Abstract
In this study, it is aimed to investigate catalytic decomposition of hydrogen peroxide for oxygen generation for a fuel-cell based air independent hydrogen production system in underwater applications for our following studies. O2 and water were generated after H2O2 was decomposed catalytically. Here, H2O2 acts as an oxidizer and pure O2 is fed on to a fuel cell and the water is used for hydrolysis reaction of sodium borohydride for clean H2 production. Due to these reasons, H2O2 was selected as an oxygen source concurrently. H2O2 is an environmentally friendly chemical because of its decomposition by-product is only water. The prepared Nb based KIT-6 silica catalysts showed high catalytic activities for the H2O2 decomposition. These catalysts were characterized by SEM, SEM–EDX, FT-IR, ICP-OES, TEM, N2 adsorption–desorption and XRD analyses.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The large specific surface area of KIT-6 is favorable for the dispersion of active metals. This property provides more active sites for the catalytic reaction to KIT-6 based catalysts [1,2,3]. KIT-6 has three-dimensional pore structure and due to its pore blockage resistance property KIT-6 is an excellent candidate for catalytic applications [4]. Mesoporous KIT-6 silicas have uniform channels, large pore size, high specific area and high thermal stability with its three-dimensional cubic la3d structure [5]. KIT-6, a mesoporous SiO2 combining the la3d structure to MCM-41 with larger pore diameters and has attracted attention in recent years due to its optimal properties which enhance metal dispersion and accessibility of reactants [6, 7]. Metal dispersion is to adopt new supports like mesostructured silica MCM-41 [8], SBA-15 [9], KIT-6 [1] etc. with appropriate good thermal and mechanical resistance, large surface area and the well ability to disperse metal active phases to attractive reaction catalysts [10]. Catalyst with high metal dispersion and good durability, many preventive measures have been taken in the catalyst preparation. In this work, Nb was introduced into KIT-6 catalyst. This study was focused on the physicochemical influence of Nb-based catalysts supported on KIT-6 and their catalytic activity in H2O2 decomposition. As consequence, they show a relatively high activity for this reaction. Metal into the framework of KIT-6 to achieve stronger solid acid catalyst for organic reactions such as esterification, sucrose hydrolysis, oxidation, epoxidation etc. [11]. In this work, unlike the literature H2O2 decomposition reaction was investigated with Nb ions into the mesoporous KIT-6 framework under hydrothermal route. To the knowledge, this is the first time to use the mesoporous silica KIT-6 for this reaction system.
Hydrogen peroxide (H2O2) is an environmentally friendly chemical because its only decomposition by-product is water [12,13,14,15]. It is a clean oxidant and generally selected as an oxidizer source [16,17,18,19,20,21]. It is used by many sectors due to its easy access and safe use possibilities [22,23,24]. It is generally used in waste water treatment or chemical industry [25,26,27]. However, rare study has focused on its decomposition reaction for O2 and water generation used in H2 production. The H2O2 decomposition reaction given below [28]:
The overall reaction is:
This catalytic decomposition process can be performed in not only homogeneous system but also heterogeneous system [29,30,31,32]. In recent years, there are some studies that used active metal catalysts for this reaction [32,33,34,35,36]. This reaction also has for long been used for redox catalytic activity of metals [37, 38].
In this study, a catalytic H2O2 decomposition reaction was used for generating oxygen and water. Nb monometallic catalysts were synthesized for the hydrogen peroxide decomposition reaction. It was shown that Nb had efficient potential for this reaction. In the following studies it is aimed that the pure oxygen is used as an oxidizer for fuel cell system. The by-product water is stored and used for hydrolysis of sodium borohydride for clean H2 production [39,40,41,42]. It is thought that at these days, O2 may also plays an important and excellent role. Mesoporous silicas as MCM, SBA, MSU, KIT-6 etc. types have great potentials for many catalytic reactions [8, 9, 43,44,45,46,47]. They are generally used as a catalyst support for chemical reactions and also used for drug delivery, radiotherapy, separation or adsorption processes [48,49,50,51]. This work has focused on Nb based catalysts due to their effective performance in comparison with other active metals [52,53,54]. The scope of the present study is to demonstrate the suitable and effective metal based catalyst for catalytic H2O2 decomposition reaction.
