Abstract
Fresh button mushrooms (Agaricus bisporus) were harvested and treated with a solution of 1.5% CaCl2 + 0.5% citric acid and stored for 16 days at 12 °C. The effects of this treatment on firmness, weight, color, cell wall compositions (cellulose and chitin) and cell wall degrading enzymes (cel1ulase, beta-1, 3 glucanase, chitinase and phenylalanine ammonialyase) were investigated during post-harvest storage. The expressions of major genes (Cel1, Glu1, Chi1 and PAL1) involved in cell wall degradation during post-harvest storage were also monitored. The results revealed that the post-harvest chemical treatment maintained better firmness, weight, color and inhibited cellulase, beta-1, 3 glucanase, chitinase and phenylalanine ammonialyase activities. These findings showed that the down-regulation of cell wall degrading enzymes is a possible mechanism that delays the softening of button mushrooms by the application of combined chemical treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Among the mushrooms, button mushroom (Agaricus bisporus) has significant importance due to its nutritional, medicinal and organoleptic properties [1]. It has been observed that mushrooms have 3 to 4 days shelf life at room temperature and up to 8 days under refrigerator conditions [2]. High respiration rate, dehydration and fast metabolic activities are the underlying reasons for a short shelf life of mushrooms [3]. Softening in button mushrooms may also have occurred by bacterial enzymes and endogenous autolysins that degrade the cell wall during storage [4]. The changes in cell wall composition mainly affect the texture of the mushroom. Fresh and stored mushrooms can easily be identified on the basis of deterioration in cell wall [5]. The mushroom has no pectin layer, therefore the textural changes are directly linked to modification in cell wall components [6]. Beta-1, 3 and beta-1, 6-glucans are present at the surface of cells, and act as an amorphous matrix in the cell wall [7], while chitin forms a layer that increases their strength. After harvesting, beta-1, 3 glucanase, beta-1, 6 glucanase, chitinase and chitosanase have been reported as key enzymes involved in the deterioration of the cell wall of mushrooms; however, it has been reported that phenylalanine ammonialyase (PAL) is responsible for the degradation of color and quality in mushrooms [8]. High expression of the PAL gene increases PAL activity during the accumulation of PAL mRNA which in turn leads to browning and deterioration in the quality of mushrooms [9]. Different preservation methods i.e., low temperature, thermal (physical treatment) and many others have been used to slow down the process of degradation in mushrooms as well as in other food products [10–12]. Recently, chemical pre-treatments for mushroom preservation have been reported [13, 14]. These chemical pre-treatments had significant effect on expression of important cell wall degrading genes that caused softening [15]. However, to the best of our knowledge; little or no information is available for chemicals likewise CaCl2 and citric acid in post-harvest storage. Hence, this work seeks to evaluate the effects of post-harvest chemical treatment on button mushroom firmness, through analysis of the relationships between texture change and expression of genes as well as enzymatic activities in deterioration of button mushroom.
Materials and Methods
Samples Preparation, Treatment, Storage, Weight, Color and Texture
Mushrooms for the experiment were selected on the basis of same size, color and with no physical damage. The selected mushrooms were then divided into two groups. One of the groups was dipped in water and used as a control (CK) while another group was dipped in a chemical solution [1.5% CaCl2 (w/v) and 0.5% citric acid (w/v)] of the same volume for two and half minutes at room temperature (25 °C) and was labeled as (T). After the treatments, samples were kept in open air for 1 h. Each treated sample was then divided into five subsamples with the average weight of 99 ± 2 g. The subsamples were packed in polyethylene bags without being tightly closed and stored at 12 °C while the humidity (RH) was kept at about of 90 ± 5% for 16 days in storage chamber. Loss in weight was measured as a percentage loss (%). Mushroom caps were used for the color and texture determination. WSC-S colorimeter (Shanghai precision instrument Co. Ltd., Shanghai, China) was used for color, and penetration test was performed for firmness, using a TA.XT2i texture analyzer (Stable Micro Systems, UK) - a 5 mm diameter cylindrical probe. The procedures were same as reported in our previous work [16].
Determination of Cellulose and Chitin
1 g for cellulose and 5 g for chitin determination, mushrooms tissue was used according to the method described in [4, 17].
Cellulase, Beta-1,3 Glucanase, Chitinase, and Phenylalanine Ammonialyase (PAL) Activities
Mushrooms cap tissues were used for the enzymatic study. A 1.5 g sample was ground with 3 mL of 0.1 M sodium phosphate buffer (pH 7.0) at 4 °C. The mixture was centrifuged, the supernatant was collected and used for measurement of enzymatic activities of cellulase, beta-1,3 glucanase, chitinase, and PAL according the methods described by [18–21].
