Abstract
This paper discusses the microbial basis and the latest research on nitrous oxide (N2O) emissions from biofilms processes for wastewater treatment. Conditions that generally promote N2O formation in biofilms include (1) low DO values, or spatial DO transitions from high to low within the biofilm; (2) DO fluctuations within biofilm due to varying bulk DO concentrations or varying substrate concentrations; (3) conditions with high reaction rates, which lead to greater formation of intermediates, e.g., hydroxylamine (NH2OH) and nitrite (NO2−), that promote N2O formation; and (4) electron donor limitation for denitrification. Formation of N2O directly results from the activities of ammonia-oxidizing bacteria (AOB), ammonia-oxidizing archaea (AOA), and heterotrophic denitrifying bacteria. More research is needed on the roles of AOA, comammox, and specialized denitrifying microorganisms. In nitrifying biofilms, higher bulk ammonia (NH3) concentrations, higher nitrite (NO2−) concentrations, lower dissolved oxygen (DO), and greater biofilm thicknesses result in higher N2O emissions. In denitrifying biofilms, N2O accumulates at low levels as an intermediate and at higher levels at the oxic/anoxic transition regions of the biofilms and where COD becomes limiting. N2O formed in the outer regions can be consumed in the inner regions if COD penetrates sufficiently. In membrane-aerated biofilms, where nitrification takes place in the inner, aerobic biofilm region, the exterior anoxic biofilm can serve as a N2O sink. Reactors that include variable aeration or air scouring, such as denitrifying filters, trickling filters, or rotating biological contactors (RBCs), can form peaks of N2O emissions during or following a scouring or aeration event. N2O emissions from biofilm processes depend on the microbial composition, biofilm thickness, substrate concentrations and variability, and reactor type and operation. Given the complexity and difficulty in quantifying many of these factors, it may be difficult to accurately predict emissions for full-scale treatment plants. However, a better understanding of the mechanisms and the impacts of process configurations can help minimize N2O emission from biofilm processes for wastewater treatment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Wastewater treatment processes can be a significant source of nitrous oxide (N2O), a powerful greenhouse gas (GHG) with a global warming potential around 300 times that of carbon dioxide (CO2) (Montzka et al. 2011). N2O is very stable and may persist in the atmosphere for over 120 years (Kampschreur et al. 2009; Schreiber et al. 2012). The U.S. Environmental Protection Agency (EPA) estimates that U.S. wastewater treatment plants emit around 5.2 Tg N2O year−1 as CO2 equivalents (Ritter & Chitikela 2014), and these amounts are expected to increase with time (Law et al. 2012; Okabe et al. 2011).
Much past research has addressed N2O emissions from suspended growth processes (Ahn et al. 2010; Kampschreur et al. 2009; Law et al. 2012). However, much less is known about emissions from biofilm processes, such as the moving bed biofilm reactor (MBBR), integrated fixed-film activated sludge (IFAS), biological aerated filter (BAF), granular sludge, and membrane-aerated biofilm reactors (MABRs) (Henze et al. 2008; Martin and Nerenberg 2012; Syron and Casey 2008). Biofilm processes are becoming increasingly popular due to their higher volumetric treatment rates, reduced operational costs, minimal need for settling, and operational simplicity (Henze et al. 2008; Khan et al. 2013; Nicolella et al. 2000; WEF 2010).
While the microbial basis of N2O formation, i.e., the microorganisms and metabolic pathways leading to its formation, are the same for suspended growth and biofilm systems, the observed behavior may be very different. This results from the microbial stratification, microbial interactions, substrate gradients, and substrate interactions unique to biofilms, as well as the biofilm reactor configuration (Henze et al. 2008; Law et al. 2012; Vlaeminck et al. 2010). Thus, the “mechanisms” leading to N2O emissions in biofilms may significantly differ from those of suspended growth systems.
Todt and Dorsch (2016) provided a comprehensive review of N2O emissions from biofilm systems. They explored the biochemistry of N2O production/consumption in relevant organisms, discussed current biofilm models, evaluated possible environmental factors affecting N2O emissions, and tabulated emission factors for different processes. Massara et al. (2017) briefly addressed biofilms as part of a comprehensive review of N2O emissions from wastewater processes. This review provides an update, considering new information on the N2O emissions from microbial systems. It also discusses new types of microbial metabolism and different biofilm reactor configurations, and their impacts on N2O emissions.
Biofilms vs. suspended growth systems
Biofilms are aggregates of microbial cells embedded in a network of self-produced extracellular polymeric substances (EPS) (Flemming et al. 2016; Stoodley et al. 2002). Biofilms are widespread in natural systems (Donlan 2002) and increasingly used in engineered treatment processes, especially for those with low substrate concentrations and high flows (Henze et al. 2008; Nicolella et al. 2000; WEF 2010). Unlike with suspended bacteria, diffusion and reaction in biofilms lead to substrate gradients. As a result, concentrations in the biofilm may differ significantly from those in the bulk liquid (Fig. 1). In addition, bacteria stratify into layers, where different types of metabolism may predominate at different depths within the biofilm.
Idealized schematics of a a floc and b a biofilm. The biofilm schematic shows the liquid diffusion layer (LDL), as well as profiles of a substrate and metabolic product. Note that real flocs are highly complex and heterogeneous in morphology, and biofilms may have rough or dendritic surfaces with internal pores
The dynamics of growth, decay, and detachment influence the microbial community structure of biofilms (Elenter et al. 2007). Slow growing organisms may be “pushed out” of the biofilm by faster growing organisms (Lackner et al. 2008; Xavier et al. 2005). Metabolic products may diffuse out of the biofilm or may be consumed by other populations. pH gradients may form due to proton-producing or consuming processes within the biofilm (Vroom et al. 1999). The greater complexity of biofilms, compared to suspended growth processes, makes their behavior more difficult to predict.
N2O and nitrogen cycle
This section discusses basic microbial transformations that affect N2O formation in wastewater treatment processes. These processes are relevant to both suspended growth and biofilm processes. The relationship between these transformations and N2O formation in biofilms is discussed in subsequent sections.
The nitrogen cycle includes a number of N species and both microbial and abiotic transformations, where N varies in redox state between − 3 and + 5. While most of the nitrogen cycle is well established, new biotic and abiotic transformation processes continue to be discovered (Daims et al. 2016; Kuypers et al. 2018; Schreiber et al. 2012; Stein and Klotz 2016). Figure 2 schematically shows key N species and biological transformations. For wastewater treatment processes, the key transformations include nitrification and denitrification, where nitrate (NO3−) is sequentially reduced to nitrogen gas (N2). Both processes can lead to N2O formation.
Key processes in the N-cycle. N2O is highlighted in gray (adapted from Daims et al. 2016 and Schreiber et al. 2012). The dashed line for comammox shows not only the formation of NO2− as intermediate but also its oxidation to NO3− by the same organism. Abbreviations in figure: DNRA is dissimilatory nitrite reduction to ammonia; assimil. is assimilatory; dissimil. is dissimilatory. Note that denitrification can produce N2O, but it is also the only known process that can reduce it
N2O from microorganisms related to nitrification
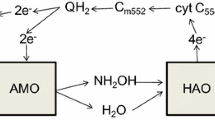
Nitrification is carried out by the sequential activity of ammonia-oxidizing bacteria (AOB) and archaea (AOA), and nitrite-oxidizing bacteria (NOB). AOB and AOA oxidize ammonia (NH3) to nitrite (NO2−), with hydroxylamine (NH2OH) as an intermediate (Fig. 3) (Daims et al. 2016; Guo et al. 2017), while NOB oxidize NO2− to NO3−. AOB directly produce N2O through two main pathways: nitrifier denitrification and NH2OH oxidation (Fig. 3). NOB, AOA, anammox, and comammox microorganisms may play an indirect role in N2O formation by affecting the availability of NH3 and NO2−.
Nitrogen transformations in AOB, NOB, and DNB. Abbreviations: AOB, ammonia-oxidizing bacteria; NOB, nitrite-oxidizing bacteria; DNB, denitrifying bacteria, AMO, ammonia monooxygenase; HAO, hydroxylamine oxidoreductase (hydroxylamine dehydrogenase in Nitrospira); NXR, nitrite oxidoreductase; NirK, copper-containing nitrite reductase; NirS, cytochrome cd1 type nitrite reductase; NOR, nitric oxide reductase; and NOS, nitrous oxide reductase. Purple arrows show intermediates potentially shared between nitrification and denitrification pathways. Abiotic reactions (gray) are further discussed in the text
In the nitrifier denitrification pathway, AOB reduce NO2− to nitric oxide (NO) and N2O (Chandran et al. 2011; Kampschreur et al. 2007; Kim et al. 2010; Tallec et al. 2006) (Fig. 3). The NH2OH oxidation pathway involves the oxidation of NH2OH to NO by hydroxylamine oxidoreductase (HAO) and subsequent reduction to N2O catalyzed by the enzyme NO reductase (Chandran et al. 2011; Law et al. 2012; Stein 2011) (Fig. 3).
Recent findings show that, in the canonical nitrifying bacteria N. europaea, two other routes for N2O production exist under anaerobic conditions. One is the direct oxidation of NH2OH to N2O by cytochrome P460 (Caranto et al. 2016) and the nitrification intermediate NO (Caranto and Lancaster 2017). Although not all AOB share the same route for N2O production, these recent findings expand on previous knowledge where chemical reactions were thought to be mainly important at higher oxygen (O2) levels (Liu et al. 2017a).
N2O can also be produced biologically or abiotically by coupling NH2OH oxidation with the reduction of NO2− (Harper et al. 2015; Terada et al. 2017), free nitrous acid (HNO2) (Soler-Jofra et al. 2016), or NO (Spott et al. 2011). These are termed N-nitrosation hybrid reactions, or simply “hybrid” reactions (Spott and Stange 2011). In addition, metals, such as copper (Harper et al. 2015) and manganese (Heil et al. 2015), can catalyze abiotic N2O production from NH2OH via the hybrid reaction. Under some conditions, the hybrid reaction can become a predominant pathway for N2O production in a partial nitrifying reactor (Soler-Jofra et al. 2018; Terada et al. 2017). N2O production via the hybrid reaction is enhanced in the presence of AOB (Liu et al. 2017a; Terada et al. 2017).
