrates for the reductase enzymes nitrate reductase (NAR), nitrite reductase (NIR), nitric oxide reductase (NOR) and N2O reductase (N2OR), respectively (Zumft 1997). The overall chemical reaction is shown in Eq. 3 for the reduction of NO3 − and Eq. 4 for the reduction of NO2 −.
Denitrification is a facultative metabolic pathway, which enables denitrifiers to respire organic carbon anoxically. Denitrification is suppressed by O2 in favour of oxic respiration. O2 tension also regulates the relative activity of the denitrification enzymes, and may thus determine the composition of the gaseous end products (Körner and Zumft 1989). In micro-aerobic zones having a DO in the range of 0.2–0.5 mg L−1, the expression of N2OR, the enzyme reducing N2O to N2, may be repressed, resulting in high N2O emissions (Bergaust et al. 2012; Bonin and Raymond 1990; Morley et al. 2008). A second factor affecting the balance of the reduction steps is the pH. It has been shown that pH below 6.1 impairs N2OR functioning, likely post-transcriptionally, i.e. no functional enzyme is synthesized at low pH unless acid tolerant denitrifiers proliferate. This was shown in pure culture studies of Paracoccus denitrificans (Bergaust et al. 2010) and in bacteria extracted from soils (Brenzinger et al. 2015; Dörsch et al. 2012; Liu et al. 2014).
Recent studies suggest that the reduction of N2O to N2 also depends on the availability of electron donors (Pan et al. 2013; Richardson et al. 2009). For instance, N2O accounting for up to 20 % of biologically reduced N was attributed to low degradability of endogenous substrates in a biofilm (Itokawa et al. 2001), which limits electron availability for the reduction of N2O to N2 (Pan et al. 2013).
Similarly to its role in nitrification, accumulation of NO2 − originating from NO3 − reduction in anoxic wastewater reactor systems has been pointed out to be an important source for increased N2O production in heterotrophic denitrification (e.g. Schreiber et al. 2012; Schulthess et al. 1995; Zhou et al. 2011). Campos et al. (2009) reported N2O emission accounting for up to 55 % of totally reduced N when heterotrophic denitrification was fed with NO2 − as an electron acceptor as compared to 0.8 % with NO3 −. The exact mechanisms behind the enhanced N2O production in the presence of NO2 − are not known, but nitrosative stress response triggered by HNO2 or NO (both of which can be formed chemically from NO2 −) leading to enzymatic or abiotic reactions have been proposed. For example, NO may be formed chemically (abiotic) through dismutation of HNO2 (Stein and Arp 1998). NO may also accumulate biotically as an intermediate of heterotrophic denitrification if NOR expression is delayed relative to NIR expression (Schulthess et al. 1995). HNO2 is toxic and suspected to inhibit periplasmatic N2OR, which would lead to incomplete denitrification and release of N2O instead of N2 (Schreiber et al. 2012; Zhou et al. 2008b). Inhibition of N2OR by HNO2 was observed in an anoxic activated sludge culture at concentrations as low as 0.2 µg HNO2–N L−1. At HNO2 concentration ranging from 0.7 to 1.0 µg N L−1, 50 % inhibition of N2O reduction was observed whereas 4 µg HNO2–N L−1 inhibited N2O reduction completely. Based on these data, and the acid-base equilibrium constant for NO2 −, a 50 % inhibition at pH 7 would take place at NO2 − concentrations of 3–4 mg N L−1 and a total inhibition at 17 mg N L−1 or higher (Zhou et al. 2008b). The presence of free NO was shown to have an immediate, irreversible inhibitory effect on the N2OR enzyme, while leading to enhanced NIR activity (Frunzke and Zumft 1986). Hence, regardless whether NO is formed biotically or abiotically during periods of high NO2 − accumulation, it might result in a suppression of N2O reduction in heterotrophic denitrification (Schulthess et al. 1995). Given the proposed irreversibility of the N2OR inhibition, high N2O production may be sustained over longer periods even if free NO only occurred as a temporary phenomenon.
2.4 Anaerobic ammonia oxidizing bacteria (Anammox)
A recently discovered group of obligate anaerobic, chemolithoautotrophic organisms gains energy for metabolism and growth from the anaerobic oxidation of NH4 + to N2 with NO2 − as electron acceptor (Jetten et al. 1998; Kuenen 2008; Mulder et al. 1995; van de Graaf et al. 1995). So far, only few Anammox species have been described, all of which belong to the class of Planctomycetes. The most studied ones are Candidatus B. anammoxidans (Strous et al. 1999a) and Candidatus K. stuttgartiensis (Schmid et al. 2000), which are also the main species used for wastewater treatment (Kuenen 2008). Anammox-bacteria were shown to grow in extreme dense colonies, preferably biofilms, at extremely low growth rates and are therefore difficult to purify or isolate (Strous et al. 1999a). The Anammox metabolism converts NH4 +, NO2 − and protons into N2, NO3 − and H2O as shown in overall Eq. 5 (Kartal et al. 2013; Strous et al. 1999b).
The coefficients for Eq. 5 where experimentally derived by Strous et al. (1999b) and should be verified by pure culture experiments (Lotti et al. 2014). NO2 − is reduced to NO in a complex reaction chain (Eq. 6) which again reacts with NH4 + to N2H4 (Eq. 7). In a final step, N2H4 is oxidized to N2 (Eq. 8). This synproportionation of NH4 + with NO2 − results in an energy gain of −357 kJ mol−1 (Eq. 9) (Kartal et al. 2011).
This reaction chain, performed by a series of recently discovered enzymes, is not yet fully clarified (Kartal et al. 2013). The concurrent oxidation of NO2 − to NO3 − is assumed to generate reducing potentials for CO2 fixation (Lotti et al. 2014). Anammox bacteria have a little investigated optional organotrophic metabolism, which can reduce organic acids (propionate, acetate) to CO2 with NO3 − as electron acceptor (Güven et al. 2005). 15N labelling experiments indicated that these electron acceptors are reduced to NH4 + with NO2 − as intermediate (Kartal et al. 2007a) rather than to N2O and N2. The purpose of this organotrophic metabolism may be energy conversation (Güven et al. 2005; Kartal et al. 2007b) or internal production of substrates (NH4 +, NO2 −) for the main metabolism (Kartal et al. 2007a). An assimilation of organic substrates into biomass was not observed (Güven et al. 2005; Kartal et al. 2007b). The organotrophic metabolism seems to be highly selective for volatile fatty acids (VFA). Other typical electron donors (e.g. glucose) had no significant effect or even inhibited (alcohols) Anammox metabolism (Güven et al. 2005).
Anammox bacteria use NH4 + as substrate (Kartal et al. 2013, 2011). This distinguishes them from AOB, which have NH3 as substrate. However, since NH4 + and NH3 are in equilibrium (pKa NH3/NH4 + 9.24 in water), the availability of and competition for substrate depends on pH, which is controlled by proton release (AOB) or proton uptake (Anammox). The Anammox metabolism is inhibited in the presence of O2, but the inhibition was shown to be reversible even after exposure to a DO as high as 8 mg O2 L−1 (Hu et al. 2013). Also NO2 − has a reversible inhibitory effect on the Anammox organism at higher concentrations (IC50 400 mg L−1) (Lotti et al. 2012). Anammox bacteria are at the same time extremely tolerant to NO, which they are able to reduce to N2, most likely via the intermediate N2H4 with NH4 + as electron donor (Kartal et al. 2010).
According to present knowledge, Anammox bacteria do not produce N2O (Hu et al. 2013). However, an earlier study showed that the heterotrophic organism Pseudomonas aeruginosa nitrosates the intermediate N2H4 and HNO2 to N2O (Kim and Hollocher 1984). It is possible that also Anammox bacteria perform a similar reaction under specific conditions. A recent pure culture study (Lotti et al. 2014) detected a small but measurable production of N2O (0.2 % of removed N), which might have been produced by this pathway. Considerable N2O emissions were reported from Anammox reactor systems, amounting to 0.4–1.3 % of the biologically transformed N (Hu et al. 2013). It was shown that emissions rates for NO and N2O increased with increasing O2 availability in the bulk liquid (Kampschreur et al. 2009a). However, these data were obtained from systems with mixed biomass cultures of both AOB and Anammox organisms and hence N2O may have been produced by AOB. This would be consistent with a reported trend of decreased N2O and NO production with increasing Anammox activity (Ni et al. 2013). More research is needed to clarify the N2O production potential by Anammox.
3 Mechanism of N2O production in biofilm systems
3.1 Biofilms in wastewater treatment application
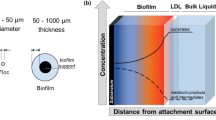
This section summarizes the current understanding of biofilm functioning by using a mass balance model as a framework for the discussion of N2O producing mechanism. A biofilm is defined as densely aggregated biomass which is typically attached to a solid surface, the so-called substratum (e.g. gravel, plastic media) (Morgenroth 2008a), but also unattached, spherical forms of biofilms, usually called granular sludge, exist (Khan et al. 2013). The mass flow of a particular compound in a biofilm can be approached with help of a diffusive/reactive mass balance equation (Eq. 10, Morgenroth 2008a; Wanner and Gujer 1986). It describes the balance between accumulation, diffusive transport and reaction over the biofilm cross section where CF is concentration, DF the diffusion coefficient, x the distance from the biofilm surface and rF the reaction rate for a particular compound in the biofilm.
The simplest types of biofilms consist of only one functional group of organisms, inert biofilm mass and interstitial void spaces. However, wastewater usually provides a range of different substrates (e.g. organics, ammonia) which support multi-species biofilms (Wanner et al. 2006; Wanner and Gujer 1985). Most of the present models describe the biofilm as a rigid, stratified biomass with fast-growing organism in the outermost layers, which are in contact to the bulk liquid where substrate availability is highest and slow-growing organism in deeper biofilm layers, where substrate availability is limited due to diffusion constraints (Morgenroth 2008a; Wanner and Gujer 1985; Wanner and Reichert 1996). However, a one-dimensional, rigid biomass distribution only partially reflects true spatial biomass dynamics. Especially biomass detachment, which typically takes place during periodical events, may impact the three-dimensional biofilm structure. Another important aspect is biomass competition that needs to be better characterized in future biofilm studies (Derlon et al. 2013; Elenter et al. 2007; Horn et al. 2003; Morgenroth and Wilderer 2000).
