Abstract
This study utilized Fenton, Fenton-like, photo-Fenton, photo-Fenton-like, sono-Fenton, and sono-Fenton-like systems for the decolorization of Reactive Red 2 (RR2) dye. Fe2+ and Fe3+ were used to catalyze the actions of oxidants H2O2 and Na2S2O8. Pseudo-first order rate constants (k) were obtained by fitting the decolorization kinetics of RR2. With added oxidant at 0.3 mM and iron ion at 0.03 mM, the k values of H2O2/Fe2+, H2O2/Fe3+, Na2S2O8/Fe2+, and Na2S2O8/Fe3+ were 13, 0.67, 6.2, and 0.080 r−1, in order. Higher oxidant and iron concentrations resulted in faster decolorization in Fenton and Fenton-like systems. Additionally, decolorization was accelerated by ultraviolet (UV) and ultrasound (US) irradiation. When ethanol was used as a scavenger for the generated hydroxyl and sulfate radicals, it significantly inhibited the decolorization process in photo-Fenton and photo-Fenton-like systems. The k values obtained for the UV/H2O2/Fe2+, UV/Na2S2O8/Fe2+, UV/H2O2/Fe2+/C2H5OH, and UV/Na2S2O8/Fe2+/C2H5OH systems were 23, 14, 0.36, and 0.69 h−1, in order.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Wastewaters from the textile dyeing industry typically contain a high concentration of color compounds. Azo dyes containing one or more azo bonds are most common among the synthetic dyes and are a major pollutant in wastewaters. Dyes are readily discernible at small quantities and adversely affect the water environment. Therefore, the removal of color from wastewater often takes priority over the treatment of other colorless organics. Reactive Red 2 (RR2), a common azo dye with the dichlorotriazine group, was selected as the contaminant compound in this study.
Conventional physical (adsorption, filtration, and flotation), chemical (coagulation, oxidation, reduction, and electrolysis), and biological processes have been applied to treat wastewaters containing organic dyes and pigments. Advanced oxidation processes (AOPs) are treatment processes based on the generation of radicals that are highly reactive and nonselective oxidants towards a wide range of organic compounds in water. These processes were significantly advanced in recent years for the treatment of textile effluents [1–14]. Among the AOPs, Fenton-type processes have shown pollutant removal efficiencies >90% [2–4, 15–22]. The Fenton-type process combines iron (Fe2+ or Fe3+) with hydrogen peroxide to produce hydroxyl radicals. The general mechanism involves Fenton reagents that utilize Fe2+ (Fenton) or Fe3+ (Fenton-like) ions as a catalyst to decompose hydrogen peroxide. Several studies have investigated the effectiveness of Fenton-type processes according to H2O2 dosage [2, 3, 15, 16, 23, 24], catalyst dosage [2, 3, 15–17, 23, 24], iron speciation [4, 15, 18], the presence or absence of ultraviolet illumination (UV) [2, 3, 15–17, 23–25], and UV intensity [3, 26].
Various AOP combinations often result in synergistic effects that significantly reduce the required reaction time [5, 6, 8–10]. In recent years, attention has been focused on the application of ultrasonic energy to meet wastewater treatment challenges. Ultrasound (US) causes acoustic cavitation, resulting in bubble collapses that cause intense local heating, pressurization, and very short lifetimes of the bubbles; these transient, localized hot spots are useful in driving high-energy chemical reactions [27]. The solute or solvent molecules in contact with or inside these cavities in the vapor phase undergo fragmentation, yielding free radicals that degrade organic compounds. Degradation of organic compounds is typically achieved via a combination of pyrolytic reactions that occur inside or near the bubble and radical reactions in the bulk liquid [28, 29]. The combinations of US with other techniques such as UV [5], Fe0 [30–32], Fe2+ [29, 30, 32, 33], Fe3+ [33], TiO2 [6, 7, 31, 34, 35], H2O2 [8], UV/ZnO [5], UV/TiO2 [6, 7], UV/H2O2 [8], H2O2/Fe2+ [36–38], \( {\text{S}}_{ 2} {\text{O}}_{ 8}^{2 - } \)/Fe2+ [37], and \( {\text{S}}_{ 2} {\text{O}}_{ 8}^{2 - } \)/H2O2/Fe2+ [39] have been shown to enhance contaminant degradation.
