Abstract
In this work, Fe3O4/SiO2 nanoparticles were synthesized according to the literature and characterized by transmission electron microscopy, powder X-ray diffraction, Fourier transform infrared spectroscopy, thermogravimetric analysis and vibrating sample magnetometer. The catalytic properties of the Fe3O4/SiO2 catalyst have been tested in the epoxidation of different cyclic and acyclic alkenes with m-chloroperbenzoic acid (oxidizing agent). Under optimized conditions, all reactions afforded the desired products in good to excellent yields. Furthermore, the effect of different additives such as pyridine N-oxide and N-methylmorpholine-N-oxide was tested on the conversion of alkenes, but in all cases additives did not improve epoxidation yields. As a result, the Fe3O4/SiO2 showed good activity and super stability in the epoxidation of various olefins. Moreover, this catalyst can be recovered by using a magnetic field and recycled for several times without a significant loss in the catalytic activity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Selective epoxidation of alkenes into high value chemicals (epoxides) which are widely used in the fine chemical industry is of great attention in chemical and pharmaceutical industries [1]. Some transition metal oxides such as NiO, CoO, MoO3, CuO, TiO2–SiO2, Au/SiO2, CuOx/SiO2 and tungstate(VI) or molybdate(VI) have been reported for the epoxidation of alkenes with organic hydroperoxides [2–8]. However, in most cases, either alkene conversion or alkene oxide selectivity/yield was poor and many of these transition metal catalysts are expensive and highly dangerous for the environment as well. From an economic point of view, the separation of ultrascaled and nanosized catalysts from the reaction system via routine methods such as free sedimentation, centrifuging and filtration is difficult, time-consuming and costly. Magnetic nanoparticles (NPs) which are economic friendly can be easily separated by applying a simple external magnet without any significant loss of activity put forward a solution to this problem. In addition, good selectivity and great stability are some especial advantageous of magnetic NPs [9–11]. Magnetic NPs have been reported to catalyze a wide range of reactions including, C–C, C–S, C–N bond formation, asymmetric synthesis and oxidations reactions [12–15]. Usually, silica was utilized to coat the Fe3O4 particles forming Fe3O4/SiO2 core–shell structure. This layer not only protects Fe3O4 from oxidizing or dissolving in the acidic reaction media, but also stabilizes NPs by preventing aggregation of the Fe3O4 particles [16]. The silica shell can also provide numerous surface Si–OH groups for further modification [17, 18].
This article presents the epoxidation of cyclic and acyclic alkenes with m-chloroperbenzoic acid (m-CPBA) in the presence of catalytic amount of Fe3O4/SiO2 NPs in dichloromethane as solvent. By using this catalyst, the corresponding products were obtained in excellent yields after 4 h and magnetic NPs, which are recovered from the reaction mixture by using external magnetic field simplified the isolation of products (Fig. 1).
Experimental
General
Tetraethoxysilane (TEOS), FeCl3·6H2O, FeCl2·4H2O, styrene, 4-chlorostyrene, cyclohexene, α-methyl styrene, indene, cis- and trans-stilbene, cyclohexene, cyclooctene, 1-octene, m-CPBA, pyridine N-oxide (PNO), pyridine (Py), N-methylmorpholine-N-oxide (NMNO), 1-methylimidazole (MI), imidazole, dichloromethane (DCM), ethyl acetate (EtOAC), ethanol (EtOH), CH3CN, CHCl3, THF, NaIO4, NH4OAC, t-BOOH, PhI(OAC)2, H2O2 (30 %) and Oxone were purchased from Merck and used without purification. Graphite powder was obtained from Aldrich. The resulting Fe3O4/SiO2 was characterized by IR, TEM, TGA, VSM and X-ray diffraction (XRD) patterns. The IR experiments were carried out on a Perkin-Elmer 783 Infrared spectrophotometer in a KBr pellet, scanning from 4,000 to 600 cm−1 at room temperature. The XRD measurements were carried out by using a Bruker D8-advance X-ray diffractometer with Cu Kα radiation (k = 1.5406 Å). The TEM images measurements were obtained using Philips CM10 instrument. Magnetization measurements were carried out at 300 K on a vibrating sample magnetometer (VSM Leak shore 7200). The TGA analysis was performed by heating the samples in an argon flow at a rate of 100 mL min−1 using a Perkin–Elmer Diamond TG/DTA thermal analyzer with a heating rate of 10 °C min−1. The conversion of products was determined by GC-17A Shimadzu with capillary column (Shimadzu, CBP5, 30 m × 25 mm × 0.25 µm).
