Abstract
Postharvest browning is the primary cause of a decrease in the shelf life of the white button mushroom (Agaricus bisporus). This study investigated the effect of postharvest brassinolide (BL) treatment on metabolism in relation to browning of the white button mushroom. Each harvested mushroom was dipped into one of three solutions containing 0, 1, or 3 μM BL for 5 min and stored in darkness at 4 °C for 16 days. Our results indicated that treatment with BL restrains browning development and reduces the total phenolic content and polyphenol oxidase activity. In addition, BL treatment maintains lower weight loss, electrolyte leakage, and malondialdehyde content and inhibits any increase in lipoxygenase activity compared with those of the control mushrooms. Furthermore, BL treatment significantly decreases the accumulation of reactive oxygen species (ROS) and induces the antioxidant enzyme system. Compared with 1 μM BL, treatment with 3 μM BL is more effective in reducing cap browning. The reduction of membrane oxidative damage and ROS levels induced by BL inhibits enzymatic browning reaction in the white button mushroom. These findings suggest that treatment with BL could have the potential of inhibiting browning and thus maintaining the mushroom’s commercial value.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The white button mushroom (Agaricus bisporus) is considered the most extensively cultivated edible mushroom in the world (Meng et al. 2012). However, it has a short postharvest life, of only less than 3 days at room temperature, mainly due to browning, senescence, high respiration, water loss, and microbial attack (Ye et al. 2012). In particular, browning determines the marketability and consumer acceptability of mushrooms (Qin et al. 2015). Postharvest browning of mushrooms is mainly presumed to occur through the oxidation of phenolic substances into quinones by enzymatic action, such as the polyphenol oxidase (PPO), which ultimately polymerize to produce the browning appearance (Mohapatra et al. 2008). To a lesser extent, some surface discoloration is also caused by microbial contamination (Jahangir et al. 2011). Therefore, delaying or reducing browning could be an important means of extending the shelf life and maintaining the quality of the white button mushroom.
Enzymatic browning is associated with membrane integrity loss during tissue deterioration and senescence (Zhu et al. 2009). Membrane integrity is negatively correlated with membrane lipid peroxidation, which is catalyzed by lipoxygenase (LOX) and leads to malondialdehyde (MDA) production (Gill and Tuteja 2010; Sharma et al. 2012). Lipid peroxidation alters membrane properties and causes cell defects, such as electrolyte leakage and cellular decompartmentation (Yang et al. 2009; Chomkitichai et al. 2014). Reactive oxygen species (ROS), such as superoxide anion (O2 ·−) and hydrogen peroxide (H2O2), play a critical role in increasing lipid peroxidation and causing membrane oxidative damage (Thompson et al. 1987; Scandalios 1993). A natural defense system, such as an enzymatic antioxidant, has the ability to protect the plant from the toxic effect of ROS. It has been shown that superoxide dismutase (SOD) catalyzes the dismutation of the superoxide free radicals into H2O2 and that H2O2 can be scavenged as a result of catalase (CAT) and ascorbate peroxidase (APX) enzyme activities (Sevillano et al. 2009). Reduced oxidative damage to the membrane apparently maintains the compartmentation of enzymes and substrates and, thereby, lessens enzymatic browning (Chomkitichai et al. 2014).
Various strategies have been attempted to retard browning and prolong shelf life of fresh whole or sliced mushrooms. Heretofore desirable results have been obtained through some chemical treatments and/or physical methods, such as citric acid (Brennan et al. 2000), hydrogen peroxide (Cliffe-Byrnes and O’Beirne 2008), calcium chloride (Miklus and Beelman 1996), ethylenediaminetetraacetic acid (EDTA) (Sapers et al. 1994), sorbitol (Anantheswaran et al. 1996), modified atmosphere packaging (Simón et al. 2010), electron-beam irradiation (Koorapati et al. 2004), and ultrasound treatment (Lagnika et al. 2014). However, the response of white button mushroom to citric acid, hydrogen peroxide, or electron-beam irradiation suggests that the alleviation of discoloration was mainly due to antibacterial activity, rather than inhibition of enzymatic browning (Brennan et al. 2000; Koorapati et al. 2004). Recently, several researchers have focused on methyl jasmonate (MeJA, a naturally occurring plant growth regulator) treatments of white button mushroom for long-term preservation. Possible mechanisms of browning alleviation by such treatments generally involve maintenance of membrane integrity, improvement of antioxidant system, and low gene expression and enzymatic activity of PPO (Jahangir et al. 2011; Meng et al. 2012).