RNA Extraction, cDNA Preparation and qPCR
The RNAiso Plus (D9108B, TaKaRa, Japan) kit was used for RNA isolation by following the instructions of the manufacturer and the quantity of RNA was determined by a Nano drop spectrophotometer (Nano Drop 2000,Thermo-Fisher scientific Inc., Wilmington, DE, USA). The RNA concentration was calculated and then reverse-transcribed to a single-strand cDNA using the RNA PCR kit (codeDRR047A, TaKaRa) following the protocol and the cDNA was further used for RT-PCR analysis with an ABI Step One RT-PCR system.
Results and Discussion
A gradual loss of weight was observed with storage time in both treated samples (T) as well as in control samples (CK), as is shown in Table 1. Maximum weight loss (7.2%) was observed in the CK samples during the post-harvest storage. The T samples showed significantly low weight loss (p < 0.05) as compared to the CK during the storage. The effects of post-harvest chemical treatment on button mushrooms have been previously studied in our laboratory [14]. It has already been reported that loss in weight affects the quality of food products as well as economics values, while dehydration is considered as key parameter in weight loss during post-harvest storage [5]. Our study showed that the application of the treatment delayed percentage weight loss. The reduction in weight loss was probably because of the CaCl2 as it has a rigidifying effect on the cell wall. Citric acid also acted as a chelating agent that prevented dehydration. This conclusion was supported by the research of Jafri et al. [13], who used chemical treatment in combination with modified atmosphere packaging (MAP). Appearance is usually the main criteria for selection. Thus, processing companies, supermarkets and consumers insist on buying white mushrooms with good looks and flavors. There was no significant difference in whiteness between the T samples and the CK samples during the first four days, but after this a sharp decrease was observed in the L* values in the CK samples. The T samples showed significantly (p < 0.05) higher L* values at 8, 12 and 16 days as compared to the CK samples (Table 1). The final whiteness of the CK (81.2) may not be acceptable for consumers since it is too close to the acceptance threshold of 80 [22]. T samples maintained better color as compared to the CK samples. There were significantly higher L* values in the T samples which may be due to post-harvest chemical treatment, because CaCl2 played a key role in maintaining good structure by sticking to the cell wall and in reducing enzymatic browning [23]. Furthermore, citric acid has metal chelating properties that can reduce oxidation [10]. These results showed that the treatment has a good potential to maintain the color of mushrooms for more days as compared to the CK samples. In this study, regular softening was observed in both the T and CK samples during the post harvesting storage (Table 1). However, a significant difference was observed between the T samples and the CK samples. On the 4th day, the T samples showed significantly higher firmness (p < 0.05) as compared to the CK. This study shows that combined chemical treatment (CaCl2 and citric acid) has a good effect on harvested button mushrooms and treated mushrooms maintained their firmness. Texture being the chief attribute of button mushrooms is not only important from qualitative point of view, but is also important for consumers in terms of selection [3, 16]. The present study demonstrated that the treatment was effective in preventing softening of button mushrooms during post-harvest storage. The ability of the CaCl2 to stick to the vacuole and strengthen the cell wall, which protects the structure of the mushrooms, was revealed in [23]. Previously, we used post-harvest chemical treatment for the preservation of button mushrooms and observed better firmness as compared to the CK [14]. Our results were consistent with findings [24], who used CaCl2 and citric acid for freshly-cut papaya. A general increase of cellulose contents was observed in both T and CK samples. Significant difference was observed in cellulose content between the T and CK samples during post-harvest storage. The treatment significantly increased cellulose contents as compared to the CK on 4, 8, 12 and 16 days of post-harvest storage (Table 1). This study showed that the treatment has the ability to inhibit the activities of cell wall degrading enzymes which have a negative effect on the firmness of mushrooms. Mushroom texture is directly affected by modifications in the cell wall compositions such as cellulose, chitin, polysaccharide and protein [5]. In the present work, it was observed that there was a significant difference in cellulose content between the T and CK samples and a general increase was observed in the T samples. The increased cellulose content in the T samples may be helpful in maintaining the firmness of button mushrooms. There was a continuous decrease in chitin content in both samples with no significant difference (p < 0.05) from 0 to 12 days, however, on 16 days of storage the T samples showed significantly higher chitin contents than the CK samples (Table 1). As a whole, higher chitin contents resulted as compared to the CK during the storage period. The T samples maintained higher chitin content. The higher chitin content in the T samples was helpful in maintaining the firmness of button mushrooms and these results were in agreement with the previous study [5].