Under aerobic conditions, N2O is mainly formed via the NH2OH pathway, and rates are relatively low. When DO concentrations decrease, the nitrifier denitrification pathway becomes more important, leading to higher rates of N2O formation (Chen et al. 2018; Kampschreur et al. 2009; Ma et al. 2017a; Park et al. 2000; Tallec et al. 2008). However, under complete anoxic conditions N2O emissions are again low due to the lack of DO for NH3 oxidation (Fig. 3). Spikes of N2O production can occur at transitions from anoxic to aerobic, or aerobic to anoxic, conditions, due to an electron imbalance (Domingo-Felez et al. 2014; Kampschreur et al. 2008; Sabba et al. 2015; Yu et al. 2010). Thus, N2O emissions can be significant in processes with anoxic/aerobic stages or intermittent aeration (Chandran et al. 2011).
Unlike AOB, which have well-elucidated N2O production pathways, the pathways for AOA are yet to be fully understood (Blum et al. 2018b). They perform NH3 oxidation in a similar way to AOB (Kozlowski et al. 2016); however, they lack the ability to produce N2O enzymatically through side reactions of NH3 oxidation or nitrifier denitrification, as mediated by AOB (Spang et al. 2012; Tourna et al. 2011; Walker et al. 2010). Stieglmeier et al. (2014) showed that Nitrososphaera viennensis, a pure culture of AOA from soil, produces N2O via a hybrid reaction. While AOA are found in WWTPs (Park et al. 2006; Sauder et al. 2012; Zhang et al. 2009), AOA are more common in marine environments (Santoro et al. 2011) and soils (Gubry-Rangin et al. 2010; Li et al. 2018; Nicol et al. 2008; Zhang et al. 2012).
Anammox bacteria convert NH3 and NO2− to N2 under anoxic conditions (Kuypers et al. 2003). NO is a key intermediate in anammox metabolism (Kartal et al. 2011), and genomic evidence suggests that anammox species have the potential to produce N2O via NO reduction (Kartal et al. 2007; Strous et al. 2006). However, research suggests that N2O production under process-relevant conditions is negligible (Blum et al. 2018a). Anammox may indirectly affect N2O formation by heterotrophs and AOB by reducing the concentrations of NH3 and NO2−.
Comammox bacteria are a subset of the genus Nitrospira capable of complete ammonia oxidation (comammox) via oxidation of NH3 to NO3− (Daims et al. 2015; van Kessel et al. 2015). Comammox are thought to have a competitive advantage over conventional ammonia oxidizers (e.g., AOA and AOB) under ammonia-limiting conditions (Costa et al. 2006; Daims et al. 2015; Kits et al. 2017; van Kessel et al. 2015). While little is known about comammox in wastewater biofilms, van Kessel et al. (2015) and Daims et al. (2015) obtained comammox enrichments in the lab by operating their systems with low NH3 concentrations. Thus, it is likely they play a role in wastewater biofilms under similar conditions.
Evidence suggests that comammox Nitrospira, as opposed to canonical Nitrospira, harbor genomic NH3 and NO2− oxidation machinery homologous to classical AOB and NOB, respectively (e.g., gene clusters encoding amo, hao, and nxr) (Daims et al. 2015; van Kessel et al. 2015). However, very little is known about their capacity for N2O production. NH2OH appears to be an obligate intermediate of comammox metabolism, analogous to AOB catabolism, and it is likely that N2O can be formed by comammox via the NH2OH pathway (Fig. 3). Comammox genomes recovered to date also harbor capacity for NO2− reduction to NO (NirK), similar to non-comammox Nitrospira (Camejo et al. 2017; Lawson and Lucker 2018). Comammox clades A and B genomes reported to date lack a known NOR or proteins related to NOx metabolism (Palomo et al. 2018), similarly to common Nitrospira taxa (Lawson and Lucker 2018) and therefore may be incapable of nitrifier denitrification. Thus, the presence of reactive nitrogen species produced by comammox biomass, e.g., NO or NH2OH, could to lead to abiotic reactions with the production of N2O as a final product.
Comammox may be detrimental to PN/A systems, where NO2− production is needed. However, they may also reduce N2O emissions by minimizing NO2− accumulation. The presence of comammox in wastewater treatment processes, both in suspended growth and biofilm processes, and the metabolic versatility of Nitrospira species including the two comammox Nitrospira clades is currently an active area of research. Future research should also address the selecting factors for partitioning between comammox and canonical Nitrospira and clarify the potential role for comammox in N2O emissions.
N2O from microorganisms related to denitrification
Denitrification is the sequential reduction of NO3− and NO2− to NO, N2O, and finally N2 (Ni and Yuan 2015). It involves four enzymes: the nitrate reductase (NAR), nitrite reductase (NIR), nitric oxide reductase (NOR), and nitrous oxide reductase (NOS). A schematic of the denitrification metabolism is shown in Fig. 3.
The formation of N2O in wastewater denitrification processes is often due to selective inhibition of the NOS enzyme (Guo et al. 2017). This can be caused by its greater sensitivity to DO (Firestone et al. 1979; Tallec et al. 2008), pH (Firestone et al. 1979; Hanaki et al. 1992), NO2− (Alinsafi et al. 2008), carbon source type and concentration (Tallec et al. 2006), carbon limitation (Alinsafi et al. 2008; Tallec et al. 2006), and hydrogen sulfide (H2S) (Schonharting et al. 1998).
While denitrifying bacteria produce N2O during denitrification, they also can reduce N2O to N2 (Read-Daily et al. 2016). Externally supplied N2O can be reduced concurrently with NO3− and NO2− (Conthe et al. 2018a; Pan et al. 2013a, 2015; Read-Daily et al. 2016).
While many denitrifying bacteria have a complete reduction pathway and can reduce NO3− and NO2− all the way to N2, less is known about bacteria that can grow with N2O but not with NO3− or NO2−. Newly classified clade II-type nosZ N2O reducing bacteria were recently discovered (Jones et al. 2013; Sanford et al. 2012). These have since been detected in a granular sludge reactor (Lawson et al. 2017), a MABR (Kinh et al. 2017b) and a biofiltration system (Yoon et al. 2017). Some isolates harboring clade II type nosZ have higher affinity for N2O reduction than those harboring clade I type nosZ (Suenaga et al. 2018; Yoon et al. 2016), whereas a contradictory finding was reported (Conthe et al. 2018a), requiring more in-depth analysis concerning bacteria as an N2O sink at a low N2O concentration. Some clade II-type nosZ bacteria appear to lack genes encoding for NIR and/or NOR, suggesting their potential as an N2O sink but not an N2O source (Graf et al. 2014). As reviewed elsewhere, these non-denitrifying N2O-reducing bacteria in wastewater engineering are yet to be explored in detail (Hallin et al. 2018). The ecophysiology of non-denitrifying N2O reducers in a biofilm system warrants further research.
There are a wide range of denitrifying microorganisms and some with special behavior with respect to N2O formation and reduction. Some can fully reduce NO3− and NO2− to NH3 in an ecologically important process called dissimilatory nitrate or nitrite reduction to ammonium (DNRA) (Stein and Klotz 2016) (Fig. 2). In this process, NO3− or NO2− is reduced to NH3, with N2O produced at the NO2− reduction stage as a by-product (Fig. 2) (Kelso et al. 1997; Rutting et al. 2011; Streminska et al. 2012). Unlike denitrification, this process conserves N in the ecosystem (Rutting et al. 2011; Tiedje et al. 1982). Many DNRA microorganisms can produce N2O as a by-product (Stevens and Laughlin 1998; Stevens et al. 1998). Some of these microorganisms employ DNRA as a detoxification mechanism in order to avoid high concentration of NO2− (Kaspar 1982). However, the actual contribution of DNRA to N2O formation in these species remains uncertain (Butterbach-Bahl et al. 2013).
Behavior regarding N2O emissions may also vary based on the type of electron donor. For example, elemental-sulfur (So)-oxidizing denitrifiers (Di Capua et al. 2015; Liu et al. 2017b), methane (CH4)-oxidizing denitrifiers (He et al. 2018), phosphate-accumulating (PAO) denitrifiers (Gao et al. 2017; Wang et al. 2011, 2014; Zhou et al. 2012), H2-oxidizing denitrifiers (Li et al. 2017), and bacteria growing with an electrode as an electron donor (Jiang et al. 2018) display different behaviors with respect to N2O emissions. Methane-oxidizing denitrifiers appear to reduce NO2− to N2 without forming N2O as an intermediate and therefore are thought to minimize N2O emissions (He et al. 2018). While the details on each of these donors are beyond the scope of this review, the kinetics for each donor can have important impacts on N2O formation and consumption.
Types of biofilm reactors and impacts on N2O emissions
This section describes different types of biofilm reactors and their special characteristics as relate to N2O emissions. Based on the analysis in the previous section, and also following Todt & Dorsch (2016) and Massara et al. (2017), conditions that promote N2O emission include (1) low DO values, or DO spatially transitioning from high to low within the biofilm, as this leads to nitrifier denitrification or incomplete heterotrophic denitrification; (2) conditions where the DO fluctuates temporally from high to low values, (3) conditions with high reaction rates, which lead to greater formation of intermediates (e.g., NH2OH, NO2−) that promote N2O formation; and (4) limiting electron donor for denitrification.
The above factors may have different impacts for different types of biofilm reactors. There is a wide range of biofilm reactors, and they can be classified based on the arrangement of their solid, liquid, and gas phases, whether the carriers are fixed or moving, their carrier specific surface area (area of carrier per unit volume of reactor), their mixing regime (completely mixed or plug flow), and the mechanisms of transfer of gases and electron donor or acceptor substrates. Typical biofilm reactor configurations are shown schematically in Fig. 4.
