The process of hydrothermal synthesis of boehmite from aluminum powder is studied at 330 – 350°C and a pressure of 15 – 17 MPa. Calculation of mechanical stresses arising in an oxide film on aluminum powder particles during heating and the volume of hydrogen formed during oxidation of aluminum under hydrothermal conditions is conducted. Liberated hydrogen puts pressure on a boehmite layer, destroys it, and leads to the formation of nanostructured powder from the initial aluminum powder with sizes of several microns. Dependences of an ideal mixing continuous-type reactor volume on boehmite productivity, temperature, and powder fineness are determined. Fast oxidation rates make it possible to reduce reactor volume to 2 liters with a capacity of up to 10 kg/h and to improve process safety.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Due to an extensive field of application aluminum hydroxide is a multitonnage product of the chemical substance market within the world. Among aluminum oxyhydroxide hydroxides is boehmite (AlOOH) and to a greater extent pseudo-boehmite, containing within the AlOOH structure and additional amount of water (15 wt.% above stoichiometric with respect to the boehmite formula) being a valuable raw material, for example in the production of catalysts and ceramics. In contrast to other hydroxides boehmite and pseudo-boehmite exhibit a capacity for peptization forming plastic pastes and ceramic materials with a highly developed surface.
The main industrial methods for preparing very fine boehmite and pseudo-boehmite are reprecipitation of aluminum trihydroxide, obtained by the Bayer method, and aluminum oxide hydrolysis. Among other numerous methods for synthesizing boehmite in our opinion the thermal method is promising using aluminum metal making it possible to create a broad spectrum of aluminum hydroxides and oxides [1]. The method is ecologically clean and provides preparation of nanostructured powder of prescribed fineness and morphology with simultaneous separation of hydrogen and heat. A feature of the method is high purity boehmite preparation and an improved crystal structure. Favorable results have been obtained in using hydrothermal synthesis in ceramic, agricultural services, and other fields [2, 3]. One of the marked disadvantages of the method is a requirement for observing precautionary measures laid down by Gosgortekhnadzor rules for high-pressure units, in particular presence of an individual location for an autoclave and automation of production. In this case automation of production is one of the most extensively used methods. An important question is that a reduction in reactor size is due to the rapid nature of the chemical reaction that makes it possible to reduce its volume and precautionary measures connected with it.
The aim of the present work is to study the process of nanostructured boehmite powder preparation and determination of preliminary relationships for reactor volume on boehmite productivity, synthesis conditions, aluminum powder fineness required for reducing reactor size, increasing safety, and governing the possibility of scale-up.
Materials and Research Procedure
In order to prepare nanostructured boehmite industrial alumina powder grade ASD-4 was used (with specific surface of 0.35 m2/g) and finer grade powder ASD-6 (with specific surface of 0.54 m2/g). The average particle size according microscope analysis data is 10.8 μm for ASD-4 powder and 6.2 μm for ASD-6 grade powder. Optimum conditions for providing good control of boehmite synthesis reactions in the form of finely dispersed powder have been provided in publication [4]. A suspension of finely dispersed aluminum powder in water (Al/H2O = 1/8) was sprayed into a reactor in which with a sub-critical water parameters (T = 330 – 350°C, P = 1517 MPa, water critical point Tcr = 374.3°C, Pcr = 22.1 MPa) there was boehmite formation. The chemical reaction for boehmite formation is heterogeneous, exothermic, with low reaction heat of about 16 MJ/kg:
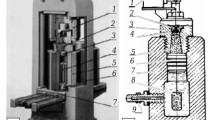
The aluminum oxidation reaction proceeds with formation of hydrogen and a significant release of energy. In view of this various technologies are developed for converting hydrogen into electrical or thermal energy depending on user requirements [5]. Within the composition of the isothermal oxidation reactor there is a power unit used for producing a high pressure steam-hydrogen mixture that increases the economic attraction of synthesizing nanostructured boehmite by this method. A process is considered in this work for preparing just aluminum oxyhydroxide. Boehmite was prepared in a hydrothermal synthesis experimental unit of nanostructured aluminum hydroxide and oxide powders, registered as a unique scientific unit (USU) [6]. The layout of the experimental unit is shown in Fig. 1. The unit operates in a periodic regime. The operating sequence includes preparation of a finely dispersed aluminum suspension in mixer 2, creation in reactor 4 of a high steam vapor pressure, supply of a suspension by means of a high-pressure dispensing pump 3 into the reactor, removal from the reactor of aluminum oxyhydroxide into a reception device 5, removal of hydrogen from the reactor and collection 13.