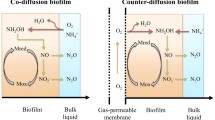
In systems loaded with organic substrate and ammonia, as typical for domestic wastewater, the outermost biofilm layer is dominated by fast growing heterotrophic bacteria. In the presence of abundant organic substrates, all O2 is consumed by heterotrophic activity and little nitrifying biomass establishes (Wanner and Gujer 1985). When the availability of organic substrate becomes limiting for heterotrophic activity, the biofilm is substrate limited and oxygen is not completely consumed by heterotrophs. Under these conditions, O2 is still present in deeper layers where slow growing nitrifying organisms (AOB, NOB) can establish. Biofilms accommodating both heterotrophic and autotrophic organisms are often called heterogeneous biofilms. In biofilm systems receiving little organic substrate, heterotrophs are not able to compensate biomass decay by growth and will therefore only represent a minor fraction of the biomass. This results in so-called autotrophic or nitrifying biofilms which are typically dominated by nitrifiers in aerobic zones (Morgenroth 2008a; Wanner and Gujer 1985) and Anammox in anoxic zones (Jin et al. 2012; Lackner and Horn 2012; Wang et al. 2010). Recently, a novel type of engineered biofilm has been introduced based on membrane aeration, a so-called counter-diffusional biofilm, which is supplied by gaseous substrates (e.g. O2, CH4) via a membrane across the substratum (growing surface), resulting in a counter-flow with corresponding gradients of gaseous and liquid components. So far, such systems have only been applied at the experimental scale and are therefore beyond the scope of this review.
3.2 Exploring critical factors leading to N2O production in biofilms and mathematical modelling
Mathematical modelling is frequently used to explore mass transport limitation resulting in substrate gradients and biomass competition in biofilms (e.g. Elenter et al. 2007; Hao et al. 2001; Wanner and Gujer 1986). In recent time, several attempts have been made to explore N2O emitting processes within the complex multi-organismal environment of biofilms with help of mathematical modelling (Table 2). All of the models are based on the general mass balance equation (Eq. 10), but they differ considerably in the way metabolic reaction chains are represented and parameterized. Table 2 gives an overview over recent model studies, which are further discussed below.
Model A (Schreiber et al. 2009) focuses on NO and N2O and considers transformation and transport processes directly related to these N species. Model B (Ni and Yuan 2013; Peng et al. 2016a) describes N conversion in four different groups of organism: AOB, NOB, heterotrophs and Anammox bacteria. AOB and heterotrophs are considered as potential sources for N2O. Model C (Sabba et al. 2015) focuses on AOB dominated biofilm, assuming AOB being the sole organism in the system. The authors adopted a novel model of AOB metabolism recently developed by Ni et al. (2014), in which the particular enzymatic reaction steps are connected via different operational electron carriers. Only Model B includes heterotrophic denitrification as a complete process. Model A only encompasses partial denitrification, i.e. the reduction of NO2 − via NO to N2O, which is activated when O2 falls below 0.3 mg L−1. All modeling studies listed in Table 2 identified AOB as the major source of N2O, albeit taking different modelling approaches. To gain more insight into these approaches, we implemented the different AOB reaction terms into Aquasim 2.1 (Reichert 1994) and tested them using combinations of different steady-state conditions. NH3 was kept constant at non-limiting concentration (50 mg L−1), while O2 was kept constant at three different concentration levels: non-limiting (4 mg O2 L−1), slightly rate-limiting (1 mg O2 L−1; given AMO/HAO affinity constants of 126–600 µg O2 L−1) and rate-limiting (0.2 mg O2 L−1) (Kester et al. 1997). NO2 − was set to either 0.2 mg N L−1 (background), 5 mg N L−1, simulating NO2 − peak concentrations in complete nitrifying wastewater treatment system, or 50 mg N L−1 as typical for partial nitrification systems.
When NO2 − was set to low (0.2 mg N L−1), all AOB models predicted greatest N2O production (relative to NH3 oxidation) under micro-aerobic conditions (Fig. 3), which is in accordance with findings from pure culture studies (Ch. 2.1.3) and a recent study on mixed AOB biomass taken from a wastewater treatment plant (Peng et al. 2014). However, when increasing O2 from 1 to 4 mg L−1, Model A predicted a larger decrease in N2O emission than Models B and C. This is in conflict with incubation studies with N. europaea, which did not show a significant change in the steady state N2O emission factor in a DO range of 0.6–4.0 mg O2 L−1 (Kester et al. 1997). Model A may therefore overestimate the impact of O2 increase above 1 mg O2 L−1 on N2O emissions. Increasing NO2 − concentration resulted in distinct, contradictory behavior among the three AOB models, especially under fully oxic conditions (DO > 1 mg L−1). Model A showed a remarkable increase in N2O production with increasing NO2 −, which was most pronounced at a DO of 4 mg L−1. Here, an increase of NO2 − from 0.2 to 50 mg N L−1 resulted in a more than one order of magnitude higher N2O emission factor (Fig. 3). These findings are in accordance with measured N2O emissions from a fully aerated biofilm where DO was measured to be 1.6 mg O2 L−1 at the substratum and around 5 mg L−1 close to the biofilm surface (Schreiber et al. 2009). Model C predicted a decrease of the N2O emission factor with increasing NO2 − (Fig. 3), which is a result of the Haldane-type substrate inhibition for NIR implemented in this model (Sabba et al. 2015). Model B calculated a slight increase of the N2O emission factor when NO2 − increased from 0.2 to 5 mg N L−1, especially under micro-aerobic conditions, but there was little change in N2O upon further increase of NO2 − (Fig. 3). The different responses to NO2 − shown by the three models reflect the variable effects of NO2 − on N2O production found in different AOB cultures (Ch. 2.3) and underlines the need to clarify the mechanism underlying N2O production by AOB in response to NO2 −.
None of the models captured the exponential increase of N2O production with increasing NH3 oxidation rates as observed in certain wastewater systems (Law et al. 2011). N2O production and NH3 oxidation rates were weakly linearly related in Model B (R2 = 0.4) and in Model A (R2 = 0.1), while slightly negatively related in Model C (R2 = 0.7). Model C accumulated large amounts of NH2OH at a DO of 4 mg L−1, accounting for 27 % of the oxidized NH3 (molar ratio) at high oxidation rates (Fig. 3), which is accordance to what was found for Model C in the surface-near layer by Sabba et al. (2015). NH2OH release to medium was reported in a recent study with N. europaea (Yu and Chandran 2010) and did not exceeded 0.1 % of the NH3 consumption. This suggests that model C probably overestimates NH2OH release.
The applicability of Model A in its present form is limited to explore NO and N2O production under the specific in vitro conditions used by its developers (Schreiber et al. 2009). Especially the lack of a complete heterotrophic denitrification reaction chain is a serious shortcoming given the frequently reported occurrence of anoxic zones in biofilms, where a reduction of N2O to N2 by N2OR may occur if sufficient electron donors are present (Ch. 2.3). For exploring N2O production in a wastewater treating biofilm system, Model B and C seem to be more adequate. Model C needs to be expanded to encompass all four main groups of organism as already suggested by its authors (Sabba et al. 2015). Model C is more complex and therefore more difficult to parameterize, especially with respect to the numerous metabolic intermediates and electron flows that are difficult to determine in a real system. The published parameterisation captures N2O production well under certain conditions but seems to overestimate NH2OH accumulation. More work is needed to determine and validate the parameters for a wider set of conditions, ideally in pure culture studies with relevant AOB organism. Model B is based on the relatively well-established activated sludge model ASM (Henze et al. 1987), in which the basic metabolic parameters are well established and validated. Model B seems therefore easier to parameterize and may be at present to most applicable model to explore N conversions associated with N2O production in biofilms, which is also reflected in the outcome of our model testing (Fig. 3).
3.2.1 O2 and its impact on N2O emissions in different biofilm zones
As a result of O2 consumption by microbial activity and diffusive mass transport resistance, aerated biofilms have pronounced O2 gradients, supporting hyp- or anoxia in deeper layers. These zones support N2O formation in the presence of NO2 − or other oxidized N-compounds by AOB (Ch. 2.1.3) and heterotrophic denitrifiers (Ch. 2.3). As shown by microprobes, hypoxic zones (<0.2 mg O2 L−1) in well aerated biofilms (bulk liquid DO > 4 mg O2 L−1) are relatively small, accounting for less than 10 % of the aerated biofilm (Okabe et al. 1999; Schramm et al. 1999). Hence, under high aeration conditions, hypoxic N2O formation is likely small, so that biofilm systems do not a priori have greater N2O emission factors than suspended biomass cultures. This is also well supported by several biofilm studies listed in Table 3, reporting N2O emission factors comparable to those of well-functioning suspended biomass systems (Kampschreur et al. 2008). However, the hypoxic fraction of the aerated biofilm increases with decreasing bulk liquid DO. Modelled O2 profiles for biofilms with a bulk liquid DO of 1 mg O2 L−1 (Sabba et al. 2015) and 0.3 mg O2 L−1 (Ni and Yuan 2013) showed hypoxic biofilm fractions of 50 and 75 %, respectively. Moreover, at low DO (<0.5 mg L−1), the stimulating effect of NO2 − accumulation on N2O production by AOB seems to be most pronounced (Peng et al. 2015a). Recent studies using Models B and C (Ni and Yuan 2013; Sabba et al. 2015) clearly indicate that poorly aerated biofilms tend to produce more N2O, especially in the presence of NO2 − or NH2OH. The latter is a result of complex interactions between different organisms, which in turn depend on O2 availability. This is discussed in more detail in chapter 3.2.2. Together with NO2 − and NH2OH accumulation, bulk liquid DO is a key factor for controlling N2O emissions in biofilm-based wastewater treatment plants (e.g. Lochmatter et al. 2013; Peng et al. 2016a; Sabba et al. 2015).