Types of biofilm reactors. a Unsubmerged filter (e.g., trickling filter or biofilter), b upflow fixed-bed reactor (e.g., biologically active filter (BAF), c downflow fixed-bed reactor (e.g., BAF), d rotating biological contactor (RBC), e suspended or airlift biofilm reactor, f fluidized-bed biofilm reactor (FBBR or granular sludge), g moving-bed biofilm reactor (MBBR), integrated fixed film activated sludge (IFAS), and h membrane-supported biofilm reactor (e.g., MBfR or MABR). Note: i = influent; e = effluent; r = recycle; w = wasting flow; g = gas flow (typically air) in or out. Black dots in panels e, f, and g are biofilm carriers. Adapted from (Morgenroth 2008) and (WEF 2010)
Trickling filters (Fig. 4a) are commonly used for COD removal and nitrification. The media is non-submerged and is kept aerobic by convective air currents within the bed. While considered aerobic, anoxic niches can form in the deeper biofilm (Dalsgaard and Revsbech 1992). The variations in DO and donor concentration in the biofilm between passes of the wastewater distributor arm can lead to N2O emissions. When used for nitrification, N2O is likely to form within the bed, with some stripped by the air currents and present in the effluent (Melse and Mosquera 2014). There is little experimental data on N2O emissions from trickling filters, possibly due to the difficulty in capturing the off-gases, and further research is needed in this area.
Biofilters (Fig. 4a) are similar to trickling filters, but used to treat gaseous contaminants such as odorous compounds in air or volatile organic compounds (VOCs). Air is passed through a non-submerged packed bed with biofilms growing on the media, and the contaminants partition into the liquid phase coating the biofilm. Yoon et al. (2017) proposed using a biofilter supplied to remove N2O in off gases from an activated sludge aeration basin. Raw wastewater was used as the electron donor. In lab tests, 99.9% of N2O was removed when supplied at 100 ppmV in N2, i.e., without any O2. However, removals decreased significantly when supplied in air. Biofilters are likely an expensive approach to mitigating N2O emissions, as they require covering aeration basin to collect off gases, treating large volumes of gas, and adding an additional process and complexity to the treatment train.
Packed bed reactors (Fig. 4b, c) are fully submerged fixed bed biofilm reactors. They can be operated in upflow or downflow mode and either aerated (e.g., for nitrification) or unaerated with electron donor addition (denitrifying filters). Upflow packed bed reactors, such as nitrifying or denitrifying filters, typically operate in plug flow fashion. Thus, the filters experience high substrate concentrations at the influent end and low concentrations at the effluent end. The concentration gradients (e.g., high NH3 at influent, low DO at effluent) can impact N2O formation processes. When used for denitrification, air pulses are periodically performed at the bottom of the filter to release N2 bubbles accumulating in the reactor. These pulses can strip N2O formed at the beginning of the bed, when normally it would be reduced to N2 further within the bed (Bollon et al. 2016). Whenever air is added to a denitrifying filter, there is potential for N2O formation at some location within the biofilm due to the greater sensitivity of N2OR to O2 inhibition. N2O may also accumulate due to insufficient electron donor supply. For nitrifying and denitrifying packed bed reactors, backwashing is carried out regularly to remove excess biomass. Thinner biofilms may not allow full treatment, leading N2O breakthrough from the reactor. For denitrifying biofilms, breakthrough can also be caused by donor limitation. Bollon et al. (2016) found that a full-scale denitrifying filter with a C/N of 3 or higher had up to 93% N2O reduction. However, during a carbon supply failure, removals lowered 26%. Similar results were found by Capodici et al. (2018) and Zhang et al. (2016). In the latter study, the authors found that a decrease of the C/N from 3 to 0.65 led to an increase of the genes encoding for NOR that would enhance the transformation of NO to N2O and lead to increased N2O emissions. Zhang et al. (2017) studied the behavior of lab-scale denitrification filters and found a complex interaction of the denitrification with anammox and DNRA. Gene abundance together with accumulation of NO2− at temperatures between 5 and 15 °C were found to be important factors for N2O accumulation. Further research is required to investigate the impact of influent NO2− and possible adaptation of bacteria to variable influent loadings of both NO2− and NO3− in denitrifying filters.
RBCs (Fig. 4d) use rotating wheels of media partially submerged in wastewater. When the wheels are outside the water, the biofilm can experience O2 concentrations in the biofilm exterior, while the DO concentrations can drop significantly when immersed in the wastewater (Pynaert et al. 2002). This cycling of high and low DO concentrations, as well variations in donor concentration when the biofilm is submerged vs. when it is out of the wastewater, can potentially lead to higher N2O emissions. There does not appear to be any published findings of N2O emissions from RBCs. Note that RBCs are often covered to prevent from UV toxicity and to protect from low temperatures in winter. In these cases, it may be possible to pump air from the enclosures through an anoxic zone or into a biofilter, such as that described above, to reduce N2O to N2.
Airlift, MBBRs, and IFAS (Fig. 4e, g) use carriers that “float” in the water and therefore have little relative velocity between the carrier and the water. They can be operated under aerobic or anoxic conditions. In continuous systems, the biofilm carriers are kept in a single zone, experiencing consistent bulk environments. This can avoid the high N2O emissions in suspended growth systems transitioning from anoxic to aerobic zones (Chandran et al. 2011). Recent research on N2O emissions from MBBRs are consistent with the factors described at the beginning of this section, depending on the application (Mannina et al. 2017, 2018a, b; Wei et al. 2017).
Fluidized bed reactors (Fig. 4f) behave similarly to a BAF, but use much finer media. This provides a high specific surface area and allows the particles to become suspended in the upward wastewater flow. These reactors also experience a somewhat higher degree of mixing, compared to packed bed reactors, but still have some plug flow behavior. Excess biofilm is continuously removed by abrasion, and biofilms typically are thinner than in BAFs. The behavior with respect to N2O emissions should be similar to the BAFs. Note that aerobic granular sludge can behave similarly to a fluidized bed reactor. However, granular sludge is typically operated in sequencing batch mode (Castro-Barros et al. 2015). Recent research on N2O emission from granular sludge also confirms the above mechanisms (Jia et al. 2018; Lu et al. 2018; Peng et al. 2017; Reino et al. 2017).
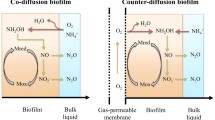
Counter-diffusional biofilms are those where one substrate diffuses from the bulk liquid, while the other penetrates the biofilm from the attachment surface. The counter-diffusion of substrates leads to a range of different behaviors with respect to conventional, co-diffusional biofilms (Nerenberg 2016). Examples of counter-diffusional biofilms include MABRs, where the membranes are used to supply air or O2; membrane-biofilm reactors (MBfRs) where membranes supply H2 or CH4 (Liu et al. 2017b); sulfur-based biofilms, where solid So particles support a biofilm (Wang et al. 2016a); and even bioelectrochemical biofilms (Jiang et al. 2018). MABR behavior is discussed in more detail in the next section.
Mechanisms of N2O formation in biofilm processes for wastewaster treatment
Because of their special layered structure and organization, biofilms allow unique niche formation with specific metabolic functions. In addition, intermediates formed in one biofilm location can diffuse to another with different environments, leading to transformations that would not normally occur in a suspended growth system (Dalsgaard et al. 1995; de Beer et al. 1997; Nielsen et al. 1990; Sabba et al. 2017b; Schreiber et al. 2009). This section discusses basic behavior of biofilms for some key processes, including nitrification, denitrification, combined nitrification and denitrification, and partial nitrification/anammox. The behavior is common for most biofilm reactors except for MABRs, which are described separately. The figures in this section are intended to illustrate typical behavior. They are only schematics, not meant to reflect an actual operating condition.
Nitrifying biofilms
Nitrifying biofilms form when NH3 is the dominant or sole electron donor. While AOB and NOB are primary population members in nitrifying biofilms, heterotrophic bacteria typically co-exist (Kindaichi et al. 2004), growing on the decay products from nitrifying microorganisms (Gieseke et al. 2005; Okabe et al. 2005). However, N2O production in nitrifying biofilms is likely dominated by AOB, with a minor contribution from heterotrophic bacteria. In this section, we focus on the mechanisms of N2O from the nitrifying population. In the subsequent section, we discuss the impact of heterotrophs on nitrifying biofilms, especially when organic carbon is present in the bulk.
Typical substrate profiles in nitrifying biofilms and zones of N2O formation and emission are shown schematically in Fig. 5. In conventional, co-diffusional biofilms, the outer biofilm is aerobic and has the highest NH3 concentrations. As a result, the NH3 oxidation rates are high, leading to high NH2OH concentrations. In addition, the nitrifier denitrification pathway is inhibited by the high DO in this zone. Thus, the NH2OH oxidation pathway is likely to dominate, and N2O formation rates are likely to be relatively low. Nitrifier denitrification may become significant in the aerobic/anoxic transition zone (Mao et al. 2008; Schreiber et al. 2008, 2009). In the anoxic zone, N2O formation rates are low. This is because NH3 oxidation, which is the source of electrons for nitrifier denitrification, requires O2. However, Sabba et al. (2015) proposed that NH2OH formed in the aerobic biofilm exterior would diffuse to the interior anoxic zones. AOB in this zone could utilize NH2OH as a rich electron source, enabling the nitrifier denitrification pathway and resulting in a spike of N2O. Further research is needed to confirm this mechanism experimentally. In Fig. 5, the N2O concentration profile slopes towards the outer biofilm, indicating diffusive mass transfer towards the bulk. If diffused aeration is used, the N2O is readily stripped from the liquid phase (Law et al. 2012; Rassamee et al. 2011; Wu et al. 2014).
MABRs are a novel biofilm process for wastewater treatment, where O2 is supplied from the membrane and NH3 from the bulk (Martin and Nerenberg 2012; Syron and Casey 2008) (Fig. 5). Because of the unique penetration of NH3 and O2 from opposite sides of the biofilm, they are called, as mentioned above, counter-diffusional biofilms (Nerenberg 2016). N2O can also occur in MABRs systems. In MABs, the highest nitrification rates usually occur in the biofilm interior, not at the outer edge. Thus, N2O formation via the NH2OH pathway is likely to occur in the deep biofilm. In addition, the aerobic/anoxic transition occurs in the biofilm interior, and the bulk is anoxic. Thus, while N2O can be stripped from suspended growth systems by bulk aeration (Law et al. 2012; Rassamee et al. 2011; Wu et al. 2014), N2O in MABRs can be consumed by denitrifying bacteria in the outer biofilm or bulk liquid. Conversely, some N2O may be stripped from MABR biofilms by air flowing through the membrane lumen, if operated with open end membranes (Kinh et al. 2017a). Stripping from the lumen is indicated in Fig. 5b by the slope of the N2O concentration profile towards the membrane in its proximity.
