Formation and sintering of nanostructured Al2O3 and boehmite powders prepared by hydrothermal synthesis from aluminum powder are studied. Formation of workpieces (green bodies) is performed by extrusion, static and explosive pressing followed by high-temperature sintering. Use of explosive pressing increases raw material density, reduces the temperature for α-Al2O3 formation, activates sintering, and improves ceramic operating properties.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Improvement of operating properties and the narrow ecological framework determines the complex set of specifications laid down currently for objects prepared from ceramic. In order to achieve good properties new materials and technology are created for their preparation, important ones of which are nanomaterials and nanotechnology. There is an increase in interest in nanosize powders of refractory compounds, including aluminum hydroxide and oxide, due to the extensive range of their application. Compared with normal powder of micron size nanopowder exhibits greater adhesive and self-adhesive activity, and this increases a tendency towards aggregation and creates problems in preparing ceramic articles from them [1, 2]. Aserious problem is molding and sintering objects from nanopowder, and without its solution nanoparticles, even with excellent individual properties, are very difficult to convert into dense nanoceramic.
The aim of this research is to study processes of molding workpieces of nanopowder of aluminum monohydroxide and oxide by extrusion, static uniaxial and pulsed pressing, and high-temperature sintering.
Research Materials and Procedure
Aluminum monohydroxide (boehmite) was prepared by hydrothermal synthesis from aluminum powder [3]. The method concerns the fact that from finely-dispersed aluminum (with a particle size up to 20 μm) a suspension in water is prepared. The suspension is sprayed into a reactor within which with almost critical parameters for water in the surface layer of aluminum particles there is formation of a new phase (aluminum hydroxide) with an increase in volume by a factor of two. Due to this with an increase in mechanical stresses at the phase boundary above critical levels there is separation of new formations from aluminum particles. As a result of this aluminum particles are converted into nanocrystalline boehmite AlOOH (size up to 50 nm). The method of hydrothermal synthesis makes it possible almost in one production operation to prepare nanopowder with simultaneous preparation of hydrogen and heat, without formation of subsidiary products and effluent. The product purity may reach 99.9% and it is determined by the purity of the original aluminum powder and water. In powder form nanocrystalline boehmite and its heat treatment products appear to be valuable additives for modifying the properties of very different materials [4, 5]. The properties of boehmite and aluminum oxide nanopowder prepared by calcining original boehmite powder are provided in Table 1.
The size of crystals was determined in a XRD-600 x-ray diffractometer from the value of the coherent scattering region (CSR) from data for the change in shape of the diffraction reflection profile [6, 7], and particle size was determined in a Solver Next scanning probe microscope by a semi-contact method. Resolution achieved by means of the Solver Next microscope was equal to the opening radius of the probe used (10 nm).
Specific surface was determined by low-temperature nitrogen absorption in a AUTOSORB-1 specific surface analyzer [8], and pore volume and average pore radius were determined by the Dubinin – Astakhov and BJH method [9].
Specimen material hardness was determined by indentation in accordance with GOST 2999–75 with loads of 613, 809.1, and 931.7 N and a holding time of 15 sec [10]. Hardness of ceramic specimens was determined in an IT 5010 instrument. Five indentations were made in each specimen at each load.
Specimens of boehmite powder were molded by extrusion, uniaxial and static pulsed pressing. For extrusion powder was previously mixed with water in a Venera (Z-shape mixer) stirrer with addition of nitric acid as a peptizing agent (1.5 ml for 100 g of boehmite powder). The moisture content of a mix was 26 – 27%. Workpieces were molded in a laboratory single-screw extruder. The diameter of nozzle opening was 5 mm. Binder (CMC) was added to the mix for static uniaxial pressing. Mix moisture content was 11 – 13%.
Explosive pressing was accomplished in HDP (hydrodynamic machine) and HPU (hydro-explosive pressing unit) class machines, operating on explosive substances (ES) [11, 12]. Explosive pressing is classified as a dynamic molding method, making possible to overcome forces of adhesion, especially significant for nano-particles with a highly developed surface, and with the same pressure to achieve considerably greater density of compact specimens than under static pressing conditions [13].
