Abstract
Commercial mushroom production can expose mushrooms post-harvest to UV light for purposes of vitamin D2 enrichment by converting the naturally occurring provitamin D2 (ergosterol). The objectives of the present study were to artificially simulate solar UV-B doses occurring naturally in Central Europe and to investigate vitamin D2 and vitamin D4 production in sliced Agaricus bisporus (button mushrooms) and to analyse and compare the agaritine content of naturally and artificially UV-irradiated mushrooms. Agaritine was measured for safety aspects even though there is no rationale for a link between UV light exposure and agaritine content. The artificial UV-B dose of 0.53 J/cm2 raised the vitamin D2 content to significantly (P < 0.001) higher levels of 67.1 ± 9.9 μg/g dry weight (DW) than sun exposure (3.9 ± 0.8 μg/g dry DW). We observed a positive correlation between vitamin D4 and vitamin D2 production (r2 = 0.96, P < 0.001) after artificial UV irradiation, with vitamin D4 levels ranging from 0 to 20.9 μg/g DW. The agaritine content varied widely but remained within normal ranges in all samples. Irrespective of the irradiation source, agaritine dropped dramatically in conjunction with all UV-B doses both artificial and natural solar, probably due to its known instability. The biological action of vitamin D from UV-exposed mushrooms reflects the activity of these two major vitamin D analogues (D2, D4). Vitamin D4 should be analysed and agaritine disregarded in future studies of UV-exposed mushrooms.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Mushrooms contain high levels of ergosterol (provitamin D2), the principal sterol in fungi, and on exposure to wavelengths <315 nm it is converted through a photochemical reaction to previtamin D2, followed by thermal isomerization to vitamin D2 [1]. In 2011, we published the first report demonstrating the efficient and safe consumption of artificially UV-irradiated vitamin D2-enriched Agaricus bisporus (white button mushrooms) in vitamin D-deficient humans [2]. A review of the safety assessment and a meta-analysis of randomised controlled trials confirmed that UV light-exposed mushrooms are safe and able to improve the vitamin D status in adults presenting low baseline status [3, 4]. Other published studies reported no significant changes in water-soluble vitamins, amino acids and fatty acids after the UV-B processing of mushrooms [5, 6].
The food safety regulations of the European Commission (EC) must be considered prior to employing artificial UV light to enrich the vitamin D2 content of commercially produced mushrooms. The use of artificial UV light falls under the EC’s regulatory control [Regulation EC No. 258/97] and applies to foods not previously used for human consumption prior to May 15, 1997 or to foods processed in a significantly different new way, thus constituting novel foods [7]. To comply with this regulation, the commercial production and introduction of vitamin D2-enriched mushrooms to the market place will require the demonstration of substantial equivalence between the two methods of vitamin D enrichment, namely artificial UV light and sunlight [3, 8].
Very few working groups have addressed the impact of sun exposure on vitamin D2 content in mushrooms or have even attempted to simulate natural conditions for indoor production. Phillips and Rasor [9] evaluated the reliability of a consumer-friendly protocol to increase the amount of vitamin D2 in sliced mushrooms via sun exposure at different durations, times of day and seasons. Kristensen et al. [10] investigated the effect of a single sun exposure duration on the vitamin D2 content of whole button mushrooms. Simon et al. compared changes in key nutritional and non-nutritional analytes in whole button mushrooms exposed to solar versus artificial UV irradiation [6]. A total of five replicate samples were exposed for 150 min to the sun on three different days, unfortunately without measuring the UV-B doses. They were the first to assess the agaritine content, finding that it did not change as a result of exposure to sunlight or artificial UV-B. In addition, Phillips et al. [11] demonstrated the omnipresence of the vitamin D4 precursor 22,23-dihydroergosterol in mushrooms and hypothesised the occurrence of vitamin D4 in UV-exposed mushrooms [11]. Agaritine [N-(gamma-L-glutamyl)-4-hydroxymethylphenylhydrazine] is an aromatic hydrazine found in mushrooms of the genus Agaricus. As in vitro and in vivo studies have shown that agaritine and its derivatives may be mutagenic [12, 13], it has been considered an undesirable substance and is mentioned in the Organisation for Economic Co-operation and Development (OECD) consensus document on compositional considerations for new varieties of button mushrooms [14]. Hence, data on agaritine are of interest, even though there is no rationale for a link between UV light exposure and its content.
A subtask of the EC-funded ODIN project consortium (food-based solutions for optimal vitamin D nutrition) consists of gathering convincing evidence of the substantial equivalence of button mushrooms exposed to artificial UV light and sunlight, as there is currently a paucity of data on this subject [15]. Our sun exposure experiments were done on a single day around summer solstice during the most abundant natural UV-B (280–315 nm) irradiance immediately next to a high performance spectroradiometer for real-time UV-B and UV-A (315–380 nm) data run by the German Solar UV Monitoring Network (Oberried, Germany, under clear sky conditions, 47.91 °N, 1205 m above sea level). The first part of the sun exposure substudy has been published, addressing the measurement of the dose-response to UV-B from sunlight and vitamin D2 content in frozen button mushrooms of different slice thicknesses during the longstanding conversation process of sun-drying mushrooms [16].