NOB can contribute indirectly to N2O emissions by scavenging DO and favoring the formation of a steeper gradient for transitioning from oxic to anoxic conditions (Sabba et al. 2015, 2017a). They also can play a key role in reducing the NO2− concentration, which reduces the rates of nitrifier denitrification (Schreiber et al. 2009). Anammox bacteria can play a similar role in decreasing N2O emissions (Pellicer-Nacher et al. 2010). As mentioned previously, NOB do not play a direct role for NO and N2O emissions, but may affect emission by modifying the NO2− concentrations (Wang et al. 2016b).
Denitrifying biofilms
Denitrifying biofilms are those where NO3− is the primary electron acceptor. We also consider biofilms with an aerobic exterior and denitrifying interior, but neglect any nitrification in the aerobic zone. In denitrifying biofilms, N2O is an obligate intermediate. It is typically present at higher concentrations in the outer biofilm region, where NO3− and NO2− reduction activity is higher, but can diffuse and be consumed in deeper regions where NO3− and NO2− concentrations are lower (Fig. 6a). Thus, biofilms can have regions that can serve as an N2O sink, mitigating N2O emissions (Dalsgaard and Revsbech 1992; Nielsen et al. 1990).
In the presence of high DO, denitrification is usually inhibited and therefore little N2O is formed (Conte et al. 2018b) (Fig. 6b). However, biofilms typically have DO gradients, and denitrification and N2O formation may occur deeper in the biofilm (Dalsgaard and Revsbech 1992; Nielsen et al. 1990). In the transition zone from oxic to anoxic, higher amounts of N2O will be formed due to the higher sensitivity of NOS to O2 inhibition (Bonin et al. 1992; Lu and Chandran 2010; Morley et al. 2008; Otte et al. 1996). When this transition zone is near the outer biofilms, more N2O may be exported to the bulk liquid. When the transition occurs deeper in the biofilm, i.e., at higher bulk DO concentrations, and when electron donor is sufficient, N2O is more likely to be reduced in the deeper biofilm and less emissions will occur (Dalsgaard and Revsbech 1992).
If N2O is formed in the outer biofilm and if sufficient electron donor is available in the deeper zones of the biofilm, denitrifying biofilms can serve as an N2O sink (Eldyasti et al. 2014; Sabba et al. 2017b). However, if sulfate reduction occurs in the deeper biofilm where NO3− has been depleted, H2S may accumulate and inhibit N2O reduction (Pan et al. 2013b). Electron donor limitation in the denitrifying zone also may result in greater N2O formation (Dalsgaard and Revsbech 1992; Nielsen et al. 1990; Todt and Dorsch 2015) (Fig. 6c).
Combined nitrifying/denitrifying biofilms
Biofilms exposed to both organic carbon and NH3 usually have an outer layer dominated by fast-growing heterotrophic bacteria (Henze et al. 2008). In the presence of non-limiting organic substrates, O2 is usually consumed by heterotrophic activity with little formation nitrifying biomass. However, in presence of low or transient organic carbon concentrations, nitrifying organisms can develop in the biofilm. These biofilms are here referred as “combined nitrifying/denitrifying biofilms.”
In combined nitrifying/denitrifying biofilms, the mechanisms of N2O formation can be quite complex. Both co- and counter-diffusional combined nitrifying/denitrifying biofilms are characterized by the presence of complex communities, where N2O not only is formed by both nitrifiers and denitrifiers but also reduced by denitrifiers (Matsumoto et al. 2007; Nerenberg 2016). Various intermediates play roles in both pathways, as indicated in Fig. 2. For example, NO2− and NO, two crucial components of both nitrifier denitrification and NH2OH oxidation pathways, also play a role as intermediates in the denitrification pathway (Todt and Dorsch 2015). Thickness is also a crucial component for both co- and counter-diffusional biofilm, if adequate thickness and COD concentrations are present, then N2O reduction can occur (Eldyasti et al. 2014; He et al. 2017).
Co-diffusional combined nitrifying/denitrifying biofilms receive both electron donor and acceptor from the bulk (Fig. 7a). In this type of biofilm, heterotroph are typically more abundant in the outer biofilm, due to their faster growth rates and the greater availability of COD. This zone is typically aerobic, so little or no denitrification or N2O reduction occurs. Nitrifiers are typically located in the aerobic zone below the heterotrophs. If enough COD is present, then N2O reduction can occur in the deeper biofilm (Fig. 7a) (Chae et al. 2012; Eldyasti et al. 2014; He et al. 2017). When the bulk is aerated in co-diffusional combined nitrifying/denitrifying biofilms, there is greater N2O mass transfer towards the bulk rather than towards the anoxic zone where it can be reduced. This translates in higher N2O emissions.
In counter-diffusional combined nitrifying/denitrifying biofilms, DO penetrates the biofilm from the attachment surface. In this case, and assuming the bulk liquid is anoxic, the nitrifiers would only be active near the membrane surface (Kinh et al. 2017a). In addition, N2O formed by the nitrifiers could potentially be reduced by the heterotrophs in outer, anoxic region of the biofilm, where the COD concentrations are highest (Cole et al. 2004; Kinh et al. 2017b; LaPara et al. 2006). As seen for nitrifying biofilms (Fig. 5b), there could also be N2O stripping by the membrane, as indicated from a negative slope of the N2O profile towards the membrane (Fig. 7b). The lack of bulk aeration reduces N2O mass transfer to the bulk. Note that MABR membranes can also strip CO2 from the biofilm, leading to pH shifts that can impact the microbial community and potentially impact N2O emissions (Ma et al. 2017b).
Based on the above, the type of biofilm (co- vs. counter-diffusional) also can affect the microbial community structure and therefore the N2O emissions. For each bulk substrate condition and detachment regime, there may be a different microbial community structure, which in turn can affect the formation/reduction and emissions of N2O. Therefore, the behavior of these biofilms is complex and hard to predict (Martin and Nerenberg 2012; Nerenberg 2016).
Partial nitritation/anammox biofilms
In combined partial nitritation/anammox (PN/A) reactors, NH3 is partially oxidized to NO2− by AOB. The remainder of the NH3 is then oxidized to N2 gas via NO2− reduction by anammox bacteria. NOB are undesirable in PN/A reactors, and diverse strategies are employed to outselect these organisms. PN/A reactors typically also harbor a diverse flanking community, many of which are capable of heterotrophic denitrification (Lawson et al. 2017).
A distinguishing feature of PN/A systems is the presence of multiple biological sinks for NO2−. Biofilm-based PN/A systems are further distinguished by strong spatial segregation of AOB (in oxic layers) and anammox and denitrifiers (in anoxic, usually deep, layers) (Hubaux et al. 2015; Laureni et al. 2016; Okabe et al. 2011). Crossfeeding within the biofilm and capacity of certain denitrifiers to act as internal N2O sinks likely differentiates N2O emissions in biofilms from suspended growth PN/A processes.
The potential of PN/A systems to act as significant N2O sources, particularly from biofilm or hybrid PN/A reactors, is poorly understood. Results suggest that emissions depend strongly on bulk O2 concentration (Harris et al. 2015), NO2− concentration (Van Hulle et al. 2012), NH3 oxidation activity (Blum et al. 2018a; Domingo-Felez et al. 2014), nitrogen loading (Yang et al. 2016), aeration regime (intermittent vs. continuous aeration) (Blum et al. 2018a; Domingo-Felez et al. 2014; Kampschreur et al. 2008; Ma 2018), presence of organic matter (Jia et al. 2018), and biofilm thickness (Vlaeminck et al. 2010).
Intermittent aeration mirrors conditions recently shown to promote N2O generation (Chandran et al. 2011; Kampschreur et al. 2008, 2009; Yu et al. 2010), but has also been suggested that appropriate intermittent aeration can facilitate control or minimization of N2O emissions from PN/A processes (Castro-Barros et al. 2015; Domingo-Felez et al. 2014; Su et al. 2017).
While sources of N2O in PN/A systems are still not well understood, multiple studies have indicated it may derive predominantly from AOB. Ali et al. (2016) provided evidence based that nitrifier denitrification and NH2OH pathways were equally important to N2O formation in the oxic surface region of granules from a PN/A reactor. However, ~ 30% of N2O emissions in this system could be attributed to the anammox dominated anoxic interior of granules due to either heterotrophic denitrification or a yet unidentified pathway. Harris et al. (2015) showed that N2O site preference data from a suspended growth PN/A reactor was inconsistent with current understanding of N2O production pathways and further suggested that N2O emissions in this system could be due in part to an unknown inorganic or anammox-associated N2O production pathway. In general, biofilm-based PN/A processes appear to emit less N2O than suspended nitrifying processes (Gilmore et al. 2013). Further research is needed to better identify sources of N2O in biofilm-based and hybrid biofilm suspended growth PN/A systems and to quantitatively evaluate how spatial structuring, biofilm thickness, and aggregate architecture influence N2O emissions in these emerging low energy N removal systems.
Conclusions
N2O formation is promoted when there are (1) low DO values, or DO spatially transitioning from high to low within the biofilm; (2) conditions where the DO fluctuates temporally from high to low values; (3) conditions with high reaction rates, which lead to greater formation of intermediates (e.g., NH2OH and NO2−) that promote N2O formation; and (4) limiting electron donor for denitrification. The microbial basis of N2O formation in biofilms and suspended growth systems are similar, yet N2O emissions in biofilm systems depend greatly on microbial stratification, the formation of substrate gradients, the exchange of intermediates within the biofilm, and the type of biofilm reactor. This can lead to different patterns and quantities of N2O emission for the same bulk environment and make it more difficult to predict N2O emissions. Co-diffusional and membrane-aerated biofilms may have substantially different behavior, due to the unique microbial and stratifications and substrate profiles. In order to predict N2O emissions from biofilm processes and develop strategies to minimize them, it is important to understand the microbiological and biochemical basis for N2O formation, the factors affecting N2O formation in biofilms, as well as the impacts of reactor configurations and operating modes. Future research should address the pathways and kinetics of N2O emissions from AOA, comammox bacteria, methane-oxidizing denitrifying bacteria, and others. It also is important to explore their abundance in biofilms. Given the complexity of biofilms and biofilm processes, empirical assessments of N2O emissions from the broad range of biofilm reactors type and operating conditions is needed, and application-specific recommendations to minimize emissions should be developed.