Several studies have explored the performance of US/Fenton [36–39], US/Fenton-like [37, 39], UV/Fenton [15–19, 23–26], and UV/Fenton-like [15, 18, 19] systems in the degradation of different compounds. Additionally, UV/US/TiO2 [6, 7]; O3 [40, 41]; UV/O3 [40]; UV/TiO2 [40, 42–44]; H2O2/Fe2+ [14, 45] and H2O2/Fe3+ [46] systems have been used to decolorize RR2. However, none have been undertaken to compare all of these systems based on the degradation of a single contaminant compound. This study compares US/Fenton, US/Fenton-like, UV/Fenton, and UV/Fenton-like systems with similar oxidant and catalyst dosages for their degradative performance on RR2. Both Fe2+ and Fe3+ were used to catalyze H2O2 and Na2S2O8. Fenton (H2O2/Fe2+), Fenton-like (H2O2/Fe3+, \( {\text{S}}_{ 2} {\text{O}}_{ 8}^{2 - } \)/Fe2+ and \( {\text{S}}_{ 2} {\text{O}}_{ 8}^{2 - } \)/Fe3+), photo-Fenton (UV/H2O2/Fe2+), photo-Fenton-like (UV/H2O2/Fe3+, UV/\( {\text{S}}_{ 2} {\text{O}}_{ 8}^{2 - } \)/Fe2+ and UV/\( {\text{S}}_{ 2} {\text{O}}_{ 8}^{2 - } \)/Fe3+), sono-Fenton (US/H2O2/Fe2+), and sono-Fenton-like (US/H2O2/Fe3+, US/\( {\text{S}}_{ 2} {\text{O}}_{ 8}^{2 - } \)/Fe2+, and US/\( {\text{S}}_{ 2} {\text{O}}_{ 8}^{2 - } \)/Fe3+) systems were tested. The objectives of this study are to: (i) determine the effects of oxidant and iron dosages in Fenton and Fenton-like systems, and (ii) evaluate the effects of UV and US irradiation in Fenton and Fenton-like systems.
Experimental
Materials
The parent compound RR2 was purchased from Aldrich. The formula, molecular weight, and maximum light absorption wavelength of RR2 were C19H10Cl2N6Na2O7S2, 615 g/mol, and 538 nm, respectively. Ferrous sulfate (FeSO4) and ferric sulfate (Fe2(SO4)3) (Merck) were used to supply Fe2+ and Fe3+, respectively. Oxidants sodium persulfate (Na2S2O8) and hydrogen peroxide (H2O2, 30% w/w) were also purchased from Merck. Ethanol (C2H5OH) was used as a hydroxyl and sulfate radical scavenger. The solution pH was controlled by adding HNO3 or NaOH through an automatic titrator. All reagents were of analytical grade and used as purchased. Deionized, double-distilled water from a Milli-Q system was used.
Decolorization experiments
The decolorization of RR2 in the UV, US, H2O2, Na2S2O8, Fe2+, and Fe3+ systems was individually tested in control experiments. RR2 at 20 mg/L was used in all experiments. Several studies had indicated pH 3 being optimal for Fenton and photo-Fenton systems [18, 23, 26] and thus pH 3 was used in all decolorization experiments. These experiments were performed with a 2.5-L hollow cylindrical glass reactor. An 8-W UV lamp (254 or 365 nm, Philips) was placed inside the quartz tube as the irradiation source. The intensity of the light from the lamps at wavelengths of 245 and 365 nm was 3.92 and 0.57 mW/cm2, respectively. The ultrasonic bath was operated at 40 kHz and 400 W (Delta, DC 400H). The distance between the reactor bottom and the ultrasonic bath was maintained at 2 cm. A calorimetric method was used to determine the acoustic power of sonication over 40 min. The ultrasonic power received by the system was calculated by [47, 48]:
where (dT/dt) is the temperature rise rate of the water during sonication (K s−1), Cp the heat capacity of water (4.2 J g−1 K−1), and M the water mass (g). The acoustic power received by the reactor was determined to be 13.0 W. Oxidant doses of 0.3, 0.5, 1, and 2 mM along with iron doses of 0.01, 0.03, and 0.05 mM were used to evaluate the dosage effects in the Fenton and Fenton-like systems. In experiments to determine the effects of US and UV irradiation, the doses of oxidant and iron were 0.3 and 0.03 mM, respectively. Ethanol was added at 1,200 mg/L as a radical scavenger to determine the involvement of free radicals in the Fenton, Fenton-like, photo-Fenton, and photo-Fenton-like systems. All experiments were stirred at 100 rpm and aerated continuously and the temperature was controlled at 25 °C using a water circulation system. Aliquots (15 mL) were withdrawn from the photoreactor at predetermined time intervals. The solution was filtered through a 0.22-μm filter (Millipore). RR2 concentration was measured using a UV–vis spectrophotometer (Hitachi U-2001) at 538 nm. Decolorization of RR2 was quantified by the difference in absorbance measured before and after treatment. Most experiments were performed in duplicate and the averages shown.