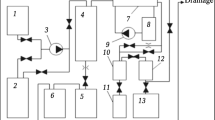
Hydrodynamic pressing was performed in a HDP-190 machine (Fig. 1). The machine operates with a powder charge of artillery propellant explosive substance, whose combustion energy within a closed volume goes into pulsed compression and molding a powder medium through a liquid transmission medium (water). The powder was pressed in a metal shell that was evacuated and sealed
External appearance of hydrodynamic unit HDP-190 (a) and its fundamental layout (b ). a: 1 ) upper cylinder; 2 ) powder charge; 3 ) piston; 4 ) assembly with powder; 5 ) container; 6 ) ram; 7 ) movable table; b: 1 ) electromagnet; 2 ) impact mechanism; 3 ) valve; 4 ) capsule; 5 ) powder charge; 6 ) piston; 7 ) container; 8 ) powder; 9 ) pressure sensor.
A distinguishing feature of the explosive pressing method from hydrodynamic pressing is the possibility of creating higher (tens of GPa) pressures with action of stronger impact waves due to use of high-explosive substances. The pressure level depends on the form of explosive and it is possible to achieve 9 – 15 and 15 – 17 GPa depending on the direction of an explosion. In this case in order to prepare charges powder ammonite 6ZhP was used with a bulk density of 10 kg/m3 and a detonation rate from 2000 to 4500 m/sec. Contact pressure at the boundary of detonation products of a punch was determined from an expression:
where P p is contact pressure; ρ0 is explosive density; V d is explosive detonation rate.
Experimental Results and Discussion
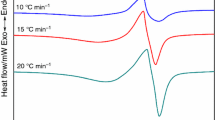
Heating of extruded workpieces (extrudates) of boehmite is accompanied by breakdown of boehmite at 500°C with formation of an anhydrous form of aluminum oxide [14]. At this temperature there is the maximum amount of specific surface. A further increase in temperature to 900°C led to an increase in strength due to diffusion in surface regions of particles, and then with a subsequent increase in temperature due to diffusion within volume of particles (Table 2). Therefore on heating to 900°C shrinkage and porosity remained almost unchanged since surface diffusion hardly led to a change in overall pore volume. In addition, there may be coagulation (coalescence) of pores, when the volume of coarse pores increases due a reduction in the amount of fine pores. More dense areas of a workpiece (areas of local compaction) start to sinter and the pore size within them decreases [15]. In areas located between local compactions, pores grow.
At all temperatures above 500°C in ceramic there is crystal growth and up to 1300°C the average particle size does not go beyond limits of nanosizes (see Table 2). Open pores located between nanoparticles have dimensions an order of magnitude less than the surrounding particles, i.e., they are nanosize (Table 3) Therefore ceramic is not only nanocrystalline, but nanoporous. During measurement of porosity it is normal to use methods based on determining the overall surface of open pores. Nanopores make the greatest contribution towards overall surface. Therefore in spite of presence due to coalescence of micron-size pores, the average pore size appears to be nanosize.
Presence of intercrystalline boundaries at the surface of pores in sintered specimens markedly affects pore coagulation. Diffusion of atoms over intercrystalline boundaries and the surface of pores occurs many time more rapidly than diffusion through the volume of a crystal. The smaller the powder particle size, the thicker is the network of intercrystalline boundaries, and there should be rapid coagulation. With an increase in temperature there is crystal growth and the thickness of a boundary network decreases. This should reduce overall diffusion mass transfer over crystal boundaries. However, an increase in temperature increases diffusion flow through intercrystalline boundaries and the surface of pores so much that this exceeds the unfavorable effect of a reduction in boundary network density. Therefore with an increase in temperature pore coagulation increases.
Increased specimen porosity is explained with a large reduction in weight and volume during phase transformation of boehmite into the final α-Al2O3 phase. As a result of this overall volumetric changes may reach 36%. Molding by extrusion is used extensively in preparing catalysts and catalyst supporters, which are normally highly porous materials. Due to the intercrystalline structure of specimens they have quite high strength. Use of boehmite powder is also promising for creating membrane layers as a result of their nanosizes and high chemical resistance of aluminum oxide. Highly porous materials prepared from boehmite may serve as high-temperature heat insulation.
Dependences are given in Fig. 2 for overall boehmite workpiece porosity and relative density of fired specimens, prepared with static and explosive pressing (last point of the curve) on pressure. Over the whole pressing pressure range for workpieces (from 50 MPa to 4 GPa) the dependence of overall porosity on pressing pressure is satisfactorily described by an A. S. Berezhnyi equation.