A recent in vitro study (Schreiber et al. 2009) showed that decreasing the O2 concentration across a biofilm induces remarkable transient N2O accumulation, especially in the boundary zones between hypoxia and anoxia. With help of Model A, the authors attributed these N2O emission peaks to a temporary metabolic imbalance of AOB, which resulted in N2O production from NO release by HAO (Ch. 2.1.2). In praxis, such N2O peaks occur when the composition of the wastewater changes, but also under batch operation conditions. Frequent changes in the DO profile may also be an explanation for the observed inverse relationship between N2O production and RPM in a rotating disk reactor (Rezić et al. 2007).
Microprobe studies (Okabe et al. 1999; Schramm et al. 1999; Schreiber et al. 2009) of biofilms in an O2 saturated bulk liquid (DO 7.5–8.5 mg O2 L−1) showed that the O2 concentration at the biofilm surface is notably lower (1.9–5.7 mg L−1) than in the bulk liquid due to the external boundary layer mass transport resistance. Given Monod terms with a typical O2 affinity constant of 0.6 mg O2 L−1 (Brockmann et al. 2008), O2 availability in the biofilm surface limits NH3 oxidation rates to 75–90 % of the maximum reaction rates for suspended biomass. The modelling studies listed in Table 3 captured the rate-limiting effect caused by O2 mass transport resistance within the biofilm. However, they do not take into account the additional mass transport resistance across the boundary layer, which seems to be partially controlled by TAN flux (Fig. 4). This negative feedback interaction between O2 consumption by AOB and O2 availability in the biofilm likely limits the maximum possible NH3 oxidation rate in a biofilm. Therefore, we believe that the frequently cited N2O formation from AOB intermediates released during high reaction rates (Ch. 2.1.3) plays only a minor role for N2O production in biofilms. This is supported by a recent study (Lochmatter et al. 2013) which found highest N2O production rates in a biofilm at low DO (1 mg O2 L−1), coinciding with accumulation of NO2 −, but not at high DO (5 mg O2 L−1) and accordingly higher NH4 + flux with almost quantitative conversion of NH3 to NO3 −.
Difference between O2 concentration in bulk liquid and biofilm surface expressed as boundary layer gradient in relation to the flux of total ammonia nitrogen (TAN) determined by microprobe studies. The bulk liquid DO was in a range of 7.5–8.5 mg L−1 in all of the studies (Okabe et al. 1999; Schramm et al. 1999; Schreiber et al. 2009)
3.2.2 Accumulation of NO2 −
Nitrite, as a highly reactive and toxic N oxyanion, which is central to the metabolism of both AOB (Ch. 2.1.3) and denitrifiers (Ch. 2.3), plays an important role in N2O formation by biofilms. A biofilm controls NO2 − accumulation, dependent on biomass composition, substrate load and O2 availability. In a balanced suspended biomass reactor supporting both AOB and NOB, NO2 − accumulation is usually small, in the range of 0–0.2 mg N L−1 (Peng et al. 2014). However, NO2 − has been reported to accumulate in nitrifying reactors when DO in bulk liquid falls below 2 mg O2 L−1 (Brockmann et al. 2008; Fux et al. 2004a; Sliekers et al. 2005). This is commonly attributed to an imbalance between NO2 − production by AOB and consumption by NOB owing the higher affinity of AMO for O2 as compared with NXR. This imbalance is utilized in partial nitrification reactors for producing NO2 − for Anammox based N removal (Ciudad et al. 2006; Hellinga et al. 1998; Sliekers et al. 2005). Recent cross-section studies of biomass distribution in nitrifying biofilm systems (Okabe et al. 1999; Schramm et al. 1999) found the highest NOB abundance in deeper zones with less O2 availability. The study of Okabe et al. (1999) showed that these NOB clusters were dominated by Nitrospira sp., while the faster growing NOB species Nitrobacter sp. were almost absent. Hence, Nitrospira sp. seems to be better adapted to oxygen limiting conditions than Nitrobacter sp. This may be related to the presence of Nitrospira-like “complete nitrifiers” in deeper biofilm layers, which have been recently shown to thrive in substrate-influx, oxygen limited zones (Daims et al. 2015). These newly discovered, slow-growing Nitrospira species are able to oxidize NH4 + all the way to NO3 − at oxygen concentrations as low as 0.1 mg O2 L−1, thereby counteracting NO2 − accumulation in deeper biofilm layers (van Kessel et al. 2015). Hence, decreased O2 availability does not necessarily lead to critical NO2 − accumulation under steady state conditions, especially when other NOB inhibiting factors such as high NH3 concentrations are absent. This is also illustrated by reported difficulties to limit the occurrence of NOB in one-stage partial-nitrification-Anammox reactors via DO as the only controlling parameter (Fux et al. 2004a).
Anoxic biofilm zones can act as a sink for NO2 − through activity of heterotrophic denitrifies or Anammox bacteria. In Anammox systems, NO2 − production by AOB and NO2 − consumption by Anammox can be kept in balance by controlling DO and ammonia loading rates (Hao et al. 2001; Peng et al. 2016b). In such well-balanced Anammox systems (Table 3), NO2 − in the bulk liquid has been reported to be below 0.5 mg N L−1 (e.g.Christensson et al. 2013; Yang et al. 2013). In systems without Anammox, heterotrophic denitrifieres may reduce NO2 − in anoxic zones of nitrifying biofilms. However, the activity of denitrifiers is strongly limited by the electron donor availability (Kindaichi et al. 2004a; Rittmann et al. 1994). Hence, the reported co-existence of heterotrophs in nitrifying biofilms (Okabe et al. 1999; Schramm et al. 1999) plays likely only a minor role in NO2 − accumulation.
3.2.3 pH and inorganic carbon
In biofilms, low pH may lead to enhanced N2O production by causing nitrosative stress for AOB (Ch. 2.1.3) and heterotrophic denitrifiers (Ch. 2.3) through protonation of NO2 − to HNO2. HNO2 is also a strong inhibitor for NOB, which again enhances accumulation of NO2 − (Ch. 2.1.3). Metabolic activity consumes or produces protons, which results in marked pH gradients in biofilms. In aerated zones, protons are produced by nitrification (Eq. 1, Ch. 2.1.1) and during dissolution of CO2 produced by respiration. In anoxic biofilm zones, acidification by CO2 dissolution may be driven by heterotrophic denitrification. In aerated suspended biomass cultures, CO2 does usually not affect the pH significantly, as it is continuously stripped out. In biofilm systems however, CO2 accumulates due to mass transport limitation, which can lead to measurable pH decrease (Xiao et al. 2013). Heterotrophic denitrification (Eqs. 3, 4, Ch. 2.3), but also Anammox (Eq. 5, Ch. 2.4) consume protons, thus counteracting pH decrease in anoxic zones. Yet, micro-probe studies have demonstrated strong pH gradients towards acidic conditions in deeper biofilm zones in completely anoxic biofilms with high heterotrophic activity (Xiao et al. 2013). This suggests that the proton release from the dissolution of CO2 exceeds proton consumption by dissimilatory NO3 − and NO2 − reduction. In biofilms dominated by autotrophic organism, CO2 may be consumed at high rates and become limiting, especially for Anammox bacteria which have a lower affinity for inorganic carbon than AOB or NOB (Ma et al. 2015). Hence, depletion of inorganic carbon in autotrophic biofilms would favour AOB and NOB and thus increase N2O production from increasing NO2 − due to lower Anammox activity. In suspended biomass cultures, CO2 limitation was shown to reduce N2O emissions from intermediates released by AOB at high reaction rates (Peng et al. 2015b). In biofilms, this effect is unlikely to occur because of O2 limitation, which impedes AOB reaction rates regardless of the availability of inorganic carbon (Ch. 3.2.2).
3.2.4 Accumulation of AOB intermediates
Several studied have suggested that high AOB activity goes along with release of intermediates (NO, HN2OH) conductive to N2O formation (Ch. 2.2.2). In biofilms, transient NO accumulation was detected in hypoxic and anoxic zones upon decrease of O2 or increase of NO2 − (Schreiber et al. 2009). NH2OH on the other hand, was proposed to accumulate in aerated zones as a result of higher AOB reaction rates (Sabba et al. 2015). By applying Model C (Table 2) to a well-aerated nitrifying biofilm, Sabba et al. (2015) simulated N2O production by nitrifier denitrification driven by excessive NH2OH release into hypoxic or anoxic zones and proposed that this could be one of the major N2O producing pathways in a well aerated, AOB dominated biofilms. However, as discussed earlier, the O2 mass transport limitation likely limits AOB activity (Ch. 3.2.1) so that extracellular NH2OH accumulation in a ratio of 1:2 in relation to produced NO2 − as proposed by Model C (Ch. 3.2) seems unlikely.
The presence of extracellular NH2OH (>3 mg N L−1) may further induce biomass detachment from biofilms as indicated by recent studies with Nitrosonomas sp., which observed a change in growth pattern from dense, spherical micro-colonies to single scattered cells (Harper et al. 2009; Kindaichi et al. 2004b). However, the NH2OH dosage applied by these studies (3.5–40 mg N L−1) is several orders of magnitudes higher than bulk liquid concentration simulated by Sabba et al. (2015) or measured by a recent study (Yu and Chandran 2010). NH2OH is difficult to measure and is therefore little investigated. Irrespective the uncertainties about magnitude and fate of NH2OH released by AOB, N2O production from extracellular NH2OH needs further investigation.
4 Reported N2O emissions in different types of wastewater treating biofilm systems
To give an overview of published N2O emission data from biofilm systems, a search was performed with the keywords “N2O” and “biofilm” in Web of Science (Thomson Reuters, New York). By December 2015, the search yielded 59 studies comprising both natural and artificial biofilm systems. The results were screened for studies of wastewater treating biofilm systems that provide data on N2O emissions under different operational conditions (Table 3). The N2O emission factor (N2O–N in % of biologically transformed N) was calculated from the data if not provided by the authors. Studies for which a calculation of the emission factor was not possible were omitted.