Layout of an experimental unit for hydrothermal synthesis of nanostructured boehmite, hydrogen, and thermal energy: 1 ) vessel with water; 2 ) mixer; 3 ) high pressure pump; 4 ) reactor; 5 ) vessel for hydroxides; 6 ) condenser; 7 ) heat exchanger; 8 ) vessel with water; 9 ) pump; 10 ) condenser; 11 ) vessel for decanting condensate; 12 ) drying column; 13 ) vessel for hydrogen.
The hydrothermal oxidation process for aluminum is determined not only by the chemical nature of the reaction, but also the effect on it of thermomechanical properties of system components. Under normal condition aluminum particles are covered by a continuous oxide film. Tensile stresses arise within a film on heating, since the LTEC for aluminum is greater by several factors than that for oxide film material. The oxide film has small thickness and stresses within it are calculated by a procedure for calculating thin-walled shells (ratio of film thickness to particle diameter does not exceed 1:20). In this case the value of the internal pressure within a film is adjusted taking account of the external pressure formed by the water vapor and water mixture (16 MPa).
The oxidation process under hydrothermal conditions differs from oxidation in an air atmosphere. Whereas in an air atmosphere the process occurs in the direction of forming a continuous aluminum oxide film, then under hydrothermal conditions there is continuous formation of a boehmite film and hydrogen that causes pressure within the boehmite layer formed. The volume of hydrogen formed has been calculated for chemical reaction (1). The volume of hydrogen formed under hydrothermal conditions has been worked out using the well-known Klaperon – Mendeleev equation. The equation concerns an ideal gas condition. However, according to published data [7, 8] hydrogen physical properties do not differ strongly from those of an ideal gas in the temperature range 0 – 500°C and pressure up to 10 MPa. For example, with a temperature of 300°C and pressure of 10 MPa deviation may be 2 – 6%. Therefore, for rough calculations we will use an ideal gas equation.
The volume of an ideal continuous type mixing reactor has been calculated corresponding the volume of chemical reaction products for aluminum hydrothermal oxidation. The volume of chemical reaction products was calculated as the sum of derived volumes of mixture components in accordance with the Amaga rule, i.e., as the sum of reaction products of components produced for the specific volume under experiment performance conditions.
Research Results and Discussion
The main equation of momentless shell theory is a Laplace equation that for particles in the form of a sphere has the following form:
where σt is meridional stress within a shell, MPa; σs is circumferential stresses within a shell, MPa; R and δ are particle radius and shell thickness taken as 10–5 and 4 × 10–8 m respectively [9] q is internal gas pressure, MPa.
Internal pressure within a film (considered to consists of α-Al2O3, for which thermomechanical properties are well known) arises due to expansion on aluminum particle heating. This pressure q within a film may be evaluated by an equation:
where δα is the average difference of aluminum LTEC and oxide film LTEC, equal for the range 20 – 340°C 24.1 × 10–6 – 7.7 × 10–6 1/deg; δT is the temperature difference 20 – 340°C; E is aluminum elasticity modulus equal to 0.7·105 MPa.
The value of internal pressure obtained σt, s should be reduced to the value of pressure of the sub-critical reactor environment (16 MPa). After substituting Eq. (3) in Eq. (2) we obtain:
After substitution of all values tensile stresses within a film comprise 3.5 × 104 MPa, which exceeds by two orders of magnitude the strength of newly formed material (for example the ultimate strength in bending for different grades of corundum ceramic is 300 – 450 MPa). Whence it is possible to conclude that there is cracking of a surface oxide layer during aluminum heating and oxidation, Analysis of stresses calculated by Eq. (20 indicates that heating even by 10°C leads to film breakage. Other researchers have come to a similar conclusion that thermal stresses calculated for ASD-4 powder with a particle size of 20 μm exceed the oxide film mechanical strength even at 10°C [10].
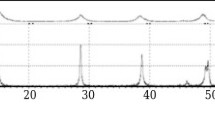
The oxidation process under hydrothermal conditions differs from oxidation in an air atmosphere. Whereas in an air atmosphere the process advances in the direction of forming a continuous aluminum oxide film, under hydrothermal conditions there is continuous formation of a boehmite layer and hydrogen that breaks down a newly formed layer. Under the hydrothermal synthesis conditions adopted the process occurs with formation of well crystallized boehmite without forming any subsidiary products (Fig. 2). A feature of powder creation under hydrothermal conditions is the fact that well recrystallized unreacted or weakly reacted nanoparticles are obtained. The material has high purity, depending on the purity of aluminum powder, water and reactor materials (weight content of the most widespread impurity elements does not exceed 0.07 wt.%), and a nanocrystalline structure, particle size does not exceed 35 nm (Fig. 3).