General procedure for the synthesis of Fe3O4/SiO2 core–shell
Black magnetic Fe3O4 NPs were prepared as described in the literature [19]. The core–shell Fe3O4/SiO2 NPs were prepared by a modified Stober method [20]. Briefly, Fe3O4 (0.50 g, 2.1 mmol) was dispersed in the mixture of ethanol/deionized water (50:5 mL). Then, TEOS (0.20 mL) was slowly added to the mixture followed by addition of 5 mL of NaOH (10 wt%). This solution was stirred mechanically for 20 h at room temperature. Then the product, Fe3O4/SiO2, was separated by an external magnet, washed with deionized water and ethanol three times and dried at 80 °C for 10 h.
General procedure for the epoxidation of olefins
In a typical procedure, the catalyst (0.06 g) was dispersed in 3 mL of dichloromethane for 10 min. Then the substrate (1 mmol) and m-CPBA (2 mmol) were added to the mixture at room temperature. The mixture was stirred at room temperature for appropriate times. The progress of the reaction was monitored by TLC. After the completion of the reaction, the catalyst was separated by external magnet. The solution was washed with 1 M NaOH (8 mL) and brine (8 mL) and dried over MgSO4. Then, the solution was concentrated by rotary evaporator to 1 mL. Finally, the conversion of the products was determined by GC. The catalyst was washed twice with ethanol and reused.
Results and discussion
Characterizations of catalyst
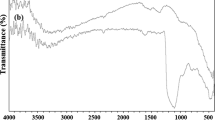
The Fe3O4 NPs (A) were simply synthesized by using of FeCl3·6H2O and FeCl2·4H2O in deionized water under nitrogen atmosphere. Then, Fe3O4/SiO2 NPs were obtained by treating the Fe3O4 NPs with TEOS (Fig. 2). The magnetic properties of the Fe3O4 and Fe3O4/SiO2 NPs (B) were studied by a VSM at 300 k. As shown in Fig. 3, both NPs have super paramagnetism at room temperature. Also, no hysteresis phenomenon was observed in Fig. 3 and the saturation magnetization values for Fe3O4 and Fe3O4/SiO2 NPs were 70.495 and 38.30 emu/g, respectively. However, despite the considerable decrease of the magnetization of Fe3O4/SiO2 NPs compared to Fe3O4, Fe3O4/SiO2 NPs can still be separated from the solution by using an external magnetic field on the sidewall of the reactor. Fig. 4a shows the IR spectrum of Fe3O4 with important vibration bands in 560–590 and 3,400 cm−1, which are due to Fe–O and OH, respectively. According to the IR spectrum of Fe3O4/SiO2 in Fig. 4b, there are several important vibration bands in 560–590, 954, and 3,400 cm−1, which are due to Fe–O, Si–OH, and OH. The vibration bands in 755 and 1,100 cm−1 are due to Si–O–Si. These vibration bands (755, 954 and 1,100 cm−1) confirmed coating of the silica shell on the surface of the Fe3O4 NPs.
