Abstract
Methamphetamine, ketamine, and morphine, found in the influent and effluent of domestic treatment plants as well as in rivers, were selected as parent compounds in this study. This investigation examined the photocatalytic removal of methamphetamine, ketamine, and morphine, from municipal wastewater effluents using illuminated TiO2 and ZnO. HPLC–MS/MS was used to measure the concentration of these drugs during reactions. UV light of 254 nm alone is capable of destroying the drugs to some extent without the TiO2 or ZnO photocatalyst, while UV light of 365 nm must be coupled with the photocatalysts to be effective. UV light of 254 nm in the presence of 0.04 g/L of TiO2 was most effective, eliminating all three drugs within 5 min; ten times as much of ZnO were required to demonstrate comparable removal. Among the three tested drugs, morphine is most readily removed by the photocatalytic treatment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Morphine and ketamine are controlled drugs and routinely dispensed at pharmacies and hospitals. However, their uses and particularly that of methamphetamine are not limited to medical needs but often abused [1–3]. In recent years, the occurrence and distribution of pharmaceutical and personal care products (PPCPs) including controlled drugs have gained much attention due to their potential risk to aquatic lives particularly through bioaccumulation and biomagnification [4, 5]. Controlled substances enter the natural waterways with human metabolic wastes. From 2008, many developed countries began examining controlled drugs in surface waters and wastewater treatment plants. Van Nuijs et al. [6] investigated the occurrence of cocaine (COC) and its metabolite benzoylecgonine (BE) in municipal wastewaters and rivers of Belgium. They found as high as 115 ng/L of COC and 520 ng/L of BE in rivers, and 680 ng/L of COC and 550 ng/L of BE in the wastewater of treatment plants. In 2008, the daily loading rate of cocaine was 40–128 g/d to Brussel–Noord wastewater treatment plants, serving 850,000 inhabitants at 2–3 m3/s [7]. In South Wales, average daily loads of amphetamine and cocaine were 2.5 and 0.9 g/day/1,000 people, respectively, primarily of abuse uses [8]. Lin et al. [9] sampled waters of three rivers and effluents from 5 regional hospitals and rwo wastewater treatment plants in Taiwan; they found concentrations of morphine, codeine, methamphetamine, and ketamine in rivers to be as high as 108, 57, 405, and 341 ng/L, in order, and 1240, 378, 260, and 206 ng/L in hospital effluents. In addition, methamphetamine and ketamine were found in wastewater treatment plants at 296 and 147 ng/L, respectively, in the influents and at 61 and 183 ng/L, respectively, in the effluents. Boleda et al. [10] reported normorphine, morphine, codeine, EDDP, and methadone up to 107, 81, 397, 1150, and 732 ng/L, in order, in the effluents of 15 wastewater treatment plants in Catalonia, Spain. Traditional domestic wastewater treatment plants are designed to remove suspended solids and biodegradable organics and not PPCPs. Based on influent and effluent concentrations, removals were estimated at 70–100 % for cocaine and amphetamine by conventional activated sludge process [11, 12]. Postigo et al. [13] reported >90 % removal of cocaine and amphetamine but no removal of 3,4-methylenedioxymethamphetamine (“ecstasy”) and methamphetamine through wastewater treatment plants. The removal of illicit drugs via biodegradation or biosorption has not been adequately addressed [11, 13, 14]. Lin et al. [9] suggested from their study that domestic wastewater treatment plants were incapable of removing controlled drugs adequately to prevent them from entering the aqueous environment.
