Abstract
In this study, UV/oxidant and UV/TiO2/oxidant systems were employed to treat textile wastewater. The parent compound was C.I. reactive red 198 (RR198). The selected oxidants were H2O2, Na2S2O8, NaBrO3, and NaIO4. The effects of oxidant dosage (1–24 mM), wavelength of UV (254 and 365 nm) and radical scavenger addition (C2H5OH) were determined in UV/oxidant systems. The experimental results revealed that all oxidants effectively decolorized RR198 under 254 nm irradiation; however, only Na2S2O8 and NaIO4 can decolorize RR198 under 365 nm irradiation. The decolorization rates fit a pseudo-first order reaction model. Under 254 nm irradiation and 6 mM oxidant addition, the decolorization rate constants (k) of H2O2, Na2S2O8, NaBrO3, and NaIO4 for RR198 were 10.24, 17.93, 13.37, and 11.90 h−1. Under 365 nm irradiation, 1 g/L TiO2 and 1 mM NaIO4 addition, the k values of the UV/TiO2, UV/NaIO4, and UV/TiO2/NaIO4 systems were 0.50, 0.52, and 11.67 h−1. The inhibition of RR198 decolorization by the addition ethanol indicates that the primary decolorization pathway involves hydroxyl radicals in UV/H2O2 and UV/Na2S2O8 systems, and that oxidation by other radicals is probably important in UV/NaBrO3 and UV/NaIO4 systems.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Wastewater from the textile dyeing industry has a high or low pH, a high temperature and a high concentration of coloring material. Several dyes are environmental hazards because they are toxic. Removing color from wastewater is important because small amounts of dye are clearly visible and adversely affect water quality. Colored wastewater is subject to strict environmental legislation because it has a negative effect on photosynthetic activity all over the world. Therefore, the decolorization of dye effluents is attracting an attention. C.I. reactive red 198 (RR198), a dye that contains two of the most commonly used anchors—monochlorotriazine and vinyl sulfone groups—was adopted as the parent compound in this study.
Conventional treatments of dye effluents include biological oxidation and adsorption. Although less expensive than other approaches, biological treatment is ineffective for decolorization because the dyes are toxic. Adsorption onto activated carbon transfers most of the contaminant from the wastewater to the solid phase. This method therefore requires further disposal of the sludge. Advanced oxidation processes (AOPs) are alternative procedures for decolorizing and reducing problematic wastewater loads from textile companies. AOPs are based on the formation of hydroxyl radicals in water, which are highly reactive and nonselective oxidants that can oxidize organic compounds. In a heterogeneous photocatalytic reaction, molecular oxygen acts as an electron acceptor to prevent the recombination of electrons and holes. To improve the photocatalytic efficiency of semiconductors, Serpone et al. [1] proposed an interparticle electron transfer process, which couples two semiconductors with different redox energy levels to increase charge separation for the corresponding conduction and valance bands. Coupled semiconductors improved charge separation and were responsible for the enhancement in the rate of organics photocatalytic degradation [1–4]. Degradation levels could also be improved using various oxidants as electron scavengers. Such inorganic oxidants as IO4 −, S2O8 2−, BrO3 −, ClO3 −, and H2O2 can be utilized as additives instead of oxygen to enhance the photodegradation rates of organic substrates by quenching the conduction band electrons and form reactive radical intermediates [5–7]. These oxidants increase the effectiveness of UV/photocatalyst in degrading organic substrates by capturing the electrons that are ejected from the photocatalyst, to reduce the probability of recombination of photogenerated electrons and photogenerated holes: the available number and survival time of the photogenerated holes is thus increased, promoting their effective reaction with organic substrates. The effect of the oxidants in the UV/TiO2 system on the degradation rate of 4-chloro-2-methylphenol were found to follow the order IO4 − > BrO3 − > H2O2 > O2 > ClO3 − [6]. Syoufian and Nakashima [8] indicated that the effectiveness of the oxidants in the UV/TiO2 system followed the order S2O8 2− > IO4 − > BrO3 − > H2O2 > ClO3 −. Selvam et al. [9] found the order of enhancement, UV/TiO2/IO4 − > UV/TiO2/BrO3 − > UV/TiO2/S2O8 2− > UV/TiO2/H2O2 > UV/TiO2/ClO3 −. Yu et al. [7] demonstrated that in UV/TiO2/oxidant systems, decolorization rate constants of C.I. reactive black 5 (RB5) varied with oxidant following the order NaIO4 > Na2S2O8 > NaBrO3 > H2O2 > absence of oxidant. Although several studies have examined the effects of adding oxidant to UV/photocatalyst systems, few data are available on the degradation ability of UV/oxidant systems. Hence, the decolorization performance of RR198 by H2O2, Na2S2O8, NaBrO3, and NaIO4 under UV irradiation was evaluated. The goals of this study were to (i) determine the influences of oxidant dosage; (ii) compare the efficiencies of 254 and 365 nm irradiation, (iii) evaluate the inhibitions of adding ethanol to UV/oxidant systems, and (iv) calculate the synergistic effects of NaIO4 addition in UV/TiO2 system.