The objectives of the present study are fourfold: to artificially simulate with UV-B tubes the solar UV-B doses naturally occurring in Central Europe, measured during the sun-exposure substudy [16]; to analyse and compare the agaritine content of naturally and artificially UV-irradiated white button mushrooms; to compare the vitamin D2 content of artificially UV-irradiated mushrooms with published data from the sun-exposure substudy [16]; to investigate the vitamin D4 production during artificial UV irradiation.
Materials and Methods
Experimental Design
A total of two batches of mushrooms were used, one for the sun-exposed and one for the artificially irradiated mushroom samples, because both experiments were conducted at a 2-month interval since planning the artificial simulation of the solar UV-B doses depended on our results from the outdoor dose-response experiments. Apart from non-exposed control samples, the UV-B doses we used were: 50, 100 and 400 % of 0.53 J/cm2 - attained after 1 h of sun exposure and leading to the highest vitamin D2 yield [16]. Each sample consisted of four replicates for all experiments. All mushrooms (first flush, genetically the same strain) issued from the same production plant were processed in the same manner as described later. The irradiation intensity was adjusted via the distance between the UV-B tubes and samples. The distance was chosen to enable a homogeneous UV-B radiant flux per unit area (or irradiance) of two samples per run, and as short exposure time as possible (experiments A). In experiment (B), the effect of the exposure time on vitamin D2 production was investigated by enlarging the distance between the tubes and samples to reduce the UV-B intensity.
Mushrooms
Twelve kg of fresh white button mushrooms were harvested in the morning at a commercial mushroom production plant in Ireland (Monaghan Mushrooms Inc., Tyholland, Ireland) and packed into insulated expanded polystyrene (EPS) boxes together with dry ice and shipped via overnight express delivery to the University Medical Center Freiburg, Germany. The mushrooms arrived frozen (−9 °C), and were then sliced manually in a frozen state using an electric bread-cutting machine (Universal Slicer 372–75, Krups, Frankfurt, Germany) in a room devoid of UV light. The whole mushrooms weighed 21.4 ± 3 g with a hat diameter of 4.2 ± 0.3 mm (n = 20). Moisture content was 91.8 ± 0.4 % determined after freeze-drying at 1 mbar for approximately two days and afterwards homogenised in a blender. Slices were 9 ± 0.3 mm thick; they were weighed, coded, carefully put in polyethylene zipperbags, and placed in EPS boxes with dry ice. Weight per sample was 70.8 ± 15.0 g of sliced mushrooms, and each zipperbag was randomly allocated to either the control or a UV-B dose.
The mushroom samples resembled those used in the sun exposure substudy in hat diameter, moisture content, and slice thickness. However, major differences were: lower weight of the whole mushrooms used in the UV-B tubes experiments (21.4 ± 3 g vs 28.7 ± 4.9 g, P < 0.001) and lower temperature of the mushrooms at the beginning and end of exposure times (−0.6 °C vs 10.4 °C and 0.9 °C vs 34.0 °C).
Artificial UV-B Irradiation of Mushroom Slices
The custom-made UV unit was equipped with UV-B tubes 176 cm in length (UV21, Waldmann, Villingen-Schwenningen, Germany). The tubes were mounted unprotected on a galvanized sheet steel enclosure, 14.5 cm apart from each other. The number of tubes, the distance applied and the intensities achieved in experiments A and B are given in Table 1. Before each exposure experiment, the tubes were switched on to warm up for 10 min. The intensities of the UV-B tubes for UV-A and UV-C (100–280 nm) were 0.51 and 0.02 mW/cm2, respectively. The mushroom slices were completely separated from each other on horizontally-positioned polyester food trays (32 cm × 46 cm) with a gray and non-reflective melaminic surface (Presswerk Köngen, Wendlingen, Germany). The beginning and end pieces of the sliced mushrooms were all placed with their slice plane facing the UV-B tubes. The trays with mushrooms were rotated after half the irradiance time had passed to ensure equal doses. Room temperature during the experiments was 19 °C. The internal temperature was monitored. Immediately after each experiment, the samples were double-sealed in polyethylene zipperbags, labelled and repacked into EPS boxes with dry ice which were stored finally at −26 °C until shipment via overnight express delivery to Technical University of Denmark, Denmark for analyses of vitamin D and to Monaghan Mushrooms, Ireland for analyses of agaritine.