Nb based KIT-6 catalysts with different metal loadings were prepared by hydrothermal method [55]. The catalysts were characterized by X-ray diffraction (XRD), N2 adsorption–desorption, Transmission electron microscopy (TEM), Fourier transform infrared spectroscopy (FT-IR), Scanning electron microscopy (SEM), energy dispersive X-ray spectroscopy (EDX), inductively coupled plasma-optical emission spectrometry (ICP-OES) analysis. The catalytic performance of the prepared catalysts was evaluated via H2O2 catalytic decomposition reaction. Nb based KIT-6 catalysts showed good catalytic performance and especially superior catalytic activity than other supported materials. Catalytic activity for H2O2 decomposition with Nb has not been reported as far as we know.
Experimental
Catalyst preparation
Nb based KIT-6 catalysts with different metal contents were prepared by the typical procedure [55]. Nb containing KIT-6 mesoporous la3d structure materials with metal loading 1.5, 3.4, 6.1 and 10.9 wt% were synthesized using Pluronic P123 (Carbosynth) tri-block copolymer and n-butanol (Merck). 5.0 g of P123 was dissolved in 161 ml of 0.5 M hydrochloric acid (Merck) at 35 °C. After dissolution was completed, 5.0 g of n-butanol was added and the resulting mixture was stirred for 1 h at 35 °C. Metal source Niobium (V) chloride (Acros) and the required amounts of TEOS (Abcr) were added to the mixture to obtain the desired molar ratio of 1.5, 3.4, 6.1 and 10.9 wt% and the mixture was stirred for 24 h. Finally, the reaction mixture was poored in a 250 ml Teflon autoclave for hydrothermal treatment (24 h at 100 °C). The final solid was seperated and dried at 100 °C overnight. The directing agent was removed by calcination in dry air at 550 °C for 5 h.
Characterization of catalysts
Surface morphology of Nb loaded KIT-6 catalysts were measured by TEM; JEOL 1220 JEM and SEM using Quanta 400F Field Emission device. FT-IR spectra of all the samples were recorded on Cary 630 Fourier transform infrared spectrometer, equipped with a single reflection diamond attenuated total reflectance (ATR) accessory between 400 and 4000 cm−1 employing diluted samples. The textural parameters (specific surface areas, porosities and pore sizes) were obtained via N2 adsorption–desorption isotherms using BET and BJH methods (Micromeritics ASAP instrument). Before the measurements samples were outgassed at 250 °C and 100 mmHg, overnight. XRD patterns of the samples were obtained by a Panalytical Empyrean instrument at 200 kV and 50 mA with 2θ values ranging between 5° and 80° and with a speed of 10 °C/min. Metal loading were determined from ICP-OES analyses; Perkin Elmer Optima 4300DV.
Catalytic activity tests
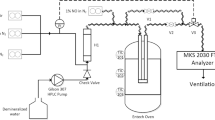
0.25 g catalyst was added to an aqueous solution of H2O2 (5.5 g, 30%) and the reaction mixture was stirred for 2 h at ambient temperature (∼25 °C). At the end of the reaction, the catalyst was separated out. The partly decomposed H2O2 was diluted to 250 ml in a standard volumetric flask. Ten milliliter of this solution was transferred in a flask and titrated with standard KMnO4 after addition of 20 ml of 2 M H2SO4 and 20 ml water [56]. The decomposition rate of hydrogen peroxide was measured by mass titration with KMnO4. In each reaction experiment, 0.25 g of catalyst was used and four simultaneous reactions were carried out at room temperature.