The results of the literature survey are summarized in Table 3. N2O emission factors vary greatly from 0.2 to 57 % N2O–N emitted per unit biologically transformed N. The main findings from this compilation can be summarized as follows: (a) both aerobic and anaerobic reactor conditions allow for high N2O emission factors; (b) heterogeneous biofilm systems fed with both organics and ammonia substrates tend to have higher N2O emission factors than autotrophic biofilms fed with ammonia alone; (c) under anoxic conditions, large N2O emissions are clearly linked to elevated NO2 − concentrations, which is not the case for aerobic conditions. No clear tendency was found for different aeration regimes and DO levels, but there is some indication that intermitting aeration or batch operation results in greater N2O emission factors than operation at constant DO.
4.1 N2O production in aerated, heterogeneous biofilms loaded with organic substrates and ammonia
Table 3 suggests that N2O emissions are greater in systems with heterogeneous biofilms receiving both organic substrates and ammonia than in nitrifying biofilms loaded with ammonia alone. This notion is supported by three studies which explored the effect of organic load on heterogeneous biofilms in more detail and showed that the addition of organic substrate increased N2O emission especially at high DO levels (>1 mg L−1) (Campos et al. 2009; Lochmatter et al. 2013; Todt and Dörsch 2015). So far, N2O producing mechanisms within the complex framework of interacting heterotrophic and autotrophic biomass in aerated heterogeneous biofilms have been little investigated. In stratified biomass, readily degradable organic substrates control the O2 consumption by heterotrophic activity and thus O2 availability in deeper biofilm zones, were nitrifiers are located (Ch. 3.1). Increase in organic load will therefore decrease O2 availability for nitrifiers and reduce the nitrification activity, but at the same time may increase NO2 − accumulation due to the lower O2 affinity of NOB (Ch. 3.2.2). Transient shifts in O2 availability across the biofilm (Ch. 3.2.1) are more frequent in systems loaded with organic substrate.
Another aspect affecting N2O emissions in heterogeneous biofilms is the size-dependent penetration depths and degradability of organic substrates. A typical wastewater contains both slowly and readily degradable organics, which to some extent correlate with molecule size (Dulekgurgen et al. 2006; Henze and Comeau 2008). Readily degradable substrates usually refer to low-molecular organic matter (e.g. volatile fatty acids), while slow-degradable substrates refer to high-molecular, colloidal or particulate organic matter. In some types of wastewater, slow-degradable organic substrates may also consist of low-molecular compounds such as humic acids (Dulekgurgen et al. 2006). With these slow-degradable substrates, the growth rate of heterotrophic organisms is too small to outcompete nitrifiers (Jin et al. 2012; Lackner and Horn 2012; Wang et al. 2010). The co-occurrence of substrate limitation and nitrification therefore depends on the presence of readily degradable compounds. If present at high concentrations, slow-degradable, low molecular substrates penetrate deeper into anoxic biofilm zones where they may fuel incomplete heterotrophic denitrification and produce N2O (Todt and Dörsch 2015). Heterogeneous biofilms are little studied for N2O producing processes and more research is needed to assess the effect of organic molecule size and distribution on N2O production.
4.2 N2O production in aerobic biofilms predominately loaded with ammonia
A combination of partial nitrification (also called nitritation) and anaerobic ammonia oxidation by Anammox bacteria has gained popularity for biological N-removal, especially for treatment of wastewaters with high ammonia content (e.g. Fux et al. 2004b; Jetten et al. 1998; Kampschreur et al. 2006; Yang et al. 2013). In biofilm systems, nitritation and Anammox can be run in a single reactor by combining AOB activity in aerobic zones with Anammox bacteria in anoxic zones (Christensson et al. 2013; Fux et al. 2004b; Ma et al. 2015; Yang et al. 2013). N removal based on one-stage partial-nitrification-Anammox seems to produce less N2O than traditional nitrification/denitrification-based N-removal (Table 3). This is due to the efficient conversion of nitrite to N2 in systems harbouring Anammox, which keeps NO2 − concentrations low. This can be achieved by keeping the NO2 − production rate of AOB below the NO2 − consumption rate of Anammox by actively controlling DO (Cema et al. 2011; Christensson et al. 2013; Peng et al. 2016a) and/or the TAN bulk concentration (Peng et al. 2016a). Modelling studies indicate that high N removal efficiency with low N2O emissions can be achieved at a DO around 0.5 mg L−1 and TAN < 50 mg N L−1. A higher DO up to 1 mg L−1 together with a larger TAN concentration result in greater AOB activity and NO2 − accumulation. If DO exceeds 2 mg L−1, N2O production declines, however N removal efficiency declines as well due to increased presence of NOB and production of NO3 − (Peng et al. 2016a).
Another critical factor for N2O emissions in autotrophic biofilms may be the temporal presence of organic substrates fuelling heterotrophic denitrifiers. Organic substrates can originate from the inlet, but also from endogenous biomass decay (Ma et al. 2015). Table 3 suggests that the occurrence of heterotrophic denitrifiers in anoxic biofilm zones increases N2O production, especially in the presence of NO2 − and/or slowly degradable substrates (Ch. 3.4). The competition between heterotrophs and Anammox bacteria in anoxic biofilm zones is determined by the concentration of organic substrates relative to the substrate affinity constant of heterotrophs, but also by the ratio of volatile fatty acids (VFA) to N. Anammox bacteria use VFA as electron donors for their organotrophic metabolism (Güven et al. 2005; Winkler et al. 2012a) and it is assumed that Anammox bacteria outcompete heterotrophs for VFA as long as NH4 + is present in ample amounts (Kartal et al. 2007b), With VFA/N ratios (expressed as COD/N) lower than 0.5 g O2 g N−1, Anammox bacteria were shown to outcompete heterotrophic bacteria (Winkler et al. 2012b). The extent of N2O production by heterotrophic denitrifiers in Anammox systems may therefore not only depend on the availability, but also on the type of organic substrates present. In autotrophic biofilms, VFA are likely short-lived, since continuous presence of these readily degradable substrates would support heterotrophic organisms in the aerobic surface-near biofilm layers and transform the biofilm into a heterogeneous biofilm.
4.3 N2O emissions from anoxic biofilms loaded with organic matter and NO2 − or NO3 −
Two research groups studied N2O emissions from heterotrophic denitrifying biofilms under anoxic conditions (Campos et al. 2009; Zhou et al. 2011, see also Table 3). Heterotrophic degradation of organic matter produces CO2 regardless whether O2 or NOx − is used as electron acceptor. CO2 may accumulate, resulting in a measurable pH drop as shown by a recent microprobe study (Ch. 3.2.3). As the pH in the inner, anoxic biofilm layers fell below 6.8, heterotrophic denitrification appeared to become incomplete resulting in greater N2O concentrations within the biofilm (Xiao et al. 2013). This can be attributed to direct impairment of N2OR by low pH itself and/or HNO2 (Ch. 2.3). A critical concentration of HNO2 was likely reached in the study of Campos et al. (2009) when supplying the biofilm system with NO2 − as electron acceptor. This would explain the more than one order of magnitude greater N2O emissions compared to a system fed with NO3 − (Campos et al. 2009). Thus, N2O formation in anoxic biofilm systems seems to be mainly controlled by NO2 − concentration levels, acidification by CO2 production and associated HNO2 formation.
5 Summary
The following key controls for N2O production in biofilms were identified:
-
N2O formation occurs mainly in hypoxic or anaerobic zones where low O2 tensions trigger production via nitrifier denitrification or incomplete heterotrophic denitrification, especially in the presence of NO2 − and other intermediates released from nitrification activity in aerated zones.
-
NO2 − accumulation is a key factor for N2O formation in biofilms. It is controlled by complex metabolic interactions between different types of organisms, which are controlled by O2 and substrate availability along the biofilm cross section.
-
Heterogeneous biofilm systems loaded with mixed wastewater seem to be particularly conductive to N2O formation, as frequent shifts in the O2 profile occur with varying loads of organic substrate and TAN which affect O2 consumption by heterotrophs and AOB.
-
Mathematical models are useful tools to explore N2O emissions in biofilms, but there are knowledge gaps about the N2O producing metabolism, especially of AOB, and consequently about the factors controlling N2O formation by AOB.