Photocatalytic degradation of organic pollutants can be an effective alternative to biological methods for removal of organic contaminants. At present, UV disinfection is widely deployed in wastewater treatment plants. Among many semiconductor materials, titanium dioxide (TiO2) has shown promise as a photocatalyst because of its high chemical and photocatalytic reactivity and stability [15, 16]. At contact with an illuminated photocatalyst, organic chemicals are oxidized and eventually mineralized to carbon dioxide. As widely accepted for the degradation of organics by UV/TiO2 [17], valence holes are created in TiO2 after absorption of sufficiently energetic photons (λ < 360 nm) that result in the oxidation of surface bound OH− or H2O to potent hydroxyl radicals at the surface, which in turn oxidize and break down contaminant compounds. The separation of valence holes from conduction band electrons are enhanced by adsorbed oxygen that acts as an electron trap for the conduction band electrons, preventing the recombination of photogenerated electrons and holes thus increasing photocatalytic efficiency. A main focus of this manuscript is to examine the possible incorporation of semiconductor material such as TiO2 for plants with existing UV disinfection process in removal of controlled substances as a means to arrest their release to the environment.
In this study, we examined the photocatalytic degradation of morphine, methamphetamine, and ketamine using illuminated TiO2 and ZnO. The degradation kinetics and optimal operating parameters were determined. The results have provided an assessment in the utility of photocatalysis for abating the release of controlled substances into the aqueous environments.
Materials and methods
Materials
TiO2 Degussa P 25 (Degussa, Germany) with 20 % rutile and 80 % anatase was used; ZnO was of 20 nm nano grade from a local supplier (Yu-Ho, Taiwan). Both photocatalysts were used as received. Ketamine hydrochloride and morphine sulfate were purchased from Sigma-Aldrich (USA), and methamphetamine hydrochloride was obtained from Pharmacopeial Convention (USA) with permission of the Department of Health, Taiwan. The chemical structures and properties of morphine, methamphetamine, and ketamine are summarized in Table 1. Other chemicals were of reagent grade. Deionized Milli-Q water with specific resistance of 18.2 MΩ cm was used in solution preparations. Stock solutions of the studied drugs were prepared at 1,000 mg/L and stored in amber glass bottles at 4 °C. Standard solutions of different concentrations were prepared by appropriate dilution of stock solutions before each experiment.
Photocatalytic experiments
A hollow, cylindrical photoreactor with an effective volume of 2 L was used. Two kinds of UV light sources were used: a 9 W UV lamp (Philips) that generated 3.61 mW/cm2 of light at 254 nm and a UVLED array of 10 LED bulbs (S-bend, SB1100UV-365) that generated 1.97 mW/cm2 of light at 365 nm. Control experiments were conducted with 100 μg/L solutions of ketamine, methamphetamine, and morphine to evaluate the extent of evaporation (without light and without catalyst), adsorption (0.4 g/L of catalyst without light and without aeration), and photolysis (without catalyst but with light at 254 or 365 nm). Photocatalytic experiments were performed with the test drugs under varied conditions of photocatalysts and UV systems. All experiments were performed at 25 ± 1 °C at 1 atm. The pH of the solution was controlled at 5.5 ± 0.2 during reaction by manually adding HNO3 or NaOH. Studied TiO2 doses were 0.001, 0.005, 0.01, 0.04, 0.05, 0.1, 0.4, 0.7, and 1.0 g/L and ZnO doses were 0.01, 0.04, 0.05, 0.1, 0.4, 0.7, and 1.0 g/L. Experiments were conducted with an initial drug concentration of 100 μg/L and continuous stirring (500 rpm), with samples periodically withdrawn at prescribed time intervals. The samples were filtered through a 0.45 μm filter and the filtrate stored at 4 °C until analysis by LC/MS–MS.