Results and discussions
Characterization of catalysts
Physical and textural properties of Nb/KIT-6 catalysts were illustrated in Table 1. Results indicated higher surface areas for the catalysts synthesized. An increase in surface area with increasing Nb amount was observed. The increase was due to the increase of porosity emanated from niobium presence resulted in the increase of surface area. The decrease in pore volumes implied incorporation of Nb inside the pores with the increasing Nb amounts on the catalysts. Pore diameters varied between 3.9 and 7.6 nm and these values were determining mesoporous structure (2 nm < pore size < 50 nm). The amount of Nb% loss from the catalyst was determined by ICP-OES analyses (Table 1). Inductively Coupled Plasma (ICP-OES) is an analytical technique in which low concentrations of elements are measured. The sample is sent to argon plasma at a temperature of 6000–10,000 K. In the plasma, molecular bonds are broken, atoms and ions are formed. Immediately after these formed atoms and ions are excited in the plasma, they return to their former energy levels by irradiating at characteristic wavelengths. Emission signals are measured by array detector system. More than one element can be determined at the same time. The determination is made according to the wavelengths depending on the optical properties of the light passed through the plasma. Results indicated a significant loss of active metal synthesized with 10.9% Nb loaded KIT-6 during synthesis. It is seen that the metal loss decreases as the loading ratio decreased. It is thought that the loss occurs during the synthesis procedure or it passed into the washing water during washing procedure.
Wide-angle XRD patterns of Nb containing KIT-6 catalysts prepared with different amounts of metal loading were given in Fig. 1. Small-angle XRD pattern of KIT-6 was at Supplementary file. The peaks of KIT-6 at 2θ of 0.99° and 1.68° indicated the (211) and (220) reflections which are due to the well-ordered cubic 3-D mesoporous arrangement [54]. The broad peak obtained at 23.5° corresponded to amorphous silica. The peaks observed at 2θ values of 34.6°, 37.1°, 48.4°, 51.9°, 58.2°, 62.3°, 68.7°, 70.1°, and 76.4° corresponded to reflections of Nb metal. XRD results are important due to confirming the presence of Nb in the catalyst structure. Similar to the literature, it was observed that the intensity of the peaks increased as the amount of Nb increased [57].
N2 adsorption–desorption isotherms of catalysts were Type IV according to IUPAC classification which indicated the formation of mesoporous structure with narrow pore size distribution [58]. The KIT-6 has the characteristic type IV isotherm according to the IUPAC classification with an average pore size of 4.5 nm. Nb/KIT-6 catalysts exhibit type II or type IV isotherms with average pore sizes between 4.7 and 5.5 nm (Fig. 2).
Fig. 3 displays the SEM images of the Nb/KIT-6 catalysts. The surface morphology and the spongy nature of Nb/KIT-6 catalysts were investigated. Niobium ions into the catalyst surface affected the smoothness of the materials and Nb/KIT-6 is agglomerated to small irregular particles.
SEM–EDX analysis was also compiled to establish the chemical composition of the Nb/KIT-6. The EDX analysis demonstrated the presence of niobium in Nb/KIT-6 framework. Niobium was successfully incorporated into the KIT-6 mesoporous silica. It was observed from the EDX results that the amount of niobium in the structure increased as the niobium loading rate increased (Supplementary File). The elementel distribution of Nb is indicated using EDX-SEM mapping. Nb particles were homogeneously dispersed on the catalysts surface (Supplementary File).
The morphology of Nb/KIT-6 was also investigated by TEM analysis (Fig. 4). The well-ordered pore structures of mesopores and arrays of mesoporous channels were observed from the images (shown in blue circle).
The FTIR spectra between 400 and 4000 cm−1 of Nb/KIT-6 catalysts are shown in Fig. 5. The characteristic peak belonging to Si–O–Si bond appeared at around 1074 cm–1 due to symmetric stretching vibrations for all samples. The peaks at 455 cm–1 and 806 cm–1 corresponded to the bending of Si–O bond and asymmetric bending of Si–O–Si bond, respectively. The symmetric stretching of Si–OH was observed at around 952 cm–1. As well as the band at about 3392 cm−1, the peaks around 1645–1650 cm–1 was attributed to –OH stretching vibrations related to adsorbed water molecules which provide surface modification easily [59].