Brassinosteroids (BRs) are a group of naturally occurring plant steroidal hormones that are important for plant growth and development and plant responses to biotic and abiotic stresses (Bajguz and Hayat 2009). BRs have also attracted much attention in the preservation of postharvest horticultural products. Treatment with brassinolide (BL) (Fig. 1), the first member of BRs isolated and shown to have biological activity, induced disease resistance and delayed senescence in jujube fruit (Zhu et al. 2010). Exogenous BL maintained membrane integrity and mitigated chilling injury in mangos and tomatoes, thus improving their storage quality (Li et al. 2012; Aghdam and Mohammadkhani 2014). Wang et al. (2012) reported that BL increased the resistance of green bell pepper to low temperature stress, possibly in conjunction with improved membrane stability and increased activities of antioxidant enzymes. Recently, Gao et al. (2015, 2016) found that 24-epibrassinolide (EBL), an active form of BR, inhibited flesh browning of eggplant and peach fruit in relation to phenol metabolism during cold storage.
Structural formula of brassinolide (BL). (Cited from Lei et al. 2015)
To the best of our knowledge, no research work has been conducted to study the effects of BL postharvest treatment on browning of the white button mushroom during storage. Therefore, the aim of this study was to investigate the effects of BL on phenol metabolism, membrane oxidative damage, and ROS accumulation that have been linked to browning development of mushroom caps.
Materials and Methods
Chemicals
BL (≥90 %) was obtained from Aladdin Industrial Co., Ltd., Shanghai, China. Folin-Ciocalteu’s phenol reagent (Vetec™ reagent grade) and thiobarbituric acid (≥98 %) were obtained from Sigma-Aldrich, Shanghai, China. All other chemicals were of analytical reagent-grade purity and obtained from Beijing Solarbio Science & Technology Co., Beijing, China.
Mushrooms and Treatment
White button mushrooms (Agaricus bisporus (J.E. Lange) Imbach stain A-15) from the first flush were harvested at commercial maturity stage (approximately 3–4 cm) from Liaoyuan Agricultural Co., Ltd., Beijing, China. The mushrooms were transported to the laboratory within 1 h and stored in darkness at 4 °C and 80–90 % relative humidity (RH) for 24 h. The mushrooms were selected based on color uniformity and absence of mechanical damage and then randomly divided into three lots of 300 sporophores each. Two lots were dipped in aqueous solutions of either 1 or 3 μM BL (prepared in ethanol/distilled water (1:1000, v/v) containing 0.1 % (v/v) Tween-20) for 5 min at 25 °C. A third lot was dipped in ethanol/distilled water (1:1000, v/v) with 0.1 % (v/v) Tween-20 under the same conditions and used as a control. Each treatment was replicated three times. The mushrooms were allowed to completely dry at 25 °C for 1 h and packed in low-density polyethylene bags (ten intact sporophores for each bag). The samples were stored in darkness at 4 °C and 80–90 % RH for 16 days. Twenty sporophores per replicate of each treatment were taken immediately following treatment (time 0) and after 4, 8, 12, and 16 days. Ten mushroom caps per replicate of each treatment were selected to assess surface color. Another ten mushroom caps per replicate of each treatment were diced, frozen in liquid nitrogen, and stored at −80 °C to assess total phenolic content, MDA content, ROS levels, and enzyme activity.
Cap Color
Mushroom cap browning was assessed by measuring the surface color using a Minolta Chroma Meter CR-400 (Konica Minolta Sensing, Inc., Osaka, Japan). The lightness (L*) value was determined after 0, 4, 8, 12, and 16 days of storage at 4 °C according to the method of Meng et al. (2012).