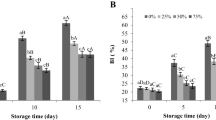
Enzymes which are involved in cell wall degradation are discussed in [25]. Beta-1, 3 glucanase, cellulase and chitinase are the key enzymes that are directly linked with the cell wall degradation [10]. It was noticed that in the initial days of storage, the activity of cellulase increased in both the CK and T samples, but in later days a gradual decrease was observed in both of the samples (Fig. 1a). There was no significant difference (p < 0.05) from 0 to 8 days between the CK and T samples, but on 12 and 16 days of storage the T samples showed significantly lower (p < 0.05) cellulase activity than the CK samples. In this study, cellulase activity gradually increased in the initial stage of softening but in later days it was decreased in both of the samples. The increase of cellulase activity in the CK samples coincided with the firmness decline. Furthermore, the low cellulase activity by the treatment was related to the preservation of mushroom firmness; these results were consistent with another study found in [26]. There was a gradual increase in beta-1, 3 glucanase activities in the first eight days of storage and then a continuous decrease was observed in both treated and untreated samples. As a whole, the T samples showed a lower level of beta-1, 3 glucanase activity, and there was also a significantly higher (p < 0.05) beta-1, 3 glucanase activity on 8 and 16 days in the T samples (Fig. 1b). It has been observed that beta-1, 3 glucanase plays a part in lentinan degradation, and that beta-1, 3 glucanase plays an important role in the cell wall degradation during post-harvest storage of mushrooms [10]. It was observed that beta-1, 3 glucanase activity increased during the initial days of storage and maximum activity was observed in the middle period of post-harvest storage (8 days) in the CK and T samples. As a whole, the treatment maintains lowered activity of beta-1, 3-glucanase activity. The higher beta-1, 3 glucanase activity in the CK samples may be responsible for texture degradation, while low beta-1, 3 glucanase in the T samples assisted in maintaining the firmness of button mushrooms. Chitinase activity in the control mushroom samples increased significantly and reached a peak value on the 4th day, and then a decrease was observed on the 8th day. Although there was an increase of chintinase activity in both CK and T samples, the treatment resulted in significantly lower chitinase activity (p < 0.05) (Fig. 1c). This investigation has similarities with the research on plums carried out by Manganaris et al. [27] which was treated with 1-methylcyclopropene. Chitin is the key component of a fungal cell wall, and chitinase is responsible for chitin degradation. It has been reported that chitinase is responsible for chitin in mushrooms [2]. In our investigation, chitinase activity increased in both of the samples with the passage of storage time, but in the T samples it was observed to be low in concentration. [28] reported an increase inactivity of chitinase during the deterioration of mushrooms. Increased chitinase activity was responsible for degradation of chitin in fungi cell wall, thus resulting in a softening of button mushrooms. There was an increase in PAL activity in both the T and CK samples from 0 to 8 days of storage but in later days a decrease was noticed in its activity (Fig. 1d). In this investigation, it was observed that the T samples maintained significantly lower values of PAL activity as compared to the CK samples on 4, 8 and 12 days but there was no significant difference on 0 and 16 days. In the browning process, phenylalanine ammonialyase serves as a first-step reaction in which phenylalanine is degraded to ammonia and trans-cinnamic acid [28]. In this study, it was noticed that the treatment has the ability to inhibit PAL activity during the post-harvest storage. There was a normal relationship between PAL activity and firmness. It has been documented that any treatment which can inhibit PAL activity and prevent free phenol compounds accumulation has also the ability to maintain firmness [24]. Reduction in PAL activity in the T samples as compared to the CK samples maybe involved in maintaining color of mushrooms during post-harvest storage. Ca ions have the ability to reduce PAL activity [28] and citric acid also helps to maintain better firmness and reduce the browning process due to its chelating properties [10].