Catalytic performance of the catalysts in decomposition of H2O2
All Nb loaded silica based KIT-6 catalysts were tested in decomposition of H2O2 reaction. The catalytic activities were observed at room temperature and four simultaneous reactions were carried out for 2 h. In each reaction experiment, 0.25 g of catalyst was used and 4 simultaneous reactions were carried out at room temperature (Run-1 to Run-4). 5.5 g 30% of hydrogen peroxide was used in the reactions and the experiments were carried out in a batch reaction system consisting of a three-necked glass balloon. The amount of hydrogen peroxide decomposed as a result of the reaction was determined as a result of titration of 10 ml samples taken from the reaction mixtures with KMnO4. The reaction was continued for a maximum of 120 min and reaction experiments were carried out under the same experimental conditions at different time intervals. At the end of each reaction, 10 ml of solution was taken and titrated with 0.01 KMnO4 solution according to the procedure applied in the literature [56]. The reaction experiment, which was continued for 120 min, was repeated 4 times, showing that the results were reproducible. The conversion of H2O2 after 2 h has shown different trends for all of the catalysts. The decomposition of H2O2 is presented in Fig. 6. 0.25 g catalyst was used in the H2O2 decomposition reactions carried out to determine the catalytic activity and the reactions were carried out in atmospheric pressure at room temperature. Before the reaction experiments, it was observed that H2O2 remained intact in the structure at the end of the highest reaction time of 120 min, in the trials without using a catalyst. The experiments carried out for 120 min were repeated 4 times, the conversion values were compared and the reproducibility of the results was examined. In Fig. 6, it was seen that at the end of 120 min, the H2O2 conversion reached the highest value of 85% conversion. Experiments performed under the same conditions reveal that the results are reproducible and that the experimental error is negligible. The results showed that the highest conversion value could be reached in a shorter time depending on the amount of active substance added. In other words, the conversion value increases depending on the amount of active substance in the catalyst structure. The results also revealed that the reactant could diffuse into the catalyst during the reaction. For the catalysts, as the niobium loading rate increases, there is a significant increase is observed. The choice of catalyst is justified by the fact that niobium metal is the most successful to catalyze the decomposition of H2O2. The catalyst synthesized has shown good activity for H2O2 decomposition. There are few studies with metal-containing KIT-6 catalyst in decomposition of H2O2 reaction. To the best of knowledge, there is no study about Nb/KIT-6 catalysts in this reaction. Literature survey showed a number of studies regarding to H2O2 decomposition. These studies were conducted in the presence of different variety of metal containing catalysts and varying experimental conditions (Table 2). It is clearly observed from the table that these Nb/KIT-6 catalysts exhibit the highest activity and efficiency. The Nb/KIT-6 catalyst synthesized in this study has shown good activity for H2O2 decomposition reaction. A comparison with literature must be made in order to better visualize the extent of its activity and efficiency. Literature survey showed a few and new number of studies regarding to H2O2 decomposition reaction with KIT-6 catalysts. These studies were conducted in the presence of a variety of catalysts with varying experimental conditions and selected studies were summarized. It was clearly seen from the table that synthesized catalyst had been among the ones with highest activity and efficiency [56, 60,61,62]. It is obvious from reaction experiments and characterization analyses that 10.9% Nb/KIT-6 catalyst had the highest activity among those synthesized by varying Nb amounts. This was among the most important results of the present study as it showed the possibility of a sustainable production.
Reaction experiments conducted in the order of 10.9% Nb (85%) > 6.1% Nb (72%) > 3.4% Nb (63%) > 1.5% (51%) Nb/KIT-6 based on H2O2 conversion. From reaction experiments and characterization studies it is obvious that 10.9% Nb/KIT-6 catalyst had the highest activity (85% H2O2 conversion) among those synthesized by varying Nb amounts. It was seen that as the amount of Nb increased, the activity increased accordingly. Nb was among the proper choices with its efficient property in this reaction.