Results and discussion
Effects of oxidant and iron doses in Fenton and Fenton-like systems
After 120 min, the extents of decolorization of RR2 by UV (254 nm), UV (365 nm), and US irradiation were 18, 4, and 19%, respectively. The extents of decolorization of RR2 when individually subjected to Fe2+, H2O2, and Na2S2O8 at the highest test concentration (i.e. 2 mM of oxidant or 0.05 mM of iron) were 2, 2, and 5%, respectively. The decolorization extents were small (i.e., <5% after 120 min) compared to subsequent results with UV and US irradiation that would demonstrate catalytic, photocatalytic, or sonocatalytic effect. The synergistic effects of added oxidants in Fenton and Fenton-like systems are shown in Table 1. RR2 decolorization in the Fenton and Fenton-like systems was found to be well described by pseudo-first order kinetics, as previously reported for dye decolorization by various investigators [5–8, 18, 31, 49].
The H2O2/Fe2+ system involves several cyclical reactions with catalyst regeneration, as shown in Eqs. 2–9. In H2O2/Fe3+, the reaction sequence begins with Eq. 3 [2, 15, 25, 37, 39]. The reaction mechanisms of Na2S2O8/Fe2+ are shown in Eqs. 10–14, while those of Na2S2O8/Fe3+ systems are shown in Eqs. 11–14 [37, 39, 50, 51].
Increasing the oxidant concentration (from 0.3 to 2 mM) enhanced generation of radicals and therefore increased RR2 decolorization in AOP systems of H2O2/Fe2+ (Eqs. 2 and 3), H2O2/Fe3+ (Eq. 3), and Na2S2O8/Fe2+ (Eqs. 10, 11, 13, and 14). In the Na2S2O8/Fe3+ system, the k value increased with increasing oxidant concentration up to 1 mM, beyond which it decreased possibly due to recombination of the free radicals (Table 1).
The effects of catalyst concentration in Fenton and Fenton-like systems are presented in Table 2. The results revealed that the k values increased with Fe concentration in all tested Fenton and Fenton-like systems. Fe served as a catalyst and initiated the decomposition of hydrogen peroxide and persulfate for the generation of HO• and HO •2 in the Fenton system and SO −•4 and HO• in the Fenton-like system. More free radicals were produced with an increase of available Fe.
When compared at the same oxidant and iron doses, the rate of RR2 decolorization (k) followed the order of H2O2/Fe2+ > Na2S2O8/Fe2+ > H2O2/Fe3+ > Na2S2O8/Fe3+ (Tables 1, 2). Sulfate radical is a very strong oxidant with redox potential of 2.6 V, next only to hydroxyl radical with redox potential of 2.8 V [51]. Generally, the oxidizing properties of peroxide oxidants are measured by the tendency of electron transfer to a lower unoccupied molecular orbital (LUMO) [52]. Miroshnichenko and Lunenok-Burmakina [52] have indicated that the energy of LUMO of \( {\text{S}}_{ 2} {\text{O}}_{ 8}^{ 2- } \) is higher than that of H2O2, implying that H2O2 accepts an electron more readily than does \( {\text{S}}_{ 2} {\text{O}}_{ 8}^{ 2- } \). It has also been suggested that the Fe catalyst activates H2O2 more readily than \( {\text{S}}_{ 2} {\text{O}}_{ 8}^{ 2- } \) [22, 52]. Hence, at the same Fe dose, H2O2-related systems would be more active than \( {\text{S}}_{ 2} {\text{O}}_{ 8}^{ 2- } \)-related systems. Based on Eqs. 3–7, 11, and 12, Fe3+-related mechanisms are side reactions of Fe2+-related mechanisms; therefore, the Fe2+-related systems had higher k values than the Fe3+-related systems. This was corroborated by results of Orozco et al. [4] that showed higher decolorization rate of azo-dye Reactive Blue 69 by H2O2/Fe2+ than by H2O2/Fe3+.