References
Ahn JH, Kwan T, Chandran K (2011) Comparison of partial and full nitrification processes applied for treating high-strength Nitrogen wastewaters: microbial ecology through nitrous oxide production. Environ Sci Technol 45:2734–2740. doi:10.1021/es103534g
Anthonisen AC, Loehr RC, Prakasam TBS, Srinath EG (1976) Inhibition of nitrification by ammonia and nitrous-acid. J Water Pollut Control Fed 48:835–852
Arciero DM, Pierce BS, Hendrich MP, Hooper AB (2002) Nitrosocyanin, a red cupredoxin-like protein from Nitrosomonas europaea. Biochemistry 41:1703–1709. doi:10.1021/bi015908w
Arp DJ, Sayavedra-Soto LA, Hommes NG (2002) Molecular biology and biochemistry of ammonia oxidation by Nitrosomonas europaea. Arch Microbiol 178:250–255. doi:10.1007/s00203-002-0452-0
Beaumont HJE et al (2002) Nitrite reductase of Nitrosomonas europaea is not essential for production of gaseous nitrogen oxides and confers tolerance to nitrite. J Bacteriol 184:2557–2560. doi:10.1128/jb.184.9.2557-2560.2002
Beaumont HJE, Lens SI, Reijnders WNM, Westerhoff HV, van Spanning RJM (2004a) Expression of nitrite reductase in Nitrosomonas europaea involves NsrR, a novel nitrite-sensitive transcription repressor. Mol Microbiol 54:148–158. doi:10.1111/j.1365-2958.2004.04248.x
Beaumont HJE, van Schooten B, Lens SI, Westerhoff HV, van Spanning RJM (2004b) Nitrosomonas europaea expresses a nitric oxide reductase during nitrification. J Bacteriol 186:4417–4421. doi:10.1128/jb.186.13.4417-4421.2004
Beaumont HJE, Lens SI, Westerhoff HV, van Spanning RJA (2005) Novel nirK cluster genes in Nitrosomonas europaea are required for NirK-dependent tolerance to nitrite. J Bacteriol 187:6849–6851. doi:10.1128/jb.187.19.6849-6851.2005
Bergaust L, Mao Y, Bakken LR, Frostegård Å (2010) Denitrification response patterns during the transition to anoxic respiration and posttranscriptional effects of suboptimal pH on nitrogen oxide reductase in Paracoccus denitrificans. Appl Environ Microbiol 76:6387–6396. doi:10.1128/aem.00608-10
Bergaust L, van Spanning RJM, Frostegard A, Bakken LR (2012) Expression of nitrous oxide reductase in Paracoccus denitrificans is regulated by oxygen and nitric oxide through FnrP and NNR. Microbiology 158:826–834. doi:10.1099/mic.0.054148-0
Beyer S, Gilch S, Meyer O, Schmidt I (2009) Transcription of genes coding for metabolic key functions in Nitrosomonas europaea during aerobic and anaerobic growth. J Mol Microbiol Biotechnol 16:187–197. doi:10.1159/000142531
Blackburne R, Vadivelu VM, Yuan Z, Keller J (2007) Kinetic characterisation of an enriched Nitrospira culture with comparison to Nitrobacter. Water Res 41:3033–3042. doi:10.1016/j.watres.2007.01.043
Bonin P, Raymond N (1990) Effects of oxygen on denitrification in marine sediments. Hydrobiologia 207:115–122. doi:10.1007/BF00041447
Bothe H, Jost G, Schloter M, Ward BB, Witzel KP (2000) Molecular analysis of ammonia oxidation and denitrification in natural environments. FEMS Microbiol Rev 24:673–690. doi:10.1111/j.1574-6976.2000.tb00566.x
Bougard D, Bernet N, Chèneby D, Delgenès JP (2006) Nitrification of a high-strength wastewater in an inverse turbulent bed reactor: effect of temperature on nitrite accumulation. Process Biochem 41:106–113. doi:10.1016/j.procbio.2005.03.064
Bremner JM, Blackmer AM, Waring SA (1980) Formation of nitrous oxide and dinitrogen by chemical decomposition of hydroxylamine in soils. Soil Biol Biochem 12:263–269. doi:10.1016/0038-0717(80)90072-3
Brenzinger K, Dörsch P, Braker G (2015) pH-driven shifts in overall and transcriptionally active denitrifiers control gaseous product stoichiometry in growth experiments with extracted bacteria from soil. Front Microbiol. doi:10.3389/fmicb.2015.00961
Brockmann D, Rosenwinkel KH, Morgenroth E (2008) Practical identifiability of biokinetic parameters of a model describing two-step nitrification in biofilms. Biotechnol Bioeng 101:497–514. doi:10.1002/bit.21932
Campos JL, Arrojo B, Vázquez-Padín JR, Mosquera-Corral A, Méndez R (2009) N2O production by nitrifying biomass under anoxic and aerobic conditions. Appl Biochem Biotechnol 152:189–198. doi:10.1007/s12010-008-8215-2
Casciotti KL, Ward BB (2001) Dissimilatory nitrite reductase genes from autotrophic ammonia-oxidizing bacteria. Appl Environ Microbiol 67:2213–2221. doi:10.1128/aem.67.5.2213-2221.2001
Castro-Barros CM, Rodríguez-Caballero A, Volcke EIP, Pijuan M (2016) Effect of nitrite on the N2O and NO production on the nitrification of low-strength ammonium wastewater. Chem Eng J 287:269–276. doi:10.1016/j.cej.2015.10.121
Cema G, Plaza E, Trela J, Surmacz-Gorska J (2011) Dissolved oxygen as a factor influencing nitrogen removal rates in a one-stage system with partial nitritation and Anammox process. Water Sci Technol 64:1009–1015. doi:10.2166/wst.2011.449
Chain P et al (2003) Complete genome sequence of the ammonia-oxidizing bacterium and obligate chemolithoautotroph Nitrosomonas europaea. J Bacteriol 185:2759–2773. doi:10.1128/jb.185.9.2759-2773.2003
Chandran K, Stein LY, Klotz MG, van Loosdrecht MC (2011) Nitrous oxide production by lithotrophic ammonia-oxidizing bacteria and implications for engineered nitrogen-removal systems. Biochem Soc Trans 39:1832–1837. doi:10.1042/bst20110717
Christensson M, Ekstrom S, Chan AA, Le Vaillant E, Lemaire R (2013) Experience from start-ups of the first ANITA Mox plants. Water Sci Technol 67:2677–2684. doi:10.2166/wst.2013.156
Ciudad G, Werner A, Bornhardt C, Muñoz C, Antileo C (2006) Differential kinetics of ammonia- and nitrite-oxidizing bacteria: a simple kinetic study based on oxygen affinity and proton release during nitrification. Process Biochem 41:1764–1772. doi:10.1016/j.procbio.2006.03.032
Colliver BB, Stephenson T (2000) Production of nitrogen oxide and dinitrogen oxide by autotrophic nitrifiers. Biotechnol Adv 18:219–232. doi:10.1016/s0734-9750(00)00035-5
Cua LS, Stein LY (2011) Effects of nitrite on ammonia-oxidizing activity and gene regulation in three ammonia-oxidizing bacteria. FEMS Microbiol Lett 319:169–175. doi:10.1111/j.1574-6968.2011.02277.x
Daims H et al (2015) Complete nitrification by nitrospira bacteria. Nature. doi:10.1038/nature16461
Derlon N, Coufort-Saudejaud C, Queinnec I, Paul E (2013) Growth limiting conditions and denitrification govern extent and frequency of volume detachment of biofilms. Chem Eng J 218:368–375. doi:10.1016/j.cej.2012.11.061
Dörsch P, Braker G, Bakken LR (2012) Community specific pH response of denitrification: experiments with cells extracted from organic soils. FEMS Microbiol Ecol 79:530–541
Dulekgurgen E, Dogruel S, Karahan Ö, Orhon D (2006) Size distribution of wastewater COD fractions as an index for biodegradability. Water Res 40:273–282
Ekama GA, Wentzel MC (2008) Nitrogen Removal. In: Henze M, van Loosdrecht MCM, Ekama GA, Brdjanovic D (eds) Biological wastewater treatment. IWA Publishing, London, pp 33–52
Eldyasti A, Nakhla G, Zhu J (2014) Influence of biofilm thickness on nitrous oxide (N2O) emissions from denitrifying fluidized bed bioreactors (DFBBRs). J Biotechnol 192(Part A):281–290. doi:10.1016/j.jbiotec.2014.10.008
Elenter D, Milferstedt K, Zhang W, Hausner M, Morgenroth E (2007) Influence of detachment on substrate removal and microbial ecology in a heterotrophic/autotrophic biofilm. Water Res 41:4657–4671. doi:10.1016/j.watres.2007.06.050
Freeman JP (1973) Less familiar reactions of oximes. Chem Rev 73:283–292. doi:10.1021/cr60284a001
Frunzke K, Zumft WG (1986) Inhibition of nitrous-oxide respiration by nitric oxide in the denitrifying bacterium Pseudomonas perfectomarina. Biochim Biophys Acta (BBA)-Bioenerg 852:119–125. doi:10.1016/0005-2728(86)90064-2
Fux C, Huang D, Monti A, Siegrist H (2004a) Difficulties in maintaining long-term partial nitritation of ammonium-rich sludge digester liquids in a moving-bed biofilm reactor (MBBR). Water Sci Technol 49:53–60
Fux C, Marchesi V, Brunner I, Siegrist H (2004b) Anaerobic ammonium oxidation of ammonium-rich waste streams in fixed-bed reactors. Water Sci Technol 49:77–82
Gujer W, Henze M, Mino T, van Loosdrecht M (1999) Activated sludge model no. 3. Water Sci Technol 39:183–193. doi:10.1016/s0273-1223(98)00785-9
Güven D et al (2005) Propionate oxidation by and Methanol inhibition of anaerobic ammonium-oxidizing bacteria. Appl Environ Microbiol 71:1066–1071. doi:10.1128/aem.71.2.1066-1071.2005
Hao X, Heijnen JJ, Van Loosdrecht MCM (2001) Sensitivity analysis of a biofilm model describing a one-stage completely autotrophic nitrogen removal (CANON) process. Biotechnol Bioeng 77:266–277. doi:10.1002/bit.10105
Harper WF, Terada A, Poly F, Le Roux X, Kristensen K, Mazher M, Smets BF (2009) The effect of hydroxylamine on the activity and aggregate structure of autotrophic nitrifying bioreactor cultures. Biotechnol Bioeng 102:714–724. doi:10.1002/bit.22121
Hellinga C, Schellen A, Mulder JW, van Loosdrecht MCM, Heijnen JJ (1998) The SHARON process: an innovative method for nitrogen removal from ammonium-rich waste water. Water Sci Technol 37:135–142. doi:10.1016/s0273-1223(98)00281-9