Conclusions
Nb/KIT-6 with different Si/Nb weight ratios was successfully synthesized. The presence of highly ordered structure was evident from SEM, SEM–EDX, TEM and N2 adsorption–desorption analysis. The absence of Nb crystals was inferred from XRD spectra. The effects of varying Nb amounts on the catalytic properties of KIT-6 catalysts were investigated during H2O2 decomposition at 25 °C. Nb based KIT-6 catalysts presented high activity. Catalytic tests and characterization studies revealed better performance. It is noteworthy that the catalytic activity of Nb/KIT-6 was increased with increasing amounts of niobium. In this study, where the catalytic activity was investigated by the H2O2 decomposition reaction, the results showed that the reactant could diffuse into the catalyst and the conversion value increased according to the increasing amount of active substance in the catalyst structure. The obtained high catalytic activity revealed that the catalyst is an important alternative for such reactions. This shows that niobium is an effective metal for this reaction. There is no previous study in the literature using this metal in this reaction. Nb-containing mesoporous silicas have attracted good consideration as catalysts in similar reactions. Nb into the KIT-6 structure generated better Lewis and Bronsted acid sites this is why it was chosen for this reaction. In the following studies it is aimed that the pure oxygen is used as an oxidizer for fuel cell system and the by-product water is used for hydrolysis of sodium borohydride for clean H2 production with a systematic reaction process.
References
Lv Y, Xin Z, Meng X, Tao M, Bian Z, Gu J, Gao W (2017) Effect of La, Mg and Mo additives on dispersion and thermostability of Ni species on KIT-6 for CO methanation. Appl Catal A 543:125–132
Bérubé F, Kaliaguine S (2008) Calcination and thermal degradation mechanisms of triblock copolymer template in SBA-15 materials. Microporous Mesoporous Mater 115(3):469–479
Mandal M, Kruk M (2012) Surfactant-templated synthesis of ordered silicas with closed cylindrical mesopores. Chem Mater 24(1):149–154
Kishor R, Ghoshal AK (2017) Understanding the hydrothermal, thermal, mechanical and hydrolytic stability of mesoporous KIT-6: a comprehensive study. Microporous Mesoporous Mater 242:127–135
Vinu A, Gokulakrishnan N, Balasubramanian VV, Alam S, Kapoor MP, Ariga K, Mori T (2008) Three‐dimensional ultralarge‐pore Ia3d mesoporous silica with various pore diameters and their application in biomolecule immobilization. Chem A Eur J 14(36):11529–11538
Wu S, Lan P (2012) A kinetic model of nano-CaO reactions with CO2 in a sorption complex catalyst. AlChE J 58:1570–1577
Broda M, Kierzkowska AM, Baudouin D et al (2012) Sorbent-enhanced methane reforming over a Ni-Ca based, bifunctional catalyst sorbent. ACS Catal 2:1635–1646
Zhang J, Xin Z, Meng X, Lv Y, Tao M (2014) Effect of MoO3 on the heat resistant performances of nickel based MCM-41 methanation catalysts. Fuel 116:25–33
Bian Z, Meng X, Tao M, Lv Y, Xin Z (2016) Uniform Ni particles on amino-functionalized SBA-16 with excellent activity and stability for syngas methanation. J Mol Catal A Chem 417:184–191
García-Sancho C, Moreno-Tost R, Mérida-Robles JM, Santamaría-González J, Jiménez-López A, Maireles-Torres P (2011) Niobium-containing MCM-41 silica catalysts for biodiesel production. Appl Catal B 108:161–167
Anilkumar M, Hoelderich WF (2015) A one step synthesis ofcaprolactam out of cyclohexanone by combinded ammoximation and Beckmann rearrangement over Nb-MCM-41 catalysts. Appl Catal B 165:87–93