UV Measurements
The UV unit’s spectral irradiance was measured by a calibrated spectral radiometer (SR600, Opsytec Dr. Groebel, Ettlingen, Germany) with spectral resolution of 1.5 nm. For the wavelength range 200–360 nm, the spectral radiometer was calibrated traceable to spectral irradiances. The calibration was removed by a relative measurement with a calibrated deuterium lamp. The calibration standard uncertainty is 3.2 %. We also used a calibrated radiometer (RM-22 with UV-B sensor, Opsytec Dr. Groebel). It was adjusted to the spectral measurement result from the spectral radiometer to take fast measurements and control the dose exactly at the position of the exposed mushroom surfaces during the experiments. Mean deviation of UV-B doses for all artificial irradiation experiments was 2.6 ± 1.9 %. Regarding the measurement devices’ reproducibility, positioning accuracy and influences such as dark signal and scattered radiation, the resulting measurement’s range of uncertainty is 8 %.
Analyses of Vitamin D2 and Vitamin D4
The contents of vitamin D2 and vitamin D4 were quantified in freeze-dried materials by a HPLC-method [17]. The analyses for vitamin D2 were performed accredited according to ISO17025. In short, to 0.1 g freeze-dried mushrooms was added vitamin D3 (C9774, Sigma-Aldrich Denmark A/S, Copenhagen, DK) as internal standard. The solution was saponified, liquid-liquid extracted, and clean-up through a silica solid phase extraction and a preparative normal phase HPLC for collection of the vitamin D fraction. No commercial standard is available for vitamin D4, therefore the identification of vitamin D4 was based on information provided by Philips et al. [11] identifying vitamin D4 in UV-exposed mushrooms. Following evaporation and dilution in mobile phase [acetonitrile:methanol (80:20)] for the analytical HPLC-system (VYDAC201TP54, 5 μm, 250 × 4.6 mm, Grace, MD) the vitamin D2, D3, and D4 were separated having retention times at 19.6, 20.0, and 23.4 min, respectively. Detection was performed by diode array detector set from 220 to 320 nm and quantification of vitamin D2 at 265 nm. Vitamin D2 was used for quantification of vitamin D4, but corrected for difference in molar absorption coefficient, 18,200 and 18,120 [18]. The precision measured in-house reference materials of vitamin D-enriched mushrooms is for vitamin D2 5.9 % (n = 13) and for vitamin D4 6.2 % (n = 4) for levels at 17.2 and 0.9 μg/100 g, respectively.
Analyses of Agaritine
All analyses of agaritine from the sun-exposed and artificially UV-B irradiated mushrooms were done simultaneously. The frozen mushrooms were homogenized for three minutes in a food grade blender (Cookwork GG500, Milton Keynes, Buckinghamshire, UK). Twenty grams were homogenized with 100 ml of methanol for 10 min in a blender (Philips HR2000, Breitner Center, Amsterdam, Netherlands), and shaken for 30 min at ambient temperatures in a temperature controlled shaker (Innova 44, New Brunswick Scientific, Hamburg, Germany), and filtrated through 0.2 μm bottle top filter (Thermo Fisher, Waltham, MA, USA). The filtrate volume was transferred to a 200 ml volumetric flask and adjusted to 200 ml methanol. 10 ml of the extract was evaporated to dryness under nitrogen and reconstituted in 2 ml of ultrapure water (18.2 Ώ Merck Millipore, Darmstadt, Germany). Afterwards the extract was filtered through a 0.45 μm filter into an amber HPLC vial. Within 6 h of preparation 20 μl injected on to the HPLC-system equipped with a diode array detector (1260 Infinity, Agilent Technologies Inc., Santa Clara, CA, USA) and equipped with a reverse phase columns (Pursuit C18, 5 μm, 250 × 4.6 mm, Agilent Technologies Inc., Santa Clara, CA, USA) and similar guard column (10 × 4.6 mm). The mobile phase was methanol-water; the mobile phase gradient being 10 % methanol held for 5 min, changing to 80 % over 15 min, held at 80 % methanol for 5 min, and finally returning to 10 % methanol over 5 min to equilibrate. The total run time was 35 min. UV detection at 237 nm and UV spectra were measured from 190 to 400 nm to confirm the analyte’s identity. Each extract was injected in duplicate. Quantification of agaritine was based on external calibration and the molar absorption coefficient of agaritine, ε, 12,000) in water [6, 19]. This method is well described elsewhere [20, 21]. The HPLC method used in this study has been used as reference method in a recent inter-laboratory study with mixed mushroom material [22]. We used the same mixed mushroom material used by Philips and Rasor [22] to validate HPLC method. Agaritine values obtained were not significantly different from those already reported. The calculated limits of detection and quantification using this method are 11.1 and 50 mg/kg, respectively.