Chemical analysis
The analysis of ketamine, methamphetamine, and morphine was carried out with HPLC–MS/MS as previously described [9]. The HPLC–MS/MS system consisted of a liquid chromatograph (Agilent 1200 module; Agilent, USA) equipped with a ZORBAX Eclipse XDB-C18 column and the tandem mass spectrometer (Sciex API 4000; Applied Biosystem, USA) with an electrospray ionization interface. Ions were acquired in multiple reaction monitoring (MRM) mode with a dwell time of 50 ms. The mass spectrometer conditions were: ion spray voltage of 5.5 kV, curtain gas at 10 L/h, nebulizer gas at 50 L/h, turbo gas at 60 L/h, heated capillary temperature of 500 °C, interface heater switched on, and collision activated dissociation at 5. Analyses were performed in positive mode. The HPLC–ESI–MS/MS conditions by MRM in positive ion modes for the drugs are listed in Table 2. Calibration curves for ketamine and methamphetamine were established at concentrations of 0.1, 0.25, 0.5, 1, 2.5, 5, 10, 25, 50, and 100 μg/L and for morphine at 0.5, 1, 2.5, 5, 10, 25, 50, and 100 μg/L.
Results and discussion
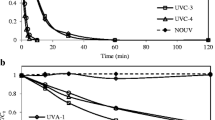
The results of control experiments to delineate the effects of evaporation, adsorption, and direct photolysis are shown in Fig. 1. Negligible losses were due to evaporation (Fig. 1a) or adsorption to TiO2 or ZnO solids (Fig. 1b) after 30 min, a typical duration of experiments. Fig. 1c shows that, after 30 min, no degradation of the drugs by UVLED light at 365 nm but significant degradation, i.e. ketamine by 93.7 %, methamphetamine by 45.1 %, and morphine by 95.8 %, by UV light at 254 nm. The ability of UV light at 254 nm to destruct compounds and the inability of the UVLED light at 365 nm to do the same was consistent with the ability to break bonds with the more energy-intense UV light of the shorter wavelength, as observed in Fig. 1c. It should be noted that the destruction of drugs with UV light at 254 nm was much more than twice that of with UVLED light at 365 nm, while the light intensity of the former (3.6 mW/cm2) was about twice that of the latter (2.0 mW/cm2). Fig. 2 shows various extents of degradation of ketamine according to different UV and catalyst conditions, specifically UV-TiO2, UV-ZnO, UVLED-TiO2, and UVLED-ZnO. The destruction of ketamine was most rapid with UV-TiO2, showing complete removal in 20 min with all tested TiO2 concentrations (0.001–0.4 g/L) and within 5 min with > 0.01 g/L of TiO2 (Fig. 2a). Similarly to UV-ZnO, destruction of ketamine was complete in 30 min, albeit with a higher required dose of ZnO (0.01–1 g/L) (Fig. 2b). The destruction of ketamine with UVLED-TiO2 was also significant, capable of achieving complete destruction in 20 min when a higher dose of TiO2 (0.04–1.0 g/L) was used (Fig. 2c). The destruction of ketamine (Fig. 2d) was modest with UVLED-ZnO, achieving 18–99 % removal in 30 min with increased removal at increased ZnO concentration (0.01–0.7 g/L). The results showed greater destruction effectiveness with UV (254 nm) than with UVLED (365 nm), as well as with TiO2 than with ZnO.
The increased contaminant degradation with UV light of shorter wavelength was previously reported by Lo et al. [18] and Lin et al. [19], both showing increased 4-chlorophenol degradation with decreased wavelength of the light source. This study confirmed it. We also found increasing destruction of ketamine with increasing photocatalyst loading, which was explained by increased adsorption of the photonic output of light by increased catalyst loading that turned a larger portion of the photonic outputs into valence holes leading to increased extent of photocatalytic oxidation of the compound. Thus, the increased destruction was attributed to increased utilization of the light’s photonic output.