Analysis of results of studying the pore structure of workpieces of boehmite specimens prepared with uniaxial pressing made it possible to conclude that already in the first pressing stage there is structure formation, typical for each specific molding mix and caused by viscous flow parameters of individual particles. With an increase in pressing pressure there is a reduction in average workpiece pore size and the distribution spectrum for effective pore diameter throughout the volume of a specimen is reduced. The material structure becomes more uniform. After sintering, during which there is phase transformation with a reduction in volume and breakdown of aggregates, pore size within specimens is an order of magnitude lower (it decreases from 50 nm after uniaxial pressing with a pressure of 150 MPa to several nanometers after sintering).
Aluminum oxide nanopowder, prepared by calcining boehmite, compacted worse than the original boehmite powder (see Figs. 2 and 3). The same result has also been obtained for industrial aluminum oxide powder of different grades [18]. Among aluminum oxide nanopowders the best compaction is for powder calcined at 1100°C. Particles of this powder are coarser and the surface is less developed.
Apparently as a result of this there is a reduction in friction between powder particles during pressing. In spite of the difference in the size of particles, crystals, specific surface, and phase composition of aluminum oxide powders, calcined at different temperature, the dependence of overall porosity on pressing pressure may also to a first approximation be described by an arithmetic dependence corresponding to the A. S. Berezhnyi equation.
With explosive pressing the structure of workpieces is fine-grained, more uniform, denser, and isotropic (less texturing), since anisotropy is partly overcome connected with orientation and the arrangement of particles with uniaxial compression. In order to orientate particles time is required, and the rapidness of compaction with explosive pressing does not provide this possibility. Achievement of higher compact density with explosive pressing compared with that for compacts prepared by a static method points to a significant reduction in friction force between powder particles under impact wave compression conditions.
The greater density of workpieces with explosive pressing is achieved with a finer grain structure. Supersonic vibrations, propagating during explosive pressing of powder, whose wavelength is commensurate with powder particle dimensions, lead to weakening of chemical bonds between particles of nano- and ultra-size range and breakdown of agglomerate that subsequently makes it possible to prepare a denser and finer grain structure. Considerable kinetic energy for a very short time interval is transmitted to an aggregate of nanoparticles. It is impossible to dissipate this energy into the surrounding by creating a pressed object dissipative structure [19], and time is inadequate. Therefore a significant part of this energy goes into creating accumulated structures. In a pressed workpiece transmitted energy is primarily accumulated in breaking chemical bonds and agglomerates, leading to breakage. This reduces friction between particles (internal friction) and makes it possible to prepare a denser workpiece. Compaction of Al2O3 nanopowder is accompanied by brittle breakage of particle agglomerates and other structural elements, rearrangement, mechanical engagement, and wedging. After sintering these specimens it is possible to obtain a finely crystalline structure.
Firing of boehmite workpieces compacted by explosive pressing does not lead to preparation of dense ceramic, the same as in the case of molding by extrusion (see Fig. 2). As is seen in Fig. 2, even with a pressure of 4 GPa and high relative workpiece density (about 75%) the relative density after sintering at 1650°C does not exceed 71%. The reason is the same, i.e., greater material volumetric changes with transition of boehmite into α-Al2O3. An increase in sintering temperature (1850°C, soaking for 1.5 h, vacuum) increased the relative density only to 76.2%.
In spite of the quite high dispersion of the Al2O3 powder used, workpiece pressing pressure (up to 4 GPa) and firing temperature (up to 1350°C, vacuum) it was impossible to obtain high density sintered ceramic (Table 4). Industrial alumina powder with a larger particle size, lower pressing pressure and lower firing temperature sintered to a higher density [18]. They also compact better during pressing. Evidently the very high activity of nanoparticles leads to rapid loss of their activity due to improvement of sintered workpiece structure and the crystals and pores comprising it. It is possible that pores acquire a uniform facet and are not healed at the sintering temperature. As a result of this compaction to high density does not even occur with a high firing temperature. In industrial powder the activity towards sintering is lost more slowly than in nanopowder. Retention of sufficient activity for sintering in industrial powder makes it possible to sinter it to a higher density. Nonetheless, an increase in workpiece density of nanopowder markedly increases the density of sintered ceramic.