Statistical Analysis
Study findings were analyzed using SPSS statistics version 22 (IBM Corp., Armonk, NY, USA) and graphs created with Microsoft Excel 2010 (Microsoft Corp., Redmond, WA, USA). For descriptive data analyses, values are presented as means ± standard deviations. Group comparisons were analyzed with two sample t-tests. Pearson’s correlation was calculated to assess the relation between variables.
Results and Discussion
Spectral UV Irradiation: Tubes Vs Sun
Solar UV intensities change during the sun’s path in the sky. Hence, the data in Fig. 1 serve to generally describe major differences in spectral irradiance between solar and artificial irradiation. The gray area represents the absorption spectrum (270–315 nm) for conversion of provitamin D to previtamin D [1, 23] For the UV-B tubes, the intensities of UV-A (315–380 nm), UV-B and UV-C (100–280 nm) were 0.51, 0.69 and 0.02 mW/cm2, respectively. For the sun, the intensities of UV-A, UV-B and UV-C were 0.91, 0.17 and 0 mW/cm2, respectively. Due to differences in spectral composition and because these experiments controlled only for total UV-B dose, mushroom samples under the UV-B tubes and the sun-exposed samples were irradiated additionally with some UV-C, and UV-A, respectively.
Spectral irradiance of the UV unit (UV-B tubes) compared with solar irradiances measured under clear sky conditions at 11:00 UTC on July 3, 2014, shortly after summer solstice with the highest natural UV-B irradiance (Oberried, Germany, 47.91 °N, 7.91 °E, 1205 m above sea level). UV unit spectra emitted by four UV-B tubes were measured at a distance of 75 cm. Gray area represents the assumed wavelengths of the formation of previtamin D from provitamin D via a photochemical reaction [1, 23]
Effect of UV-B Doses on Vitamin D2 Content
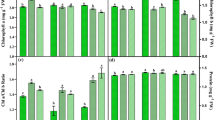
Simulating UV-B dose after 1 h of sun exposure (0.53 J/cm2) with UV-B tubes raised the vitamin D2 content to a significantly (P < 0.001) higher levels [67.1 ± 9.9 μg/g dry weight (DW)] than the highest yields reported in our previous article (3.9 ± 0.8 μg/g dry DW) [16]. Vitamin D2 production under laboratory conditions with UV-B tubes was almost 20 times higher than vitamin D2 production (2.5 ± 0.3 μg/g DW) at the same UV-B dose of 0.26 J/cm2 after 30 min of sun exposure (Table 1, Fig. 2). Further UV-B doses of 0.53 and 2.01 J/cm2 from UV-B tubes increased significantly the vitamin D2 production (P < 0.05, P < 0.001). We noted the highest vitamin D2 content after the largest UV-B dose of 2.01 J/cm2 and consisted of 101.5 ± 4.3 μg/g DW, equalling 874 μg vitamin D2
per 100 g of fresh mushroom slices (Fig. 2). These levels are more than 58 times the current Dietary Reference Intake (600 IU, or 15 μg) [24]. Similarly high vitamin D2 yields with artificial UV-B exposure in button mushrooms are cited in studies using UV-B doses between 1.5 and 1.8 J/cm2 and reporting vitamin D2 contents of 55–60 μg/g DW [2, 25]. It is noteworthy that we used mushroom slices with a larger surface area for irradiation; this may be why the vitamin D2 contents are so much higher than those in other reports on button mushrooms using UV-B tubes [6, 16, 26, 27].
Vitamin D2 content (μg/g DW) of button mushroom slices after UV irradiation via sun and UV-B tubes at identical UV-B doses. Vitamin D2 data on the sun-exposed mushrooms have been retrieved from our previous article [16]. Error bars indicate standard errors. Group comparisons: the signs above the error bars indicate the P values for the corresponding bar with a lower UV-B dose; the signs above the curly brackets indicate the P values between the two bars of each UV-B dose. † Not significantly different, * P < 0.05, *** P < 0.001 (two sample t-tests)
Simon et al. [6] compared vitamin D2 production in whole button mushrooms exposed to the sun or to UV-B tubes. In contrast to our results, first they achieved low contents of vitamin D2 (4.1 ± 0.6 μg/g DW) after an artificial UV-B dose of 1.06 J/cm2, and second, their samples exposed 2.5 h to the sun produced similar vitamin D2 contents (3.7 ± 0.9 μg/g DW), but without monitoring the UV-B dose.
We did not anticipate such a high magnitude of the different vitamin D2 yields after the same UV-B doses from sunlight or UV-B tubes. Besides the uniform conditions regarding the origin of the mushroom samples, slice thickness, initial moisture content, same UV-B doses and analytical laboratory, there were several inevitable differences originating from the study design: exposure time, total spectral irradiance, final sample temperature and moisture content. The only evitable error was the 12 °C lower initial temperature of the samples under the UV-B tubes. However, the higher vitamin D2 content of the samples treated with artificial UV-B cannot be explained by the lower temperature as the optimal reaction temperatures for production of vitamin D2 under UV-B irradiation in oyster and shiitake mushrooms has been reported to be 28 and 35 °C, respectively [28, 29]. Another difference based on study design between samples irradiated by the UV-B tubes vs. the sun was the exposure time: it was always ≈80 % shorter for the UV-B samples, thereby limiting the period for degradation.