We try to present the mechanism for nanostructured powder formation. In accordance with the chemical reaction equation the volume of hydrogen formed under normal conditions (Vn.c., m3) may be determined by the following equation:
where PAl is weight of aluminium particles; MH is hydrogen molecular weight equal to 2.016; 22.4 is volume of 1 kg mole of gas, m3; AAl is aluminum atomic weight equal to 26.98.
An expression has been obtained for the volume of hydrogen formed during oxidation of a single aluminum particles under hydrothermal conditions Vh.c
where P1 is gas pressure under normal conditions; T1 is gas temperature under normal conditions; T2 and P2 are temperature and pressure under synthesis conditions (613 K and 16 MPa respectively); ∅ and ρAl are average aluminum spherical particle diameter and its density equal correspondingly to 7.2 × 10–6 m (for aluminum powder grade ASD-6) and 2700 kg/m3.
The volume of hydrogen calculated by Eq. (3) formed during oxidation of aluminum particles is 9.21 × 10–15 m3, which exceeds by a factor of 47 the aluminum particle volume. Hydrogen liberated has a pressure exceeding the boehmite layer strength and breaks (refines) it to almost individual nanoparticles. In our opinion this mechanism is responsible for transformation of aluminum particles of micron size into nanosize boehmite particles (Fig. 4).
The calculation is rough since it does not take account of a change in aluminum particle volume on heating, deviation of the reaction gas atmosphere condition from an ideal gas, and possibly some other factors, but it is possible understand the process of nanosize particle formation. Under hydrothermal conditions there is continuous formation of a boehmite film and its breakdown. The diffusion stage of reacting mixtures through a boehmite layer is not limiting since film breakdown reveals a fresh surface of an aluminum particles and process continues to complete aluminum oxidation. This explains the high rate of the process. As a result of this complete oxidation of powder grade ASD-4 it proceeds in 320 sec, and for finer grade powder ASD-6 in 55 sec [4]. The average particle size is 10.8 μm for powder grade ASD-4 and 7.2 μm for powder grade ASD-6.
We calculate reactor volume for a continuously operating ideal mixing reactor within the process temperature and concentration of all substances within the reactor volume is constant in time. For this reactor the overall volume of the original suspension and steam and hydrogen mixture corresponds to the reactor volume. In order to define a continuous ideal mixing reactor (IMR-C) this is a reactor with a stirrer to which reagents are fed continuously and reaction products emerge from it. Due to intense mixing of flows there is instantaneous establishment of a reagent concentration uniform through the whole reactor volume, equal to its concentration at the outlet from the reactor. The authors used a reactor without a stirrer, but the fast diffusion rate and small size of aluminum particles leads to a situation that as a result of a fast chemical reaction rate (total transformation proceeds for 55 – 320 sec) approximates to an IMR-C reactor. The ratio of the weight of water to aluminum within a suspension is 8:1.From equality of the sum of the weight of starting components and reaction products the equation for the aluminum oxidation chemical reaction material balance has the form:
where mAl + mHO2 are weights of aluminum and water respectively; mH2 + mAlOOH are weights of hydrogen and boehmite; m1 and m2 are weights of water in liquid phase and saturated water vapor respectively.
The reaction product volume is greater than that of the starting substances since component composition changes with formation of a steam and hydrogen mixture. The volume chemical reaction product volume V may be determined (to a first approximation) as the sum of the derived volumes of mixture components in accordance with the Amaga rule, i.e., as the sum of the weight of components produced or their specific volume under hydrothermal conditions according to an equation
where vH2 is hydrogen specific volume, m3/kg; ρAlOOH is boehmite density equal to 3100 kg/m3; m1 and m2 are weight of water in liquid phase and saturated water vapor respectively; v1 and v2 are specific volume correspondingly for boiling water and dry saturated water vapor, m3/kg.
A temperature range was selected for calculation providing a nanostructured boehmite state under hydrothermal synthesis conditions. The region for coherent dissipation according to which boehmite nanoparticle size was evaluated corresponding to 32 – 55 nm [35]. This size range corresponds to a reactor temperature of 300 – 350°C. At lower temperature the crystal size is even smaller, but in this case there is an unjustifiable increase in typical reaction time and reactor volume. In addition, complete aluminum transformation into nanosize boehmite cannot be achieved.
The specific volume of hydrogen has been calculated for hydrothermal conditions from tabulated density values [7, 8]. For water the specific volumes for boiling water and dry saturated water vapor under synthesis conditions is 0.03 – 0.9%, and therefore their volumes cannot be considered separately but were taken as their sum [11]. Taking account of this equation (8) is transformed into a relationship for the volume of reaction space V, m3, on productivity with respect to boehmite:
where G is boehmite productivity, kg/sec; treac is chemical reaction time, sec.