Total Phenolic Content
Total phenolic content was determined according to Folin-Ciocalteu’s method (Singleton and Rossi 1965) with modifications. One gram of frozen tissue was homogenized at 4 °C with 5 mL of ice-cold methanol, centrifuged, and filtered, and the filtrate was diluted with 5 mL of distilled water. A sample of 150 μL was transferred in a test tube and added 750 μL Folin-Ciocalteu’s phenol reagent and 600 μL sodium carbonate solution (7.5 %, w/v); the tube was vortexed and incubated at 25 °C for 1 h, after which the absorbance was measured at 760 nm using a TU-1901 spectrophotometer (Beijing Purkinje General Instrument Co. Ltd., Beijing, China). Gallic acid was used as the calibration standard, and the data were expressed as gallic acid equivalents in mg g−1 fresh weight (FW).
Weight Loss
Weight loss was determined at time 0 and after 4, 8, 12, and 16 days of storage at 4 °C. Weight loss was expressed as a percentage using Eq. (1), where W0 and W1 are the initial and final sample weights, respectively.
Electrolyte Leakage
Electrolyte leakage was measured using the method of Meng et al. (2012) with minor modifications. Disks (3 mm thick) of the pileus tissue were excised using a stainless steel cork borer (1 cm in diameter) from the top and middle part of the cap. Five disks of each replicate, excised from five caps, were washed with distilled water and put into 30 mL deionized water in a 50-mL plastic centrifuge tube. The electrical conductivity of the suspended solution was measured immediately (P 0) and again after being shaken for 2 h (P 1) with an MP513 conductivity meter (Sanxin, Shanghai, China). The samples were then boiled for 10 min and cooled to room temperature, and a final conductivity measurement (P 2) was taken. Electrolyte leakage was expressed as a percentage using Eq. (2):
MDA Content
MDA content was measured using the thiobarbituric acid (TBA) method described by Ding et al. (2007) with modifications. One gram of frozen tissue was homogenized with 3 mL of 10 % (w/v) trichloroacetic acid and then centrifuged at 12,000×g for 30 min at 4 °C. One milliliter of the supernatant was mixed with 3 mL of 0.6 % (w/v) TBA. The mixture was heated to 100 °C for 20 min, cooled and centrifuged at 12,000×g for 15 min at 4 °C. The absorbance of the supernatant was determined at 560 nm and subtracted from the absorbance at 600 nm. MDA concentration was calculated using the extinction coefficient 155 mM−1 cm−1 and expressed as μmol g−1 FW.
O2 ·− Production Rate
O2 ·− production rate was determined according to the method of Jiang et al. (2010) with modifications. One gram of frozen tissue was homogenized in 6 mL 65 mM potassium phosphate buffer (pH 7.8), 2 mL 10 mM hydroxylamine hydrochloride, and 2 mL 0.1 M EDTA at 4 °C. The homogenate was centrifuged at 12,000×g for 30 min at 4 °C. Two milliliters of supernatant was mixed with 2 mL 17 mM 4-aminobenzenesulfonic acid and 2 mL 7 mM α-naphthylamine. After incubation at 40 °C for 15 min, 2 mL ehylether was added and the mixture was centrifuged at 3000×g for 15 min. The absorbance was recorded at 530 nm. A standard curve with potassium nitrite was used to calculate the O2 ·− production rate from the reaction equation of O2 ·− with hydroxylamine. O2 ·− production rate was expressed as nmol min−1 g−1 FW.
H2O2 Content
H2O2 content was determined according to the method described by Patterson et al. (1984). One gram of frozen tissue was homogenized at 4 °C with 2 mL ice-cold acetone. The homogenate was centrifuged at 12,000×g for 30 min at 4 °C, after which 1 mL supernatant was mixed with 0.1 mL of 5 % (w/v) titanium sulfate and 0.2 mL 17 M ammonia. The precipitate was washed five times with ice-cold acetone by resuspension, drained, and dissolved in 5 mL 2 M H2SO4. The absorbance was measured at 410 nm. H2O2 content was calculated using H2O2 as a standard curve and then expressed as μmol g−1 FW.
Enzyme Assessment
Enzyme extracts for enzyme activity assays were prepared according to the method of Zhao et al. (2011) with modifications. The enzyme extraction procedure was conducted at 4 °C. Five grams of frozen tissue was extracted with 10 mL 50 mM potassium phosphate buffer (pH 7.0) containing 1 mM EDTA and 5 % (w/v) polyvinyl polypyrrolidone. After centrifuging the homogenate at 12,000×g for 30 min at 4 °C, the supernatant was used for the enzymatic assays.
