Abstract
The selective catalytic reduction (SCR) of NOx using NH3 was studied at pressures up to 5 bar over a vanadium-based SCR catalyst (~1 wt% V2O5 and 10 wt% WO3/TiO2), relevant for the installation of SCR reactors upstream of the turbocharger at marine engines. Experiments were performed using both granulated catalyst in a lab-scale fixed-bed reactor and a monolith catalyst in a bench-scale setup. The residence time across the catalytic bed was kept constant, by increasing the (normalized (0 °C, 1 atm)) volumetric flow rate proportionally to the pressure. The results show that for the granulated catalyst, the NOx conversion was independent of the pressure, indicating that the SCR kinetics are not affected by the increased pressure up to 5 bar. NH3 temperature-programmed desorption experiments showed that the catalyst NH3 adsorption increased with more than 30% when the pressure was increased from 1 bar to 4.5 bar. On the other hand, when the adsorption temperature was increased from 150 to 300 °C, the adsorption capacity decreased by approximately 60% independent on the pressure. The SCR reaction was unaffected by the increased NH3 uptake caused by the increased pressure, because only a certain fraction of the sites (\( {\theta}_{N{H}_3}^{\ast } \) = 0.14) was found to be active in the SCR reaction, and these are filled up at lower NH3 partial pressure than the total number of sites. Experiments using a monolithic catalyst showed that at temperatures above 250 °C, the NOx conversion was lower at an increased pressure (3.1 bar) when the residence time was held constant. This decrease was ascribed to increased internal and external diffusion limitations at the elevated pressure.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Currently, more than 80% of the international transport of goods is covered by ships [1, 2], and the majority of these (> 90% [3]), are powered by diesel engines. The diesel engine benefits from low emissions of CO2 (g/km); however, pollutants causing acidification and smog such as SOx (SO2, SO3, and H2SO4) and NOx (NO and NO2) are produced [4,5,6,7,8]. It has been estimated that approximately 30% of the global anthropogenic NOx emissions originate from the overall transport sector, and 5–15% originate from the marine industry alone [4, 9]. The emissions of NOx and SOx are therefore targeted by the emission legislation presented by the international maritime organization (IMO) through the protocol of MARPOL 73/78 Annex VI [10].
SOx emissions are produced due to the sulfur content of the fuel oil used at a ship, and MARPOL Annex VI regulation 14 therefore limits the fuel oil sulfur content to 3.5 wt% until 2020 [11], after which the global value is further reduced to 0.5 wt% sulfur in the fuel oil or an equivalent treatment of the exhaust gas. Furthermore, since 2015 when sailing within SOx emission control areas (SECAs), the sulfur emissions from a ship must be equivalent to using a fuel oil containing no more than 0.1 wt% sulfur. The currently known SECAs are the Baltic Sea, the North Sea, the North American sea area, and the US Caribbean Sea area [12].
NOx emissions are produced in high-temperature combustion zones within the combustion chamber, mainly through the Zeldovich mechanism, independent of the fuel oil used [13, 14]. NO is the main contributor (> 90%) to the NOx emissions from diesel engines [15,16,17,18,19].
NOx emissions are targeted through the introduction of MARPOL Annex VI, regulation 13, Tier I (2000) and Tier II (2011), both of which were reachable by the use of primary methods which reduces the combustion temperature, and therefore, also the production of NOx. With the introduction of MARPOL Annex VI Tier III (2016), 80% NOx reduction is required compared to the Tier I regulation. Tier III applies to all ships constructed after 2016 and when sailing within NOx emission control areas (NECAs), which currently is the North American sea area and the US Caribbean Sea area [12].
The two leading global engine manufacturers, MAN Energy Solutions and Wärtsilä, have stated that multiple solutions exist to comply with the new NOx regulation. At the moment, the most favorable solutions are the use of either exhaust gas recirculation (EGR) [3, 20], selective catalytic reduction (SCR) of NOx, or dual-fuel engines fueled by either conventional liquid marine fuels or liquid natural gas (LNG) [21]. In this article, the focus will be on the SCR of NOx.
SCR of NOx was first introduced in the 1970s and 1980s for the removal of NOx from stationary applications such as power plants, waste incinerators, and within the chemical industry [15, 22, 23]. NOx is typically reduced over a catalyst containing 1–5 wt% V2O5/~10 wt% WO3/TiO2 (V-SCR catalyst) using an N-containing reductant such as ammonia (NH3) on stationary units or an aqueous urea solution on mobile units, which decomposes into NH3 upon heating [15, 16, 24, 25]. The SCR of NOx typically proceeds at temperatures between 200 and 500 °C, during which NO is reduced according to reaction (1) into harmless nitrogen and water. Depending on the applied ammonia to NOx ratio (ANR), NOx reductions above 90% is reachable.
A highs sulfur fuel oil (approx. 2.5 wt% sulfur [26]) is typically used within the marine industry, which results in concentrations of up to 600 ppm SO2 in the flue gas. Up to 10% of the SO2 is further oxidized into SO3 within the combustion chamber [27], and a small part of the remaining SO2 (1–3% [28, 29]) is oxidized across the V-SCR catalyst according to reaction (2). The V-SCR catalyst is not deactivated by the presence of SO2; however, when both SO3, H2O, and NH3 are present in the exhaust gas, ammonium bisulfate (ABS, reaction (3)), and ammonium sulfate (AS, reaction (4)) may form when the exhaust gas is cooled [30,31,32].
The sulfates condense, creating solid residues, at temperatures below 260–300 °C, depending on the partial pressures of NH3 and SO3. If the sulfates condense at the surface or within the pores of the catalyst [30], the catalyst is deactivated by the fouling, and hence, temperatures higher than about 300 °C are required in order to keep the catalyst active in high sulfur environments, such as a ship. For the highly effective two-stroke marine diesel engine, such high temperatures are only continuously present upstream of the turbocharger, where an increased pressure of up to 5 bar also can be present depending on the engine load [20, 33,34,35,36,37,38].
The research within SCR at elevated pressure is scarce and only a few previous studies have been published. Kröcher et al. [35] measured the effect of increased pressure on NOx conversion over two monolith V-SCR catalysts (1.47 wt% V2O5) with either 87 or 225 channels per square inch (CPSI). The experiments were performed with 1000 ppm NOx, ANR > 1, at pressures of 1 bar, 2 bar, and 4 bar. They kept the partial pressure of NO and NH3 constant, and decreased the amount of catalyst inversely proportional to the pressure, so the actual gas residence time was independent of the reaction pressure. They noted that at low temperature (200 °C), the pressure had no effect on the NOx conversion. However, at higher temperatures (> 250 °C), increased pressure resulted in a lower NOx conversion, from 70% at 1 bar to 50% at 4 bar at 250 °C using the 225 CPSI catalyst. The loss in NOx conversion was attributed to increased diffusion limitations, due to the inverse proportionality between pressure and the binary diffusion coefficient [39].
Rammelt et al. [36] also investigated the effects of increased pressure on two V-SCR (1.9 wt% V2O5) monolith catalysts with either 300 or 25 CPSI. In line with Kröcher et al. [35], they observed a drop in NOx conversion with increasing pressure which was more pronounced for the low CPSI catalyst, due to longer diffusion pathways.
The present study investigates SCR at elevated pressure, using both lab-scale packed bed reactors and bench-scale monoliths to test the effects of pressure on SCR kinetics. Furthermore, the transient adsorption and desorption of NH3 at increased pressures up to 4.5 bar are investigated to reveal information of the changes in NH3 adsorption with pressure. Based on the experiments, both steady-state SCR kinetics and dynamic adsorption and desorption processes are modeled. The side reaction of SO2 oxidation was studied in a previous publication by Christensen et al. [40].