Experimental
Materials
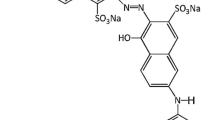
The parent compound, RR198, was obtained from Aldrich Chemical Company, and was used without further purification. The formula, molecular weight and maximum wavelength of light absorption of RR198 were C27H18ClN7Na4O15S5, 967.5 g/mol and 520 nm, respectively. Fig. 1 shows the chemical structure of RR198. TiO2 (Degussa P-25) was used directly without treatment. The crystal phases of Degussa P-25 were anatase/rutile at a ratio of 3/1. The specific surface area, average particle size and pH of zero point of charge (pHzpc) of Degussa P-25 were 50 m2/g, 30 nm and 6.6 [10]. H2O2, Na2S2O8, NaBrO3, and NaIO4 were applied as oxidants to evaluate the decolorization efficiency. The hydroxyl radical scavenger was ethanol (C2H5OH). The pH of the solution was controlled by adding HNO3 and NaOH via an automatic titrator. HNO3, NaOH, H2O2, Na2S2O8, NaBrO3, NaIO4, and ethanol were obtained from Merck. All reagents were of analytical grade and used as purchased.
Decolorization experiments
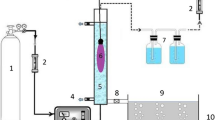
The RR198 concentration was 20 mg/L in all experiments. The dosage of TiO2 in UV/TiO2 and UV/TiO2/NaIO4 systems was 1 g/L. The solution was maintained at pH 7 during the reaction. Decolorization experiments were conducted in a 3 L hollow cylindrical glass reactor. The inner tube was made of quartz, and an 8 W, 254 nm or 365 nm UV-lamp (Philips) was placed inside the tube as the source of irradiation. In the ethanol addition experiments, 1,200 mg/L ethanol was added. All of the systems were stirred continuously at 300 rpm and the temperature was controlled at 25 °C. A 15-mL aliquot was withdrawn from the photoreactor at pre-specified intervals. The RR198 concentration was measured using a spectrophotometer (Hitachi U-2001) at 520 nm. The decolorization efficiency was calculated from the difference between the dye concentrations before and after the experiment.
Results and discussion
Effects of oxidant dosage
Figs. 2 and 3 plot the effects of Na2S2O8 dosage in a UV/Na2S2O8 system under 254 and 365 nm irradiation. For both wavelengths, 254 and 365 nm, the experimental results show that the decolorization rate constant (k) increased with the dose of Na2S2O8 (Table 1). The k values of RR198 in the UV/oxidant systems followed pseudo-first order kinetics, and various works have found that dye decolorization rates can generally be approximated using pseudo-first order kinetics [7, 11–15]. Ivanov et al. [16] indicated that persulfate ions undergo photolysis under light irradiation, forming sulfate ion radicals (Eq. 1). Sulfate ion radicals react with water molecules to generate hydroxyl radicals (HO•) (Eq. 2) [17]. According to Eqs. 1 and 2, increasing the amounts of S2O8 2− increased the additional sulfate ion radicals and HO•, accelerating decolorization. Since the k values increased with the dose of Na2S2O8, the effects of HO• scavenging by S2O8 2− were not observed herein (Eq. 3). Peternel et al. [18] indicated the optimal operating conditions of UV/S2O8 2− to decolorize C.I. reactive red 45 were pH 5 to pH 7 and [S2O8 2−] = 15 mM.