PPO (EC1.10.3.1) activity was determined according to the method of Yingsanga et al. (2008). One unit of enzyme activity was defined as a 0.01 increase in absorbance at 420 nm per minute and was expressed as units (U) mg−1 protein. LOX (EC 1.13.1.12) activity was determined according to the method of Todd et al. (1990). One unit of LOX was defined as a 0.01 increase in absorbance at 234 nm per minute and was expressed as U mg−1 protein. SOD (EC 1.15.1.1) activity was determined according to the method of Dhindsa et al. (1981). One unit of SOD activity was defined as the amount of enzyme that caused 50 % inhibition of nitroblue tetrazolium and was expressed as U mg−1 protein. CAT (EC 1.11.1.6) was determined according to the method of Aebi (1984). One unit of CAT activity was defined as the amount of enzyme that caused a 0.01 decrease in absorbance at 240 nm per minute and was expressed as U mg−1 protein. APX (EC 1.11.1.11) activity was determined according to the method of Nakano and Asada (1981). One unit of APX activity was defined as the amount of enzyme that caused a 0.1 decrease in absorbance at 290 nm per minute and was expressed as U mg−1 protein.
The activity of each enzyme was expressed based on the amount of protein, and protein content was determined according to Bradford (1976) using bovine serum albumin as a standard.
Statistical Analyses
The experiments were arranged in a completely randomized design with three replicates. Error bars showed standard errors (SE) of the means for three replicates. The data were analyzed statistically with one-way analysis of variance (ANOVA) using SPSS (version 19.0) statistical analysis software (IBM SPSS, Inc., Chicago, IL, USA). Differences between means were assessed by Duncan’s multiple range tests. Correlation analysis was carried out using Pearson’s test (two-tailed). A difference was considered to be statistically significant when P < 0.05.
Results and Discussion
Cap Color
Mushrooms with an L* value <80 or <69 were considered as unacceptable from a whiteness point of view at wholesale or consumer levels, respectively (Gormley 1975; Meng et al. 2012). The L* value in the control mushrooms sharply decreased from 89.71 ± 1.80 at day 0 to 79.03 ± 1.28 at day 8 and then to 68.78 ± 1.43 at day 16, which might not be considered as wholesale acceptable (Fig. 2a). Compared with the control, the 1 μM BL treatment significantly inhibited mushroom cap browning as the L* value for these mushrooms was 79.94 ± 1.17 at day 12. Finally, the application of 3 μM BL remarkably retarded the decrease in L* value, as the luminosity remained above the acceptance limit until day 16, with a value of 80.13 ± 1.42. These results were also supported by visual inspection at day 16 (Fig. 2b). As noted in this figure, the treatment with 3 μM BL leads to a mushroom cap that was lighter in color than either the control or those treated with 1 μM BL.
Total Phenolic Content
The total phenolic content in the control mushrooms peaked at day 8 and then gradually decreased (Fig. 3). Compared with the control, both 1 and 3 μM BL-treated mushrooms maintained lower levels of total phenolic content from day 8 till the end of the storage period, which were 8 and 13 % lower than those in the control mushrooms at day 16, respectively. These data reveal that the accumulation of phenolic compounds in white button mushrooms during storage is effectively inhibited by BL. Gao et al. (2015) also reported that EBL treatment reduced total phenolic content in eggplant fruits during the postharvest period.
PPO Activity, Weight Loss, Membrane Oxidative Damage, and ROS Levels
PPO activity in all mushrooms progressively increased throughout the storage. However, the PPO activities were still 19 and 30 % lower in the mushrooms treated with 1 and 3 μM BL, respectively, than that in the control mushrooms at day 16 (Table 1). Our results are in conformity with the findings of earlier researchers who reported that phytohormone MeJA was effective in decreasing PPO activity in white button mushrooms (Jahangir et al. 2011; Meng et al. 2012). The L* value was negatively correlated with the PPO activity after 3 μM BL treatment with Pearson’s coefficient of 0.95 (P < 0.05). The concomitant reduction of total phenols (Fig. 3) suggests that the inhibition of browning could be due to the lower level of phenols available to be oxidized in conjunction with reduced PPO activity.