2 Experimental Methods
2.1 Lab-Scale Setup
2.1.1 Apparatus
The packed bed reactor shown schematically in Fig. 1 was used for kinetic SCR studies up to 5 bar. Four mass flow controllers from Brooks (SLA5850) controlled the gas composition. Water was added by the use of a HPLC pump (Gilson 307) and carried together with air, nitrogen, and ammonia into the homemade evaporator (H1), which consisted of 0.5 m 1/8 in 316 steel tube which was coiled together with heat tracing (HSS from Lund & Sørensen) and operated at a temperature of 350 °C. After the evaporator, all tubes were heat traced to 150 °C to ensure that water vapor did not condense. NO was added to the hot gas downstream of the evaporator, to ensure that the oxidation of NO into NO2 was low, similar to diesel engine conditions (NO > 90% NOx [41]).
The synthetic gas mixture was passed through a packed bed reactor, contained within a U-type quartz reactor. The position of the catalytic bed was maintained by a quartz wool plug on both sides, and a constriction of the glass tube, beneath the catalyst bed. A thermocouple was placed before the catalytic bed, to measure the temperature. After the reactor, the pressure was reduced by two automatic back pressure valves (Fisher Fieldvue DVC 2000), and gas concentrations were measured at atmospheric pressure using an MKS Multigas 2030 FTIR analyzer.
2.1.2 Catalyst
An SCR catalyst containing approximately 1 wt% V2O5/10 wt% WO3/TiO2 (V-SCR) was supplied by Umicore Denmark ApS. The catalyst was produced as a monolith, from which the catalyst powder was obtained by crushing and removing of visible fibers. The powder was pressed into self-supporting pellets, which were crushed and sieved into catalyst particles with a size fraction of 150–300 μm, and loaded in the reactor setup. The fraction of 150–300 μm was chosen to diminish diffusion limitations, as shown in the online supplementary material..
2.1.3 Steady-State SCR—Experimental Methodology
Approximately 20 mg of catalyst was loaded in a U-type quartz reactor (as shown in Fig. 2a) with an inner diameter of 3 mm, resulting in a bed height of 4 mm. Catalysts were degreened for 20 hours at 410 °C and 4.8 bar using a volumetric flow rate of 1200 NmL/min, containing 10% O2, 8% H2O, 600 ppm NOx, and 720 ppm NH3 in N2.
After degreening, steady-state SCR experiments were carried out with typical gas concentrations of 10% O2, 8% H2O, 600 ppm NOx, and an ammonia to NOx ratio (ANR) of 0.8–1.2 in N2. The pressure was varied in steps of 1.2 bar up to 4.8 bar. The residence time within the catalyst bed was kept independent of pressure by increasing the total volumetric flow rate proportional with the pressure as shown in Eq. (5):
Here, Q0 is the design total volumetric flow rate (normal (0 °C, 1 atm)) used at 1 atm, Preactor is the reactor pressure, and Qtotal@P is the total volumetric flow rate calculated for the specific pressure. At 1.2 bar, a total volumetric flow rate of 300 NmL/min was used and at 4.8 bar a total volumetric flow rate of 1200 NmL/min was used. The gas outlet concentrations were continuously measured by the FTIR analyzer using the mean results of 16 spectra per sample resulting in gas concentration outputs every 16th second.
2.1.4 NH3 TPD—Experimental Methodology
Approximately 115 mg of catalyst loaded in a U-type quartz reactor, with an inner diameter of 4 mm. The catalyst was degreened for 20 hours at 410 °C and 4.5 bar using a total volumetric flow rate of 600 NmL/min containing 9% O2, 8% H2O in N2. The increased reactor diameter was used to decrease the pressure drop over the increased amount of catalyst, compared to the steady-state SCR experiments.
The NH3 temperature programmed desorption (NH3 TPD) experiments consisted of 1 hour of NH3 adsorption at a specific temperature (150 °C, 200 °C, 250 °C, and 300 °C) and pressure using a flow of 300 NmL/min containing 9% O2, 8% H2O, and 600 ppm NH3 in N2. During NH3 TPD experiments, the total volumetric flow rate was kept constant at 300 NmL/min. After 1 hour, the outlet concentration of NH3 was the same as the inlet concentration (typically already after 5-10 min.), i.e., the catalyst surface was saturated, and the flow of NH3 and H2O was stopped. Feeding of H2O was stopped to remove small pulsations from the pumping and evaporation of H2O, so the desorption curve was smooth. During desorption, a total volumetric flow rate of 300 NmL/min was used at 1 bar, while in all other experiments, a total volumetric flow rate of 276 NmL/min was used due to slightly different set points of the nitrogen MFC setting. After flushing for 1 hour, a temperature ramp (10 K/min) was performed from the saturation temperature and until 420 °C. The temperature was maintained at 420 °C for 20 min before the reactor was cooled to the next adsorption temperature. The gas outlet concentrations were continuously measured by the FTIR analyzer using the mean of two spectra per sample resulting in gas concentration outputs every 2 s.
During NH3 adsorption, the outlet concentration was observed to correspond to the inlet concentration indicating that no NH3 oxidation was present at any adsorption temperatures (150–300 °C). Ammonia oxidation could occur under the desorption; however, for the applied low vanadium-based catalyst, NH3 oxidation is first expected at temperatures well above 350 °C, during which most of the NH3 has been desorbed. Therefore, NH3 oxidation has been disregarded.
Blank experiments (i.e., no catalyst loaded) were performed to examine the response of the setup when NH3 addition was stopped. The first blank experiment was performed at 1.2 bar, with NH3 adsorption steps at 150 °C, 200 °C, 250 °C, and 300 °C all followed by the described heating ramp. However, since no change was observed between the different temperatures, the ramp was skipped, and the blank experiments were performed without the ramp to save time and only at a temperature of 150 °C. Because the ramp was skipped, the blank experiments were shorter than the actual experiment.
2.2 Bench-Scale Setup
2.2.1 Apparatus
Experiments using monolith catalyst elements at elevated pressure were performed with the setup previously described in detail, for the measurements of pressurized SO2 oxidation over a V-SCR catalyst by Christensen et al. [40]. The setup is a bench-scale setup for testing monolith catalysts using Brooks smart flow controllers for gasses (Air, N2, NO, and NH3) and Brooks liquid mass flow controller (model 5882) for water addition. Air, nitrogen, and water were passed through a preheater, in which water was evaporated. The gas mixture was further heated in a second heater, after which NH3 and NO were added upstream of a static mixer from Sulzer, from which the hot mixed gas was passed through the monolith catalyst. The catalyst was placed within a square electrically heated furnace, and gas analysis was performed using a Gasmet FTIR analyzer (GASMET DX - 4000) by the withdrawal of gas before and after the catalyst.
2.2.2 Catalyst
A V-SCR monolith catalyst (~1 wt% V2O5/10 wt% WO3/TiO2) was cut into a square cross-sectional area (43.5 mm), and a length of 99.1 mm and the sides were sealed with quartz wool. Furthermore, to reduce external mass transfer limitations and the NOx conversion, all but nine channels of the monolith were sealed resulting in an open area of 223 mm2, i.e., area without the channel walls, as shown in Fig. 2b.
2.2.3 Steady-State SCR—Experimental Methodology
The monolith was loaded into the reactor setup, and the catalyst was degreened at 410 °C in a flow of air and 5% H2O for 15 hours. The SCR reaction was performed at 1 and 3 bar while keeping the residence time independent of the pressure as shown in Eq. (5). At 1 bar, a flow of 4.9 Nm3/h was used containing 8% O2, 5% H2O, 720 ppm NOx, and ANR = 0.75 or 1.16 in N2.
3 Results
3.1 High-Pressure Steady-State SCR Experiments
The SCR experiments were carried out at steady-state conditions, and the measured NOx conversion as a function of temperature is shown for ANR = 1.2 and ANR = 0.8 in Fig. 3.