Schematic illustration for synthesis of Fe3O4 (a) and Fe3O4/SiO2 (b). Reaction conditions: FeCl3·6H2O (4.8 mmol g), FeCl2·4H2O (4.5 mmol), water (30 mL), NH4OH (pH 10), PEG (1 g), N2, 80 °C, 1 h (a) and Fe3O4 (2.1 mmol), ethanol/deionized water (50:5 mL), TEOS (0.20 mL), of NaOH (10 wt%, 5 mL) 20 h at room temperature (b)
The XRD pattern of Fe3O4 and Fe3O4/SiO2 NPs were determined by powder XRD. As shown in Fig. 5a, the pattern of Fe3O4 indicates a crystallized structure at 2θ: 30.2°, 35.4°, 43.3°, 53.6°, 57.5° and 63.1° which are assigned to the (220), (311), (400), (422), (511) and (440) crystallographic faces of magnetite, which is in good agreement with the literature value (JCPDS Card No. 19-0629). The XRD pattern of Fe3O4/SiO2 presents almost the same feature as shown in Fig. 5b. The broad peak at 2θ = 15–27°, which depends on amorphous silica. The average diameter of Fe3O4 was about 11 nm, while the diameter of Fe3O4/SiO2 was about 14 nm, which is due to the agglomeration of Fe3O4 inside nanospheres and surface growth of silica on the shell. The SEM image of Fe3O4 shows the morphology and average product size of Fe3O4 NPs (Fig. 6a). The TEM images of Fe3O4 and Fe3O4/SiO2 NPs are shown in Fig. 6b, c. The results showed the average product size of Fe3O4 and Fe3O4/SiO2 NPs 10 and 13 nm similar to the results of XRD patterns. The TEM images of Fe3O4/SiO2 NPs indicate the successful coating of magnetic Fe3O4 NPs (Fig. 6c). The TGA curve of Fe3O4/SiO2 shows a weight loss over the range of 90–160 °C of about 3 %. These losses can be attributed to the loss of adsorbed water and dehydroxylation of internal OH groups. The second weight loss step is over the range 250–590 °C, which can be ascribed to even further decomposition of the materials. The total weight losses are approximately 10 % (Fig. 7).
Catalytic activity
In this study, Fe3O4/SiO2 NPs were tested to catalyze the selective oxidation of alkenes using m-CPBA as an oxidant. Styrene was selected as model substrate and treated with Fe3O4/SiO2 NPs in the presence of m-CPBA as the oxygen donor at room temperature under different conditions. This reaction was best carried out using 2 equiv. of m-CPBA for 4 h at room temperature in dichloromethane. The catalytic activity of the Fe3O4 NPs was also investigated in the epoxidation of styrene, and low yield (45 %) was observed. Blank experiments showed that Fe3O4 and Fe3O4/SiO2 NPs alone are inactive towards the styrene epoxidation. To optimize the amount of catalyst, the reaction was carried out in the presence of different amount of Fe3O4/SiO2 NPs (0.01–0.08 g) at room temperature. It was found that 0.06 g of Fe3O4/SiO2 was sufficient enough to afford styrene oxide with 97 % isolated yield (Table 1, entry 6).
To explore the effect of solvent on the reaction, the same reactions were performed in different solvents. Despite the moderate yields in some solvent such as EtOAC and CH3CN (Table 2, entries 1, 2), the best conversion was observed when the reaction was performed in DCM (Table 1, entry 7, 97 %).
The effect of different oxidants and additives in the epoxidation of styrene
The effect of various oxidants such as NaIO4, m-CPBA, UHP, H2O2, Oxone, PhIO, PhI(OAC)2 and tert-BuOOH was investigated in the epoxidation of styrene. The results showed that m-CPBA is the best oxygen source because this oxidant can give better yield (Table 3, entry 1) while other oxidants such as UHP, H2O2, PhIO, PhI(OAC)2 gave low yields (Table 3, entries 5–8). In addition, different equivalents of the oxidant were tested, and the best catalytic activity was obtained with 2 equivalents of the oxidant which provide moderate source of oxygen for the catalytic reaction. Also, dichloromethane was chosen as the best reaction medium. Consequently, the optimum molar ratio of olefin to oxidant is 1:2.
The effect of various additives such as NMNO, PNO, NH4OAC, MI, Py and imidazole was investigated in the epoxidation of styrene in the DCM/m-CPBA system. Generally, additives such as NMNO and imidazole in the Mn(III) salen reaction mixture facilitate faster reaction rates and higher epoxide yields. However, in this test, the catalytic activity did not increase in the presence of PNO and other additives.