Hellinga C, Van Loosdrecht MCM, Heijnen JJ (1999) Model based design of a novel process for nitrogen removal from concentrated flows. Math Computer Model Dyn Syst 5:351–371. doi:10.1076/mcmd.5.4.351.3678
Henze M, Comeau Y (2008) Wastewater characterization. In: Henze M, van Loosdrecht MCM, Ekama GA, Brdjanovic D (eds) Biological wastewater treatment. IWA Publishing, London, pp 33–52
Henze M, Grady CPL, Gujer W, Marais GvR, Matsuo T (1987) Activated sludge model no. 1. IAWPRC Scientific and Technical Report No. 1. IAWPRC, London
Hooper AB (1968) A nitrite-reducing enzyme from Nitrosomonas europaea-preliminary characterization with hydroxylamine as electron donor. Biochim Biophys Acta 162:49–65. doi:10.1016/0005-2728(68)90213-2
Hooper AB, Terry KR (1979) Hydroxylamine oxidoreductase of nitrosomonas production of nitric-oxide from hydroxylamine. Biochim Biophys Acta 571:12–20. doi:10.1016/0005-2744(79)90220-1
Horn H, Reiff H, Morgenroth E (2003) Simulation of growth and detachment in biofilm systems under defined hydrodynamic conditions. Biotechnol Bioeng 81:607–617. doi:10.1002/bit.10503
Hu ZY, Lotti T, van Loosdrecht M, Kartal B (2013) Nitrogen removal with the anaerobic ammonium oxidation process. Biotechnol Lett 35:1145–1154. doi:10.1007/s10529-013-1196-4
Hyman MR, Arp DJ (1995) Effects of ammonia on the de novo synthesis of polypeptides in cells of Nitrosomonas europaea denied ammonia as an energy source. J Bacteriol 177:4974–4979
Itokawa H, Hanaki K, Matsuo T (2001) Nitrous oxide production in high-loading biological nitrogen removal process under low COD/N ratio condition. Water Res 35:657–664. doi:10.1016/s0043-1354(00)00309-2
Jason J, Cantera L, Stein LY (2007) Role of nitrite reductase in the ammonia-oxidizing pathway of Nitrosomonas europaea. Arch Microbiol 188:349–354. doi:10.1007/s00203-007-0255-4
Jetten MSM et al (1998) The anaerobic oxidation of ammonium. FEMS Microbiol Rev 22:421–437. doi:10.1111/j.1574-6976.1998.tb00379.x
Jia WL, Liang S, Zhang J, Ngo HH, Guo WS, Yan YJ, Zou YN (2013) Nitrous oxide emission in low-oxygen simultaneous nitrification and denitrification process: sources and mechanisms. Bioresour Technol 136:444–451. doi:10.1016/j.biortech.2013.02.117
Jiang QQ, Bakken LR (1999) Nitrous oxide production and methane oxidation by different ammonia-oxidizing bacteria. Appl Environ Microbiol 65:2679–2684
Jin RC, Yang GF, Yu JJ, Zheng P (2012) The inhibition of the Anammox process: a review. Chem Eng J 197:67–79. doi:10.1016/j.cej.2012.05.014
Kaelin D, Manser R, Rieger L, Eugster J, Rottermann K, Siegrist H (2009) Extension of ASM3 for two-step nitrification and denitrification and its calibration and validation with batch tests and pilot scale data. Water Res 43:1680–1692. doi:10.1016/j.watres.2008.12.039
Kampschreur MJ, Tan NCG, Picioreanu C, Jetten MSM, Schmidt I, van Loosdrecht MCM (2006) Role of nitrogen oxides in the metabolism of ammonia-oxidizing bacteria. Biochem Soc Trans 34:179–181
Kampschreur MJ, van der Star WRL, Wielders HA, Mulder JW, Jetten MSM, van Loosdrecht MCM (2008) Dynamics of nitric oxide and nitrous oxide emission during full-scale reject water treatment. Water Res 42:812–826. doi:10.1016/j.watres.2007.08.022
Kampschreur MJ et al (2009a) Emission of nitrous oxide and nitric oxide from a full-scale single-stage nitritation-anammox reactor. Water Sci Technol 60:3211–3217. doi:10.2166/wst.2009.608
Kampschreur MJ, Temmink H, Kleerebezem R, Jetten MSM, van Loosdrecht MCM (2009b) Nitrous oxide emission during wastewater treatment. Water Res 43:4093–4103. doi:10.1016/j.watres.2009.03.001
Kartal B, Kuypers MM, Lavik G, Schalk J, Op den Camp HJM, Jetten MSM, Strous M (2007a) Anammox bacteria disguised as denitrifiers: nitrate reduction to dinitrogen gas via nitrite and ammonium. Environ Microbiol 9:635–642. doi:10.1111/j.1462-2920.2006.01183.x
Kartal B et al (2007b) Candidatus “Anammoxoglobus propionicus” a new propionate oxidizing species of anaerobic ammonium oxidizing bacteria. Syst Appl Microbiol 30:39–49. doi:10.1016/j.syapm.2006.03.004
Kartal B, Tan NCG, Van de Biezen E, Kampschreur MJ, Van Loosdrecht MCM, Jetten MSM (2010) Effect of nitric oxide on anammox bacteria. Appl Environ Microbiol 76:6304–6306. doi:10.1128/aem.00991-10
Kartal B et al (2011) Molecular mechanism of anaerobic ammonium oxidation. Nature 479:127–130. doi:10.1038/nature10453
Kartal B, de Almeida NM, Maalcke WJ, Op den Camp HJM, Jetten MSM, Keltjens JT (2013) How to make a living from anaerobic ammonium oxidation. FEMS Microbiol Rev 37:428–461. doi:10.1111/1574-6976.12014
Kester RA, De Boer W, Laanbroek HJ (1997) Production of NO and N2O by pure cultures of nitrifying and denitrifying bacteria during changes in aeration. Appl Environ Microbiol 63:3872–3877
Khan MZ, Mondal PK, Sabir S (2013) Aerobic granulation for wastewater bioremediation: a review. Can J Chem Eng 91:1045–1058. doi:10.1002/cjce.21729
Kim CH, Hollocher TC (1984) Catalysis of nitrosyl transfer reactions by a dissimilatory nitrite reductase (cytochrome c, d1). J Biol Chem 259:2092–2099
Kim SW, Miyahara M, Fushinobu S, Wakagi T, Shoun H (2010) Nitrous oxide emission from nitrifying activated sludge dependent on denitrification by ammonia-oxidizing bacteria. Bioresour Technol 101:3958–3963. doi:10.1016/j.biortech.2010.01.030
Kindaichi T, Ito T, Okabe S (2004a) Ecophysiological interaction between nitrifying bacteria and heterotrophic bacteria in autotrophic nitrifying biofilms as determined by microautoradiography-fluorescence in situ hybridization. Appl Environ Microbiol 70:1641–1650. doi:10.1128/aem.70.3.1641-1650.2004
Kindaichi T, Okabe S, Satoh H, Watanabe Y (2004b) Effects of hydroxylamine on microbial community structure and function of autotrophic nitrifying biofilms determined by in situ hybridization and the use of microelectrodes. Water Sci Technol 49:61–68
Kong Q, Zhang J, Miao M, Tian L, Guo N, Liang S (2013) Partial nitrification and nitrous oxide emission in an intermittently aerated sequencing batch biofilm reactor. Chem Eng J 217:435–441. doi:10.1016/j.cej.2012.10.093
Körner H, Zumft WG (1989) Expression of denitrification enzymes in response to the dissolved oxygen level and respiratory substrate in continuous culture of Pseudomonas stutzeri. Appl Environ Microbiol 55:1670–1676
Kostera J, Youngblut MD, Slosarczyk JM, Pacheco AA (2008) Kinetic and product distribution analysis of NO center dot reductase activity in Nitrosomonas europaea hydroxylamine oxidoreductase. J Biol Inorg Chem 13:1073–1083. doi:10.1007/s00775-008-0393-4
Kostera J, McGarry J, Pacheco AA (2010) Enzymatic interconversion of ammonia and nitrite: the right tool for the job. Biochemistry 49:8546–8553. doi:10.1021/bi1006783
Kozlowski JA, Price J, Stein LY (2014) Revision of N2O-producing pathways in the ammonia-oxidizing bacterium Nitrosomonas europaea ATCC 19718. Appl Environ Microbiol 80:4930–4935. doi:10.1128/aem.01061-14
Kuenen JG (2008) Anammox bacteria: from discovery to application. Nat Rev Microbiol 6:320–326
Lackner S, Horn H (2012) Comparing the performance and operation stability of an SBR and MBBR for single-stage nitritation-anammox treating wastewater with high organic load. Environ Technol 34:1319–1328. doi:10.1080/09593330.2012.746735
Law Y, Lant P, Yuan ZG (2011) The effect of pH on N2O production under aerobic conditions in a partial nitritation system. Water Res 45:5934–5944. doi:10.1016/j.watres.2011.08.055
Law Y, Lant P, Yuan Z (2013) The confounding effect of nitrite on N2O production by an enriched ammonia-oxidizing culture. Environ Sci Technol 47:7186–7194. doi:10.1021/es4009689
Law Y, Ni BJ, Lant P, Yuan ZG (2012a) N2O production rate of an enriched ammonia-oxidising bacteria culture exponentially correlates to its ammonia oxidation rate. Water Res 46:3409–3419. doi:10.1016/j.watres.2012.03.043
Law Y, Ye L, Pan Y, Yuan Z (2012b) Nitrous oxide emissions from wastewater treatment processes. Philos Trans R Soc Lond B Biol Sci 367:1265–1277. doi:10.1098/rstb.2011.0317
Liu B, Frostegård Å, Bakken LR (2014) Impaired reduction of N2O to N2 in acid soils is due to a posttranscriptional interference with the expression of nosZ. MBio. doi:10.1128/mBio.01383-14
Lo IW, Lo KV, Mavinic DS, Shiskowski D, Ramey W (2010) Contributions of biofilm and suspended sludge to nitrogen transformation and nitrous oxide emission in hybrid sequencing batch system. J Environ Sci 22:953–960. doi:10.1016/S1001-0742(09)60204-7
Lochmatter S, Gonzalez-Gil G, Holliger C (2013) Optimized aeration strategies for nitrogen and phosphorus removal with aerobic granular sludge. Water Res 47:6187–6197. doi:10.1016/j.watres.2013.07.030
Lotti T, van der Star WRL, Kleerebezem R, Lubello C, van Loosdrecht MCM (2012) The effect of nitrite inhibition on the anammox process. Water Res 46:2559–2569. doi:10.1016/j.watres.2012.02.011
Lotti T, Kleerebezem R, Lubello C, van Loosdrecht MCM (2014) Physiological and kinetic characterization of a suspended cell anammox culture. Water Res 60:1–14. doi:10.1016/j.watres.2014.04.017