References
Ahn JH, Kim S, Park H, Rahm B, Pagilla K, Chandran K (2010) N2O emissions from activated sludge processes, 2008–2009: results of a national monitoring survey in the United States. Environ Sci Technol 44(12):4505–4511
Ali M, Rathnayake RMLD, Zhang L, Ishii S, Kindaichi T, Satoh H, Toyoda S, Yoshida N, Okabe S (2016) Source identification of nitrous oxide emission pathways from a single-stage nitritation-anammox granular reactor. Water Res 102:147–157
Alinsafi A, Adouani N, Beline F, Lendormi T, Limousy L, Sire O (2008) Nitrite effect on nitrous oxide emission from denitrifying activated sludge. Process Biochem 43(6):683–689
Blum J-M, Jensen MM, Smets BF (2018a) Nitrous oxide production in intermittently aerated partial nitritation-anammox reactor: oxic N2O production dominates and relates with ammonia removal rate. Chem Eng J 335:458–466
Blum JM, Su Q, Ma Y, Valverde-Perez B, Domingo-Felez C, Jensen MM, Smets BF (2018b) The pH dependency of N-converting enzymatic processes, pathways and microbes: effect on net N2O production. Environ Microbiol 20(5):1623–1640
Bollon J, Filali A, Fayolle Y, Guerin S, Rocher V, Gillot S (2016) Full-scale post denitrifying biofilters: sinks of dissolved N2O? Sci Total Environ 563-564:320–328
Bonin P, Gilewicz M, Bertrand JC (1992) Effects of oxygen on Pseudomonas nautica growth on N-alkane with or without nitrate. Arch Microbiol 157(6):538–545
Butterbach-Bahl K, Baggs EM, Dannenmann M, Kiese R, Zechmeister-Boltenstern S (2013) Nitrous oxide emissions from soils: how well do we understand the processes and their controls? Philos Trans R Soc Lond Ser B Biol Sci 368(1621):20130122
Camejo PY, Santo Domingo J, McMahon KD, Noguera DR (2017) Genome-enabled insights into the ecophysiology of the comammox bacterium “Candidatus Nitrospira nitrosa”. mSystems 2(5):e00059–e00017
Capodici M, Avona A, Laudicina VA, Viviani G (2018) Biological groundwater denitrification systems: lab-scale trials aimed at nitrous oxide production and emission assessment. Sci Total Environ 630:462–468
Caranto JD, Lancaster KM (2017) Nitric oxide is an obligate bacterial nitrification intermediate produced by hydroxylamine oxidoreductase. Proc Natl Acad Sci U S A 114(31):8217–8222
Caranto JD, Vilbert AC, Lancaster KM (2016) Nitrosomonas europaea cytochrome P460 is a direct link between nitrification and nitrous oxide emission. Proc Natl Acad Sci U S A 113(51):14704–14709
Castro-Barros CM, Daelman MR, Mampaey KE, van Loosdrecht MC, Volcke EI (2015) Effect of aeration regime on N(2)O emission from partial nitritation-anammox in a full-scale granular sludge reactor. Water Res 68:793–803
Chae KJ, Kim SM, Oh SE, Ren X, Lee J, Kim IS (2012) Spatial distribution and viability of nitrifying, denitrifying and ANAMMOX bacteria in biofilms of sponge media retrieved from a full-scale biological nutrient removal plant. Bioprocess Biosyst Eng 35(7):1157–1165
Chandran K, Stein LY, Klotz MG, van Loosdrecht MC (2011) Nitrous oxide production by lithotrophic ammonia-oxidizing bacteria and implications for engineered nitrogen-removal systems. Biochem Soc Trans 39(6):1832–1837
Chen X, Yuan Z, Ni BJ (2018) Nitrite accumulation inside sludge flocs significantly influencing nitrous oxide production by ammoniumoxidizing bacteria. Water Res 143:99–108
Cole AC, Semmens MJ, LaPara TM (2004) Stratification of activity and bacterial community structure in biofilms grown on membranes transferring oxygen. Appl Environ Microbiol 70(4):1982–1989
Conthe M, Wittorf L, Kuenen JG, Kleerebezem R, van Loosdrecht MCM, Hallin S (2018a) Life on N2O: deciphering the ecophysiology of N2O respiring bacterial communities in a continuous culture. ISME J 12(4):1142–1153
Conthe M, Parchen C, Stouten G, Kleerebezem R, van Loosdrecht MCM (2018b) O2 versus N2O respiration in a continuous microbial enrichment. Appl Microbiol Biotechnol. https://doi.org/10.1007/s00253-018-9247-3
Costa EC, Pèrez J, Kreft J-U (2006) Why is metabolic labour divided in nitrification? Trends Microbiol 14(5):213–219
Daims H, Lebedeva EV, Pjevac P, Han P, Herbold C, Albertsen M, Jehmlich N, Palatinszky M, Vierheilig J, Bulaev A, Kirkegaard RH, von Bergen M, Rattei T, Bendinger B, Nielsen PH, Wagner M (2015) Complete nitrification by Nitrospira bacteria. Nature 528(7583):504–509
Daims H, Lucker S, Wagner M (2016) A new perspective on microbes formerly known as nitrite-oxidizing bacteria. Trends Microbiol 24(9):699–712
Dalsgaard T, Revsbech NP (1992) Regulating factors of denitrification in trickling filter biofilms as measured with the oxygen nitrous-oxide microsensor. FEMS Microbiol Ecol 101(3):151–164
Dalsgaard T, Dezwart J, Robertson LA, Kuenen JG, Revsbech NP (1995) Nitrification, denitrification and growth in artificial Thiosphaera pantotropha biofilms as measured with a combined microsensor for oxygen and nitrous-oxide. FEMS Microbiol Ecol 17(2):137–147
de Beer D, Stoodley P, Lewandowski Z (1997) Measurement of local diffusion coefficients in biofilms by microinjection and confocal microscopy. Biotechnol Bioeng 53(2):151–158
Di Capua F, Papirio S, Lens PNL, Esposito G (2015) Chemolithotrophic denitrification in biofilm reactors. Chem Eng J 280:643–657
Domingo-Felez C, Mutlu AG, Jensen MM, Smets BF (2014) Aeration strategies to mitigate nitrous oxide emissions from single-stage nitritation/anammox reactors. Environ Sci Technol 48(15):8679–8687
Donlan RM (2002) Biofilms: microbial life on surfaces. Emerg Infect Dis 8(9):881–890
Eldyasti A, Nakhla G, Zhu J (2014) Influence of biofilm thickness on nitrous oxide (N2O) emissions from denitrifying fluidized bed bioreactors (DFBBRs). J Biotechnol 192(Pt A):281–290
Elenter D, Milferstedt K, Zhang W, Hausner M, Morgenroth E (2007) Influence of detachment on substrate removal and microbial ecology in a heterotrophic/autotrophic biofilm. Water Res 41(20):4657–4671
Firestone MK, Smith MS, Firestone RB, Tiedje JM (1979) The influence of nitrate, nitrite, and oxygen on the composition of the gaseous products of denitrification in Soil1. Soil Sci Soc Am J 43(6):1140–1144
Flemming HC, Wingender J, Szewzyk U, Steinberg P, Rice SA, Kjelleberg S (2016) Biofilms: an emergent form of bacterial life. Nat Rev Microbiol 14(9):563–575
Gao H, Liu M, Griffin JS, Xu L, Xiang D, Scherson YD, Liu WT, Wells GF (2017) Complete nutrient removal coupled to nitrous oxide production as a bioenergy source by denitrifying polyphosphate-accumulating organisms. Environ Sci Technol 51(8):4531–4540
Gieseke A, Nielsen JL, Amann R, Nielsen PH, de Beer D (2005) In situ substrate conversion and assimilation by nitrifying bacteria in a model biofilm. Environ Microbiol 7(9):1392–1404
Gilmore KR, Terada A, Smets BF, Love NG, Garland JL (2013) Autotrophic nitrogen removal in a membrane-aerated biofilm reactor under continuous aeration: a demonstration. Environ Eng Sci 30(1):38–45
Graf DR, Jones CM, Hallin S (2014) Intergenomic comparisons highlight modularity of the denitrification pathway and underpin the importance of community structure for N2O emissions. PLoS One 9(12):e114118
Gubry-Rangin C, Nicol GW, Prosser JI (2010) Archaea rather than bacteria control nitrification in two agricultural acidic soils. FEMS Microbiol Ecol 74(3):566–574
Guo G, Wang Y, Hao T, Wu D, Chen G-H (2017) Enzymatic nitrous oxide emissions from wastewater treatment. Front Env Sci Eng 12(1)
Hallin S, Philippot L, Loffler FE, Sanford RA, Jones CM (2018) Genomics and ecology of novel N2O-reducing microorganisms. Trends Microbiol 26(1):43–55
Hanaki K, Hong Z, Matsuo T (1992) Production of nitrous-oxide gas during denitrification of waste-water. Water Sci Technol 26(5–6):1027–1036
Harper WF, Takeuchi Y, Riya S, Hosomi M, Terada A (2015) Novel abiotic reactions increase nitrous oxide production during partial nitrification: modeling and experiments. Chem Eng J 281:1017–1023
Harris E, Joss A, Emmenegger L, Kipf M, Wolf B, Mohn J, Wunderlin P (2015) Isotopic evidence for nitrous oxide production pathways in a partial nitritation-anammox reactor. Water Res 83:258–270
He Q, Zhu Y, Fan L, Ai H, Huangfu X, Chen M (2017) Effects of C/N ratio on nitrous oxide production from nitrification in a laboratory-scale biological aerated filter reactor. Water Sci Technol 75(5–6):1270–1280
He Z, Feng Y, Zhang S, Wang X, Wu S, Pan X (2018) Oxygenic denitrification for nitrogen removal with less greenhouse gas emissions: microbiology and potential applications. Sci Total Environ 621:453–464
Heil J, Liu SR, Vereecken H, Bruggemann N (2015) Abiotic nitrous oxide production from hydroxylamine in soils and their dependence on soil properties. Soil Biol Biochem 84:107–115