Packed bed steady-state SCR experiments using 20.8 mg (~1 wt% V2O5) V-SCR catalyst and a flow of 300 NmL/min at 1.2 bar containing 10% O2, 8% H2O, 600 ppm NOx, ANR = 1.2 (a), or ANR = 0.8 (b) in N2. The residence time was kept constant by increasing the total volumetric flow rate according to Eq. (5)
Figure 3 shows that the NOx conversion is independent of the pressure when the residence time is kept constant. The NH3 and NOx consumption was found to follow the stoichiometry according to reaction (1) (1:1), within 5% of the total NOx concentration, or at low concentration (< 100 ppm) where differences in gas measurements give a large error within 15 ppm. Formation of other byproducts such as N2O from NH3 oxidation was not observed at any conditions. At atmospheric pressure water is known to limit the formation of N2O across V-SCR due to competitive adsorption [7, 24], which also seems to apply for increased pressure. Figure 3 shows that the kinetics of the SCR reaction is not affected by pressures up to 4.8 bar. The small variations observed are within the experimental uncertainty. Furthermore, Fig. 3b shows that when NH3 becomes the limiting reactant at high temperatures, the conversion of NOx levels off at a NOx conversion close to the ANR value, as also typically reported for atmospheric pressures. The fact that the SCR reaction is not affected by the increased pressure also means that a regular kinetic expression [15, 24, 42, 43] taking into account the NH3 surface coverage can be used, either in more complicated forms of Temkin isotherms as discussed later or using regular Langmuir isotherms as in the simplified Eley-Rideal mechanism [42] as given in Eq. (6). Figure 3a also indicates that the reaction can be treated as a first-order reaction at ANR > 1, because the conversion does not change when the pressure is increased, i.e., no changes at increased partial pressures.
The addition of NO to the hot gas ensured that the maximum concentration of NO2 in the feed gas was 40 ppm NO2 (at 4.8 bar) out of a total NOx concentration of 600 ppm, which was not observed to change the NOx reduction, so the reaction can be modeled as only the standard SCR reaction, i.e., considering all NOx as NO.
To investigate the NO reaction order, inlet NO concentrations of 300, 600, and 900 ppm NO were used, with constant residence time and using an ANR of 0.8 or 1.2 as shown in Fig. 4.
Packed bed steady-state SCR experiments using 20.5 mg (~1 wt% V2O5) V-SCR catalyst and a flow rate of 300 NmL/min at 1.2 bar containing 10% O2, 5% H2O, 300, 600, or 900 ppm NOx, ANR = 1.2 (a) or ANR = 0.8 (b) in N2. The residence time was kept constant by increasing the total volumetric flow rate according to Eq. (5)
Figure 4 shows that changing the inlet NOx concentration from 600 to 900 ppm did not affect the NOx conversion as expected for a reaction that is first order in NO. The NO2 concentration increased to 50 ppm for the case of 900 ppm NOx at 3.6 bar, i.e., 94% of NOx is NO, and the assumption of only standard SCR is still applicable. When the inlet NOx concentration was lowered to 300 ppm (at 3.6 bar), the NOx conversion unexpectedly increased at the lower temperature range which is expected to be due to an unintended increased residence time within the catalyst. A concentration of 300 ppm of NOx is well within normal atmospheric SCR operation [24, 44]. The expected effects of increased pressure include increased NH3 adsorption, decreased diffusivity, and the possibility of increased formation of NO2. The increased NH3 adsorption at increased pressure should not affect an experiment with ANR = 1.2, because the rate of NO reaction is independent of NH3 coverage, as also will be shown later. Furthermore, the behavior should have been visible independently of the NOx inlet concentration. A decrease in diffusivity would lower the NOx conversion and is first observed in diffusion-controlled operation, such as a monolith, and again should be present independently of the NOx inlet concentration. Lastly, the increased formation of NO2 at increased pressure was observed, however, never exceeding 6% of the total NOx and the effect is more pronounced at increased NOx concentrations due to a second-order reaction dependency in NO for the NO oxidation [45]. Therefore, the change in conversion observed with an inlet concentration of 300 ppm NOx at an ANR of 1.2 or 0.8 is likely due to an unintended lower total volumetric flow rate, resulting in increased residence time.
3.2 NH3 TPD at Elevated Pressure
NH3 TPD experiments were performed at 1.2, 2.4, 3.6, and 4.5 bar using a total volumetric flow rate during adsorption of 300 NmL/min containing 9% O2, 8% H2O, and 600 ppm NH3 in N2. A typical set of data for TPD at 1.2 bar is shown in Fig. 5. Similar plots for 2.4, 3.6, and 4.5 bar and a zoom of the NH3 peaks can be found in the online supplementary material.
Figure 5 shows that loosely bound NH3 desorbs during the 1-hour flush, while the rest of the NH3 is released during the temperature ramp. No NH3 was released during the 20-min isotherm at 420 °C. Integration of the data for the desorption of NH3 was performed at each pressure and temperature, to calculate the amount of NH3 adsorbed on the surface of the catalyst. Integration of the full desorption curve was performed, but also the heating ramp peak only. The blank experiments, performed without a catalyst, resulted in full desorption backgrounds of 97, 150, 166, and 166 μmol/gcat NH3 at 150 °C, and a pressure of 1.2, 2.4, 3.6, and 4.5 bar, respectively. The background integration was divided by the amount of catalyst used in the actual experiments, to ease comparison. The backgrounds were withdrawn from the integration, and the NH3 adsorbed on the surface of the catalyst was calculated as shown in Fig. 6.
Figure 6 shows that in general the ammonia, adsorption increases as the pressure increases, and that the ammonia adsorption decreases as the adsorption temperature increases. Figure 6a shows that at the high adsorption temperature of 300 °C, the adsorption capacity of the catalyst is similar at pressures above 2.4 bar. At this high temperature, the amount of NH3 stored on the catalyst is low, and hence, the small increase, at increased pressure, is likely hidden within the uncertainty (± 5% of the mean) of the blank experiments. Figure 6b shows more clearly the increased adsorption at increased pressure, also at 300 °C. It should be noted that even though the NH3 adsorption increases with the increasing pressure, the steady-state NOx reduction was found to be independent of the pressure, and hence does not affect the SCR reaction, as will be further discussed during the kinetic modeling.
Figure 6a shows that by increasing the pressure 3.8 times (from 1.2 to 4.5 bar), the amount of NH3 stored on the catalyst increases by 36% at a temperature of 150 °C. Bank et al. [34] reported for a washcoated monolith that the integral ammonia storage for a 100 cpsi vanadium-based SCR catalyst increased by approximately 70% by changing the pressure from 1 to 2 bar at 215 °C. The reason for the difference between the results reported by Bank et al., and the results obtained here is not clear, but may be related to the catalyst composition which was not disclosed in their study. Furthermore, Bank et al. [34] did not report if blank experiments were performed and subtracted, which is an important issue due to the increased mass of gas contained within the setup at increased pressure. For instance, if the blank is not subtracted, the adsorption at 150 °C increases by 46% instead of 35% when the pressure is increased by a factor 4. The blank experiments depend on the dynamics of the experimental facility, and for larger facilities, for instance using monoliths such as that used by Bank et al. [34], the effect of the blank experiment is expected to increase.
3.2.1 Modeling of the Transient Adsorption/Desorption Experiments
A transient model was set up in order to obtain the kinetics describing the adsorption-desorption processes. The gas phase concentration of NH3 was modeled similarly to Lietti et al. [46] as an isothermal plug flow reactor, which was simplified in this study as CSTRs in series. Diffusion limitations and axial dispersion were estimated to be negligible as shown in the online supplementary material. The gas phase concentration and the surface coverage of NH3 are therefore given by Eqs. (7) and (8).
Here, N is the CSTR number, CN is the NH3 concentration out of the Nth CSTR, CN-1 is the inlet concentration of NH3, v0 is the volumetric flow rate, VN is the volume of the Nth CSTR, ε is the interparticle porosity of the reactor volume, Ω’ is the NH3 adsorption capacity (mol/m3 particles), the density of the particles are 1236 kg/m3 particles, θN is the surface coverage of NH3, and ra, N and rd, N are the rate of adsorption and the rate of desorption, respectively. The rate of adsorption and desorption is given by Eq. (9) and Eq. (10), respectively.