Effects of UV and US irradiation in Fenton and Fenton-like systems
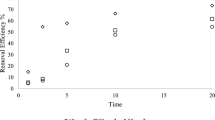
The decolorization of RR2 in the H2O2/Fe2+, H2O2/Fe3+, Na2S2O8/Fe2+, and Na2S2O8/Fe3+ systems when coupled with US and UV (254 and 365 nm) irradiation is shown in Figs. 1, 2, 3, and 4. In 5 min, the extent of RR2 decolorization was 70, 84, 80, 73, 42, 67, 58, and 46% for the H2O2/Fe2+, UV (254 nm)/H2O2/Fe2+, UV (365 nm)/H2O2/Fe2+, US/H2O2/Fe2+, Na2S2O8/Fe2+, UV (254 nm)/Na2S2O8/Fe2+, UV (365 nm)/Na2S2O8/Fe2+, and US/Na2S2O8/Fe2+ systems, respectively (Figs. 1, 3). After 20 min, the extent of decolorization was 10, 83, 20, 54, 2, 70, 31, and 45% for the H2O2/Fe3+, UV (254 nm)/H2O2/Fe3+, UV (365 nm)/H2O2/Fe3+, US/H2O2/Fe3+, Na2S2O8/Fe3+, UV (254 nm)/Na2S2O8/Fe3+, UV (365 nm)/Na2S2O8/Fe3+, and US/Na2S2O8/Fe3+ systems, respectively (Figs. 2, 4). The Fe2+-related systems were more effective in decolorizing RR2 than were Fe3+-related systems, as shown by higher decolorization extents within a shorter contact time in the former. Kusic et al. [15], Neamtu et al. [18], and Orozco et al. [4] also reported that the degradation rate with UV/H2O2/Fe2+ was higher than with the UV/H2O2/Fe3+ system. In Fe2+-related systems, the k values followed the order of UV (254 nm)/oxidant/Fe2+ > UV (365 nm)/oxidant/Fe2+ > US/oxidant/Fe2+ > oxidant/Fe2+. Moreover, the trend of UV (254 nm)/oxidant/Fe3+ > US/oxidant/Fe3+ > UV (365 nm)/oxidant/Fe3+ > oxidant/Fe3+ was also found in Fe3+-related systems (Table 3). Therefore, these experimental results indicate that UV (254 nm) activates these systems more than US. In some cases (e.g. UV (365 nm)/oxidant/Fe2+), UV (365 nm) irradiation was better as well.
Wang et al. [34, 35] proposed that US irradiation caused the formation of US-induced luminescence at wavelength below 375 nm, decompose H2O2 and \( {\text{S}}_{ 2} {\text{O}}_{ 8}^{ 2- } \), and produce HO•and \( {\text{S}}_{ 2} {\text{O}}_{ 8}^{ - \bullet } \). In Fenton and Fenton-like systems with US or UV, the decomposition of H2O2, S2O8 2−, Fe-O2H2+, and FeOH2+ to generate the reactive free radicals is shown by Eqs. 15–18:
US or UV apparently favored the formation of HO •2 and HO• in the Fenton or Fenton-like cycle (Eqs. (17) and (18)), accelerating decolorization. Sun et al. [36] and Pradhan and Gogate [38] also reported more effective oxidation with US/H2O2/Fe2+ than with H2O2/Fe2+.
In photo-Fenton, photo-Fenton-like, sono-Fenton, and sono-Fenton-like systems, decolorization was accelerated with a higher Fe dose (Table 3). Additionally, the decolorization rate at 254 nm exceeded that at 365 nm in the photo-Fenton and photo-Fenton-like systems (Table 3). This finding was attributed to the fact that the energy and intensity of UV (254 nm) light exceeded those of UV (365 nm). Lo et al. [53] and Lin et al. [54] found increasing photodegradation of 4-chlorophenol with decreasing illumination wavelength. Similar results were reported for the photodegradative decolorization of C.I. Reactive Red 198 by Wu and Wu [10].