In a UV/H2O2 system, the decolorization efficiency increases with the H2O2 concentration from 1 to 6 mM; however, at concentrations >6 mM, no further improvement occurred (Table 1). Eq. 4 describes the reaction of the UV/H2O2 system. Moreover, H2O2 may act as a HO• scavenger to form hydroperoxyl radicals, which detrimentally affect photolysis (Eq. 5). Numerous works have indicated that the rate of degradation of organic compounds increases with H2O2 concentration up to a threshold; as the H2O2 concentration increases further, the degradation efficiency declines as H2O2 scavenges HO•, when H2O2 is present at a high concentration [13, 19, 20], generating hydroperoxyl radicals, which have lower oxidation capacity than hydroxyl radicals. Accordingly, the dose of H2O2 in the UV/H2O2 system must be carefully controlled.
Under 254 nm irradiation, the k values obtained by adding 1, 3, 6, 12, and 24 mM NaBrO3 to UV/NaBrO3 were 2.38, 7.34, 13.37, 24.71, and 35.68 h−1 (Table 1). Zuo and Katsumura [21] investigated the mechanisms of UV/NaBrO3, as in Eqs. 6–11. However, the scavenging of HO• by BrO3 − was not identified herein (Eq. 11). Hence, the k values increased with the dose of NaBrO3 (Table 1).
Weavers et al. [5] proposed Eq. 10 and Eqs. 12–16 as UV/NaIO4 mechanisms. The high activity of UV/IO4 − is attributable to the formation of numerous highly reactive radicals (O−•, HO•, IO •3 , and IO •4 ) and non-radical (O3, IO4 −, and IO3 −) intermediates. Increasing the concentration of IO4 − increases the number of radicals formed. Under 254 nm irradiation, the k value increases with NaIO4 dose up to a threshold (3 mM); as the NaIO4 dose increases beyond this threshold, the degradation efficiency declines as NaIO4 scavenges HO• (Eq. 16) when NaIO4 is present at a high concentration (Table 1). However, the scavenging of HO• by IO4 − was not observed under 365 nm irradiation. Hence, the k values increased with the NaIO4 dose. Lee and Yoon [22] found that the k values of RB5 increased with low IO4 − concentrations (0–5 mM), but slightly fell as the concentration of IO4 − increased to high values (>5 mM) in a UV/NaIO4 system. The excess IO4 − ions in a solution may scavenge HO•, which may attack dye; therefore, the decolorization rate declined when a high concentration of IO4 − was added [22].
Effects of UV wavelength and ethanol addition
The wavelength of UVA and UVC was 320–400 and 200–290 nm, respectively. This study selected 254 and 365 nm UV light to clarify the effects of UVA and UVC in UV/TiO2 and UV/oxidant systems. All oxidants that were adopted herein can be photolyzed under 254 nm irradiation; however, only Na2S2O8 and NaIO4 can be photolyzed by 365 nm irradiation (Table 1). The k value of 254 nm exceeded that of 365 nm. Lo et al. [3] and Lin et al. [4] both indicated that the photodegradation efficiency of 4-chlorophenol increased with a decrease in light wavelength. Additionally, the k values of C.I. reactive red 2 decolorization by 254 nm irradiation exceeded those by 365 nm in the photo-fenton and photo-Fenton-like systems [23]. The dosage of H2O2 and NaIO4 has a threshold value, suggesting that the HO• was scavenged by excess oxidant under 254 nm irradiation; however, no such scavenging occurred under 365 nm irradiation. This work suggests that the UV wavelength affected not only the decolorization efficiency but also the optimal dosage of oxidant.