Previtamin D3 cannot absorb UV radiation beyond 315 nm to be converted photochemically to previtamin D3 [30]. Two articles by Jasinghe et al. [28, 31] demonstrate the efficacy of UV-A (315–380 nm) to produce vitamin D2 in different edible mushroom species, however, conversion was much higher under UV-B (280–315 nm) and UV-C (100–280 nm) irradiation, respectively [32]. Wu and Ahn [29] investigated optimum vitamin D2 synthesis conditions in oyster mushrooms including the effect of different UV spectra alone and in combination. UV-A alone had no effect on vitamin D2 production, whereas UV-A together with UV-B resulted surprisingly in significantly lower vitamin D2 content than UV-B alone. These results concur with an earlier study on the effects of sunlight on vitamin D3 in human skin samples, revealing that UV-A at 330 nm can photodegrade vitamin D3 into various photoproducts e.g., suprasterol I, suprasterol II, and 5,6-transvitamin D3 [33]. The proportion of photons with wavelengths in the 315–330 nm range able to photodegrade previously produced vitamin D was considerably higher in our sun-exposed mushroom samples (Fig. 1). Another difference in spectral composition of sunlight and UV-B tubes regarding vitamin D2 production was the minor UV-C intensity the tubes emitted. Moreover, the significantly prolonged exposure in the sun-exposed samples could have produced irreversible “over-exposed products” by dimerization and ring cleavage of previtamin D2 and tachysterol [30].
The moisture contents found in the present study are in agreement with published data [11], however a limitation of the study was the use of the freeze-drying factor for determination of moisture content as this method does not account for possible residual moisture, which could be up to 6 % [34].
Effect of Artificial UV-B Doses on Vitamin D4 Content
Vitamin D4 is reported to be about 60 % as active as vitamin D3 in healing rickets in rats [18]. There is published evidence that the vitamin D4 precursor sterol is detectable in all mushrooms (varying widely among species) and comparable to vitamin D2 precursor convertible to vitamin D4 via a photochemical reaction [11]. Figure 3 illustrates vitamin D4 content as a function of the vitamin D2 content in sliced mushrooms exposed to irradiation from UV-B tubes. We detected no vitamin D4 in the non-exposed control samples, concurring with analyses of button mushrooms not detecting vitamin D4 in 10 of 12 samples [11]. Those researchers observed vitamin D4 in 41 % of non-exposed mushroom samples of different species, with higher contents in wild-grown mushrooms. Overall we found a positive correlation between vitamin D4 and vitamin D2 (r2 = 0.96, P < 0.001), as did Phillips et al. [11]. All our UV-exposed samples contained vitamin D4 at an average 14.1 ± 2.9 (min-max: 9.6–20.9) μg/g DW. Our data strengthen the position of Phillips et al. [11] that vitamin D4 is formed in all mushrooms exposed to UV light during the commercial production of vitamin D2-enriched products due to the omnipresence of the precursor 22,23-dihydroergosterol [35].
Effect of Exposure Time on Vitamin D2 and D4
Experiment A was designed to obtain the shortest exposure time. Experiment B served to examine the impact on vitamin D2 and vitamin D4 contents by increasing the exposure by a factor of 5 from 12:14 min to 60:00 min with a consistent UV-B dose of 0.53 J/cm2 under laboratory conditions (Fig. 4). No difference in the contents of either analyte was detected between the two exposure times (vitamin D2: 77.9 ± 2.4 vs 67.1 ± 9.9 μg/g DW, P = 0.08; vitamin D4:13.9 ± 1.0 vs 13.2 ± 2.0 μg/g DW, P = 0.5).
Impact of a short (min:s 12:14) vs long (min:s 60:00) irradiation time with a consistent artificial UV-B dose of 0.53 J/cm2 on vitamin D2 and vitamin D4 contents [experiment (B)]. Error bars indicate standard errors. No statistical difference in the production of vitamin D2 and vitamin D4 was found between both irradiation times
Effect of UV-B Doses on Agaritine Content
Content of agaritine in the control groups (n = 4) showed a huge variation from 121 to 1063 mg/kg fresh weight (FW), and a mean of 515 ± 275 mg/kg FW. A review of OECD data on agaritine content of fresh cultivated button mushrooms revealed a range between 80 and 1730 mg/kg FW [14], but usually 200–500 mg/kg FW [19]. The mushrooms used in our experiments displayed agaritine contents within the normal range. Figure 5 shows agaritine content in control, after solar and artificial UV exposure of the sliced mushroom to different UV-B doses. Figure 5 reveals a significantly different agaritine content in the non-exposed control samples of both substudies (P < 0.05) and confirms the naturally highly variable agaritine content of button mushrooms others have reported [6, 19].