Fig. 3 shows the degradation results of methamphetamine with varied combinations of UV lights and the photocatalysts. The UV-TiO2 system (Fig. 3a) facilitated the most rapid degradation, with complete removal of methamphetamine in less than 5 min in some cases. The UVLED-ZnO system offered the least degradation of methamphetamine, with the target compound remaining after 30 min (Fig. 3d). By comparison, TiO2 played a more important role in the rapid degradation than ZnO did, even when UVLED of a longer wavelength was used (Fig. 3b, c). Wu [20] found the rate of organic degradation using UV-ZnO at pH 4 markedly slower than at pH 7 or 10. However, the rate obtained using UV-TiO2 contradicted it with the order of pH 4 > pH 7 > pH 10 [21, 22]. Gouvea et al. [23] and Sakthivel et al. [24] found ZnO to be a more powerful photocatalyst than TiO2. Conversely, Kanmoni et al. [25] indicated that TiO2 exhibited a better photocatalytic activity than ZnO. However, ZnO was less effective for oxidative degradation under acidic conditions because it corroded readily under such conditions [26]. As the reaction pH was controlled at 5.5 in the present study, the higher photocatalytic activity observed of TiO2 relative to ZnO was reasonable. Overall, the photocatalytic degradation of methamphetamine (Fig. 3) exhibited the same patterns as of ketamine (Fig. 2) in terms of the catalyst kind and dose.
The photocatalytic degradation results of morphine are presented in Fig. 4. Again, the UV-TiO2 (Fig. 4a) provided clearly superior efficiency; morphine was completely destroyed within 5 min. The UVLED-TiO2 system also completely removed morphine in 5 min under the same conditions (Fig. 4c), asserting TiO2 being a more effective photocatalyst than ZnO as contrasted in Fig. 4b, d. In general, TiO2 provided stronger photocatalytic power than did ZnO even under illumination by UVLED. When TiO2 was used, all three target compounds were removed in 30 min regardless of the light source. However, ZnO had to be coupled with the stronger UV source (254 nm) in order to be effective.
The degradation results under different conditions can be approximated by the pseudo-first order kinetics with fitted pseudo-first order rate constants k (min−1) [21, 22, 27, 28]. The fitted rate constants k under different conditions are summarized in Table 3. The k values first increased with an increasing catalyst dose and then decreased with further increase in dose (Table 3). The optimal dose of TiO2 with UV was 0.04 g/L, while the optimal dose in UVLED-TiO2, UV-ZnO, and UVLED-ZnO systems was 0.4 g/L for all test compounds. The amount of valence holes was increased with increased catalyst loading that captured more photons resulting in enhanced contaminant degradation. However, adding an excess amount beyond the level necessary to capture the light output reduced light penetration and produced a screening effect on the UV light, resulting in reduced degradation [29–32]. This explained the optimal dose observed for catalyst loading.
To compare the degradation rates of different contaminants under different conditions of light and catalyst loading, the fitted pseudo-first order rate constants are plotted in Fig. 5 along with half-lives calculated for each target compound under different conditions. The plots reveal that morphine was most rapidly removed, and methamphetamine and ketamine were removed at roughly equal rates. The order of catalyst activity follows the decreasing order of UV-TiO2 > UVLED-TiO2 > UV-ZnO > UVLED-ZnO, confirming the catalyst kind being the most important factor (i.e. TiO2 being more active than ZnO). The direct photolytic results also showed the highest removal rate for morphine, which suggested that morphine more readily absorbed UV light resulting in more extensive degradation than ketamine and methamphetamine. As a result, morphine showed the highest rate of removal by photocatalysis as well as by photolysis. TiO2 exhibited a stronger capability for degradation of methamphetamine than for ketamine (Fig. 5a, b), while ZnO was a better catalyst for degradation of ketamine than for methamphetamine (Fig. 5c, d). Calculated times to reach 3-log destruction efficiency for each drug are shown in Table 4, which show clearly that the removal of morphine is fastest among these three drugs.
Conclusions
Controlled amounts of ketamine, methamphetamine, and morphine can be removed from wastewater by direct photolysis using UV light of 254 nm even without the presence of TiO2 or ZnO catalyst. However, UVLED light of 365 nm must be coupled with TiO2 or ZnO in order to be effective. The UV-TiO2 (3.6 mW/cm2, 0.04 g/L) system was most effective, achieving 99.9 % removal of all compounds in 1–6 min, with pseudo-first order rate constants of 1.7 min−1 for katamine, 2.7 min−1 for methamphetamine, and 6.2 min−1 for morphine. The results suggest a possible technique of removing controlled substances from municipal wastewater by photocatalysis.