Henze M, Loosdrecht MCM, Ekama GA, Brdjanovic D (2008) Biological wastewater treatment—principles. Modelling and Design IWA Publishing, London
Hubaux N, Wells G, Morgenroth E (2015) Impact of coexistence of flocs and biofilm on performance of combined nitritation-anammox granular sludge reactors. Water Res 68:127–139
Jia MS, Castro-Barros CM, Winkler MKH, Volcke EIP (2018) Effect of organic matter on the performance and N2O emission of a granular sludge anammox reactor. Front Env Sci Eng 4(7):1035–1046
Jiang X, Ying D, Ye D, Zhang R, Guo Q, Wang Y, Jia J (2018) Electrochemical study of enhanced nitrate removal in wastewater treatment using biofilm electrode. Bioresour Technol 252:134–142
Jones CM, Graf DR, Bru D, Philippot L, Hallin S (2013) The unaccounted yet abundant nitrous oxide-reducing microbial community: a potential nitrous oxide sink. ISME J 7(2):417–426
Kampschreur MJ, Picioreanu C, Tan N, Kleerebezem R, Jetten MS, van Loosdrecht MC (2007) Unraveling the source of nitric oxide emission during nitrification. Water Environ Res 79(13):2499–2509
Kampschreur MJ, Tan NC, Kleerebezem R, Picioreanu C, Jetten MS, Van Loosdrecht MC (2008) Effect of dynamic process conditions on nitrogen oxides emission from a nitrifying culture. Environ Sci Technol 42(2):429–435
Kampschreur MJ, Temmink H, Kleerebezem R, Jetten MS, van Loosdrecht MC (2009) Nitrous oxide emission during wastewater treatment. Water Res 43(17):4093–4103
Kartal B, Kuypers MM, Lavik G, Schalk J, Op den Camp HJ, Jetten MS, Strous M (2007) Anammox bacteria disguised as denitrifiers: nitrate reduction to dinitrogen gas via nitrite and ammonium. Environ Microbiol 9(3):635–642
Kartal B, Maalcke WJ, de Almeida NM, Cirpus I, Gloerich J, Geerts W, den Camp HJO, Harhangi HR, Janssen-Megens EM, Francoijs K-J (2011) Molecular mechanism of anaerobic ammonium oxidation. Nature 479(7371):127–130
Kaspar HF (1982) Nitrite reduction to nitrous-oxide by Propionibacteria—detoxication mechanism. Arch Microbiol 133(2):126–130
Kelso B, Smith RV, Laughlin RJ, Lennox SD (1997) Dissimilatory nitrate reduction in anaerobic sediments leading to river nitrite accumulation. Appl Environ Microbiol 63(12):4679–4685
Khan MZ, Mondal PK, Sabir S (2013) Aerobic granulation for wastewater bioremediation: a review. Can J Chem Eng 91(6):1045–1058
Kim SW, Miyahara M, Fushinobu S, Wakagi T, Shoun H (2010) Nitrous oxide emission from nitrifying activated sludge dependent on denitrification by ammonia-oxidizing bacteria. Bioresour Technol 101(11):3958–3963
Kindaichi T, Ito T, Okabe S (2004) Ecophysiological interaction between nitrifying bacteria and heterotrophic bacteria in autotrophic nitrifying biofilms as determined by microautoradiography-fluorescence in situ hybridization. Appl Environ Microbiol 70(3):1641–1650
Kinh CT, Riya S, Hosomi M, Terada A (2017a) Identification of hotspots for NO and N2O production and consumption in counter- and co-diffusion biofilms for simultaneous nitrification and denitrification. Bioresour Technol 245(Pt A):318–324
Kinh CT, Suenaga T, Hori T, Riya S, Hosomi M, Smets BF, Terada A (2017b) Counter-diffusion biofilms have lower N2O emissions than co-diffusion biofilms during simultaneous nitrification and denitrification: insights from depth-profile analysis. Water Res 124:363–371
Kits KD, Sedlacek CJ, Lebedeva EV, Han P, Bulaev A, Pjevac P, Daebeler A, Romano S, Albertsen M, Stein LY, Daims H, Wagner M (2017) Kinetic analysis of a complete nitrifier reveals an oligotrophic lifestyle. Nature 549(7671):269–272
Kozlowski JA, Stieglmeier M, Schleper C, Klotz MG, Stein LY (2016) Pathways and key intermediates required for obligate aerobic ammonia-dependent chemolithotrophy in bacteria and Thaumarchaeota. ISME J 10(8):1836–1845
Kuypers MM, Sliekers AO, Lavik G, Schmid M, Jorgensen BB, Kuenen JG, Sinninghe Damste JS, Strous M, Jetten MS (2003) Anaerobic ammonium oxidation by anammox bacteria in the Black Sea. Nature 422(6932):608–611
Kuypers MMM, Marchant HK, Kartal B (2018) The microbial nitrogen-cycling network. Nat Rev Microbiol 16(5):263–276
Lackner S, Terada A, Smets BF (2008) Heterotrophic activity compromises autotrophic nitrogen removal in membrane-aerated biofilms: results of a modeling study. Water Res 42(4–5):1102–1112
LaPara TM, Cole AC, Shanahan JW, Semmens MJ (2006) The effects of organic carbon, ammoniacal-nitrogen, and oxygen partial pressure on the stratification of membrane-aerated biofilms. J Ind Microbiol Biotechnol 33(4):315–323
Laureni M, Falas P, Robin O, Wick A, Weissbrodt DG, Nielsen JL, Ternes TA, Morgenroth E, Joss A (2016) Mainstream partial nitritation and anammox: long-term process stability and effluent quality at low temperatures. Water Res 101:628–639
Law Y, Ye L, Pan Y, Yuan Z (2012) Nitrous oxide emissions from wastewater treatment processes. Philos Trans R Soc Lond Ser B Biol Sci 367(1593):1265–1277
Lawson CE, Lucker S (2018) Complete ammonia oxidation: an important control on nitrification in engineered ecosystems? Curr Opin Biotechnol 50:158–165
Lawson CE, Wu S, Bhattacharjee AS, Hamilton JJ, McMahon KD, Goel R, Noguera DR (2017) Metabolic network analysis reveals microbial community interactions in anammox granules. Nat Commun 8:15416
Li P, Wang Y, Zuo J, Wang R, Zhao J, Du Y (2017) Nitrogen removal and N2O accumulation during hydrogenotrophic denitrification: influence of environmental factors and microbial community characteristics. Environ Sci Technol 51(2):870–879
Li YY, Chapman SJ, Nicol GW, Yao HY (2018) Nitrification and nitrifiers in acidic soils. Soil Biol Biochem 116:290–301
Liu S, Han P, Hink L, Prosser JI, Wagner M, Bruggemann N (2017a) Abiotic conversion of extracellular NH2OH contributes to N2O emission during ammonia oxidation. Environ Sci Technol 51(22):13122–13132
Liu YW, Ngo HH, Guo WS, Zhou JL, Peng L, Wang DB, Chen XM, Sun J, Ni BJ (2017b) Optimizing sulfur-driven mixotrophic denitrification process: system performance and nitrous oxide emission. Chem Eng Sci 172:414–422
Lu H, Chandran K (2010) Factors promoting emissions of nitrous oxide and nitric oxide from denitrifying sequencing batch reactors operated with methanol and ethanol as electron donors. Biotechnol Bioeng 106(3):390–398
Lu X, T DSP, Al-Hazmi HE, Majtacz J, Zhou Q, Xie L, Makinia J (2018) Model-based evaluation of N2O production pathways in the anammox-enriched granular sludge cultivated in a sequencing batch reactor. Environ Sci Technol 52(5):2800–2809
Ma Y (2018) Monitoring and modeling of nitrogen conversions in membrane-aerated biofilm reactors: effects of intermittent aeration. Department of Environmental Engineering Technical University of Denmark (DTU)
Ma C, Jensen MM, Smets BF, Thamdrup B (2017a) Pathways and controls of N2O production in nitritation-anammox biomass. Environ Sci Technol 51(16):8981–8991
Ma Y, Domingo-Felez C, Plosz BG, Smets BF (2017b) Intermittent aeration suppresses nitrite-oxidizing bacteria in membrane-aerated biofilms: a model-based explanation. Environ Sci Technol 51(11):6146–6155
Mannina G, Capodici M, Cosenza A, Laudicina VA, Di Trapani D (2017) The influence of solid retention time on IFAS-MBR systems: assessment of nitrous oxide emission. J Environ Manag 203(Pt 1):391–399
Mannina G, Capodici M, Cosenza A, Di Trapani D (2018a) Nitrous oxide from integrated fixed-film activated sludge membrane bioreactor: assessing the influence of operational variables. Bioresour Technol 247:1221–1227
Mannina G, Ekama GA, Capodici M, Cosenza A, Di Trapani D, Odegaard H, van Loosdrecht MMC (2018b) Influence of carbon to nitrogen ratio on nitrous oxide emission in an integrated fixed film activated sludge membrane bioreactor plant. J Clean Prod 176:1078–1090
Mao Y, Bakken LR, Zhao L, Frostegard A (2008) Functional robustness and gene pools of a wastewater nitrification reactor: comparison of dispersed and intact biofilms when stressed by low oxygen and low pH. FEMS Microbiol Ecol 66(1):167–180
Martin KJ, Nerenberg R (2012) The membrane biofilm reactor (MBfR) for water and wastewater treatment: principles, applications, and recent developments. Bioresour Technol 122:83–94
Massara TM, Malamis S, Guisasola A, Baeza JA, Noutsopoulos C, Katsou E (2017) A review on nitrous oxide (N2O) emissions during biological nutrient removal from municipal wastewater and sludge reject water. Sci Total Environ 596-597:106–123
Matsumoto S, Terada A, Tsuneda S (2007) Modeling of membrane-aerated biofilm: effects of C/N ratio, biofilm thickness and surface loading of oxygen on feasibility of simultaneous nitrification and denitrification. Biochem Eng J 37(1):98–107