Ma Y, Sundar S, Park H, Chandran K (2015) The effect of inorganic carbon on microbial interactions in a biofilm nitritation–anammox process. Water Res 70:246–254. doi:10.1016/j.watres.2014.12.006
Maia LB, Moura JJG (2014) How biology handles nitrite. Chem Rev 114:5273–5357. doi:10.1021/cr400518y
Morgenroth E (2008a) Modelling biofilm. In: Henze M, van Loosdrecht MCM, Ekama GA, Brdjanovic D (eds) Biological wastewater treatment. IWA Publishing, London, pp 457–492
Morgenroth E (2008b) Biofilm reactors. In: Henze M, van Loosdrecht MCM, Ekama GA, Brdjanovic D (eds) Biological wastewater treatment. IWA Publishing, London, pp 457–492
Morgenroth E, Wilderer PA (2000) Influence of detachment mechanisms on competition in biofilms. Water Res 34:417–426. doi:10.1016/S0043-1354(99)00157-8
Morley N, Baggs EM, Dörsch P, Bakken L (2008) Production of NO, N2O and N2 by extracted soil bacteria, regulation by NO2 − and O2 concentrations. FEMS Microbiol Ecol 65:102–112. doi:10.1111/j.1574-6941.2008.00495.x
Mulder A, Vandegraaf AA, Robertson LA, Kuenen JG (1995) Anaerobic ammonium oxidation discovered in a denitrifying fluidized-bed reactor. FEMS Microbiol Ecol 16:177–183. doi:10.1111/j.1574-6941.1995.tb00281.x
Ni B-J, Yuan Z (2013) A model-based assessment of nitric oxide and nitrous oxide production in membrane-aerated autotrophic nitrogen removal biofilm systems. J Membr Sci 428:163–171. doi:10.1016/j.memsci.2012.10.049
Ni B-J, Ruscalleda M, Pellicer-Nàcher C, Smets BF (2011) Modeling nitrous oxide production during biological nitrogen removal via nitrification and denitrification: extensions to the general ASM models. Environ Sci Technol 45:7768–7776. doi:10.1021/es201489n
Ni B-J, Smets BF, Yuan Z, Pellicer-Nàcher C (2013) Model-based evaluation of the role of anammox on nitric oxide and nitrous oxide productions in membrane aerated biofilm reactor. J Membr Sci 446:332–340. doi:10.1016/j.memsci.2013.06.047
Ni BJ, Peng L, Law Y, Guo J, Yuan Z (2014) Modeling of nitrous oxide production by autotrophic ammonia-oxidizing bacteria with multiple production pathways. Environ Sci Technol 48:3916–3924. doi:10.1021/es405592h
Okabe S, Satoh H, Watanabe Y (1999) In situ analysis of nitrifying biofilms as determined by in situ hybridization and the use of microelectrodes. Appl Environ Microbiol 65:3182–3191
Pan YT, Ni BJ, Yuan ZG (2013) Modeling electron competition among nitrogen oxides reduction and N2O accumulation in denitrification. Environ Sci Technol 47:11083–11091. doi:10.1021/es402348n
Park S, Bae W (2009) Modeling kinetics of ammonium oxidation and nitrite oxidation under simultaneous inhibition by free ammonia and free nitrous acid. Process Biochem 44:631–640. doi:10.1016/j.procbio.2009.02.002
Park KY, Inamori Y, Mizuochi M, Ahn KH (2000) Emission and control of nitrous oxide from a biological wastewater treatment system with intermittent aeration. J Biosci Bioeng 90:247–252. doi:10.1016/S1389-1723(00)80077-8
Peng L, Ni B-J, Erler D, Ye L, Yuan Z (2014) The effect of dissolved oxygen on N2O production by ammonia-oxidizing bacteria in an enriched nitrifying sludge. Water Res 66:12–21. doi:10.1016/j.watres.2014.08.009
Peng L, Ni B-J, Ye L, Yuan Z (2015a) The combined effect of dissolved oxygen and nitrite on N2O production by ammonia oxidizing bacteria in an enriched nitrifying sludge. Water Res 73:29–36. doi:10.1016/j.watres.2015.01.021
Peng L, Ni BJ, Ye L, Yuan Z (2015b) N2O production by ammonia oxidizing bacteria in an enriched nitrifying sludge linearly depends on inorganic carbon concentration. Water Res 74:58–66. doi:10.1016/j.watres.2015.02.003
Peng L, Liu Y, Ni B-J (2016a) Nitrous oxide production in completely autotrophic nitrogen removal biofilm process: a simulation study. Chem Eng J 287:217–224. doi:10.1016/j.cej.2015.11.026
Peng L, Ni B-J, Law Y, Yuan Z (2016b) Modeling N2O production by ammonia oxidizing bacteria at varying inorganic carbon concentrations by coupling the catabolic and anabolic processes. Chem Eng Sci 144:386–394. doi:10.1016/j.ces.2016.01.033
Pijuan M, Torà J, Rodríguez-Caballero A, César E, Carrera J, Pérez J (2014) Effect of process parameters and operational mode on nitrous oxide emissions from a nitritation reactor treating reject wastewater. Water Res 49:23–33. doi:10.1016/j.watres.2013.11.009
Poth M, Focht DD (1985) N-15 kinetic-analysis of N2O production by Nitrosomonas europaea-an examination of nitrifier dentrification. Appl Environ Microbiol 49:1134–1141
Poughon L, Dussap C-G, Gros J-B (2001) Energy model and metabolic flux analysis for autotrophic nitrifiers. Biotechnol Bioeng 72:416–433. doi:10.1002/1097-0290(20000220)72:4<416:AID-BIT1004>3.0.CO;2-D
Quinlan AV (1984) Prediction of the optimum pH for ammonia-n oxidation by Nitrosomonas europaea in well-aerated natural and domestic-waste waters. Water Res 18:561–566. doi:10.1016/0043-1354(84)90204-5
Ravishankara AR, Daniel JS, Portmann RW (2009) Nitrous Oxide (N2O): the dominant ozone-depleting substance emitted in the 21st century. Science 326:123–125. doi:10.1126/science.1176985
Reichert P (1994) AQUASIM-a tool for simulation and data-analysis of aquatic systems. Water Sci Technol 30:21–30
Rezić T, Šantek B, Novak S, Marić V (2007) Heterotrophic cultivation of Paracoccus denitrificans in a horizontal rotating tubular bioreactor. World J Microbiol Biotechnol 23:987–996. doi:10.1007/s11274-006-9324-0
Richardson D, Felgate H, Watmough N, Thomson A, Baggs E (2009) Mitigating release of the potent greenhouse gas N2O from the nitrogen cycle-could enzymic regulation hold the key? Trends Biotechnol 27:388–397. doi:10.1016/j.tibtech.2009.03.009
Rittmann BE, Regan JM, Stahl DA (1994) Nitrification as a source of soluble organic substrate in biological treatment. Water Sci Technol 30:1–8
Sabba F, Picioreanu C, Pérez J, Nerenberg R (2015) Hydroxylamine diffusion can enhance N2O emissions in nitrifying biofilms: a modeling study. Environ Sci Technol 49:1486–1494. doi:10.1021/es5046919
Schmid M et al (2000) Molecular evidence for genus level diversity of bacteria capable of catalyzing anaerobic ammonium oxidation. Syst Appl Microbiol 23:93–106. doi:10.1016/S0723-2020(00)80050-8
Schmidt I (2008) Nitric oxide: interaction with the ammonia monooxygenase and regulation of metabolic activities in ammonia oxidizers. In: Cadenas E, Packer L (eds) Nitric oxide, Part F: oxidative and nitrosative stress in redox regulation of cell signaling, vol 440. Methods in Enzymology. Elsevier Academic Press Inc, San Diego, pp 121–135. doi:10.1016/s0076-6879(07)00807-5
Schmidt I (2009) Chemoorganoheterotrophic growth of Nitrosomonas europaea and Nitrosomonas eutropha. Curr Microbiol 59:130–138. doi:10.1007/s00284-009-9409-8
Schmidt I, Bock E (1997) Anaerobic ammonia oxidation with nitrogen dioxide by Nitrosomonas eutropha. Arch Microbiol 167:106–111. doi:10.1007/s002030050422
Schmidt I, Bock E (1998) Anaerobic ammonia oxidation by cell-free extracts of Nitrosomonas eutropha Antonie Van Leeuwenhoek. Int J Gen Mol Microbiol 73:271–278. doi:10.1023/a:1001572121053
Schmidt I, Bock E, Jetten MSM (2001a) Ammonia oxidation by Nitrosomonas eutropha with NO2 as oxidant is not inhibited by acetylene. Microbiology-Sgm 147:2247–2253
Schmidt I, Zart D, Bock E (2001b) Effects of gaseous NO2 on cells of Nitrosomonas eutropha previously incapable of using ammonia as an energy source Antonie Van Leeuwenhoek. Int J Gen Mol Microbiol 79:39–47. doi:10.1023/a:1010269331350
Schmidt I, Steenbakkers PJM, Op den Camp HJM, Schmidt K, Jetten MSM (2004a) Physiologic and proteomic evidence for a role of nitric oxide in biofilm formation by Nitrosomonas europaea and other ammonia oxidizers. J Bacteriol 186:2781–2788. doi:10.1128/jb.186.9.2781-2788.2004
Schmidt I, van Spanning RJM, Jetten MSM (2004b) Denitrification and ammonia oxidation by Nitrosomonas europaea wild-type, and NirK- and NorB-deficient mutants. Microbiology-Sgm 150:4107–4114. doi:10.1099/mic.0.27382-0
Schramm A, de Beer D, van den Heuvel JC, Ottengraf S, Amann R (1999) Microscale distribution of populations and activities of Nitrosospira and Nitrospira spp. along a macroscale gradient in a nitrifying bioreactor: quantification by in situ hybridization and the use of microsensors. Appl Environ Microbiol 65:3690–3696
Schreiber F, Loeffler B, Polerecky L, Kuypers MMM, de Beer D (2009) Mechanisms of transient nitric oxide and nitrous oxide production in a complex biofilm. ISME J 3:1301–1313. doi:10.1038/ismej.2009.55
Schreiber F, Wunderlin P, Udert KM, Wells GF (2012) Nitric oxide and nitrous oxide turnover in natural and engineered microbial communities: biological pathways, chemical reactions, and novel technologies. Front Microbiol 3:24. doi:10.3389/fmicb.2012.00372
Schulthess RV, Kühni M, Gujer W (1995) Release of nitric and nitrous oxides from denitrifying activated sludge. Water Res 29:215–226. doi:10.1016/0043-1354(94)E0108-I