Gudarzi D, Ratchananusorn W, Turunen I, Heinonen M (2015) Factors affecting catalytic destruction of H2O2 by hydrogenation and decomposition over Pd catalysts supported on activated carbon cloth (ACC). Catal Today 248:69–79
Wang Y, Guo Z, Xia Y (2013) A thin-film direct hydrogen peroxide/borohydride micro fuel cell. Adv Energy Mater 3(6):713–717
Zaki MI, Katrib A, Muftah AI, Jagadale TC, Ikram M, Ogale SB (2013) Exploring anatase-TiO2 doped dilutely with transition metal ions as nano-catalyst for H2O2 decomposition: Spectroscopic and kinetic studies. Appl Catal A 452:214–221
Amirfakhri SJ, Binny D, Meunier JL, Berk D (2014) Investigation of hydrogen peroxide reduction reaction on graphene and nitrogen doped graphene nanoflakes in neutral solution. J Power Sources 257:356–363
Yi Y, Wang L, Li G, Guo H (2016) A review on research progress in the direct synthesis of hydrogen peroxide from hydrogen and oxygen: noble-metal catalytic method, fuel-cell method and plasma method. Catal Sci Technol 6(6):1593–1610
Mase K, Yoneda M, Yamada Y, Fukuzumi S (2016) Seawater usable for production and consumption of hydrogen peroxide as a solar fuel. Nat Commun 7(1):1–7
Moon GH, Fujitsuka M, Kim S, Majima T, Wang X, Choi W (2017) Eco-friendly photochemical production of H2O2 through O2 reduction over carbon nitride frameworks incorporated with multiple heteroelements. ACS Catal 7(4):2886–2895
Kim HI, Kwon OS, Kim S, Choi W, Kim JH (2016) Harnessing low energy photons (635nm) for the production of H2O2 using up conversion nanohybrid photocatalysts. Energy Environ Sci 9(3):1063–1073
Shiraishi Y, Kofuji Y, Sakamoto H, Tanaka S, Ichikawa S, Hirai T (2015) Effects of surface defects on photocatalytic H2O2 production by mesoporous graphitic carbon nitride under visible light irradiation. ACS Catal 5(5):3058–3066
Song H, Wei L, Chen C, Wen C, Han F (2019) Photocatalytic production of H2O2 and its in situ utilization over atomic-scale Au modified MoS2 nanosheets. J Catal 376:198–208
Liu X, Zhu T, Lv Q, Li Y, Che D (2019) Simultaneous removal of NOx and SO2 from coal-fired flue gas based on the catalytic decomposition of H2O2 over Fe2(MoO4)3. Chem Eng J 371:486–499
Qi Y, Ge P, Wang M, Shan X, Ma R, Huang J, Wu J (2020) Experimental investigation and numerical simulation of simultaneous desulfurization and denitrification by H2O2 solution assisted with microwave and additive. Chem Eng J 391:123559
Yang B, Ma S, Cui R, Sun S, Wang J, Li S (2019) Simultaneous removal of NOx and SO2 with H2O2 catalyzed by alkali/magnetism-modified fly ash: high efficiency, low cost and catalytic mechanism. Chem Eng J 359:233–243
Anilkumar M, Hoelderich WF (2012) Gas phase Beckmann rearrangement of cyclohexanone oxime to ɛ-caprolactam over mesoporous, microporous and amorphous Nb2O5/silica catalysts: a comparative study. Catal Today 198(1):289–299
Yan W, Ramanathan A, Patel PD, Maiti SK, Laird BB, Thompson WH, Subramaniam B (2016) Mechanistic insights for enhancing activity and stability of Nb-incorporated silicates for selective ethylene epoxidation. J Catal 336:75–84
Thornburg NE, Nauert SL, Thompson AB, Notestein JM (2016) Synthesis−structure–function relationships of silica-supported niobium (V) catalysts for alkene epoxidation with H2O2. ACS Catal 6(9):6124–6134
Hiroki A, LaVerne JA (2005) Decomposition of hydrogen peroxide at water− ceramic oxide interfaces. J Phys Chem B 109(8):3364–3370