Melse RW, Mosquera J (2014) Nitrous oxide (N2O) emissions from biotrickling filters used for ammonia removal at livestock facilities. Water Sci Technol 69(5):994–1003
Montzka SA, Dlugokencky EJ, Butler JH (2011) Non-CO2 greenhouse gases and climate change. Nature 476(7358):43–50
Morgenroth E (2008) Biofilm reactors. In: Henze M, van Loosdrecht MCM, Ekama GA, Brdjanovic D (eds) Biological wastewater treatment. IWA Publishing, London, pp 457–492
Morley N, Baggs EM, Dorsch P, Bakken L (2008) Production of NO, N2O and N2 by extracted soil bacteria, regulation by NO2(−) and O2 concentrations. FEMS Microbiol Ecol 65(1):102–112
Nerenberg R (2016) The membrane-biofilm reactor (MBfR) as a counter-diffusional biofilm process. Curr Opin Biotechnol 38:131–136
Ni BJ, Yuan Z (2015) Recent advances in mathematical modeling of nitrous oxides emissions from wastewater treatment processes. Water Res 87:336–346
Nicol GW, Leininger S, Schleper C, Prosser JI (2008) The influence of soil pH on the diversity, abundance and transcriptional activity of ammonia oxidizing archaea and bacteria. Environ Microbiol 10(11):2966–2978
Nicolella C, van Loosdrecht MC, Heijnen JJ (2000) Wastewater treatment with particulate biofilm reactors. J Biotechnol 80(1):1–33
Nielsen LP, Christensen PB, Revsbech NP, Sorensen J (1990) Denitrification and oxygen respiration in biofilms studied with a microsensor for nitrous oxide and oxygen. Microb Ecol 19(1):63–72
Okabe S, Kindaichi T, Ito T (2005) Fate of 14C-labeled microbial products derived from nitrifying bacteria in autotrophic nitrifying biofilms. Appl Environ Microbiol 71(7):3987–3994
Okabe S, Oshiki M, Takahashi Y, Satoh H (2011) N2O emission from a partial nitrification-anammox process and identification of a key biological process of N2O emission from anammox granules. Water Res 45(19):6461–6470
Otte S, Grobben NG, Robertson LA, Jetten MS, Kuenen JG (1996) Nitrous oxide production by Alcaligenes faecalis under transient and dynamic aerobic and anaerobic conditions. Appl Environ Microbiol 62(7):2421–2426
Palomo A, Pedersen AG, Fowler SJ, Dechesne A, Sicheritz-Ponten T, Smets BF (2018) Comparative genomics sheds light on niche differentiation and the evolutionary history of comammox Nitrospira. ISME J 12(7):1779–1793
Pan Y, Ni BJ, Yuan Z (2013a) Modeling electron competition among nitrogen oxides reduction and N2O accumulation in denitrification. Environ Sci Technol 47(19):11083–11091
Pan Y, Ye L, Yuan Z (2013b) Effect of H2S on N2O reduction and accumulation during denitrification by methanol utilizing denitrifiers. Environ Sci Technol 47(15):8408–8415
Pan Y, Ni BJ, Lu H, Chandran K, Richardson D, Yuan Z (2015) Evaluating two concepts for the modelling of intermediates accumulation during biological denitrification in wastewater treatment. Water Res 71:21–31
Park KY, Inamori Y, Mizuochi M, Ahn KH (2000) Emission and control of nitrous oxide from a biological wastewater treatment system with intermittent aeration. J Biosci Bioeng 90(3):247–252
Park HD, Wells GF, Bae H, Criddle CS, Francis CA (2006) Occurrence of ammonia-oxidizing archaea in wastewater treatment plant bioreactors. Appl Environ Microbiol 72(8):5643–5647
Pellicer-Nacher C, Sun S, Lackner S, Terada A, Schreiber F, Zhou Q, Smets BF (2010) Sequential aeration of membrane-aerated biofilm reactors for high-rate autotrophic nitrogen removal: experimental demonstration. Environ Sci Technol 44(19):7628–7634
Peng L, Sun J, Liu Y, Dai X, Ni BJ (2017) Nitrous oxide production in a granule-based partial nitritation reactor: a model-based evaluation. Sci Rep 7:45609
Pynaert K, Sprengers R, Laenen J, Verstraete W (2002) Oxygen-limited nitrification and denitrification in a lab-scale rotating biological contactor. Environ Technol 23(3):353–362
Rassamee V, Sattayatewa C, Pagilla K, Chandran K (2011) Effect of oxic and anoxic conditions on nitrous oxide emissions from nitrification and denitrification processes. Biotechnol Bioeng 108(9):2036–2045
Read-Daily BL, Sabba F, Pavissich JP, Nerenberg R (2016) Kinetics of nitrous oxide (N2O) formation and reduction by Paracoccus pantotrophus. AMB Express 6(1):85
Reino C, van Loosdrecht MCM, Carrera J, Perez J (2017) Effect of temperature on N2O emissions from a highly enriched nitrifying granular sludge performing partial nitritation of a low-strength wastewater. Chemosphere 185:336–343
Ritter WF, Chitikela SR (2014) Greenhouse gas emissions from wastewater treatment plants and by-product operations—a comprehensive review. World Environmental and Water Resources Congress 2014
Rutting T, Boeckx P, Muller C, Klemedtsson L (2011) Assessment of the importance of dissimilatory nitrate reduction to ammonium for the terrestrial nitrogen cycle. Biogeosciences 8(7):1779–1791
Sabba F, Picioreanu C, Perez J, Nerenberg R (2015) Hydroxylamine diffusion can enhance N(2)O emissions in nitrifying biofilms: a modeling study. Environ Sci Technol 49(3):1486–1494
Sabba F, Picioreanu C, Boltz JP, Nerenberg R (2017a) Predicting N2O emissions from nitrifying and denitrifying biofilms: a modeling study. Water Sci Technol 75(3–4):530–538
Sabba F, Picioreanu C, Nerenberg R (2017b) Mechanisms of nitrous oxide (N2O) formation and reduction in denitrifying biofilms. Biotechnol Bioeng 114(12):2753–2761
Sanford RA, Wagner DD, Wu Q, Chee-Sanford JC, Thomas SH, Cruz-Garcia C, Rodriguez G, Massol-Deya A, Krishnani KK, Ritalahti KM, Nissen S, Konstantinidis KT, Loffler FE (2012) Unexpected nondenitrifier nitrous oxide reductase gene diversity and abundance in soils. Proc Natl Acad Sci U S A 109(48):19709–19714
Santoro AE, Buchwald C, McIlvin MR, Casciotti KL (2011) Isotopic signature of N(2)O produced by marine ammonia-oxidizing archaea. Science 333(6047):1282–1285
Sauder LA, Peterse F, Schouten S, Neufeld JD (2012) Low-ammonia niche of ammonia-oxidizing archaea in rotating biological contactors of a municipal wastewater treatment plant. Environ Microbiol 14(9):2589–2600
Schonharting B, Rehner R, Metzger JW, Krauth K, Rizzi M (1998) Release of nitrous oxide (NO) from denitrifying activated sludge caused by HS-containing wastewater: quantification and application of a new mathematical model. Water Sci Technol 38(1):237–246
Schreiber F, Polerecky L, de Beer D (2008) Nitric oxide microsensor for high spatial resolution measurements in biofilms and sediments. Anal Chem 80(4):1152–1158
Schreiber F, Loeffler B, Polerecky L, Kuypers MM, de Beer D (2009) Mechanisms of transient nitric oxide and nitrous oxide production in a complex biofilm. ISME J 3(11):1301–1313
Schreiber F, Wunderlin P, Udert KM, Wells GF (2012) Nitric oxide and nitrous oxide turnover in natural and engineered microbial communities: biological pathways, chemical reactions, and novel technologies. Front Microbiol 3:372
Soler-Jofra A, Stevens B, Hoekstra M, Picioreanu C, Sorokin D, van Loosdrecht MCM, Perez J (2016) Importance of abiotic hydroxylamine conversion on nitrous oxide emissions during nitritation of reject water. Chem Eng J 287:720–726
Soler-Jofra A, Picioreanu C, Yu R, Chandran K, van Loosdrecht MCM, Pérez J (2018) Importance of hydroxylamine in abiotic N2O production during transient anoxia in planktonic axenic Nitrosomonas cultures. Chem Eng J 335:756–762
Spang A, Poehlein A, Offre P, Zumbragel S, Haider S, Rychlik N, Nowka B, Schmeisser C, Lebedeva EV, Rattei T, Bohm C, Schmid M, Galushko A, Hatzenpichler R, Weinmaier T, Daniel R, Schleper C, Spieck E, Streit W, Wagner M (2012) The genome of the ammonia-oxidizing Candidatus Nitrososphaera gargensis: insights into metabolic versatility and environmental adaptations. Environ Microbiol 14(12):3122–3145
Spott O, Stange CF (2011) Formation of hybrid N2O in a suspended soil due to co-denitrification of NH2OH. J Plant Nutr Soil Sci 174(4):554–567
Spott O, Russow R, Stange CF (2011) Formation of hybrid N2O and hybrid N2 due to codenitrification: first review of a barely considered process of microbially mediated N-nitrosation. Soil Biol Biochem 43(10):1995–2011
Stein LY (2011) Surveying N2O-producing pathways in bacteria. Methods Enzymol 486:131–152
Stein LY, Klotz MG (2016) The nitrogen cycle. Curr Biol 26(3):R94–R98
Stevens RJ, Laughlin RJ (1998) Measurement of nitrous oxide and di-nitrogen emissions from agricultural soils. Nutr Cycl Agroecosyst 52(2–3):131–139
Stevens RJ, Laughlin RJ, Malone JP (1998) Soil pH affects the processes reducing nitrate to nitrous oxide and di-nitrogen. Soil Biol Biochem 30(8–9):1119–1126
Stieglmeier M, Mooshammer M, Kitzler B, Wanek W, Zechmeister-Boltenstern S, Richter A, Schleper C (2014) Aerobic nitrous oxide production through N-nitrosating hybrid formation in ammonia-oxidizing archaea. ISME J 8(5):1135–1146