Shaw LJ, Nicol GW, Smith Z, Fear J, Prosser JI, Baggs EM (2006) Nitrosospira spp. can produce nitrous oxide via a nitrifier denitrification pathway. Environ Microbiol 8:214–222. doi:10.1111/j.1462-2920.2005.00882.x
Shen T, Stieglmeier M, Dai J, Urich T, Schleper C (2013) Responses of the terrestrial ammonia-oxidizing archaeon Ca. Nitrososphaera viennensis and the ammonia-oxidizing bacterium Nitrosospira multiformis to nitrification inhibitors. FEMS Microbiol Lett 344(2):121–129. doi:10.1111/1574-6968.12164
Sliekers AO, Haaijer SCM, Stafsnes MH, Kuenen JG, Jetten MSM (2005) Competition and coexistence of aerobic ammonium- and nitrite-oxidizing bacteria at low oxygen concentrations. Appl Microbiol Biotechnol 68:808–817. doi:10.1007/s00253-005-1974-6
Spieck E, Lipski A (2011) Chapter five-cultivation, growth physiology, and chemotaxonomy of Nitrite-Oxidizing bacteria. In: Martin GK (ed) Methods in enzymology, vol 486. Academic Press, London, pp 109–130. doi:10.1016/B978-0-12-381294-0.00005-5
Spott O, Russow R, Stange CF (2011) Formation of hybrid N2O and hybrid N2 due to codenitrification: first review of a barely considered process of microbially mediated N-nitrosation. Soil Biol Biochem 43:1995–2011. doi:10.1016/j.soilbio.2011.06.014
Starkenburg SR, Arp DJ, Bottomley PJ (2008) Expression of a putative nitrite reductase and the reversible inhibition of nitrite-dependent respiration by nitric oxide in Nitrobacter winogradskyi Nb-255. Environ Microbiol 10:3036–3042. doi:10.1111/j.1462-2920.2008.01763.x
Stein LY, Arp DJ (1998) Loss of ammonia monooxygenase activity in Nitrosomonas europaea upon exposure to nitrite. Appl Environ Microbiol 64:4098–4102
Strous M et al (1999a) Missing lithotroph identified as new planctomycete. Nature 400:446–449
Strous M, Kuenen JG, Jetten MSM (1999b) Key physiology of anaerobic ammonium oxidation. Appl Environ Microbiol 65:3248–3250
Stuven R, Bock E (2001) Nitrification and denitrification as a source for NO and NO2 production in high-strength wastewater. Water Res 35:1905–1914. doi:10.1016/s0043-1354(00)00471-1
Sundermeyer-Klinger H, Meyer W, Warninghoff B, Bock E (1984) Membrane-bound nitrite oxidoreductase of Nitrobacter: evidence for a nitrate reductase system. Arch Microbiol 140:153–158. doi:10.1007/BF00454918
Suzuki I, Dular U, Kwok SC (1974) Ammonia or ammonium ion as substrate for oxidation by Nitrosomonas europaea cells and extracts. J Bacteriol 120:556–558
Tallec G, Garnier J, Gousailles M (2006) Nitrogen removal in a wastewater treatment plant through biofilters: nitrous oxide emissions during nitrification and denitrification. Bioprocess Biosyst Eng 29:323–333. doi:10.1007/s00449-006-0081-0
Todt D, Dörsch P (2015) Nitrous oxide emissions in a biofilm loaded with different mixtures of concentrated household wastewater. Int J Environ Sci Technol. doi:10.1007/s13762-015-0778-1
Udert KM, Jenni S (2013) Biological nitrogen conversion processes. In: Larsen TA, Udert KM, Lienert J (eds) Source separation and decentralization for wastewater management. IWA Publishing, London, pp 291–305
Udert KM, Larsen TA, Gujer W (2005) Chemical nitrite oxidation in acid solutions as a consequence of microbial ammonium oxidation. Environ Sci Technol 39:4066–4075. doi:10.1021/es048422m
Upadhyay AK, Hooper AB, Hendrich MP (2006) NO reductase activity of the tetraheme cytochrome C(554) of Nitrosomonas europaea. J Am Chem Soc 128:4330–4337. doi:10.1021/ja055183+
van de Graaf AA, Mulder A, de Bruijn P, Jetten MS, Robertson LA, Kuenen JG (1995) Anaerobic oxidation of ammonium is a biologically mediated process. Appl Environ Microbiol 61:1246–1251
van Kessel MAHJ et al (2015) Complete nitrification by a single microorganism. Nature. doi:10.1038/nature16459
Wang CC, Lee PH, Kumar M, Huang YT, Sung SW, Lin JG (2010) Simultaneous partial nitrification, anaerobic ammonium oxidation and denitrification (SNAD) in a full-scale landfill-leachate treatment plant. J Hazard Mater 175:622–628. doi:10.1016/j.jhazmat.2009.10.052
Wanner O, Gujer W (1985) Competition in biofilms. Water Sci Technol 17:27–44
Wanner O, Gujer W (1986) A multispecies biofilm model. Biotechnol Bioeng 28:314–328. doi:10.1002/bit.260280304
Wanner O, Reichert P (1996) Mathematical modeling of mixed-culture biofilms. Biotechnol Bioeng 49:172–184
Wanner O, Eberl HJ, Morgenroth E, Noguera DR, Picioreanu C, Rittmann BE, van Loosdrecht MCM (2006) Mathematical modeling of biofilms. IWA Publishing, London
Whittaker M, Bergmann D, Arciero D, Hooper AB (2000) Electron transfer during the oxidation of ammonia by the chemolithotrophic bacterium Nitrosomonas europaea. Biochim Biophys Acta-Bioenerg 1459:346–355. doi:10.1016/s0005-2728(00)00171-7
Winkler MKH, Kleerebezem R, van Loosdrecht MCM (2012a) Integration of anammox into the aerobic granular sludge process for main stream wastewater treatment at ambient temperatures. Water Res 46:136–144. doi:10.1016/j.watres.2011.10.034
Winkler MKH, Yang J, Kleerebezem R, Plaza E, Trela J, Hultman B, van Loosdrecht MCM (2012b) Nitrate reduction by organotrophic anammox bacteria in a nitritation/anammox granular sludge and a moving bed biofilm reactor. Bioresour Technol 114:217–223. doi:10.1016/j.biortech.2012.03.070
Wood PM (1986) Nitrification as a bacterial energy source. In: Prosser JI (ed) Nitrification. Society for general microbiology. IRL Press, Oxford, pp 39–62
Wunderlin P, Mohn J, Joss A, Emmenegger L, Siegrist H (2012) Mechanisms of N2O production in biological wastewater treatment under nitrifying and denitrifying conditions. Water Res 46:1027–1037. doi:10.1016/j.watres.2011.11.080
Wunderlin P, Lehmann MF, Siegrist H, Tuzson B, Joss A, Emmenegger L, Mohn J (2013) Isotope signatures of N2O in a mixed microbial population system: constraints on N2O producing pathways in wastewater treatment. Environ Sci Technol 47:1339–1348. doi:10.1021/es303174x
Xiao Y, Wu S, Yang Z-H, Wang Z-J, Yan C-Z, Zhao F (2013) In situ probing the effect of potentials on the microenvironment of heterotrophic denitrification biofilm with microelectrodes. Chemosphere 93:1295–1300. doi:10.1016/j.chemosphere.2013.06.065
Yamanaka T, Shinra M (1974) Cytochrome C-552 and cytochrome C-554 derived from Nitrosomonas-Europaes-purification, properties, and their function in hydroxylamine oxidation. J Biochem 75:1265–1273
Yang JJ, Trela J, Plaza E, Tjus K (2013) N2O emissions from a one stage partial nitrification/anammox process in moving bed biofilm reactors. Water Sci Technol 68:144–152. doi:10.2166/wst.2013.232
Yoo H, Ahn K-H, Lee H-J, Lee K-H, Kwak Y-J, Song K-G (1999) Nitrogen removal from synthetic wastewater by simultaneous nitrification and denitrification (SND) via nitrite in an intermittently-aerated reactor. Water Res 33:145–154. doi:10.1016/S0043-1354(98)00159-6
Yoshinari T (1990) Emissions of N2O from various environments—the use of stable isotope composition of N2O as tracer for the studies of N2O biogeochemical cycling. In: Revsbech N, Sørensen J (eds) Denitrification in soil and sediment, vol 56. Federation of European microbiological societies symposium series. Springer, US, pp 129–150. doi:10.1007/978-1-4757-9969-9_8
Yu R, Chandran K (2010) Strategies of Nitrosomonas europaea 19718 to counter low dissolved oxygen and high nitrite concentrations. BMC Microbiol 10:11. doi:10.1186/1471-2180-10-70
Yu R, Kampschreur MJ, van Loosdrecht MCM, Chandran K (2010) Mechanisms and specific directionality of autotrophic nitrous oxide and nitric oxide generation during transient anoxia. Environ Sci Technol 44:1313–1319. doi:10.1021/es902794a
Zhang DJ, Cai Q, Zu B, Bai C, Zhang P (2010) The influence of trace NO(2) on the kinetics of ammonia oxidation and the characteristics of nitrogen removal from wastewater. Water Sci Technol 62:1037–1044. doi:10.2166/wst.2010.319
Zhou Y, Pijuan M, Yuan Z (2008a) Development of a 2-sludge, 3-stage system for nitrogen and phosphorous removal from nutrient-rich wastewater using granular sludge and biofilms. Water Res 42:3207–3217. doi:10.1016/j.watres.2008.04.012
Zhou Y, Pijuan M, Zeng RJ, Yuan Z (2008b) Free nitrous acid inhibition on nitrous oxide reduction by a denitrifying-enhanced biological phosphorus removal sludge. Environ Sci Technol 42:8260–8265. doi:10.1021/es800650j
Zhou Y, Oehmen A, Lim M, Vadivelu V, Ng WJ (2011) The role of nitrite and free nitrous acid (FNA) in wastewater treatment plants. Water Res 45:4672–4682. doi:10.1016/j.watres.2011.06.025
Zumft WG (1997) Cell biology and molecular basis of denitrification. Microbiol Mol Biol Rev 61:533–616
Acknowledgments
This study was supported by the Norwegian Research Council (Grant No skattefunn-226774) and Ecomotive AS, the present employer of the corresponding author. We thank Linda Hink (University of Aberdeen) for reviewing the chapter on AOB metabolism.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Todt, D., Dörsch, P. Mechanism leading to N2O production in wastewater treating biofilm systems. Rev Environ Sci Biotechnol 15, 355–378 (2016). https://doi.org/10.1007/s11157-016-9401-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11157-016-9401-2