The reactions were also carried out in 0, 40 and 60 °C. The yield was lower at 0 °C than at room temperature, but both of them showed good selectivity. When the reactions were carried out in 40 and 60 °C, the reaction rates increased but the selectivity of epoxides decreased. So, considering the economic point of view and selectivity, room temperature was chosen as the best condition for this reaction. Therefore, we employed the optimized conditions (0.06 g nanocatalyst, 2 mmol m-CPBA and DCM at room temperature) for the conversion of several alkenes into the corresponding products. Table 4 lists a group of alkenes that were investigated by magnetic NPs catalysts. The catalyst showed excellent activity toward alkenes oxidation with an average isolation yield of 95 %.
The efficiency of Fe3O4/SiO2 is compared in Table 5 with the earlier reported ones for their styrene epoxidation activity, expressed in terms of selectivity and yields for the styrene oxide formation. Usually, transition metals are not very highly efficient catalysts for alkene epoxidation (Table 5, entries 1–12). The comparisons of the catalysts reveal that Fe3O4/SiO2 catalyst with faster reaction rate, higher yield and selectivity shows better performance as compared to the earlier ones (Table 5, entry 13).
We also investigated the possibility of reusing of recovered catalysts for new reaction. Therefore, the catalyst was separated by external magnet, washed with EtOH, dried and reused directly for a subsequent round of reaction without further purification with no significant loss of activity, which validates its recyclability (Fig. 8). Moreover, the IR spectrum of Fe3O4/SiO2 NPs after five reuses show that IR bands of the original skeletal vibration of Fe3O4/SiO2 have no obvious change compared to fresh catalyst (Fig. 4c).
Conclusions
In summary, we developed a facile, highly efficient, and eco-friendly procedure for the epoxidation of olefins in the presence of Fe3O4/SiO2 as a heterogeneous catalyst at mild reaction conditions. The prepared catalyst is found to be efficient catalyst for the selective epoxidation of olefins to their corresponding products. This heterogeneous catalyst is highly reactive in the epoxidation of a wide range of alkenes such as linear and cyclic ones. Moreover, easy preparation, handling and recovery, reusability and long-term stability of the catalyst, as well as excellent yields in shorter reaction time under mild reaction conditions, which are some advantages of this heterogeneous catalyst, make it a useful catalyst for further applications in the area of catalysis.
References
Cui HT, Zhang Y, Qiu ZG, Zhao LF, Zhu YL (2010) Synthesis and characterization of cobalt-substituted SBA-15 and its high activity in epoxidation of styrene with molecular oxygen. Applied Catal B 101:45–53
Choudhary VR, Jha R, Jana P (2008) Selective epoxidation of styrene to styrene oxide by TBHP using simple transition metal oxides (NiO, CoO or MoO3) as highly active environmentally-friendly catalyst. Catal Commun 10:205–207
Choudhary VR, Jha R, Jana P (2006) Epoxidation of styrene by TBHP to styrene oxide using barium oxide as a highly active/selective and reusable solid catalyst. Green Chem 8:689–690
Choudhary VR, Jha R, Chaudhari NK, Jana P (2007) Supported copper oxide as a highly active/selective catalyst for the epoxidation of styrene by TBHP to styrene oxide. Catal Commun 8:1556–1560
Khouw CB, Dartt CB, Labinger JA, Davis ME (1994) Studies on the catalytic oxidation of alkanes and alkenes by titanium silicates. J Catal 149:195–205
Xie J, Wang Y, Li Y, Wei Y (2011) Self-assembly preparation of Au/SiO2 catalyst and its catalysis for cyclohexane oxidation with air. React Kinet Mech Cat 102:143–154
He J, Zhai Q, Zhang Q, Deng W, Wang Y (2013) Active site and reaction mechanism for the epoxidation of propylene by oxygen over CuOx/SiO2 catalysts with and without Cs+ modification. J Catal 299:53–66