References
Chong MY, Chan KW, Cheng ATA (1999) Substance use disorders among adolescents in Taiwan: prevalence, sociodemographic correlates and psychiatric co-morbidity. Psychol Med 29:1387–1396
Kuo PH, Yang HJ, Soong WT, Chen WJ (2002) Substance use among adolescents in Taiwan: associated personality traits, incompetence, and behavioral/emotional problems. Drug Alcohol Depend 67:27–39
Metcalfe C, Tindale K, Li H, Rodayan A, Yargeau V (2010) Illicit drugs in Canadian municipal wastewater and estimates of community drug use. Environ Pollut 158:3179–3185
Daughton CG, Ternes TA (1999) Pharmaceuticals and personal care products in the environment: agents of subtle change? Environ Health Perspect 107:907–938
Loganathan B, Phillips M, Mowery H, Jones-Lepp TL (2009) Contamination profiles and mass loadings of macrolide antibiotics and illicit drugs from a small urban wastewater treatment plant. Chemosphere 75:70–77
Van-Nuijs ALN, Pecceu B, Theunis L, Dubois N, Charlier C, Jorens PG, Bervoets L, Blust R, Neels H, Covaci A (2009) Spatial and temporal variations in the occurrence of cocaine and benzoylecgonine in waste and surface water from Belgium and removal during wastewater treatment. Water Res 43:1341–1349
Van-Nuijs ALN, Pecceu B, Theunis L, Dubois N, Charlier C, Jorens PG, Bervoets L, Blust R, Neels H, Covaci A (2009) Cocaine and metabolites in waste and surface water across Belgium. Environ Pollut 157:123–129
Kasprzyk-Hordern B, Dinsdale RM, Guwy AJ (2009) The removal pharmaceuticals, personal care products, endocrine disruptors and illicit drugs during wastewater treatment and its impact on the quality of receiving waters. Water Res 43:363–380
Lin AYC, Wang XH, Lin CF (2010) Impact of wastewaters and hospital effluents on the occurrence of controlled substances in surface waters. Chemosphere 81:562–570
Boleda MR, Galceran MT, Ventura F (2009) Monitoring of opiates, cannabinoids and their metabolites in wastewater, surface water and finished water in Catalonia, Spain. Water Res 43:1126–1136
Castiglioni S, Zuccato E, Crisci E, Chiabrando C, Fanelli R, Bagnati R (2006) Identification and measurement of illicit drugs and their metabolites in urban wastewater by liquid chromatography-tandem mass spectrometry. Anal Chem 78:8421–8429
Kasprzyk-Hordern B, Dinsdale RM, Guwy AJ (2009) Illicit drugs and pharmaceuticals in the environment—forensic applications of environmental data. Part 1: estimation of the usage of drugs in local communities. Environ Pollut 157:1773–1777
Posttigo C, de Alda MJL, Barcelo D (2010) Drugs of abuse and their metabolites in the Ebro river basin: occurrence in sewage and surface water, sewage treatment plants removal efficiency, and collective drug usage estimation. Environ Internal 36:75–84
Kasprzyk-Hordern B, Dinsdale RM, Guwy AJ (2009) Illicit drugs and pharmaceuticals in the environment—forensic applications of environmental data. Part 2: pharmaceuticals as chemical markers of faecal water contamination. Environ Pollut 157:1778–1786
Linsebigler A, Lu G Jr, Yates JT (1995) Photocatalysis on TiO2 surfaces: principles, mechanisms, and selected results. Chem Rev 95:735–758