Stoodley P, Sauer K, Davies DG, Costerton JW (2002) Biofilms as complex differentiated communities. Annu Rev Microbiol 56:187–209
Streminska MA, Felgate H, Rowley G, Richardson DJ, Baggs EM (2012) Nitrous oxide production in soil isolates of nitrate-ammonifying bacteria. Environ Microbiol Rep 4(1):66–71
Strous M, Pelletier E, Mangenot S, Rattei T, Lehner A, Taylor MW, Horn M, Daims H, Bartol-Mavel D, Wincker P, Barbe V, Fonknechten N, Vallenet D, Segurens B, Schenowitz-Truong C, Medigue C, Collingro A, Snel B, Dutilh BE, Op den Camp HJM, van der Drift C, Cirpus I, van de Pas-Schoonen KT, Harhangi HR, van Niftrik L, Schmid M, Keltjens J, van de Vossenberg J, Kartal B, Meier H, Frishman D, Huynen MA, Mewes HW, Weissenbach J, Jetten MSM, Wagner M, Le Paslier D (2006) Deciphering the evolution and metabolism of an anammox bacterium from a community genome. Nature 440(7085):790–794
Su Q, Ma C, Domingo-Felez C, Kiil AS, Thamdrup B, Jensen MM, Smets BF (2017) Low nitrous oxide production through nitrifier-denitrification in intermittent-feed high-rate nitritation reactors. Water Res 123:429–438
Suenaga T, Riya S, Hosomi M, Terada A (2018) Biokinetic characterization and activities of N2O-reducing bacteria in response to various oxygen levels. Front Microbiol 9:697
Syron E, Casey E (2008) Membrane-aerated biofilms for high rate biotreatment: performance appraisal, engineering principles, scale-up, and development requirements. Environ Sci Technol 42(6):1833–1844
Tallec G, Garnier J, Billen G, Gousailles M (2006) Nitrous oxide emissions from secondary activated sludge in nitrifying conditions of urban wastewater treatment plants: effect of oxygenation level. Water Res 40(15):2972–2980
Tallec G, Garnier J, Billen G, Gousailles M (2008) Nitrous oxide emissions from denitrifying activated sludge of urban wastewater treatment plants, under anoxia and low oxygenation. Bioresour Technol 99(7):2200–2209
Terada A, Sugawara S, Hojo K, Takeuchi Y, Riya S, Harper WF Jr, Yamamoto T, Kuroiwa M, Isobe K, Katsuyama C, Suwa Y, Koba K, Hosomi M (2017) Hybrid nitrous oxide production from a partial nitrifying bioreactor: hydroxylamine interactions with nitrite. Environ Sci Technol 51(5):2748–2756
Tiedje JM, Sexstone AJ, Myrold DD, Robinson JA (1982) Denitrification: ecological niches, competition and survival. Antonie Van Leeuwenhoek 48(6):569–583
Todt D, Dorsch P (2015) Nitrous oxide emissions in a biofilm loaded with different mixtures of concentrated household wastewater. Int J Environ Sci Technol 12(11):3405–3416
Todt D, Dorsch P (2016) Mechanism leading to N2O production in wastewater treating biofilm systems. Rev Environ Sci Biotechnol 15(3):355–378
Tourna M, Stieglmeier M, Spang A, Konneke M, Schintlmeister A, Urich T, Engel M, Schloter M, Wagner M, Richter A, Schleper C (2011) Nitrososphaera viennensis, an ammonia oxidizing archaeon from soil. Proc Natl Acad Sci U S A 108(20):8420–8425
Van Hulle SWH, Callens J, Mampaey KE, van Loosdrecht MCM, Volcke EIP (2012) N2O and NO emissions during autotrophic nitrogen removal in a granular sludge reactor—a simulation study. Environ Technol 33(20):2281–2290
van Kessel MAHJ, Speth DR, Albertsen M, Nielsen PH, Op den Camp HJM, Kartal B, Jetten MSM, Lücker S (2015) Complete nitrification by a single microorganism. Nature 528(7583):555–559
Vlaeminck SE, Terada A, Smets BF, De Clippeleir H, Schaubroeck T, Bolca S, Demeestere L, Mast J, Boon N, Carballa M, Verstraete W (2010) Aggregate size and architecture determine microbial activity balance for one-stage partial nitritation and anammox. Appl Environ Microbiol 76(3):900–909
Vroom JM, De Grauw KJ, Gerritsen HC, Bradshaw DJ, Marsh PD, Watson GK, Birmingham JJ, Allison C (1999) Depth penetration and detection of pH gradients in biofilms by two-photon excitation microscopy. Appl Environ Microbiol 65(8):3502–3511
Walker CB, de la Torre JR, Klotz MG, Urakawa H, Pinel N, Arp DJ, Brochier-Armanet C, Chain PS, Chan PP, Gollabgir A, Hemp J, Hugler M, Karr EA, Konneke M, Shin M, Lawton TJ, Lowe T, Martens-Habbena W, Sayavedra-Soto LA, Lang D, Sievert SM, Rosenzweig AC, Manning G, Stahl DA (2010) Nitrosopumilus maritimus genome reveals unique mechanisms for nitrification and autotrophy in globally distributed marine crenarchaea. Proc Natl Acad Sci U S A 107(19):8818–8823
Wang Y, Geng J, Ren Z, He W, Xing M, Wu M, Chen S (2011) Effect of anaerobic reaction time on denitrifying phosphorus removal and N2O production. Bioresour Technol 102(10):5674–5684
Wang Y, Zhou S, Ye L, Wang H, Stephenson T, Jiang X (2014) Nitrite survival and nitrous oxide production of denitrifying phosphorus removal sludges in long-term nitrite/nitrate-fed sequencing batch reactors. Water Res 67:33–45
Wang Y, Bott C, Nerenberg R (2016a) Sulfur-based denitrification: effect of biofilm development on denitrification fluxes. Water Res 100:184–193
Wang YY, Fang HY, Zhou D, Han HC, Chen J (2016b) Characterization of nitrous oxide and nitric oxide emissions from a full-scale biological aerated filter for secondary nitrification. Chem Eng J 299:304–313
WEF (2010) Biofilm reactors WEF MOP 35. McGraw-Hill Education
Wei D, Zhang K, Ngo HH, Guo W, Wang S, Li J, Han F, Du B, Wei Q (2017) Nitrogen removal via nitrite in a partial nitrification sequencing batch biofilm reactor treating high strength ammonia wastewater and its greenhouse gas emission. Bioresour Technol 230:49–55
Wu GX, Zheng DR, Xing LZ (2014) Nitritation and N2O emission in a denitrification and nitrification two-sludge system treating high ammonium containing wastewater. Water-Sui 6(10):2978–2992
Xavier JB, Picioreanu C, van Loosdrecht MC (2005) A framework for multidimensional modelling of activity and structure of multispecies biofilms. Environ Microbiol 7(8):1085–1103
Yang J, Trela J, Plaza E (2016) Nitrous oxide emissions from one-step partial nitritation/anammox processes. Water Sci Technol 74(12):2870–2878
Yoon S, Nissen S, Park D, Sanford RA, Loffler FE (2016) Nitrous oxide reduction kinetics distinguish bacteria harboring clade I NosZ from those harboring clade II NosZ. Appl Environ Microbiol 82(13):3793–3800
Yoon H, Song MJ, Yoon S (2017) Design and feasibility analysis of a self-sustaining biofiltration system for removal of low concentration N2O emitted from wastewater treatment plants. Environ Sci Technol 51(18):10736–10745
Yu R, Kampschreur MJ, van Loosdrecht MCM, Chandran K (2010) Mechanisms and specific directionality of autotrophic nitrous oxide and nitric oxide generation during transient anoxia. Environ Sci Technol 44(4):1313–1319
Zhang T, Jin T, Yan Q, Shao M, Wells G, Criddle C, HH PF (2009) Occurrence of ammonia-oxidizing archaea in activated sludges of a laboratory scale reactor and two wastewater treatment plants. J Appl Microbiol 107(3):970–977
Zhang LM, Hu HW, Shen JP, He JZ (2012) Ammonia-oxidizing archaea have more important role than ammonia-oxidizing bacteria in ammonia oxidation of strongly acidic soils. ISME J 6(5):1032–1045
Zhang Y, Ji G, Wang R (2016) Drivers of nitrous oxide accumulation in denitrification biofilters with low carbon:nitrogen ratios. Water Res 106:79–85
Zhang Y, Ji GD, Wang RJ (2017) Quantitative responses of nitrous oxide accumulation to genetic associations across a temperature gradient within denitrification biofilters. Ecol Eng 102:145–151
Zhou Y, Lim M, Harjono S, Ng WJ (2012) Nitrous oxide emission by denitrifying phosphorus removal culture using polyhydroxyalkanoates as carbon source. J Environ Sci 24(9):1616–1623
Funding
This study was funded by the Water Environment Research Foundation (grant U2R10), the USA National Science Foundation (grant CBET0954918), the Japanese Society for the Promotion of Science (grant 17H01893), and the Danish Council for Independent (Project N2OMan, File No. 1335-00100B). F.S. and R.N. were partially supported by NSF project CBET0954918 and WERF project U2R10. A.T. was partially funded by Grant-in-Aid for Scientific Research (17H01893)—Japan Society for the Promotion of Science and BFSM was funded by the DFF project N2OMan.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
F. Sabba declares he has no conflict of interest.
A. Terada declares he has no conflict of interest.
G. Wells declares he has no conflict of interest.
B. F. Smets declares he has no conflict of interest.
R. Nerenberg declares he has no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Sabba, F., Terada, A., Wells, G. et al. Nitrous oxide emissions from biofilm processes for wastewater treatment. Appl Microbiol Biotechnol 102, 9815–9829 (2018). https://doi.org/10.1007/s00253-018-9332-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-018-9332-7