Agaritine content (mg/kg fresh weight) after solar and artificial UV exposure of button mushroom slices at different UV-B doses. Error bars indicate standard errors. Group comparisons: the signs above the error bars indicate the P values for the corresponding bar with a lower UV-B dose; the signs above the curly brackets indicate the P values between the two bars of each UV-B dose. † Not significantly different, * P < 0.05, ** P < 0.01, *** P < 0.001 (two sample t-tests)
Irrespective of the irradiation source, agaritine content dropped significantly after an UV-B dose of 0.53 J/cm2 from sun (P < 0.001) and UV-B tubes (P < 0.05, Fig. 2). These results are contrary to those of Simon et al. [6] who detected no significant difference in agaritine contents between control samples and samples exposed for 150 min to the sun or irradiated artificially with a UV-B dose of 1.08 J/cm2, respectively.
Liu et al. [36] (white and brown button mushrooms) and Schulzová et al. [21] (53 white mushrooms of the genus Agaricus) investigated the influence of storage and household processing on the agaritine content of button mushrooms and detected a pronounced reduction in agaritine content while cooking, refrigerating or freezing mushrooms, as well as after drying (20–75 %). The drop in agaritine content compared to the controls samples at a UV-B dose of 0.53 J/cm2 after 60 min of solar irradiation and ~12 min of artificial irradiation was 99 and 69 %, respectively. The extraordinary high degradation of agaritine detected here can be attributed to using mushroom slices and increasing the ratio of the mushroom’s surface area to its mass - compared to whole mushrooms used in the stability studies. The reduction in agaritine content is mainly triggered by well-known oxidation process and other influencing factors, but there is no rationale for a photochemical reaction being involved [20].
A limitation due to our study design consisted of the use of two different batches of mushrooms for the sun-exposed and artificially irradiated mushrooms, wherefore we cannot rule out the possible impact of variables (e.g., initial agaritine or provitamin D2 content) related to the mushrooms themselves.
Conclusions
There is evidence that agaritine content is not influenced by UV light, and that it is naturally highly variable. In this investigation we observed that the agaritine content remained within normal ranges in all samples, and that it was significantly degraded during the experiments due to the processing of the mushrooms and its own instability. We confirm that there are no reasons to assume that the safety concerning the agaritine content of artificially or naturally UV-exposed button mushrooms could differ from that in non-exposed button mushrooms. We believe that the agaritine content should be disregarded in future studies with UV-exposed and vitamin D2-enriched mushrooms. Furthermore, agaritine is only found in mushrooms belonging to the genus Agaricus and its levels are insignificant in edible mushrooms of other genera.
We found that the vitamin D2 produced in mushroom slices under laboratory conditions with UV-B tubes was, surprisingly, much higher than it was at the same UV-B dose of sun exposure. Because we only controlled for total UV-B doses, the spectral composition of the radiation hitting the artificially UV-exposed mushrooms differed from the sun-exposed samples: some UV-C and much less UV-A. An investigation of the various photoproducts of vitamin D2 in conjunction with various UV ranges could be enlightening. We found a positive correlation between vitamin D2 and vitamin D4 production during the artificial UV irradiation of mushroom slices, although to a much lower but nevertheless relevant degree. The biological action of vitamin D from UV-exposed mushrooms is the sum of the activity of these two major vitamin D analogues. Further studies on the precise biological activity of both vitamin D analogues relative to vitamin D3 are warranted, and vitamin D4 contents should be analysed in future studies employing UV-exposed and vitamin D2-enriched mushrooms.