Shi H-C, Wang X-Y, Hua R, Zhang Z-G, Tang J (2005) Epoxidation of α, β-unsaturated acids catalyzed by tungstate (VI) or molybdate (VI) in aqueous solvents: a specific direct oxygen transfer mechanism. Tetrahedron 61:1297–1307
Polshettiwar V, Varma RS (2010) Green chemistry by nano-catalysis. Green Chem 12:743–754
Shylesh S, Schunemann V, Thiel WR (2010) Magneticaly separable nanocatalysts: bridges between homogeneous and heterogeneous catalysis. Ange Chem Inter Edit 49:3428–3459
Lu AH, Salabas EL, Schuth F (2007) Magnetic nanoparticles: synthesis, protection, functionalization, and application. Ange Chem Inter Edit 46:1222–1244
Lu HC, Yi GS, Zhao SY (2004) Synthesis and characterization of multi-functional nanoparticles possessing magnetic, up-conversion fluorescence and bio-affinity properties. J Mater Chem 14:1336–1341
Grasset F, Dorson F, Molard Y (2008) One-pot synthesis and characterizations of bi-functional phosphor–magnetic @SiO2 nanoparticles: controlled and structured association of Mo6 cluster units and γ-Fe2O3 nanocrystals. Chem Commun 39:4729–4731
Gonzalez-Fernandez MA, Torres TE, Andr-Verg M, Costo R, de la Presa P, Serna CJ, Morales MP, Marquina C, Ibarra MR, Goya GF (2009) Magnetic nanoparticles for powerabsorption: optimizing size, shape and magnetic properties. J Solid State Chem 182:2779–2784
Polshettiwar V, Luque R, Fihri A, Zhu H, Bouhrara M, Basset J-M (2011) Magnetically recoverable nanocatalysts. Chem Rev 111:3036–3075
Lu ZY, Dai J, Song XN, Wang G, Yang WS (2008) Facile synthesis of Fe3O4/SiO2 composite nanoparticles from primary silica particles. Colloids Surf 317:450–456
Leng Y, Sato K, Shi Y, Li J-G, Ishigaki T, Yoshida T, Kamiya H (2009) Oxidation-resistant silica coating on gas-phase-reduced iron nanoparticles and influence on magnetic properties. J Phys Chem C 113:16681–16685
Morel A-L, Nikitenko SI, Gionnet K, Wattiaux A, Lai-Kee-Him J, Labrugere C, Chevalier B, Deleris G, Petibois C, Brisson A, Simonoff M (2008) Sonochemical Approach to the Synthesis of Fe3O4@SiO2 Core − Shell Nanoparticles with Tunable Properties. ACS Nano 2:847–856
Liu Y, Jia L (2008) Analysis of estrogens in water by magnetic octadecylsilane particles extraction and sweeping micellar electro kinetic chromatography. Microchem J 89:72–76
Wu C, He H, Gao H, Liu G, Ma R, An Y, Shi L (2010) Synthesis of Fe3O4@SiO2@polymer nanoparticles for controlled drug release. Sci China Chem 53:514–518
Ren S, Yang C, Sun C, Hui Y, Dong Z, Wang J, Su X (2012) Novel NiO nanodisks and hollow nanodisks derived from Ni(OH)2 nanostructures and their catalytic performance in epoxidation of styrene. Materials Lett 80:23–25
Huang C, Zhang H, Sun Z, Zhao Y, Chen S, Tao R, Liu Z (2011) Porous Fe3O4 nanoparticles: synthesis and application in catalyzing epoxidation of styrene. J Colloid Interface Sci 364:298–303
Choudhary VR, Dumbre DK, Patil NS, Uphade BS, Bhargava SK (2013) Epoxidation of styrene by t-butyl hydroperoxide over gold nanoparticles supported on Yb2O3: effect of gold deposition method, gold loading, and calcination temperature of the catalyst on its surface properties and catalytic performance. J Catal 300:217–224
Patil NS, Uphade BS, Jana P, Bharagava SK, Choudhary VR (2004) Epoxidation of styrene by anhydrous t-butyl hydroperoxide over reusable gold supported on MgO and other alkaline earth oxides. J Catal 223:236–239
Yin D, Qin L, Liu J, Li C, Jin Y (2005) Gold nanoparticles deposited on mesoporous alumina for epoxidation of styrene: effects of the surface basicity of the supports. J Mole Catal A 240:40–48
Acknowledgments
The authors are grateful to the University of Birjand for financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nasseri, M.A., Allahresani, A. & Raissi, H. Mild oxidation of alkenes catalyzed by Fe3O4/SiO2 nanoparticles. Reac Kinet Mech Cat 112, 397–408 (2014). https://doi.org/10.1007/s11144-014-0715-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-014-0715-1