Here, \( {k}_a^0 \) and Ea are the pre-exponential factor and the activation energy of the adsorption process, and \( {k}_d^0 \) and \( {E}_d^0 \) are the pre-exponential factor and the activation energy for the desorption process. α is a parameter in the Temkin isotherm. The Temkin isotherm was reported by Lietti et al. [46] to provide a better fit compared to a regular Langmuir isotherm (α = 0). A non-zero value of alpha induces a linear decrease in activation energy for the desorption process as the surface of the catalyst is filled by the adsorbate (θ → 1). Lietti et al. [46] found that the rate of adsorption was well represented by a non-activated (Ea = 0) process in line with the adsorption of an alkaline specie, such as NH3, on the acidic surface cites of the catalyst, which will also be used in this study.
3.3 Transient Model Fitting
The unknown adsorption and desorption kinetic parameters, i.e., \( {k}_a^0 \), \( {k}_d^0 \), \( {E}_d^0 \), α, and Ω’, were fitted by the use of MATLAB’s® function “fmincon” using 30 CSTRs in series. According to Levenspiel [47], the number of CSTRs in series that should be used to approximate the flow profile in a fixed bed is given by Eq. (11)
With a bed height of 12 mm and a particle diameter of 150–300 μm, this results in 40–80 tanks in series. Thirty CSTRs in series were initially chosen to ensure fast simulation, and the final fitting was verified against a run with 80 tanks in series which did not change the fitting results. The fitting procedure minimizes the function given by Eq. (12)
in which ymeas and ymodel are vectors containing the measured and modeled gas phase NH3 concentration as a function of time. The blank experiments were difficult to subtract as a vector from the actual experiment, because the time signals do not fully overlay each other, and so it was decided to only fit the NH3 peak during the ramp of temperature, during which the background was negligible (< 3 ppm NH3).
The NH3 capacity of the catalyst (Ω’) was calculated based on the data shown in Fig. 6a, from which it was found that the NH3 capacity was highest at the lowest temperature (150 °C) and the highest pressure (4.5 bar) tested. Therefore, the total NH3 adsorption capacity was calculated based on the measured total NH3 adsorption at 150 °C and 4.5 bar to Ω’ = 228 mol NH3/m3 particle. It is thus assumed that the surface of the catalyst is completely filled with NH3 (θ = 1) at a pressure of 4.5 bar and at a temperature of 150 °C. In case the model predicts a non-saturated surface, a new NH3 capacity should be calculated and a new fitting performed, hence the calculation would be iterative. As shown later, the surface coverage is found to be close to 1 (0.98). The NH3 capacity reported by Lietti et al. [46] was 270 mol NH3/m3 reactor for a similar ternary 1.47 wt% V2O5–9 wt% WO3-TiO2 catalyst. As a comparison, the NH3 capacity found in this study in the same units gives 372 mol NH3/m3 reactor based upon the bed porosity, as shown in Eq. (14). The bed porosity was calculated under the assumption of spherical particles based on the empirical formula presented by Pushnov [48] and shown in Eq. (13). Pushnov reported an average deviation of the formula of ± 5.26%.
The calculated NH3 capacity in the case of the pressurized experiments reported here is, therefore, higher than the one reported by Lietti et al. [46]. This is possibly due to the increased pressure used in this work and the fact that the catalysts are different. Lietti et al. [46] also reported the NH3 capacity for a binary catalyst (V2O5-TiO2), 209 mol/m3 reactor, and explained the difference due to changes in surface area, i.e., 46 m2/gcat for the binary catalyst vs. 80 m2/gcat for the ternary catalyst. For the catalyst used in this study, a similar catalyst surface area as that for the ternary catalyst was found by N2 adsorption calculations (BET method). The difference in NH3 capacity can therefore not be explained by different surface areas.
With the NH3 capacity fixed at 228 mol NH3/m3 particle (372 mol NH3/m3 reactor), the four last fitting parameters (\( {k}_a^0 \), \( {k}_d^0 \), \( {E}_d^0 \), and α) were fitted using 30 CSTRs in series, only fitting the NH3 peaks, however, using the combined data with all pressures and temperatures. Based on different initial guesses, it was found that the model was able to find different parameter values that fitted the data equally well. Therefore, the quality of an individual fit was evaluated based on how well the NH3 adsorption and desorption kinetics were able to predict the steady-state NO conversion presented earlier. It was found that the adsorption constant (\( {k}_a^0 \)) should be large in order to predict a correct surface coverage of the catalyst, which also was able to predict the observed NOx reduction during steady-state SCR. The only solution that was found to both fit the NH3 TPD experiments and the steady-state SCR experiments is shown in Table 1 in which the values reported in the article by Lietti et al. [46] are also shown for comparison.
Most of the fitted values in this study are close to those reported by Lietti et al. [46], with the main difference being the rate of adsorption, which is more than ten times larger than that reported by Lietti et al. The increased rate of adsorption could be related to a more acidic catalyst, which is also in line with the higher NH3 capacity. It was noted that a similar kinetic constant was found across the different adsorption temperatures, again indicating that the pressure does not affect the kinetics. A plot of how well the model predicts the measured data is shown in Figs. 7 and 8 for 1.2 bar and 4.5 bar, respectively. In the online supplementary material, a graph for each pressure (1.2 bar, 2.4 bar, 3.6 bar, and 4.5 bar) is shown together with a zoom of the NH3 peaks.
Measured and predicted NH3 adsorption and desorption at 1.2 bar, using an NH3 concentration of 610 ppm, at four different temperatures (150, 200, 250, and 300 °C). The model is based on the fitted parameters shown in Table 1. A zoom of the desorption peeks can be found in the online supplementary material
Measured and predicted NH3 adsorption and desorption at 4.5 bar, using an NH3 concentration of 600 ppm, at four different temperatures (150, 200, 250, and 300 °C). The model is based on the fitted parameters shown in Table 1. A zoom of the desorption peeks can be found in the online supplementary material
From Fig. 7 (and the online supplementary material), it is observed that the model does not predict the desorption peak at 300 °C and 1.2 bar that well since most of the NH3 is predicted to desorb during the 1-hour flush. At 4.5 bar (Fig. 8); however, the high-temperature peak at 300 °C is well fitted. Furthermore, from Fig. 8, it is observed that at a temperature of 150 °C and 4.5 bar, the model predicts a surface coverage of 0.98 which is close to 1, and so the fixed NH3 adsorption capacity (Ω) of 372 mol NH3/m3 reactor is correct.
3.4 Modeling the Steady-State SCR Experiments Using NH3 TPD Kinetics
The fitted NH3 adsorption and desorption kinetics (see Table 1) were used to model the steady-state SCR experiments shown in Fig. 3. The steady-state gas phase concentration of NH3 and NO calculated for an isothermal plug flow reactor can be written as given by Eq. (15) and Eq. (16), respectively.
In which the rate of NO reduction is given by Eq. (17) [46].
The rate given by Eq. (17) takes into account that only a certain fraction of the sites (\( {\theta}_{{\mathrm{NH}}_3}^{\ast } \)) are active in the SCR reaction. These active sites are filled up at a lower partial pressure of NH3 than the total number of sites. For example, at a total coverage of 0.5, and with \( {\theta}_{\mathrm{NH}3}^{\ast }=0.14 \), as will be shown later, the fraction of covered active sites (\( {\theta}_{\mathrm{NH}3}^{\ast } \)) is 97%. The fraction of active sites is a constant that is fitted to the experimental data within a range of 0 to 1. The NO rate constant is assumed to follow a regular Arrhenius expression written in modified form as shown in Eq. (18).
When the Arrhenius equation is written in the form shown in Eq. (18), in which k(Tref) and EA,NO are fitted, the parameters correlates less [49]. Tref was chosen as 230 °C.
The steady-state surface coverage of NH3 is now also a function of the NO reaction rate due to the equimolar consumption of NH3 on the surface of the catalyst by NO, as given by Eq. (19).
As shown in the online supplementary material, the effectiveness factor is above 0.9 for all pressures and for temperatures below 380 °C, and hence, diffusion limitations were not taken into account. Furthermore, the axial and radial dispersion is also calculated in the online supplementary material, showing that the assumption of plug flow is valid.