Ethanol is known to act as an HO• scavenger [11]. Buxton et al. [24] suggested the mechanism on the HO• scavenger was as Eq. 17. In the experiments of ethanol addition, the concentration of ethanol and oxidant was 26 and 1 mM. On a mole basis, the amount of added ethanol must exceed the amount of oxidant in order to suppress the generated reactive free radicals; therefore, a high ethanol dose (1,200 mg/L) was used. Notably, adding ethanol reduced the decolorization rate in UV/oxidant systems (Fig. 4). At 1,200 mg/L ethanol addition, the k values for UV/H2O2 and UV/Na2S2O8 systems (Table 1) were approximately 100 times less than those in the absence of ethanol, revealing that the decolorization proceeded mainly via HO•. However, adding 1,200 mg/L ethanol to UV/NaBrO3 and UV/NaIO4 systems does not completely prevent decolorization. This experimental finding suggests that another reactive species, which does not react with ethanol, participates in decolorization. This species is most likely one of the BrO•, BrO •2 , and BrO •3 that are formed in UV/NaBrO3 and the O−•, IO •3 , IO •4 , and O3 that are produced in UV/NaIO4.
Effects of added NaIO4 in UV/TiO2 system
Under both of 254 and 365 nm irradiation, decolorization rates of RR198 followed the order UV/TiO2/NaIO4 > UV/NaIO4 > UV/TiO2 (Table 2). Adding inorganic oxidants increased the rate of decolorization by various means, such as, (i) prevention of electron–hole recombination via capturing conduction band electrons; (ii) increased formation of hydroxyl radicals formed, and (iii) production of other species that oxidize intermediate compounds [7]. The net effect is to increase the rate of formation of hydroxyl radicals and, thus, the effective rate of degradation of RR198, according to Eq. 18 [5, 6, 25]. Numerous studies also found that the rate of photodegradation using UV/TiO2/IO4 − exceeded that using UV/TiO2 [6, 9, 25, 26].
Conclusion
The decolorization efficiency of RR198 in UV/H2O2, UV/Na2S2O8, UV/NaBrO3, and UV/NaIO4 systems was determined. All oxidants can be photolyzed by 254 nm irradiation; however, only Na2S2O8 and NaIO4 can be photolyzed by 365 nm irradiation. At 1 mM oxidant addition, the k values followed the order UV/NaIO4 > UV/Na2S2O8 > UV/H2O2 > UV/NaBrO3. Experimental results indicate that the k values increased with the dose of Na2S2O8 and NaBrO3 under 254 nm irradiation. This study suggests that HO• dominate decolorization in UV/H2O2 and UV/Na2S2O8 systems. Additionally, BrO•, BrO •2 , and BrO •3 , which are produced in UV/NaBrO3, and O−•, IO •3 , IO •4 and O3, which are produced in UV/NaIO4, are important reactive species in UV/NaBrO3 and UV/NaIO4 systems. Addition of NaIO4 to UV/TiO2 resulted in quenching of conduction band electrons and generation of reactive radical intermediates, enhancing decolorization of RR198.
References
Serpone N, Maruthamuthu P, Pichat P, Pelizzetti E, Hidaka H (1995) Exploiting the interparticle electron transfer process in the photocatalysed oxidation of phenol, 2-chlorophenol and pentachlorophenol: chemical evidence for electron and hole transfer between coupled semiconductors. J Photochem Photobiol A 85:247–255
Bojinova A, Dushkin C (2011) Photodegradation of malachite green in water solutions by means of thin films of TiO2/WO3 under visible light. Reac Kinet Mech Cat 103:239–250
Lo SC, Lin CF, Wu CH, Hsieh PH (2004) Capability of coupled CdSe/TiO2 for photocatalytic degradation of 4-chlorophenol. J Hazard Mater 114:183–190
Lin CF, Wu CH, Onn SN (2008) Degradation of 4-chlorophenol in TiO2, WO3, SnO2, TiO2/WO3 and TiO2/SnO2 systems. J Hazard Mater 154:1033–1039
Weavers LK, Hao I, Hofmann MR (1997) Degradation of triethanolamine and chemical oxygen demand reduction in wastewater by photoactivated periodate. Water Environ Res 69:1112–1119
Irmak S, Kusvuran E, Erbatur O (2004) Degradation of 4-chloro-2-methylphenol in aqueous solution by UV irradiation in the presence of titanium dioxide. Appl Catal B 54:85–91