Dong C, Ji J, Shen B, Xing M, Zhang J (2018) Enhancement of H2O2 decomposition by the co-catalytic effect of WS2 on the Fenton reaction for the synchronous reduction of Cr (VI) and remediation of phenol. Environ Sci Technol 52(19):11297–11308
Ma C, Feng S, Zhou J, Chen R, Wei Y, Liu H, Wang S (2019) Enhancement of H2O2 decomposition efficiency by the co-catalytic effect of iron phosphide on the Fenton reaction for the degradation of methylene blue. Appl Catal B 259:118015
Gunduz-Meric G (2021) Fe/KIT-6 Katalizörlerinin Sentezi, Karakterizasyonu ve H2O2 Bozunma Reaksiyonunda Aktivitelerinin İncelenmesi. Afyon Kocatepe Üniv Fen ve Mühendis Bilim Derg 2021(2):442–448
Gunduz G, Degirmenci L (2016) Silika ile Mikroenkapsüle Edilmiş Fe2O3 İçerikli Kürelerin Üretim Prosesinin İyileştirilmesi ve Katalitik Aktivitelerinin Belirlenmesi. Gazi Üniv Mühendis Mimar Fakültesi Derg. https://doi.org/10.17341/gummfd.84263
Ali N, Zaman H, Bilal M, Nazir MS, Iqbal HM (2019) Environmental perspectives of interfacially active and magnetically recoverable composite materials—a review. Sci Total Environ 670:523–538
Ji X, Han Z, Li J, Deng Y, Han X, Zhao J et al (2019) MoSx co-catalytic activation of H2O2 by heterogeneous hemin catalyst under visible light irradiation. J Colloid Interface Sci 557:301–310
Xing M, Xu W, Dong C, Bai Y, Zeng J, Zhou Y et al (2018) Metal sulfides as excellent co-catalysts for H2O2 decomposition in advanced oxidation processes. Chem 4(6):1359–1372
Luo H, Cheng Y, Zeng Y, Luo K, Pan X (2020) Enhanced decomposition of H2O2 by molybdenum disulfide in a Fenton-like process for abatement of organic micropollutants. Sci Total Environ 732:139335
Spear EB (1908) Catalytic decomposition of hydrogen peroxide under high pressures of oxygen. 2. J Am Chem Soc 30(2):195–209
Satterfield C, Stein T (1957) Decomposition of hydrogen peroxide vapor on relatively inert surfaces. Ind Eng Chem 49(7):1173–1180
Park T, Chang I, Jung JH, Lee HB, Ko SH, O’Hayre R et al (2017) Effect of assembly pressure on the performance of a bendable polymer electrolyte fuel cell based on a silver nanowire current collector. Energy 134:412–419
Kim T (2009) Micro methanol reformer combined with a catalytic combustor for a PEM fuel cell. Int J Hydrogen Energy 34(16):6790–6798
Joh HI, Ha TJ, Hwang SY, Kim JH, Chae SH, Cho JH et al (2010) A direct methanol fuel cell system to power a humanoid robot. J Power Sources 195(1):293–298
Schlesinger HI, Brown HC, Finholt AE, Gilbreath JR, Hoekstra HR, Hyde EK (1953) Sodium borohydride, its hydrolysis and its use as a reducing agent and in the generation of hydrogen1. J Am Chem Soc 75(1):215–219
He Q, Shi J, Cui X, Zhao J, Chen Y, Zhou J (2009) Rhodamine B-co-condensed spherical SBA-15 nanoparticles: facile co-condensation synthesis and excellent fluorescence features. J Mater Chem 19(21):3395–3403
Kim TW, Kleitz F, Paul B, Ryoo R (2005) MCM-48-like large mesoporous silicas with tailored pore structure: facile synthesis domain in a ternary triblock copolymer− butanol−water system. J Am Chem Soc 127(20):7601–7610
Park DH, Kim SS, Pinnavaia TJ, Tzompantzi F, Prince J, Valente JS (2011) Selective isobutene oligomerization by mesoporous MSU-SBEA catalysts. J Phys Chem C 115(13):5809–5816
Shen S, Chen J, Koodali RT, Hu Y, Xiao Q, Zhou J et al (2014) Activation of MCM-41 mesoporous silica by transition-metal incorporation for photocatalytic hydrogen production. Appl Catal B 150:138–146
Prathap MA, Kaur B, Srivastava R (2012) Direct synthesis of metal oxide incorporated mesoporous SBA-15, and their applications in non-enzymatic sensing of glucose. J Colloid Interface Sci 381(1):143–151