Hadjiivanov KI, Klissurski DG (1996) Surface chemistry of titania (anatase) and titania-supported catalysts. Chem Soc Rev 25:61–69
Konstantinou IK, Albanis TA (2004) TiO2-assisted photocatalytic degradation of azo dyes in aqueous solution: kinetic and mechanistic investigations: a review. Appl Catal B: Environ 49:1–14
Lo SC, Lin CF, Wu CH, Hsieh PH (2004) Capability of coupled CdSe/TiO2 for photocatalytic degradation of 4-chlorophenol. J Hazard Mater 114:183–190
Lin CF, Wu CH, Onn SN (2008) Degradation of 4-chlorophenol in TiO2, WO3, SnO2, TiO2/WO3 and TiO2/SnO2 systems. J Hazard Mater 154:1033–1039
Wu CH (2008) Effects of sonication on decolorization of C.I. Reactive Red 198 in UV/ZnO system. J Hazard Mater 153:1254–1261
Wu CH (2008) Effects of operational parameters on the decolorization of C.I. Reactive Red 198 in UV/TiO2-based systems. Dyes Pigm 77:31–38
Yu CH, Wu CH, Ho TH, Hong PKA (2010) Decolorization of C.I. Reactive Black 5 in UV/TiO2, UV/oxidant and UV/TiO2/oxidant systems: a comparative study. Chem Eng J 158:578–583
Gouvea CAK, Wypych F, Moraes SG, Duran N, Nagata N, Peralta-Zamora P (2000) Semiconductor-assisted photocatalytic degradation of reactive dyes in aqueous solution. Chemosphere 40:433–440
Sakthivel S, Neppolian B, Shankar MV, Arabindoo B, Palanichamy M, Murugesan V (2003) Solar photocatalytic degradation of azo dye: comparison of photocatalytic efficiency of ZnO and TiO2. Solar Energy Mater Solar Cells 77:65–82
Kanmoni VGG, Daniel S, Raj GAG (2012) Photocatalytic degradation of chlorpyrifos in aqueous suspensions using nanocrystals of ZnO and TiO2. Reac Kinet Mech Cat 106:325–339
Khodja AA, Sehili T, Pilichowski JF, Boule P (2001) Photocatalytic degradation of 2-phenyphenol on TiO2 and ZnO in aqueous suspensions. J Photochem Photobiol A 141:231–239
Wang J, Pan Z, Zhang Z, Zhang X, Wen F, Ma T, Jiang Y, Wang L, Xu L, Kang P (2006) Sonocatalytic degradation of methyl parathion in the presence of nanometer and ordinary anatase titanium dioxide catalysts and comparison of their sonocatalytic abilities. Ultrason Sonochem 13:493–500
Ince NH, Tezcanli G (2001) Reactive dyestuff degradation by combined sonolysis and ozonation. Dyes Pigm 49:145–153
So CM, Cheng MY, Yu JC, Wong PK (2002) Degradation of azo dye Procion Red MX-5B by photocatalytic oxidation. Chemosphere 46:905–912
Wu CH, Kuo CY, Hong PKA (2011) Effects of operational parameters on decolorization of C.I. Reactive Black 5 in UV/TiO2 system. Water Sci Technol 63:1032–1036
Wu MC, Wu CH (2011) Decolorization of C.I. Reactive Red 198 in UV/oxidant and UV/TiO2/oxidant systems. React Kinet Mech Catal 104:281–290
Wu CH, Hong PKA, Jian MY (2012) Decolorization of Reactive Red 2 in Fenton and Fenton-like systems: effects of ultrasound and ultraviolet irradiation. React Kinet Mech Catal 106:11–24
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lin, CF., Shiu, YJ., Kuo, CS. et al. Photocatalytic degradation of morphine, methamphetamine, and ketamine by illuminated TiO2 and ZnO. Reac Kinet Mech Cat 110, 559–574 (2013). https://doi.org/10.1007/s11144-013-0621-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-013-0621-y