The three remaining unknown SCR-related parameters, i.e., k(Tref), EA,NO, and \( {\theta}_{{\mathrm{NH}}_3}^{\ast } \), were fitted to the steady-state SCR data shown in Fig. 3, using MATLAB’s function “lsqcurvefit” which minimizes on the residual sum of squares (RSS). The fitted parameters are shown in Table 2 together with the parameters fitted by Lietti et al. [46] at atmospheric pressure.
The fitted kinetics, as shown in Table 2, are similar to those reported by Lietti et al. [46], and similar activation energies can be found for the SCR reaction in the literature, e.g., 55 kJ/mol [36] and 67 kJ/mol [50]; however, higher activation energies are also reported, e.g., 80 kJ/mol [51] and 94 kJ/mol [42]. Furthermore, Table 2 shows that the estimated fraction of active sites is low for both this study (\( {\theta}_{{\mathrm{NH}}_3}^{\ast }=0.14 \)) and the study by Lietti et al. [46] (\( {\theta}_{{\mathrm{NH}}_3}^{\ast }=0.12 \)). The low fraction of active sites means that the rate of NO disappearance will quickly be independent of the total amount of NH3 adsorbed on the surface of the catalyst. This indicates that the active sites of the catalyst are easily covered by NH3 and that the increased adsorption observed at increased pressure adsorbs on non-active sites, most likely on the TiO2 support and to some extent the WO3 promoter. Titania is known to adsorb NH3 without having any activity in the SCR reaction, while tungsten on the other hand does exhibit SCR activity; however, the activity is in the order of 10 times below that of vanadium [46]. It is therefore expected that Ti and W mainly act as a reservoir for the NH3 storage and that the active sites for the SCR reaction correlate to the surface coverage of vanadium as also proposed by Lietti et al. [46]. They measured the surface coverage of vanadium (θv) for a ternary catalyst to be \( {\theta}_{{\mathrm{NH}}_3}^{\ast }=0.12\ vs.{\theta}_v=0.12 \) and for a binary catalyst \( {\theta}_{{\mathrm{NH}}_3}^{\ast }=0.08\ vs.{\theta}_v=0.21 \) and explained that the active sites were expected to be V-OH and V=O sites as also proposed by Dumesic et al. [52]. The reservoir adsorbed NH3 can then redistribute either through desorption-adsorption processes or through diffusion on the surface of the catalyst, which could affect the transient behavior of the catalytic system. This also explains why the increased NH3 storage on the surface of the catalyst at increased pressure (see Fig. 6) does not result in an increased NOx reduction since the active sites are already covered with NH3.
3.4.1 Simulations Using the Fitted Temkin Kinetics
An experiment with a constant flow rate of 600 NmL/min containing 10% O2, 8% H2O, 600 ppm NOx, and ANR = 0.8 in N2 was performed, and the pressure was changed from 1.4 to 4.4 bar, this time with the residence time as a function of the pressure, i.e., using a flow of 600 NmL/min at all pressures. All four experiments were simulated using the Temkin isotherm, using the parameters given in Table 1 and Table 2 to test how well the model fit another set of experimental data. The results are shown in Fig. 9.
Packed bed steady-state SCR experiment using 20.5 mg (~1 wt% V2O5) V-SCR catalyst and a total volumetric flow rate of 600 NmL/min containing 10% O2, 8% H2O, 600 ppm NOx, and ANR = 0.8 in N2 and changing the pressure without changing the total flow. Dashed lines are based on the Temkin parameters shown in Table 1 and Table 2
Figure 9 shows that at temperatures below 300 °C, the model predicts the experimental data well. At this low conversion of NOx, and low temperature, the NO reduction rate is not dependent on the NH3 concentration, i.e., the active sites \( \left({\theta}_{{\mathrm{NH}}_3}^{\ast}\right) \) are fully covered and the model relies on the NO kinetics, which was fitted for the similar steady-state experiments and hence should predict the data. At temperatures above 300 °C, the NH3 available for the SCR reaction starts to become limiting, i.e., \( \left[{\theta}_{{\mathrm{NH}}_3}^{\ast}\cdot \left(1-\exp \kern0.2em \left(\frac{-{\theta}_{{\mathrm{NH}}_3}}{\theta_{{\mathrm{NH}}_3}^{\ast }}\right)\right)<{\theta}_{{\mathrm{NH}}_3}^{\ast}\right] \) and the NOx conversion becomes more and more dependent on the NH3 coverage, which was fitted to the separate ammonia TPD experiments. At 1.2 bar, the fitted Temkin isotherm was unable to fit the observed NH3 desorption profile at an adsorption temperature of 300 °C (Fig. 7), and therefore, at steady-state NOx reduction, this also becomes visible, due to a predicted too low overall NH3 coverage and therefore also a too low coverage on the active sites. As the pressure increases, the Temkin isotherm was observed to predict the NH3 coverage better, and as shown in Fig. 9, this is also the case in steady state NOx reduction, as expected.
3.5 Full Monolith Experiment
SCR experiments were carried out using a V-SCR catalyst (~1 wt% V2O5), in the form of a monolith with an open inlet area of 223 mm2 (see Fig. 2b). The NOx reduction measured across the monolith as a function of temperature for the two pressures and two ANRs is shown in Fig. 10.
Figure 10 shows that at the low temperature of 200 °C, similar NOx conversions are observed independently of the pressure when the residence time is constant. As the temperature is increased, a lower NOx conversion is observed for the 3.1 bar experiment, which was not observed in the packed bed reactor experiments. The lower NOx conversion at elevated pressures becomes visible around 250 °C and more pronounced as the temperature is increased. At a temperature of approximately 250 °C, the SCR reaction across a monolith starts to become influenced by external and internal diffusion limitations, and as the temperature is increased, these limitations become more pronounced [24]. The increased diffusion limitations at increased pressure are linked to the inverse proportionality between pressure and the binary diffusion coefficient which will limit the SCR reaction. The same trends were reported by Kröcher et al. [35] for a similar monolith experiment.
To simulate the monolith experiments, including the increased diffusion limitations observed at increased pressure, a single-channel monolith model developed by Olsen [53] was used. The model is a 1D-1D single channel monolith model, which calculates the concentration profiles of NH3 and NO both in the radial direction within the catalytic wall and in the axial direction. The model was developed to simulate the deactivation of SCR monoliths caused by KCl deposition; however, in this study, only the SCR reaction part of the model is used. The model is based on Eley-Rideal kinetics (see Eq. (6)), and therefore, the steady-state SCR experiments from the packed bed reactor (Fig. 3) were re-fitted under the assumption of a plug flow reactor and following the Eley-Rideal mechanism as shown in Eq. (20).
Both the NO rate constant and the NH3 adsorption equilibrium constant were assumed to follow an Arrhenius equation. The fitted parameters are shown in Table 3, and in Fig. 11, a plot similar to Fig. 9 is shown, but now also displaying how well the Eley-Rideal kinetics fit the experimental data.
Steady-state SCR experiment using 20.5 mg (~1 wt% V2O5) V-SCR catalyst and a total volumetric flow rate of 600 NmL/min containing 10% O2, 8% H2O, 600 ppm NOx, and ANR = 0.8 in N2 and changing the pressure without changing the flow. Dashed lines are based on the Temkin parameters shown in Table 1 and Table 2, and full lines are based on Eley-Rideal kinetics using the parameters shown in Table 3
In Table 3, the pre-exponential factor and the activation energy for the NO rate constant are shown together with the pre-exponential factor and the adsorption enthalpy of NH3, both of which are used to calculate the rate of NO reduction in the monolith model.
When modeling reactions in a monolith, a correlation for the external mass transfer coefficient for developing laminar flow is often applied, such as that proposed by Tronconi et al. [42, 55], based upon the work of Shah et al. [56] and London et al. [57] as shown in Eq. (21).