Pirez C, Caderon JM, Dacquin JP, Lee AF, Wilson K (2012) Tunable KIT-6 mesoporous sulfonic acid catalysts for fatty acid esterification. ACS Catal 2(8):1607–1614
Xia Y, Yang Z, Mokaya R (2004) Mesostructured hollow spheres of graphitic N-doped carbon nanocast from spherical mesoporous silica. J Phys Chem B 108(50):19293–19298
Argyo C, Weiss V, Bräuchle C, Bein T (2014) Multifunctional mesoporous silica nanoparticles as a universal platform for drug delivery. Chem Mater 26(1):435–451
Kong L, Mume E, Triani G, Smith SV (2013) Optimizing radiolabeling amine-functionalized silica nanoparticles using SarAr-NCS for applications in imaging and radiotherapy. Langmuir 29(18):5609–5616
Wang W, Qi R, Shan W, Wang X, Ji Q, Zhao J et al (2014) Synthesis of KIT-6 type mesoporous silicas with tunable pore sizes, wall thickness and particle sizes via the partitioned cooperative self-assembly process. Microporous Mesoporous Mater 194:167–173
Ramanathan A, Subramaniam B, Maheswari R, Hanefeld U (2013) Synthesis and characterization of Zirconium incorporated ultra large pore mesoporous silicate, Zr–KIT-6. Microporous Mesoporous Mater 167:207–212
Ramanathan A, Maheswari R, Barich DH, Subramaniam B (2014) Niobium incorporated mesoporous silicate, Nb-KIT-6: synthesis and characterization. Microporous Mesoporous Mater 190:240–247
Ghohe NM, Tayebee R, Amini MM (2019) Synthesis and characterization of mesoporous Nb-Zr/KIT-6 as a productive catalyst for the synthesis of benzylpyrazolyl coumarins. Mater Chem Phys 223:268–276
Maurya MR, Titinchi SJJ, Chand S, Mishra IM (2002) Zeolite-encapsulated Cr (III), Fe (III), Ni (II), Zn (II) and Bi (III) salpn complexes as catalysts for the decomposition of H2O2 and oxidation of phenol. J Mol Catal A Chem 180(1–2):201–209
Bermúdez JM, Arenillas A, Menéndez JA (2011) Syngas from CO2 reforming of coke oven gas: synergetic effect of activated carbon/Ni–γAl2O3 catalyst. Int J Hydrogen Energy 36:13361–13368
Sing KS (1985) Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (Recommendations 1984). Pure Appl Chem 57(4):603–619
Xu L, Wang C, Guan J (2014) Preparation of acid-base bifunctional mesoporous KIT-6 (KIT: Korea Advanced Institute of Science and Technology) and its catalytic performance in Knoevenagel reaction. J Solid State Chem 213:250–255
Zhang H, Deng X, Jiao C, Lu L, Zhang S (2016) Preparation and catalytic activities for H2O2 decomposition of Rh/Au bimetallic nanoparticles. Mater Res Bull 79:29–35
Voitko K, Tóth A, Demianenko E, Dobos G, Berke B, Bakalinska O et al (2015) Catalytic performance of carbon nanotubes in H2O2 decomposition: experimental and quantum chemical study. J Colloid Interface Sci 437:283–290
Wang X, Li D, Nan Z (2019) Effect of N content in g-C3N4 as metal-free catalyst on H2O2 decomposition for MB degradation. Sep Purif Technol 224:152–162
Acknowledgements
Bilecik Seyh Edebali University, Yıldız Technical University and Eskisehir Osmangazi University Central Research Laboratories are gratefully acknowledged for characterization studies. Part of the study was supported by the BAP 2019-02.BŞEÜ.03-04 project.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares that they have no known competing financial interest or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gunduz-Meric, G. Catalytic decomposition of H2O2 over Nb/KIT-6 catalyst for environmental applications. Reac Kinet Mech Cat 135, 2059–2071 (2022). https://doi.org/10.1007/s11144-022-02235-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-022-02235-5