The microhardness of sintered specimens is high and reaches 15.9 – 16.3 GPa (calcining temperature 800 and 1100°C, explosive pressing pressure 4 GPa, firing in a vacuum for 1.5 h at 1800°C). For comparison the microhardness of ceramic of industrial powder does not exceed 13 – 14 GPa.
In conducting research it was established that high energy action of a molded mass containing γ-Al2O3 leads during sintering to a reduction in temperature of the γ → α-transformation in Al2O3 by 100 – 150°C. With high shear strains in the space between particles there is formation of α-Al2O3 nuclei. It is possible to explain this effect by rapid elastic deformation of γ-Al2O3 particles with impact wave treatment. Energy supplied to a pressed workpiece is very rapidly dissipated in heat and accumulates in the form of new surface (nanosize cracks), dislocations, and point defects. The temperature at individual points may increase to values required for forming α-Al2O3. Acceleration of α-Al2O3 formation is facilitated by the possibility of a local increase in pressure at these points with explosive pressing. As a result of this α-Al2O3 nuclei form for a short time, creation of α-Al2O3 nuclei in the pressing stage facilitates γ → α-transformation in Al2O3, which proceeds within nanoparticles. A reduction in volume with γ → α-transformation in α-Al2O3 may reach 14%, although due to the nanosize scale this does not lead to formation of cracks and coarse pores, and causes occurrence within a workpiece of predominantly fine pores between particles and facilitates retention of open pores between individual nanoparticles.
According to the results of an experiment and calculation of the level of impact wave loading these transformations in powder materials proceed in the range of dynamic pressures of 0.5 – 2.5 GPa. Defect formation in powder particles and especially the interparticle contacts in the granular-porous macrostructure formed for an object activate sintering, reduce its temperature, and makes it possible to prepare objects of industrial ceramic in lower capacity furnace equipment. Therefore, high-energy pulsed loading of powder bodies may be used as a production method for introduction of point (vacancies and their associates), linear (dislocations), and planar (crystal, block and twin boundaries) defects into powder particles during workpiece molding. During sintering these defects facilitate diffusion mass transfer, providing ceramic sintering.
In order to confirm the efficiency of seed formation during explosive pressing we have studied the γ → α-phase transformation in Al2O3 with addition of α-Al2O3 seeds in the form of additive to γ-Al2O3 powder. It has been established that the kinetic equation for γ → α-transformation in Al2O3 may be presented in the form
where α is the degree of transformation of γ-Al2O3 into α-Al2O3 for time τ; k is a transformation rate constant, whose temperature dependence is described by an Arrhenius equation; n is an empirical content.
where k o is pre-exponential multiple; E is activation energy; T is temperature for completion of γ → α-transformation into Al2O3.
The rate of γ → α-transformation into Al2O3 depends considerably on addition to original powder, consisting of γ-Al2O3, of α-Al2O3 particles, which is reflected in the value of kinetic parameters and a reduction in temperature for completion of transformation (Table 5).
Formation of α-Al2O3, nuclei with explosive pressing accelerates phase transformation and reduces its temperature. A reduction in temperature for γ → α-transformation into Al2O3 facilitates retention in the course of heating with high powder activity for sintering, which is required in order to create a fine-grained structure within sintered material.
Conclusion
Formation of nanostructured boehmite powder, prepared by hydrothermal synthesis from industrial aluminum powder, by extrusion, static and explosive pressing, and also high-temperature sintering of the workpieces obtained, have been studied. As a result of phase transformations during heating sintered specimens have high porosity of a nanosize range (relative ceramic density not more than 71%). The materials obtained are promising for application as catalysts, heat insulation, and ceramic membranes.
Compaction of nanostructured boehmite and aluminum oxide nanopowder with static and explosive pressing obeys general features of compaction for ceramic powders up to a pressure of 4 GPa. The effect of the nanosize nature of boehmite and aluminum oxide nanopowder prepared from is inadequate for preparing dense ceramic. In order to increase ceramic density obtained from nanopowder it is necessary to increase workpiece density.