The typical chemical reaction time has been calculated by an equation [5]
where K1 and K2 are equation coefficients equal correspondingly to 1 × 10–5 sec (with a temperature of 9.2·103K for powder grade ASD-6) and 4 × 10–8 sec (with temperature 12.9 × 103 K for powder grade ASD-4).
Calculated dependences are shown in Fig. 5 for the reactor volume on aluminum powder grade and temperature in the reactor. It is seen that a reduction in reactor volume is possible due to using aluminum powder of grater fineness and an increase in temperature. In order to fulfil the specifications of reactor safety operation at elevated temperature and pressure, it is expedient to reduce the volume with retention of an adequate process rate. In this case it was possible. A fast aluminum micropowder particle oxidation rate made it possible to reduce the reactor volume to 2 liters with productivity for nanopowder up to 10 kg/h.
Conclusion
The process of forming nanostructured boehmite powder from aluminum powder under hydrothermal conditions has been studied. Under heating conditions there is cracking of an oxide film at an aluminum particle surface as a result of thermal stresses and oxidation a fresh aluminum particle surface. During oxidation under hydrothermal condition at 330 – 350°C and a pressure of 15 – 17 MPa there is formation of a volume of hydrogen that exceeds by a factor of 47 the volume of particles and the hydrogen liberated is a destructive pressure ion a boehmite layer. This mechanism concerns transformation of aluminum particles of micron size into nanosize boehmite particles.
A model has been developed for the volume of an ideal continuous type mixing reactor for hydrothermal oxidation of aluminum. Fast oxidation rates make it possible to minimize reactor volume to 2 liters during production of up to 10 kg/h that reduces specifications for the safety of these reactors.
References
A. V. Bersh, N. N. Zhukov, Yu. L. Ivanov, V. K. Ikonnikov, Yu. A. Mazalov, V. Yu Ryzhkin, and O. A. Trubachev, RF Patent 2223221, MPK C 01 F 7/42, C 01 B 3/1. Method for preparing hydroxides or oxides and hydrogen; Claim 11.02.2003; Publ. 10.02.2004, Bull. No. 4.
A. V. Bersh, A. V. Belyakov, D. Yu. Mazalov, et al., “Formation and sintering of boehmite and aluminum oxide nanopowders,” Refract. Ind. Ceram., 57(6), 609 – 613 (2017).
A. V. Fedotov, “Use of aluminum oxide-hydroxide and oxide in agricultural service,” Tekhnika Oborud. Dlya Sela, No. 10, 38 – 42 (2019).
A. V. Bersh, A. V. Lisitsyn, A. I. Sorokovikov, et al., “Study of the processes of steam-hydrogen mixture generation in a reaction hydrothermal aluminum oxidation for power units,” High Temperature, 48(6), 866 – 873 (2010).
M. S. Vlaskin, Continuous operation hydrothermal aluminum oxide reactor and energy equipment based upon it,” Author’s Abstr. Diss. Cand. Techn. Sci; 05.14.01, OIVT RAN Moscow (2009).
P. A. Vityaz’, A. F. Il’yushenko, L. V. Sudnik, Yu. A. Mazalov, and A, V. Bersh, Fractionated Material Based on Aluminum Oxide [in Russian], Belaruskaya Navuka, Minsk (2010).
D. Yu. Gamburg, V. P. Semenov, N. F. Dubovkin, and D. N. Smirnova, Hydrogen. Properties, Preparation, Storage, Transportation, Application [in Russian], Khimya, Moscow (1989).
I. A. Babichev, N. A. Babushkina, A. M. Btratkovskii, et al., Physical Values: Handbook [in Russian], Énergoatomizdat, Moscow (1991).
A. P. Nechitailov, I. A. Platokhnikova, and T. A. Shiyova, “Evaluation of aluminum power and powder surface film thickness,” Lit. Obrab, Aluminii: Tr. VIAM, No. 99, 96 – 100 1977).
V. G. Shevchenko, M. A. Bulatov, V. I. Kononenko, et. al., “Influence of the properties of surface oxide layers on the oxidation of aluminum powders,” Powder Metallurgy and Metal Ceramics, 27(2), 89 – 92 (1988).
A. A. Aleksandrov and B. A. Grigor’ev, “Tables of standard reference data GSSSD 187–99. Water. Specific volume and enthalpy at 0 – 1000°C and pressure 0.001 – 1000 MPa,” All-Russia Sci. Res. Center for Raw Material, Materials, and Substance Standardization, Information, and Certification, Gosstandart, Moscow (1990).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Novye Ogneupory, No.11, pp. 36 – 41, November, 2021.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fedotov, A.V., Belyakov, A.V. Features of Nanosize Boehmite Hydrothermal Synthesis. Refract Ind Ceram 62, 658–662 (2022). https://doi.org/10.1007/s11148-022-00657-1
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11148-022-00657-1