Fig. 5a, b display the UV–Vis spectral changes of RR2 in UV (254 nm)/H2O2/Fe2+ and UV (254 nm)/Na2S2O8/Fe2+ systems, respectively. RR2 had a major absorption peak at 538 nm, whose intensity declined after 20 min of photocatalytic reaction, suggesting that some intermediates may have been generated following the photocatalytic reaction, but this work did not further elucidate this possibility. Wu et al. [55] found that the degradation products of RR2 under photooxidation treatment were substituted benzene and naphthalene. Hu et al. [42] concluded that the intermediates of RR2 photodegradation were mainly organic aromatic and aliphatic carboxylic acids. Moreover, acetyl benzoic acid, diethyl phthalate and phthalic anhydride have also been identified as intermediates in RR2 photodegradation [41]. Both Wu et al. [55] and Hu et al. [42] found that the byproducts can be further oxidized and finally mineralized to form carbon dioxide.
Effects of ethanol addition
Fig. 6 presents the effects of added ethanol in the H2O2/Fe2+, UV/H2O2/Fe2+, Na2S2O8/Fe2+, and UV/Na2S2O8/Fe2+ systems. After 60 min, RR2 was decolorized by 3.7, 15, 32, and 45% in the H2O2/Fe2+/C2H5OH, Na2S2O8/Fe2+/C2H5OH, UV/H2O2/Fe2+/C2H5OH, and UV/Na2S2O8/Fe2+/C2H5OH systems, respectively. Ethanol is known to scavenge hydroxyl [56–58] and sulfate radicals [59]. Excess ethanol (1,200 mg/L) suppressed decolorization of RR2 in all tested systems. The results suggest that hydroxyl and sulfate radicals have been significantly involved in RR2 decolorization. Furthermore, the fact that RR2 decolorization was almost completely inhibited with ethanol in the H2O2/Fe2+ system indicated a major role of HO• in decolorization. The fitted k values of the UV/H2O2/Fe2+, UV/Na2S2O8/Fe2+, UV/H2O2/Fe2+/C2H5OH, and UV/Na2S2O8/Fe2+/C2H5OH systems were 23, 14, 0.36, and 0.69 h−1, in order. For the UV/H2O2/Fe2+ system, the k value with ethanol was approximately 60 times lower, which was similarly observed for the H2O2/Fe2+ system, suggesting again a major decolorization pathway via the attack by HO•. However, decolorization was only partially inhibited by ethanol even at 1,200 mg/L in the UV/Na2S2O8/Fe2+ system, as evidenced by k values reduced by only 20 times. The second-order rate constants of hydroxyl radicals and sulfate radicals with ethanol were reported to be 1.9 × 109 M−1 s−1 [58] and 7.8 × 107 M−1 s−1 [59], respectively. This indicates that ethanol is more inhibitive toward hydroxyl radicals than toward sulfate radicals. Therefore, inhibition by added ethanol was more significant in the UV/H2O2/Fe2+ system than in the UV/Na2S2O8/Fe2+ system. Sulfate radicals are produced in Na2S2O8/Fe2+ (Eqs. 10, 11, and 14) and UV/Na2S2O8/Fe2+ (Eqs. 10, 11, 14, and 16) systems. The results suggest that decolorization proceeds primarily via HO• in the H2O2/Fe2+, UV/H2O2/Fe2+, Na2S2O8/Fe2+, and UV/Na2S2O8/Fe2+ systems; however, \( {\text{S}}_{ 2} {\text{O}}_{ 4}^{ - \bullet } \) is also be involved in the Na2S2O8/Fe2+ and UV/Na2S2O8/Fe2+ systems.
Conclusion
Increasing the concentration of oxidant or catalyst increased the generation of reactive free radicals and thus enhanced RR2 decolorization in Fenton and Fenton-like systems. At the same oxidant and Fe doses, the fitted k values reveal the trend of H2O2/Fe2+ > Na2S2O8/Fe2+ > H2O2/Fe3+ > Na2S2O8/Fe3+. Fe2+-related systems are more effective than Fe3+-related systems in decolorizing RR2. At the same kind and dose of Fe, H2O2-related systems are more effective than \( {\text{S}}_{ 2} {\text{O}}_{ 8}^{2 - } \)-related systems. Both US and UV promote the effectiveness of Fenton and Fenton-like systems.