It has been established that explosive pressing of powder containing γ-Al2O3 leads during sintering to a reduction in temperature of the γ > α-transformation into Al2O3 by 100 – 150°C. Formation of α-Al2O3 nuclei during explosive pressing accelerates phase transformation and reduces its temperature, and also facilitates preparation of denser ceramic.
References
A. V. Belyakov, “Problems in making a dense nanoceramic,” Refract. Indust. Ceram., 50(2), 136 – 141 (2009).
A. V. Belyakov, “Introduction of nanomaterials and nanotechnologies in ceramics plants,” Glass and Ceramics, 67(7/8), 203 – 208 (2010).
Yu. A. Mazalov, A. V. Bersh, and Yu. L. Ivanov, RF Patent 2278077. Method for preparing hydroxide from aluminum oxide and hydrogen. No. 2005121562; Claim 07.1.05; Publ. 06.20..06.
V. I. Chernoivanov, N. N. Sergeev, A. V. Dunaev, and A. V. Fedotov, RF Patent 2472848. Addition to oil for running-in and internal combustion engine and running-in oil. No. 2011137828/04; Claim 109.14.11; Publ. 01.20.13.
V. I. Chernoivanov, P. A. Vityaz’, L. V. Sudnik, et al., RF Patent 2443502. Antiscale paint for casting molds and rods. No. 2010136065 / 02; Claim 08.31.10; Publ. 02.27.12.
M. Lee, x-ray Diffraction for Materials Research from Fundamentals to Applications, Apple Academic Press, Inc., Oakville (2016).
V. A. Liopo and V. V. Voina, X-ray Diffractometry, Grodno, 31 – 35 (2013).
GOST 23401–90. Metal Powders. Catalysts and Carriers. Determination of the Specific Surface Area, Izd. Standartov (1991).
E. P. Barrett, L. G. Joyner, and P. P. Halenda, “The determination of pore volume and area distributions in porous substances. I. Computations from nitrogen isotherms,” J. Amer. Chem. Soc., 73, 373 – 380 (1973).
GOST 2999–75, Metals and Alloys. Methods for Vickers hardness measurement, Izd. Standartov (1987). Instead of GOST 2999–59, intro. 07.01.1976.
V. F. Nesterenko, Pulsed Loading of Heterogeneous Materials [in Russian], Nauka, Novosibirsk (1992).
V. D. Rogozin, Explosive Treatment of Powder materials [in Russian], Politekhnik, Volgograd (2002).
T. J. Vogler and M. Y. Lee, “Grady static and dynamic compaction of ceramic powders,” Int. J. Solids and Structures, 44(2), 636 – 658 (2007).
A. V. Bersh, D. Yu. Mazalov, R. Yu. Solov’ev, et al., “Nanostructured aluminum hydroxide and oxide degassed powder,” Novye Ogneupory, No. 7, 37 – 42 (2016).
A. V. Belyakov, E. A. Brygina, “Local compaction areas in sintering of ceramics and structural reproducibility,” Glass and Ceramics, 55, No. 9, 307 – 309 (1998).
GOST 23775–79. Carbon objects. Methods for determining ultimate strength in compression, bending, and breaking (diametric compression. Izd. Standartov (2001).
S. W. Freiman and J. Mecholsky, The Fracture of Brittle Materials: Testing and Analysis. Wiley, USA : (2012). (ISBN 978 – 0-470 – 15586 – 8).
A. V. Bersh, A. V. Belyakov, D. Yu. Mazalov, et al., “Corundum composite ceramic, preparation with use of boehmite nanoparticles,” Novye Ogneupory, No. 10, 52 – 57 (2016).
A. V. Belyakov, “Synergetic and quasichemical approaches in ceramic technology (a review),” Glass and Ceramics, 60(9/10), 274 – 279 (2003).
The work was carried with financial support of the RF Ministry of Education and Science (Agreement for supply of a subsidy No. 14.613.21.0004 of 08.22.2014. Project unique identifier RFMEF161314X0004).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Novye Ogneupory, No. 12, pp. 46 – 51, December, 2016.
Rights and permissions
About this article
Cite this article
Bersh, A.V., Belyakov, A.V., Mazalov, D.Y. et al. Formation and Sintering of Boehmite and Aluminum Oxide Nanopowders. Refract Ind Ceram 57, 655–660 (2017). https://doi.org/10.1007/s11148-017-0040-0
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11148-017-0040-0