Generally, mRNA concentrations of Cel1, Glu1 and Chi1 genes in the T samples were not significantly changed as compared to the CK in the present study, (Fig. 2a, b, c). However, the treatment significantly (p < 0.05) inhibited the expression of PAL gene as compared to the CK from 4 to 12 days of storage (Fig. 2d). The effects of CaCl2 and citric acid treatment on mRNA expression of four genes encoding the cell wall degrading enzymes (Fig. 2) were analyzed. According to the study carried out by [26], Cel1, Cel2 and Cel3 genes encode cellulase enzyme, which are directly involved in cellulose degradation. Lentinan is an important cell wall component that is degraded by increased glucanase activity during post-harvest storage. Glu1 gene encoding glucanase is involved in mushroom cell wall degradation [8]. Chi1 gene up-regulation expression induces the autolysis process of mushrooms. It is assumed that chitinase is responsible for mushroom cell wall degradation [9]. It was noticed that the treatment did not significantly alter the Cel1, Glu1 and Chi1 gene expression in most of post-harvest storage days. However, the treated samples maintained good firmness as compared to the CK samples; this may be due to the Ca ions that help to protect the cell from enzymatic attacks and to citric acid which reduces the oxidation process. However, suppression of PAL mRNA was observed in the treated samples as compared to the CK. [27] found that lower PAL mRNA was directly associated with PAL activity suppression. PAL1 gene is involved in tissue browning and phenolic compound accumulation. This is the precursor of semi-cellulose and lignin, which affects the firmness of mushrooms. These results suggested that post-transcriptional regulation could be the major mechanism of the treatment in suppressing these enzyme activities.
Conclusion
In conclusion, we have observed that post-harvest chemical treatment delays the softening of button mushrooms during post-harvest storage. This study showed the relationships among firmness change, variations in cell wall composition, activities of main cell wall degrading enzymes and expression of key encoding genes. Although Cel1, Glu1 and Chi genes were not significantly different at most of the storage days, it was observed that the cell wall degrading enzymes like cellulase, beta-1,3 glucanase, and chintinase were down-regulated, and the firmness in button mushrooms was better maintained. This might be one of the mechanisms of post-harvest chemical treatment in delaying softening and maintaining firmness of button mushrooms.
Abbreviations
- PAL:
-
Phenylalaine ammonialyse
- CK:
-
Control sample
- T:
-
Treated sample
- MAP:
-
Modified atmospheric packing
- w/v :
-
Weight/Volume
- qPCR:
-
Quantitative polymerase chain reaction
- cDNA:
-
Complimentary deoxyribonucleic acid
- RNA:
-
Ribonucleic acid
References
Beelman RB, Royse DJ, Chikthimmah N (2003) Bioactive components in button mushroom (Agaricus bisporus) (J. Lge) Imbach (Agaricomycetideae) of nutritional, medicinal, and biological importance (Review). Int J Med Mush 5(4):321–327
Kamada T, Hamada Y, Takemaru T (1982) Autolysis in vitro of the stipe cell wall in Coprinus rnacrorhizus. J Gen Microbiol 128:1041–1046
Ares G, Lareo C, Lema P (2007) Modified atmosphere packaging for postharvest storage of mushrooms. A review. Fresh Produce 1(1):32–40
Zivanovic S, Busher R, Kim K (2000) Textural changes in mushrooms (Agaricus bisporus) associated with tissue ultrastructure and composition. J Food Sci 65:1404–1408
Jiang T, Wang Q, Xu S, Jahangir MM, Ying T (2010) Structure and composition changes in the cell wall in relation to texture of shiitake mushrooms (Lentinula edodes) stored in modified atmosphere packaging. J Sci Food Agric 90:742–749
Zivanovic S, Buescher R, Kim S (2003) Mushroom texture, cell wall composition, color, and ultrastructure as affected by pH and temperature. J Food Sci 68:1860–1865
Wessels J, Mol P, Sietsma J, Vermeulen C (1990) Wall structure, wall growth, and fungal cell morphogenesis, biochemistry of cell walls and membranes in fungi. Springer, pp 81–95.