References
Lee C, Yoon J (2004) Application of photoactivated periodate to the decolorization of reactive dye: reaction parameters and mechanism. J Photochem Photobiol A Chem 165:35–41
Lucas S, Peres JA (2006) Decolorization of the azo dye Reactive Black 5 by Fenton and photo-Fenton oxidation. Dyes Pigment 71:236–244
Tokumura M, Ohta A, Znad HT, Kawase Y (2006) UV light assisted decolorization of dark brown colored coffee effluent by photo-Fenton reaction. Water Res 40:3775–3784
Orozco SL, Bandala ER, Arancibia-Bulnes CA, Serrano B, Suarez-Parra R, Hernadez-Perez I (2008) Effect of iron salt on the color removal of water containing the azo-dye reactive blue 69 using photo-assisted Fe(II)/H2O2 and Fe(III)/H2O2 systems. J Photochem Photobiol A Chem 198:144–149
Wu CH (2008) Effects of sonication on decolorization of C.I. Reactive Red 198 in UV/ZnO system. J Hazard Mater 153:1254–1261
Wu CH (2009) Photodegradation of C.I. Reactive Red 2 in UV/TiO2-based systems: effects of ultrasound irradiation. J Hazard Mater 167:434–439
Wu CH, Yu CH (2009) Effects of TiO2 dosage, pH and temperature on decolorization of C.I. Reactive Red 2 in a UV/US/TiO2 system. J Hazard Mater 169:1179–1183
Wu CH (2007) Sonocatalytic degradation of C.I. Reactive Red 198 in H2O2-based systems. React Kinet Catal Lett 92:377–384
Yu CH, Wu CH, Ho TH, Hong PKA (2010) Decolorization of C.I. Reactive Black 5 in UV/TiO2, UV/oxidant and UV/TiO2/oxidant systems: a comparative study. Chem Eng J 158:578–583
Wu MC, Wu CH (2011) Decolorization of C.I. Reactive Red 198 in UV/oxidant and UV/TiO2/oxidant systems. Reac Kinet Mech Cat 104:281–290
Peternel I, Grcic I, Koprivanac N (2010) Degradation of reactive azo dye by UV/peroxodisulfate system: an experimental design approach. Reac Kinet Mech Cat 100:33–44
Zhang J, Hu FT, Liu QQ, Zhao X, Liu SQ (2011) Application of heterogenous catalyst of tris(1,10)-phenanthroline iron(II) loaded on zeolite for the photo-Fenton degradation of methylene blue. Reac Kinet Mech Cat 103:299–310
Beninca C, Peralta-Zamora P, Camargo RC, Tavares CRG, Zanoelo EF, Igarashi-Mafra L (2012) Kinetics of oxidation of ponceau 4R in aqueous solutions by Fenton and photo-Fenton processes. Reac Kinet Mech Cat. doi:10.1007/s11144-011-0392-2
Dutta K, Bhattacharjee S, Chaudhuri B, Mukhopadhyay S (2002) Chemical oxidation of C. I. Reactive Red 2 using Fenton-like reactions. J Environ Monit 4:754–760
Kusic H, Koprivanac N, Bozic AL, Selanec I (2006) Photo-assisted Fenton type processes for the degradation of phenol: a kinetic study. J Hazard Mater 136:632–644
Xu XR, Li XY, Li XZ, Li HB (2009) Degradation of melatonin by UV, UV/H2O2, Fe2+/H2O2, UV/Fe2+/H2O2 processes. Sep Purif Technol 68:261–266
Shemer H, Kunukcu YK, Linden KG (2006) Degradation of the pharmaceutical Metronidazole via UV, Fenton and photo-Fenton processes. Chemosphere 63:269–276
Neamtu M, Yediler A, Siminiceanu I, Kettrup A (2003) Oxidation of commercial reactive azo dye aqueous solutions by the photo-Fenton and Fenton-like processes. J Photochem Photobiol A Chem 161:87–93
Anipsitakis GP, Dionysiou DD (2004) Transition metal/UV-based advanced oxidation technologies for water decontamination. Appl Catal B Environ 54:155–163
Burbano AA, Dionysiou DD, Suidan MT, Richardson TL (2005) Oxidation kinetics and effect of pH on the degradation of MTBE with Fenton reagent. Water Res 39:107–118
Burbano AA, Dionysiou DD, Suidan MT (2008) Effect of oxidant-to-substrate ratios on the degradation of MTBE with Fenton reagent. Water Res 42:3225–3239