Here, Sh∞ is the asymptotic Sherwood number, which depends on the channel geometry, and Z* is the dimensionless axial coordinate given by Eq. (22), in which z is the axial coordinate, DAB is the binary diffusion coefficient, U is the linear velocity, and dh is the hydraulic diameter. Equation (21) was found to give poor agreement with the experimental results, predicting a too low NOx conversion as shown for 3.1 bar in Fig. 12. Since the powder used in the packed bed experiments was obtained from a similar monolith, this discrepancy was attributed to a too low mass transfer coefficient. It was therefore considered that the assumption of a developing laminar flow was not applicable to this experiment using a short monolith (10 cm) and a high flow rate. The Reynolds number within the monolith channel was calculated to be approximately 1000 at 1 bar and 3000 at 3 bar. Therefore, a mean mass transfer coefficient for the channel was instead calculated based upon laminar flow in tubes, as given by Eq. (23) [47] at 1 bar. At high pressure however, this correlation, valid for laminar flow, was found to be inadequate. Since the Reynolds number is in the intermediate range between turbulent and laminar flow, a turbulent model for flow in tubes (Eq. (24) [47]) was tested. An estimate of the roughness of the catalyst channel surface of 0.03 mm was found to result in a friction factor (f) of approximately 0.012, which was calculated based upon the Churchill equations [58], which gave a good fit for the NOx conversion.
The monolith experiment also is shown in Fig. 10. The model developed by Olsen et al. [53] was used to predict the experimental data, using the PBR kinetics as shown in Table 3. The external mass transfer coefficient was calculated assuming pure laminar flow at 1 bar (Eq. (23)) and as both developing laminar flow (Eq. (21)), laminar flow (Eq. (23)), and turbulent flow at 3.1 bar (Eq. (24))
Here, Re is the Reynolds number, Sc is the Schmidt’s number, dh is the hydraulic diameter, and L is the length of the monolith. The model prediction using these expressions for external mass transfer limitations is shown in Fig. 12.
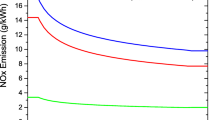
Figure 12 shows that the use of developing laminar flow results in a large error as high as 15% points in the calculated NOx conversion compared to the observed NOx reduction. The more general correlations as given in Eq. (23) at a Reynolds number of 1000 (1 bar) and Eq. (24) at a Reynolds number of 3000 (3.1 bar) were found to give better agreement. The model predicts a drop in NOx conversion when the pressure is increased due to increased diffusion limitations. Regular length, full open monoliths should be tested in a future study, to see how well mass transfer correlations for developing laminar flow predicts the external mass transfer at increased pressure when a more typical channel velocity (3–5 m/s) and length of catalyst ( 0.5 m) is used. From this study, it is concluded that at an increased pressure, when the residence time is kept constant, a decrease in NOx reduction efficiency is observed, due to increasing external and internal mass transfer limitations, as also discussed by Kröcher et al. [35].
It should be noted that the drop in NOx reduction is only observed because the residence time is kept constant with variations in the pressure. If an SCR reactor was installed on a ship, the residence time would increase with increasing pressure, and the total effect of increasing pressure becomes positive. Kröcher et al. [35] measured that to achieve 70% NOx reduction at 350 °C and having an NH3 slip of 10 ppm, the volume of a 87 cpsi catalyst could be reduced from 100% at 1 bar, to 80% at 2 bar and 70% at 4 bar, which shows that in total a positive effect of pressure is obtained. If no diffusion limitations had been present, the catalyst volume could have been reduced to 50% and 25% at 2 and 4 bar, respectively.
4 Conclusion
The following conclusions can be drawn from this study of SCR of NOx at pressures up to 5 bar for a ~ 1 wt% V2O5/10 wt% WO3/TiO2 catalyst.
-
The measured NOx conversion was independent of the pressure in the range 1.2–4.8 bar over a packed bed reactor with catalyst particles in the sieve range 150–300 μm with no diffusion limitations, when the residence time was kept constant. This shows that the kinetics are independent of pressure up to 5 bar.
-
A transient model for NH3 adsorption/desorption on the catalyst based on a Temkin isotherm was fitted to the experimental data. The data indicates that only a certain fraction of the total sites onto which NH3 adsorbs are truly active in the SCR reaction. This fraction was found to be about 14%. It was further found that these sites fill up at lower NH3 partial pressure compared to all sites.
-
The adsorption of NH3 on the catalyst was found to increase with increasing pressure and decrease with increasing temperature. The increased adsorption at increased pressure was found to be on non-active sites, and the rate of NOx reduction was therefore not promoted by the increased adsorption of NH3.
-
Experiments using a monolith showed that at an increased pressure of 3.1 bar, keeping the gas residence time constant relative to at 1 bar, a decrease in NOx reduction was observed at temperatures above 250 °C. This decrease was attributed to a decrease in the binary diffusion coefficient, which is inversely proportional to the pressure. The drop in NOx conversion was confirmed by a 1D-1D model.
-
Estimation of the external mass transfer coefficient using correlations for developing laminar flow lead to a poor agreement with the experimental data, and instead, a correlation for turbulent flow was required, probably due to the high channel velocity (10–13 m/s) used in this work.
-
This study has shown that the intrinsic kinetics of the SCR reaction is independent on pressure, and so, kinetics determined at 1 bar can be used for the design of SCR reactors also at elevated pressures, such as upstream of the turbocharger on a two-stroke marine diesel engine. The increased pressure does affect the binary diffusion coefficient and hence increased mass transfer limitations will take effect on monolithic catalysts at temperatures above approximately 250 °C.
Abbreviations
- ABS:
-
Ammonium bisulfate
- ANR:
-
Ammonia to NOx ratio
- AS:
-
Ammonium sulfate
- CPSI:
-
Channels per square inch
- CSTR:
-
Continuous stirred tank reactor
- EGR:
-
Exhaust gas recirculation
- IMO:
-
International maritime organization
- LNG:
-
Liquid natural gas
- NECA:
-
NOx emission control area
- NOx :
-
Nitrogen oxides, the sum of NO and NO2
- PBR:
-
Packed bed reactor
- RSS:
-
Residual sum of squares
- SCR:
-
Selective catalytic reduction
- SECA:
-
SOx emission control area
- SOx :
-
Sulfur oxides, the sum of SO2, SO3, and H2SO4
- V-SCR:
-
Vanadium-based SCR catalyst
- α :
-
Temkin kinetics parameter [−]
- C NH3 :
-
NH3 concentration [mol/m3]
- d particle :
-
Catalyst particle diameter [m]
- D reactor :
-
Reactor tube diameter [m]
- D AB :
-
Binary diffusion coefficient [m/s2]
- d h :
-
Hydraulic diameter [m]
- ε :
-
Porosity [−]
- E a :
-
Activation energy of the adsorption process of NH3 [J/mol]
- \( {E}_d^0 \) :
-
Activation energy for the desorption process of NH3 [J/mol]
- f :
-
Friction factor [−]
- G z :
-
Graetz dimensional number [−]
- k NO :
-
NO first order rate constant [1/s]
- k′NO :
-
Mass based NO first order rate constant [m3/s/kg]
- \( {k}_a^0 \) :
-
Pre-exponential factor of the adsorption process of NH3 [m3/mol/s]
- \( {k}_d^0 \) :
-
Pre-exponential factor for the desorption process of NH3 [1/s]
- \( {K}_{{\mathrm{NH}}_3} \) :
-
NH3 adsorption equilibrium constant [m3/mol]
- k(T ref):
-
Reaction rate constant calculate at the temperature Tref
- L :
-
Length of catalyst [m]
- Ω’:
-
NH3 adsorption capacity (mol/m3 particles)
- \( \varOmega ={\varOmega}^{\prime}\cdot \frac{1-\varepsilon }{\varepsilon } \) :
-
NH3 adsorption capacity (mol/m3 reactor)
- P reactor :
-
Reactor pressure [Pa]
- Q 0 :
-
Volumetric flow rate (normal (0 °C, 1 atm)) [Nm3/s]
- r a :
-
Rate of adsorption of NH3 [1/s]
- r d :
-
Rate of desorption of NH3 [1/s]
- Re:
-
Reynolds dimensional number [−]
- ρ :
-
Density of catalyst [kg/m3]
- r NO :
-
Rate of NO disappearance [1/s]
- S c :
-
Schmidts dimensional number [−]
- Sh:
-
Sherwood dimensional number [−]
- Sh∞ :
-
Asymptotic Sherwood number [−]
- θ :
-
Surface coverage of NH3 [−]
- \( {\theta}_{{\mathrm{NH}}_3}^{\ast } \) :
-
Fraction of active sites in the SCR reaction [−]
- θ v :
-
Surface coverage of vanadium [−]
- U :
-
Linear velocity [m/s]
- V :
-
Volume [m3]
- v 0 :
-
volumetric flow rate [m3/s]
- W :
-
Weight of catalyst [kg]
- y meas :
-
Vectors containing the measured gas phase mole fraction [ppm]
- y model :
-
Vectors containing the modeled gas phase mole fraction [ppm]
- z :
-
Axial coordinate [m]
- Z*:
-
Dimensionless axial coordinate [−]
References
Hallquist, Å.M., Fridell, E., Westerlund, J., Hallquist, M.: Onboard measurements of nanoparticles from a SCR-equipped marine diesel engine. Environ. Sci. Technol. 47(2), 773–780 (2013). https://doi.org/10.1021/es302712a
Man Diesel & Turbo, Exhaust gas emission control today and tomorrow. http://marine.man.eu/docs/librariesprovider6/technical-papers/exhaust-gas-emission-control-today-and-tomorrow.pdf?sfvrsn=22 (accessed September 10, 2015).