Sakamoto Y, Nakade K, Konno N, Sato T (2012) Senescence of the Lentinula edodes fruiting body after harvesting. Food quality. InTech North America, New York
Rosales R, Fernandez-Caballero C, Romero I, Escribano MI, Merodio C, Sanchez-Ballesta MT (2013) Molecular analysis of the improvement in rachis quality by high CO2 levels in table grapes stored at low temperature. Postharvest Biol Technol 77:50–58
Brennan MH, Gormley RT (1998) Extending the shelf life of fresh sliced mushrooms. The National Food Centre Research Report No. 2. Martine H. Brennan and T. Ronan Gormley. Dublin; Teagasc, ISBN 1901138402
Paul U, Juan V, Jette J (2016) Impact on vitamin D2, vitamin D4 and Agaritine in Agaricus bisporus mushrooms after artificial and natural solar UV light exposure. Plant Foods Hum Nutr 71:314–321
Kang MK, Jeong HC, Mosbah MK, Elizabeth HJ, John AJ (2013) Pre-harvest methyl jasmonate treatment enhances cauliflower chemoprotective attributes without a loss in postharvest quality. Plant Foods Hum Nutr 68:113–117
Jafri M, Jha A, Bunkar DS, Ram RC (2013) Quality retention of oyster mushrooms (Pleurotus florida) by a combination of chemical treatments and modified atmosphere packaging. Postharvest Biol Technol 76:112–118
Khan ZU, Bu J, Khan NM, Khan RU, Jiang Z, Mou W, Lou Z, Mao L, Ying T (2014) Integrated treatment of CaCl2, citric acid and sorbitol reduces loss of quality of button mushroom (Agaricus bisporus) during postharvest storage. J Food Process Preserv 39:2008–2016. doi:10.1111/jfpp.12441
Figueroa CR, Opazo MC, Vera P, Arriagada O, Diaz M, Moya-Leon MA (2012) Effect of postharvest treatment of calcium and auxin on cell wall composition and expression of cell wall-modifying genes in the Chilean strawberry (Fragaria chiloensis) fruit. Food Chem 32:2014–2022
Khan ZU, Aisikaer G, Khan RU, Bu J, Jiang Z, Ni Z, Ying T (2014) Effects of composite chemical pretreatment on maintaining quality in button mushrooms (Agaricus bisporus) during postharvest storage. Postharvest Biol Technol 95:36–41
Dm U (1969) Semimicro determination of cellulose in biological materials. Anal Biochem 32(3):420–424
Durbin ML, Lewis LN (1988) Cellulases in Phaseolus vulgaris. Methods Enzymol 160:342–351
Pan SQ, Ye XS, Kuc J (1991) Association of beta-1,3-glucanase activity and isoform pattern with systemic resistance to blue mold in tobacco induced by stem injection with Peronospora tabacina or leaf inoculation with tobacco mosaic virus. Physiol Mol Plant Pathol 39:25–39
Boller T, Mauch F (1988) Colorimetric assay for chitinase. Methods Enzymol 161:430–435
Koukol J, Conn EE (1961) The metabolism of aromatic compounds in higher plants. IV. Purification and properties of the phenylalanine deaminase of Hordeum vulgare. J Biol Chem 236:2692–2698
Briones GL, Varoquaux P, Chambroy Y, Bouquant J, Bureau G, Pascat B (1992) Storage of common mushroom under controlled atmospheres. Int J Food Sci Technol 27:493–505
Koushki M, Abras SK, Mohammadi M, Hadian Z, Poorfallah NB, Sharayei P, Mortazavian AM (2011) Physicochemical properties of mushrooms as affected by modified atmosphere packaging and CaCl2 dipping. Afr J Agric Res 6(24):5414–5421
Waghmare RB, Annapure US (2013) Combined effect of chemical treatment and/or modified atmosphere packaging (MAP) on quality of fresh-cut papaya. Postharvest Biol Technol 85:147–153
Brummell DA, Harpster MH (2001) Cell wall metabolism in fruit softening and quality and its manipulation in transgenic plants. Plant Mol Biol 47:311–340
Eissa HAA (2007) Effect of chitosan coating on shelf life and quality of fresh-cut mushroom. J Food Qual 30:623–645
Manganaris GA, Vicente AR, Crisosto CH, Labavitch JM (2007) Effect of dips in a 1-methylcyclopropene-generating solution on 'Harrow Sun' plums stored under different temperature regimes. J Sci Food Agric 55:7015–7020
Artes F, Castaner M, Gil MI (1998) Review: enzymatic browning in minimally processed fruit and vegetables. Food Sci Technol Int 4:377–389
Acknowledgments
We are grateful for the financial supports of National Natural Science Foundation of China (Project Number: 31171766) and the Foundation for the Program of Key Innovative Research Team of Zhejiang Province (2009R50036).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors have no conflict of interest.
Electronic supplementary material
ESM 1
(DOCX 11 kb)
Rights and permissions
About this article
Cite this article
Khan, Z.U., Jiayin, L., Khan, N.M. et al. Suppression of Cell Wall Degrading Enzymes and their Encoding Genes in Button Mushrooms (Agaricus bisporus) by CaCl2 and Citric Acid. Plant Foods Hum Nutr 72, 54–59 (2017). https://doi.org/10.1007/s11130-016-0588-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11130-016-0588-8