Antoniou MG, de la Cruz AA, Dionysion DD (2010) Degradation of microcystin-LR using sulfate radicals generated through photolysis, thermolysis and e− transfer mechanisms. Appl Catal B Environ 96:290–298
Saritha P, Aparna C, Himabindu V, Anjaneyulu Y (2007) Comparison of various advanced oxidation processes for the degradation of 4-chloro-2 nitrophenol. J Hazard Mater 149:609–614
Jamil TS, Ghaly MY, El-Seesy IE, Souaya ER, Nasr RA (2011) A comparative study among different photochemical oxidation processes to enhance the biodegradability of paper mill wastewater. J Hazard Mater 185:353–358
Kusic H, Koprivanac N, Horvat S, Bakija S, Bozic AL (2009) Modeling dye degradation kinetic using dark- and photo-Fenton type processes. Chem Eng J 155:144–154
Muruganandham M, Swaminathan M (2004) Decolourisation of Reactive Orange 4 by Fenton and photo-Fenton oxidation technology. Dyes Pigments 63:315–321
Suslick KS (1988) Ultrasound: its chemical, physical and biological effects. VCH, New York
Ince NH, Tezcanli-Guyer G, Belen RK, Apikyan IG (2001) Ultrasound as a catalyzer of aqueous reaction systems: the state of the art and environmental applications. Appl Catal B Environ 29:167–176
Papadaki M, Emery RJ, Abu-Hassan MA, Diaz-Bustos A, Metcalfe IS, Mantzavinos D (2004) Sonocatalytic oxidation processes for the removal of contaminants containing aromatic rings from aqueous effluents. Sep Purif Technol 34:35–42
Son HS, Choi SB, Khan E, Zoh KD (2006) Removal of 1, 4-dioxane from water using sonication: effect of adding oxidants on the degradation kinetics. Water Res 40:692–698
Eren Z, Ince NH (2010) Sonolytic and sonocatalytic degradation of azo dyes by low and high frequency ultrasound. J Hazard Mater 177:1019–1024
Guyer GT, Ince NH (2011) Degradation of diclofenac in water by homogeneous and heterogeneous sonolysis. Ultrason Sonochem 18:114–119
Yim B, Yoo Y, Maeda Y (2003) Sonolysis of alkylphenols in aqueous solution with Fe(II) and Fe(III). Chemosphere 50:1015–1023
Wang J, Ma T, Zhang Z, Zhang X, Jiang Y, Dong D, Zhang P, Li Y (2006) Investigation on the sonocatalytic degradation of parathion in the presence of nanometer rutile titanium dioxide (TiO2) catalyst. J Hazard Mater 137:972–980
Wang J, Pan Z, Zhang Z, Zhang X, Wen F, Ma T, Jiang Y, Wang L, Xu L, Kang P (2006) Sonocatalytic degradation of methyl parathion in the presence of nanometer and ordinary anatase titanium dioxide catalysts and comparison of their sonocatalytic abilities. Ultrason Sonochem 13:493–500
Sun JH, Sun SP, Sun JY, Sun RX, Qiao LP, Guo HQ, Fan MH (2007) Degradation of azo dye Acid black 1 using low concentration iron of Fenton process facilitated by ultrasonic irradiation. Ultrason Sonochem 14:761–766
Grcic I, Obradovic M, Vujevic D, Koprivanac N (2010) Sono-Fenton oxidation of formic acid/formate ions in an aqueous solution: from an experimental design to the mechanistic modeling. Chem Eng J 164:196–207
Pradhan AA, Gogate PR (2010) Degradation of p-nitrophenol using acoustic cavitation and Fenton chemistry. J Hazard Mater 173:517–522
Grcic I, Vujevic D, Koprivanac N (2010) Modeling the mineralization and discoloration in colored system by (US)Fe2+/H2O2/S2O8 2− processes: a proposed degradation pathway. Chem Eng J 157:35–44
Wu CH, Chang CL (2006) Decolorization of Reactive Red 2 by advanced oxidation processes: comparative studies of the homogeneous and heterogeneous systems. J Hazard Mater 128:265–272
Pachhade K, Sandhya S, Swaminathan K (2009) Ozonation of reactive dye, Procion red MX-5B catalyzed by metal ions. J Hazard Mater 167:313–318
Hu C, Yu JC, Hao Z, Wong PK (2003) Photocatalytic degradation of triazine-containing azo dyes in aqueous TiO2 suspensions. Appl Catal B Environ 42:47–55