Lamas, M.I., Rodríguez, C.G.: Emissions from marine engines and NOx reduction methods. J. Marit. Res. 9, 77–81 (2012)
Briggs, J., Mccarney, J.: Field Experience of Marine SCR. CIMAC Congr (2013)
Niki, Y. Hirata, K. Kishi, T. Inaba, T. Takagi, M. Fukuda, T. Nagai, T. Muraoka, E.: SCR System for NOx reduction of medium speed marine diesel engine, in: CIMAC Congress, Vol. 22 p. 12 (2010)
G. Lövblad, E. Fridell, Experiences from use of some techniques to reduce emissions from ships, Göteborg. http://cleantech.cnss.no/wp-content/uploads/2011/09/2006-Lovblad-and-Fridell-Experiences-from-use-of-some-techniques-to-reduce-emissions-from-ships.pdf. (2006). Accessed 16 Dec 2015
Magnusson, M., Fridell, E., Ingelsten, H.H.: The influence of sulfur dioxide and water on the performance of a marine SCR catalyst. Appl. Catal. B Environ. 111–112, 20–26 (2012). https://doi.org/10.1016/j.apcatb.2011.09.010
Österman, C., Magnusson, M.: A systemic review of shipboard SCR installations in practice. WMU J. Marit. Aff. 12(1), 63–85 (2013). https://doi.org/10.1007/s13437-012-0034-1
Lehtoranta, K., Vesala, H., Koponen, P., Korhonen, S.: Selective catalytic reduction operation with heavy fuel oil: NOx , NH3, and particle emissions. Environ. Sci. & Technol. 49(7), 4735–4741 (2015). https://doi.org/10.1021/es506185x
Det Norske Veritas (DNV), Marpol 73/78 Annex VI, (2009). http://hulpinnood.nl/wp-content/uploads/2015/03/BIJLAGE3_Marpol-annex-VI.pdf (accessed May 9, 2016)
IMO, The 2020 Global Sulfur Limit. http://www.imo.org/en/MediaCentre/HotTopics/GHG/Documents/FAQ_2020_English.pdf (accessed October 12, 2017)
IMO, Emission Control Areas designated under MARPOL Annex VI, (2018). http://www.imo.org/en/OurWork/Environment/PollutionPrevention/AirPollution/Pages/Emission-Control-Areas-(ECAs)-designated-under-regulation-13-of-MARPOL-Annex-VI-(NOx-emission-control).aspx (accessed January 12, 2019)
Mollenhauer, K., Tschöke, H.: Handbook of Diesel Engines. Springer Berlin Heidelberg, Berlin (2010). https://doi.org/10.1007/978-3-540-89083-6
Turns, S.R.: An introduction to combustion: concepts and applications. New York, NY: McGraw-Hill (2012)
Forzatti, P., Lietti, L.: Recent advances in DeNOxing catalysis for stationary applications. Heterog. Chem. Rev. 3(1), 33–51 (1996)
Koebel, M., Elsener, M., Madia, G.: Recent advances in the development of urea-SCR for automotive applications. Sae Tech. Pap. (2001). https://doi.org/10.4271/2001-01-3625
P. Blakeman, K. Arnby, P. Marsh, C. Newman, G. Smedler, Optimization of an SCR catalyst system to meet EUIV heavy duty diesel legislation, SAE Tech. Pap. 2 (2008). https://doi.org/10.4271/2008-01-1542
Guan, B., Zhan, R., Lin, H., Huang, Z.: Review of state of the art technologies of selective catalytic reduction of NOx from diesel engine exhaust. Appl. Therm. Eng. 66(1-2), 395–414 (2014). https://doi.org/10.1016/j.applthermaleng.2014.02.021
Kröcher, O.: Aspects of catalyst development for mobile urea-SCR systems—from Vanadia-Titania catalysts to metal-exchanged zeolites. Stud. Surf. Sci. Catal. 171, 261-289 (2007) https://doi.org/10.1016/S0167-2991(07)80210-2.
Man Diesel & Turbo, Tier III Two-Stroke Technology, (2012). http://marine.man.eu/docs/librariesprovider6/technical-papers/tier-iii-two-stroke-technology.pdf?sfvrsn=12 (accessed September 10, 2015)
IMO, IMO MEPC 66/6/15, (2014). http://www.worldshipping.org/industry-issues/environment/air-emissions/MEPC_66-6-15_-_Comments_concerning_potential_amendments_to_the_effective___.pdf (accessed November 20, 2017).
H. Bosch, F. Janssen, Preface, Catal. Today. 2 v. https://doi.org/10.1016/0920-5861(88)80001-4 (1988)
G. Centi, S. Perathoner: Chapter 1 introduction: state of the art in the development of catalytic processes for the selective catalytic reduction of NOx into N2. in: Stud. Surf. Sci. Catal., pp. 1–23. https://doi.org/10.1016/S0167-2991(07)80202-3. 2007
Gabrielsson, P., Pedersen, H.G.: Flue gas from stationary sources. In: Ertl, G., Knözinger, H., Schüth, F., Weitkamp, J. (eds.) Handb. Heterog. Catal, pp. 2345–2385. Wiley-VCH (2008) http://findit.dtu.dk/en/catalog/2342172429 (accessed March 29, 2017)
I. Nova, E. Tronconi: Urea-SCR Technology for deNOx After Treatment of Diesel Exhausts, Springer. https://doi.org/10.1007/978-1-4899-8071-7 (2014)
Marine Fuels 2020. https://www.marinefuels2020.com/marine-fuels/background/ (accessed April 6, 2019).
CIMAC Working Group 8, CIMAC Guideline: Cold Corrosion in Marine Two Stroke, CIMAC Guidel. (2017) 1–36. https://cimac.com/cms/upload/Publication_Press/WG_Publications/CIMAC_WG8_Guideline_2017_Two_Stroke_Engine_Cold_Corrosion.pdf. Accessed 27 June 2018
Orsenigo, C., Beretta, A., Forzatti, P., Svachula, J., Tronconi, E., Bregani, F., Baldacci, A.: Theoretical and experimental study of the interaction between NOx reduction and SO2 oxidation over DeNOx-SCR catalysts. Catal. Today. 27(1-2), 15–21 (1996). https://doi.org/10.1016/0920-5861(95)00168-9
Tronconi, E., Cavanna, A., Orsenigo, C., Forzatti, P.: Transient kinetics of SO2 oxidation over SCR-DeNOx monolith catalysts. Ind. Eng. Chem. Res. 38(7), 2593–2598 (1999). https://doi.org/10.1021/ie980673e.