Abbreviations
- EPS:
-
Expanded polystyrene
- EC:
-
European Commission
- DW:
-
Dry weight
- FW:
-
Fresh weight
- HPLC:
-
High-performance liquid chromatography
- IU:
-
International unit
- OECD:
-
Organisation for Economic Co-operation and Development
- r:
-
Correlation coefficient
- UTC:
-
Universal Time Coordinated
- UV:
-
Ultraviolet
References
Havinga E, de Kock RJ, Rappoldt MP (1960) The photochemical interconversions of provitamin D, lumisterol, previtamin D and tachysterol. Tetrahedron 11:276–284. doi:10.1016/S0040-4020(01)93178-3
Urbain P, Singler F, Ihorst G, et al. (2011) Bioavailability of vitamin D2 from UV-B-irradiated button mushrooms in healthy adults deficient in serum 25-hydroxyvitamin D: a randomized controlled trial. Eur J Clin Nutr 65:965–971. doi:10.1038/ejcn.2011.53
Simon RR, Borzelleca JF, DeLuca HF, Weaver CM (2013) Safety assessment of the post-harvest treatment of button mushrooms (Agaricus bisporus) using ultraviolet light. Food Chem Toxicol 56:278–289. doi:10.1016/j.fct.2013.02.009
Cashman KD, Kiely M, Seamans KM, Urbain P (2016) Effect of ultraviolet light-exposed mushrooms on vitamin D status: liquid chromatography-tandem mass spectrometry reanalysis of Biobanked sera from a randomized controlled trial and a systematic review plus meta-analysis. J Nutr 146:565–575. doi:10.3945/jn.115.223784
Krings U, Berger RG (2014) Dynamics of sterols and fatty acids during UV-B treatment of oyster mushroom. Food Chem 149:10–14. doi:10.1016/j.foodchem.2013.10.064
Simon RR, Phillips KM, Horst RL, Munro IC (2011) Vitamin D mushrooms: comparison of the composition of button mushrooms (Agaricus bisporus) treated postharvest with UVB light or sunlight. J Agric Food Chem 59:8724–8732. doi:10.1021/jf201255b
Regulation (EC) (1997) No 258/97 of the European Parliament and of the Council of 27 January 1997 concerning novel foods and novel food ingredients. Off J Eur Union 1–6. ISSN: 0378–6978
Joint FAO/WHO Expert Consultation on Biotechnology and Food Safety (1996) Biotechnology and food safety / report of a joint FAO/WHO consultation. FAO/WHO, Rome
Phillips KM, Rasor A (2013) A nutritionally meaningful increase in vitamin D in retail mushrooms is attainable by exposure to sunlight prior to consumption. J Nutr Food Sci 3:236. doi:10.4172/2155-9600.1000236
Kristensen HL, Rosenqvist E, Jakobsen J (2012) Increase of vitamin D2 by UV-B exposure during the growth phase of white button mushroom (Agaricus bisporus). Food Nutr Res 56. doi:10.3402/fnr.v56i0.7114
Phillips KM, Horst RL, Koszewski NJ, Simon RR (2012) Vitamin D4 in mushrooms. PLoS One 7:e40702. doi:10.1371/journal.pone.0040702
Shephard SE, Gunz D, Schlatter C (1995) Genotoxicity of agaritine in the lacI transgenic mouse mutation assay: evaluation of the health risk of mushroom consumption. Food Chem Toxicol 33:257–264. doi:10.1016/0278-6915(94)00142-B
Friederich U, Fischer B, Lüthy J, et al. (1986) The mutagenic activity of agaritine--a constituent of the cultivated mushroom Agaricus bisporus--and its derivatives detected with the salmonella/mammalian microsome assay (Ames test). Z Lebensm Unters Forsch 183:85–89
Organisation for Economic Co-operation and Development (2007) Consensus document on compositional considerations for new varieties of the cultivated mushrooms “Agaricus bisporus” key food and feed nutrients, anti-nutrients and toxicants. OECD Publishing, Paris
Kiely M, Cashman KD, the ODIN Consortium (2015) The ODIN project: development of food-based approaches for prevention of vitamin D deficiency throughout life: the ODIN project. Nutr Bull 40:235–246. doi:10.1111/nbu.12159
Urbain P, Jakobsen J (2015) Dose–response effect of sunlight on vitamin D2 production in Agaricus bisporus mushrooms. J Agric Food Chem 63:8156–8161. doi:10.1021/acs.jafc.5b02945
Burild A, Frandsen HL, Jakobsen J (2014) Simultaneous quantification of vitamin D3, 25-hydroxyvitamin D3 and 24,25-dihydroxyvitamin D3 in human serum by LC-MS/MS. Scand J Clin Lab Invest 74:418–423. doi:10.3109/00365513.2014.900694
De Luca HF, Weller M, Blunt JW, Neville PF (1968) Synthesis, biological activity, and metabolism of 22,23-3H vitamin D4. Arch Biochem Biophys 124:122–128
Andersson HC, Gry J (2004) Phenylhydrazines in the cultivated mushroom (Agaricus bisporus): occurrence, biological properties. Risk Assessment and Recommendations, Nordic Council of Ministers
Hajslová J, Hájková L, Schulzová V, et al. (2002) Stability of agaritine - a natural toxicant of Agaricus mushrooms. Food Addit Contam 19:1028–1033. doi:10.1080/02652030210157691
Schulzová V, Hajslová J, Peroutka R, et al. (2002) Influence of storage and household processing on the agaritine content of the cultivated Agaricus mushroom. Food Addit Contam 19:853–862. doi:10.1080/02652030210156340