Sahel K, Perol N, Chermette H, Bordes C, Derriche Z, Guillard C (2007) Photocatalytic decolorization of Remazol Black 5 (RB5) and Procion Red MX-5B—Isotherm of adsorption, kinetic of decolorization and mineralization. Appl Catal B Environ 77:100–109
Sahel K, Perol N, Dappozze F, Bouhent M, Derriche Z, Guillard C (2010) Photocatalytic degradation of a mixture of two anionic dyes: Procion Red MX-5B and Remazol Black 5 (RB5). J Photochem Photobiol A Chem 212:107–112
Swaminathan K, Sandhya S, Sophia AC, Pachhade K, Subrahmanyam YV (2003) Decolorization and degradation of H-acid and other dyes using ferrous-hydrogen peroxide system. Chemosphere 50:619–625
Wu CH (2008) Decolorization of C.I. Reactive Red 2 in O3, Fenton-like and O3/Fenton-like Hybrid systems. Dyes Pigment 77:24–30
Kimura T, Sakamoto T, Leveque JM, Sohmiya H, Fujita M, Ikeda S, Ando T (1996) Standardization of ultrasonic power for sonochemical reaction. Ultrason Sonochem 3:157–161
d’Auzay SDLR, Blais JF, Naffrechoux E (2010) Comparison of characterization methods in high frequency sonochemical reactors of differing configurations. Ultrason Sonochem 17:547–554
Xu XR, Li XZ (2010) Degradation of azo dye Orange G in aqueous solutions by persulfate with ferrous ion. Sep Purif Technol 72:105–111
Anipsitakis GP, Dionysiou DD (2004) Radical generation by the interaction of transition metals with common oxidants. Environ Sci Technol 38:3705–3712
Devi LG, Rajashekhar KE, Raju KSA, Kumar SG (2009) Kinetic modeling based on the non-linear regression analysis for the degradation of Alizarin Red S by advanced photo Fenton process using zero valent metallic iron as the catalyst. J Molec Catal A Chem 314:88–94
Miroshnichenko AG, Lunenok-Burmakina VA (1976) A quantum-mechanical study of the structure of inorganic derivatives of hydrogen peroxide. Theor Exp Chem 11:320–325
Lo SC, Lin CF, Wu CH, Hsieh PH (2004) Capability of coupled CdSe/TiO2 for photocatalytic degradation of 4-chlorophenol. J Hazard Mater 114:183–190
Lin CF, Wu CH, Onn SN (2008) Degradation of 4-chlorophenol in TiO2, WO3, SnO2, TiO2/WO3 and TiO2/SnO2 systems. J Hazard Mater 154:1033–1039
Wu F, Deng N, Hua H (2000) Degradation mechanism of azo dye C.I. Reactive Red 2 by iron powder reduction and photooxidation in aqueous solutions. Chemosphere 41:1233–1238
Daneshvar N, Salari D, Khataee AR (2003) Photocatalytic degradation of azo dye acid red 14 in water: investigation of the effect of operational parameters. J Photochem Photobiol A Chem 157:111–116
Daneshvar N, Salari D, Khataee AR (2004) Photocatalytic degradation of azo dye acid red 14 in water on ZnO as an alternative catalyst to TiO2. J Photochem Photobiol A Chem 162:317–322
Buxton GV, Greenstock CL, Helman WP, Ross AB (1988) Critical review of rate constants for reactions of hydrated electrons, hydrogen atoms and hydroxyl radicals (OH/O−) in aqueous solution. J Phys Chem Ref Data 17:513–759
George Ch, Rassy HEl, Chovelon JM (2001) Reactivity of selected volatile organic compounds (VOCs) toward the sulfate radical (SO4 −). Int J Chem Kinet 33:539–547
Acknowledgments
The authors would like to thank the National Science Council of the Republic of China, Taiwan, for financially supporting this research under Contract No. NSC 98-2221-E-212-002-MY3.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wu, CH., Andy Hong, P.K. & Jian, MY. Decolorization of Reactive Red 2 in Fenton and Fenton-like systems: effects of ultrasound and ultraviolet irradiation. Reac Kinet Mech Cat 106, 11–24 (2012). https://doi.org/10.1007/s11144-012-0420-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-012-0420-x