Muzio, L., Bogseth, S., Himes, R., Chien, Y.-C., Dunn-Rankin, D.: Ammonium bisulfate formation and reduced load SCR operation. Fuel. 206, 180–189 (2017). https://doi.org/10.1016/j.fuel.2017.05.081
J.M. Burke, K.L. Johnson: Ammonium sulfate and bisulfate formation in air preheaters (project summary). http://nepis.epa.gov/Exe/ZyPURL.cgi?Dockey=2000TU1N.txt.1982. Accessed 21 Sept 2015
Matsuda, S., Kamo, T., Kato, A., Nakajima, F., Kumura, T., Kuroda, H.: Deposition of ammonium bisulfate in the selective catalytic reduction of nitrogen oxides with ammonia. Ind. Eng. Chem. Prod. Res. Dev. 21(1), 48–52 (1982). https://doi.org/10.1021/i300005a009
T. Fujibayashi, S. Baba, H. Tanaka: Development of Marine SCR System for Large Two-Stroke Diesel Engines Complying with IMO NOx Tier III. in: CIMAC Congr. (2013)
R. Bank, B. Buchholz, H. Harndorf, R. Rabe, U. Etzien: High-Pressure SCR at Large Diesel Engines for Reliable NOx - Reduction and Compliance with IMO Tier III Standards, in: CIMAC Congr. 2013
Kröcher, O., Elsener, M., Bothien, M.-R., Dölling, W.: Pre-Turbo SCR - influence of pressure on NOx reduction. MTZ Worldw. 75(4), 46–51 (2014). https://doi.org/10.1007/s38313-014-0140-x
Rammelt, T., Torkashvand, B., Hauck, C., Böhm, J., Gläser, R., Deutschmann, O.: Nitric oxide reduction of heavy-duty diesel off-gas by NH3-SCR in front of the turbocharger. Emiss. Control Sci. Technol. 3(4), 275–288 (2017). https://doi.org/10.1007/s40825-017-0078-y
K. Sandelin, D. Peitz: SCR under pressure - pre-turbocharger NOx abatement for marine 2-stroke diesel engines, in: CIMAC Congr. (2016)
Schüttenhelm, W., Günther, C., Jürgens, R.: High pressure SCR for large two-stroke engines and comparison to conventional SCR high dust applications. VGB Powertech. 8, 58–62 (2017)
Bird, R.B., Stewart, W.E., Lightfoot, E.N.: Transport phenomena. J. Wiley pp. xii, 905 s (2007)
Christensen, S.R., Hansen, B.B., Johansen, K., Pedersen, K.H., Thøgersen, J.R., Jensen, A.D.: SO2 oxidation across marine V2O5-WO3-TiO2 SCR catalysts: a study at elevated pressure for preturbine SCR configuration. Emiss. Control Sci. Technol. 4(4), 289–299 (2018). https://doi.org/10.1007/s40825-018-0092-8
Koebel, M., Madia, G., Elsener, M.: Selective catalytic reduction of NO and NO2 at low temperatures. Catal. Today. 73(3-4), 239–247 (2002). https://doi.org/10.1016/S0920-5861(02)00006-8
Tronconi, E., Forzatti, P., Gomez Martin, J.P., Mallogi, S.: Selective catalytic removal of NOx: a mathematical model for design of catalyst and reactor. Chem. Eng. Sci. 47(9-11), 2401–2406 (1992). https://doi.org/10.1016/0009-2509(92)87067-Z
Nova, I., Lietti, L., Beretta, A., Forzatti, P.: Study of the sintering of a deNOx commercial catalyst. Stud. Surf. Sci. Catal. 139, 149–156 (2001). https://doi.org/10.1016/S0167-2991(01)80192-0
Dumesic, J.A., Topsøe, N.-Y., Topsøe, H., Chen, Y., Slabiak, T.: Kinetics of selective catalytic reduction of nitric oxide by Ammonia over Vanadia/Titania. J. Catal. 163(2), 409–417 (1996). https://doi.org/10.1006/jcat.1996.0342
Tsukahara, H., Ishida, T., Mayumi, M.: Gas-phase oxidation of nitric oxide: chemical kinetics and rate constant. Nitric Oxide. 3(3), 191–198 (1999). https://doi.org/10.1006/niox.1999.0232
Lietti, L., Nova, I., Camurri, S., Tronconi, E., Forzatti, P.: Dynamics of the SCR-DeNOx reaction by the transient-response method. AICHE J. 43(10), 2559–2570 (1997). https://doi.org/10.1002/aic.690431017
Levenspiel, O.: The chemical reactor omnibook, Distributed by OSU Book Stores, (1989)
Pushnov, A.S.: Calculation of average bed porosity. Chem. Pet. Eng. 42(1-2), 14–17 (2006). https://doi.org/10.1007/s10556-006-0045-x
Rawlings, J.B., Ekerdt, J.G.: Chemical reactor analysis and design fundamentals, 2. Edition, Madison, Wis.: Nob Hill Pub (2002)
Forzatti, P., Nova, I., Beretta, A.: Catalytic properties in deNOx and SO2–SO3 reactions. Catal. Today. 56(4), 431–441 (2000). https://doi.org/10.1016/S0920-5861(99)00302-8
Beeckman, J.W., Hegedus, L.L.: Design of monolith catalysts for power plant NOx emission control. Ind. Eng. Chem. Res. 30(5), 969–978 (1991). https://doi.org/10.1021/ie00053a020
J.A. Dumesic, N.-Y. Topsoe, T. Slabiak, P. Morsing, B.S. Clausen, E. Törqvist, H. Topsoe: Microiunetic analysis of the selective catalytic reduction (SCR) of nitric oxide over Vanadia/Titania-based Catalysts, in. pp. 1325–1337. https://doi.org/10.1016/S0167-2991(08)64454-7 (1993)
Olsen, B.K., Castellino, F., Jensen, A.D.: Modeling deactivation of catalysts for selective catalytic reduction of NOx by KCl aerosols. Ind. Eng. Chem. Res. 56(45), 13020–13033 (2017). https://doi.org/10.1021/acs.iecr.7b01239.
Koebel, M., Elsener, M.: Selective catalytic reduction of NO over commercial DeNOx catalysts: comparison of the measured and calculated performance. Ind. Eng. Chem. Res. 37(2), 327–335 (1998). https://doi.org/10.1021/ie970569h.
Tronconi, E., Forzatti, P.: Adequacy of lumped parameter models for SCR reactors with monolith structure. AICHE J. 38(2), 201–210 (1992). https://doi.org/10.1002/aic.690380205
Shah, R.K., London, A.L.: Laminar flow forced convection in ducts. New York: Academic Press (1978)
Tratz, H., Grigull, U.: Thermischer Einlauf in Ausgebildeter Laminarer Rohrströmung. Int. J. Heat Mass Transf. 8, 669–678 (1965)
Clement, K.H. Fangel, P. Jensen, A.D. Thomsen, K.: Kemiske enhedsoperationer, Polyteknisk Forlag. (2004)
Acknowledgments
This work is part of the Danish societal partnership, Blue INNOship, and partly funded by the Innovation Fund Denmark (IFD) under File No: 155-2014-10 and the Danish Maritime Fund. The authors gratefully acknowledge the funding support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
ESM 1
(DOCX 4763 kb)
Rights and permissions
About this article
Cite this article
Christensen, S.R., Hansen, B.B., Pedersen, K.H. et al. Selective Catalytic Reduction of NOx over V2O5-WO3-TiO2 SCR Catalysts—A Study at Elevated Pressure for Maritime Pre-turbine SCR Configuration. Emiss. Control Sci. Technol. 5, 263–278 (2019). https://doi.org/10.1007/s40825-019-00127-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40825-019-00127-0