Yu CH, Wu CH, Ho TH, Hong PKA (2010) Decolorization of C.I. Reactive Black 5 in UV/TiO2, UV/oxidant and UV/TiO2/oxidant systems: a comparative study. Chem Eng J 158:578–583
Syoufian A, Nakashima K (2008) Degradation of methylene blue in aqueous dispersion of hollow titania photocatalyst: study of reaction enhancement by various electron scavengers. J Colloid Interface Sci 317:507–512
Selvam K, Muruganandham M, Muthuvel I, Swaminathan M (2007) The influence of inorganic oxidants and metal ions on semiconductor sensitized photodegradation of 4-fluorophenol. Chem Eng J 128:51–57
Wu CH (2007) Photodegradation of toluic acid isomers by UV/TiO2. React Kinet Catal Lett 90:301–308
Daneshvar N, Salari D, Khataee AR (2004) Photocatalytic degradation of azo dye acid red 14 in water on ZnO as an alternative catalyst to TiO2. J Photochem Photobiol A 162:317–322
Wu CH, Chang CL (2006) Decolorization of Procion Red MX-5B by advanced oxidation processes: comparative studies of the homogeneous and heterogeneous systems. J Hazard Mater 128:265–272
Wu CH (2008) Decolorization of C.I. reactive red 2 in O3, fenton-like and O3/fenton-like hybrid systems. Dyes Pigments 77:24–30
Wu CH (2009) Photodegradation of C.I. reactive red 2 in UV/TiO2-based systems: effects of ultrasound irradiation. J Hazard Mater 167:434–439
Curkovic L, Ljubas D, Juretic H (2010) Photocatalytic decolorization kinetics of diazo dye congo red aqueous solution by UV/TiO2 nanoparticles. Reac Kinet Mech Cat 99:201–208
Ivanov KL, Glebov EM, Plyusnin VF, Ivanov YV, Grivin VP, Bazhin NM (2000) Laser flash photolysis of sodium persulfate in aqueous solution with addition of dimethylformamide. J Photochem Photobiol A 133:99–104
Konstantinou IK, Albanis TA (2004) TiO2-assisted photocatalytic degradation of azo dyes in aqueous solution: kinetic and mechanistic investigations—a review. Appl Catal B 49:1–14
Peternel I, Grcic I, Koprivanac N (2010) Degradation of reactive azo dye by UV/peroxodisulfate system: an experimental design approach. Reac Kinet Mech Cat 100:33–44
Kusic H, Koprivanac N, Bozic AL, Selanec I (2006) Photo-assisted fenton type processes for the degradation of phenol: a kinetic study. J Hazard Mater 136:632–644
Tokumura M, Ohta A, Znad HT, Kawase Y (2006) UV light assisted decolorization of dark brown colored coffee effluent by photo-fenton reaction. Water Res 40:3775–3784
Zuo Z, Katsumura Y (1998) Formation of hydrated electron and BrO •3 radical from laser photolysis of BrO3 − aqueous solution. J Chem Soc Faraday Trans 94:3577–3580
Lee C, Yoon J (2004) Application of photoactivated periodate to the decolorization of reactive dye: reaction parameters and mechanism. J Photochem Photobiol A 165:35–41
Wu MC, Wu CH, Hong PKA, Jian MY (2011) Decolorization of C.I. reactive red 2 in fenton and fenton-like systems: effects of ultrasound and ultraviolet irradiation. Chem Eng J (revised)
Buxton GV, Greenstock CL, Helman WP, Ross AB (1988) Critical review of rate constants for reactions of hydrated electrons, hydrogen atoms and hydroxyl radicals (•OH/•O−) in aqueous solution. J Phys Chem Ref Data 17:513–759
Sadik WA (2007) Effect of inorganic oxidants in photodegradation of an azo dye. J Photochem Photobiol A 191:132–137
Gozmen B, Turabik M, Hesenov A (2009) Photocatalytic degradation of basic red 46 and basic yellow 28 in single and binary mixture by UV/TiO2/periodate system. J Hazard Mater 164:1487–1495
Acknowledgments
The authors would like to thank the National Science Council of the Republic of China, Taiwan, for financially supporting this research under contract no. NSC 98-2221-E-212-002-MY3. Ms. Jhan of Da-Yeh University is appreciated for performing some of the experiments.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wu, MC., Wu, CH. Decolorization of C.I. reactive red 198 in UV/oxidant and UV/TiO2/oxidant systems. Reac Kinet Mech Cat 104, 281–290 (2011). https://doi.org/10.1007/s11144-011-0346-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-011-0346-8