Phillips KM, Rasor AS (2016) A mixed mushroom control material to facilitate inter-laboratory harmonization of mushroom composition analyses. J Food Compos Anal 48:48–66. doi:10.1016/j.jfca.2016.01.001
Holick MF (2003) Vitamin D: a millenium perspective. J Cell Biochem 88:296–307. doi:10.1002/jcb.10338
Institute of Medicine (2011) Dietary reference intakes for calcium and vitamin D. National Academies Press, Washington, DC, USA
Jasinghe VJ, Perera CO, Barlow PJ (2006) Vitamin D2 from irradiated mushrooms significantly increases femur bone mineral density in rats. J Toxic Environ Health A 69:1979–1985. doi:10.1080/15287390600751413
Roberts JS, Teichert A, McHugh TH (2008) Vitamin D2 formation from post-harvest UV-B treatment of mushrooms (Agaricus bisporus) and retention during storage. J Agric Food Chem 56:4541–4544. doi:10.1021/jf0732511
Ko JA, Lee BH, Lee JS, Park HJ (2008) Effect of UV-B exposure on the concentration of vitamin D2 in sliced shiitake mushroom (Lentinus edodes) and white button mushroom (Agaricus bisporus). J Agric Food Chem 56:3671–3674. doi:10.1021/jf073398s
Jasinghe VJ, Perera CO (2005) Distribution of ergosterol in different tissues of mushrooms and its effect on the conversion of ergosterol to vitamin D2 by UV irradiation. Food Chem 92:541–546. doi:10.1016/j.foodchem.2004.08.022
Wu W-J, Ahn B-Y (2014) Statistical optimization of ultraviolet irradiate conditions for vitamin D2 synthesis in oyster mushrooms (Pleurotus ostreatus) using response surface methodology. PLoS One 9:e95359. doi:10.1371/journal.pone.0095359
Boomsma F, Jacobs HJC, Havinga E, van der Gen A (1977) The “overirradiation products” of previtamin D and tachysterol: toxisterols. Recl Trav Chim Pays-Bas 96:104–112. doi:10.1002/recl.19770960405
Jasinghe VJ, Perera CO (2006) Ultraviolet irradiation: the generator of vitamin D2 in edible mushrooms. Food Chem 95:638–643. doi:10.1016/j.foodchem.2005.01.046
Teichmann A, Dutta PC, Staffas A, Jägerstad M (2007) Sterol and vitamin D2 concentrations in cultivated and wild grown mushrooms: effects of UV irradiation. LWT – Food Sci Technol 40:815–822. doi:10.1016/j.lwt.2006.04.003
Webb AR, Decosta BR, Holick MF (1989) Sunlight regulates the cutaneous production of vitamin D3 by causing its photodegradation. J Clin Endocrinol Metab 68:882–887. doi:10.1210/jcem-68-5-882
Giri SK, Prasad S (2009) Quality and moisture sorption characteristics of microwave-vacuum, air and freeze-dried button mushroom (Agaricus bisporus). J Food Process Preserv 33:237–251. doi:10.1111/j.1745-4549.2008.00338.x
Keegan R-JH LZ, Bogusz JM, et al. (2013) Photobiology of vitamin D in mushrooms and its bioavailability in humans. Dermatologica 5:165–176. doi:10.4161/derm.23321
Liu J-W, Beelman RB, Lineback DR, Speroni JJ (1982) Agaritine content of fresh and processed mushrooms [Agaricus bisporus (Lange) Imbach]. J Food Sci 47:1542–1544. doi:10.1111/j.1365-2621.1982.tb04978.x
Acknowledgments
We are grateful to C. Cuerten for editorial assistance, to D. McConnon, A. Clancy and C. Beirne from Monaghan Mushrooms for their assistance on preparing samples for analysis and conducting analyses of agaritine, to I. Mayer and F. Meinhardt form the German Federal Office for Radiation Protection for receiving complete data of spectral irradiance, and to E. Maier, F. Jaeger and F. Lorenz from University Medical Center’s central kitchen for providing material for the experiments and storage of samples, and to K. Saliger for helping out with sample preparation.
Authors Contribution
P.U., study concept, study performance and statistical analysis; J.V., analytics, critical review; J.J., analytics, critical review; all authors interpreted the data, drafted the manuscript, saw and approved the final version of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
P.U. and J.J declare no conflict of interest. J.V. is an employee of Monaghan Mushrooms, which is a commercial mushroom producer and is interested in understanding the impact of sunlight exposure into the conversion of ergosterol into vitamin D2. The mushroom samples were blinded by P.U. before shipment to J.V. for agaritine analyses.
Funding/Support
This project has received funding from the European Union’s Seventh Framework Programme (FP7/2007–2013) under grant agreement no. 613,977 (ODIN).
Rights and permissions
About this article
Cite this article
Urbain, P., Valverde, J. & Jakobsen, J. Impact on Vitamin D2, Vitamin D4 and Agaritine in Agaricus bisporus Mushrooms after Artificial and Natural Solar UV Light Exposure. Plant Foods Hum Nutr 71, 314–321 (2016). https://doi.org/10.1007/s11130-016-0562-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11130-016-0562-5