The percentage of weight loss with respect to storage time and treatments in the mushrooms was calculated from Eq. (1). The weight loss in both the control and BL-treated mushrooms increased gradually with storage duration (Table 1). The mushrooms treated with 1 and 3 μM BL showed relatively lower weight loss in comparison with the control after 8 days of storage, especially the mushrooms treated with 3 μM BL (P < 0.05). The reason for low weight loss in the BL-treated mushrooms might be due to the enhancing effects of BL to membrane integrity that could slow down the dehydration process (Maalekuu et al. 2006; Aghdam and Mohammadkhani 2014). Our results are consistent with findings in composite chemical-pretreated button mushrooms (Khan et al. 2014).
Electrolyte leakage of the control and BL-treated mushrooms during storage was calculated from Eq. (2). The electrolyte leakage increased rapidly in the control mushrooms over the entire storage period, indicating a reduction in membrane integrity of flesh cells. Even when the electrolyte leakage in the mushrooms treated with 1 and 3 μM BL presented a similar trend, the increase in electrolyte leakage in the BL-treated mushrooms was only 71 and 44 %, respectively, of that in the control mushrooms at day 16 (Table 1). Similar results were reported for green bell peppers, mangos, and eggplant fruits treated with BRs under chill stress (Wang et al. 2012; Li et al. 2012; Gao et al. 2015). Meng et al. (2012) also pointed out that 100 μM MeJA slowed the increase rate in electrolyte leakage of white button mushrooms. The L* value was negatively correlated with the electrolyte leakage after 3 μM BL treatment with Pearson’s coefficient of 0.96 (P < 0.05). These results show that the reduced electrolyte leakage found in BL-treated mushrooms is indicative of BL assisting in maintaining membrane integrity and thereby inhibiting membrane permeability. It is possible that this decreases cellular contact between enzymes and substrates (such as phenols) and further contributes to improved color maintenance.
MDA content and LOX activity in all the treatments increased continuously during storage. Treatment with 3 μM BL slowed down the formation of MDA and the increase of LOX activity during the storage. One micro-molar BL treatment was not as effective in inhibiting this increase as 3 μM BL treatment during the whole storage period, but MDA content and LOX activity in the 1 μM BL-treated mushrooms were significantly lower than those in the control mushrooms from day 8 till day 16 (Table 1). This indicates that BL reduced membrane lipid peroxidation of white button mushrooms by inhibiting LOX activity, resulting in decreased MDA production. Similar results were also mentioned by Aghdam and Mohammadkhani (2014) in BL-treated tomato fruits at low temperature. The L* value was negatively correlated with MDA content and LOX activity after 3 μM BL treatment with Pearson’s coefficients of 0.91 and 0.93, respectively (P < 0.05). These results suggest that the reduced membrane lipid peroxidation by BL might contribute to inhibition of cap browning in white button mushrooms.
In general, the O2 ·− production rate in all the samples increased with storage time. Consistently lower O2 ·− production was observed in the BL-treated mushrooms compared with that of the control, especially in mushrooms treated with 3 μM BL. H2O2 content in both the control and BL-treated mushrooms showed a pattern similar to O2 ·− production throughout the storage period (Table 1). Such decreases in both O2 ·− production rate and H2O2 content are also reported in the mushrooms treated with MeJA (Jahangir et al. 2011). These results indicate that BL helps to reduce ROS levels that are directly involved in the oxidation process and actively participate in cell degradation. The L* value was negatively correlated with O2 ·− production rate and H2O2 content after 3 μM BL treatment with Pearson’s coefficients of 0.90 and 0.91, respectively (P < 0.05). The results indicate that inhibition of ROS generation by BL might be correlated with reduced cap browning of white button mushrooms.
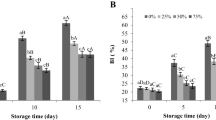
Antioxidant Enzyme Activities
As shown in Fig. 4a, an increase in SOD activity in all the samples was observed during the first 4 days of storage, after which it gradually decreased. SOD activity in the mushrooms treated with 1 and 3 μM BL was 11 and 19 % higher, respectively, than that of the control at day 8. CAT activity in the control mushrooms gradually decreased since day 4 till day 16(Fig. 4b). BL treatments enhanced CAT activity during the postharvest storage period, and notably higher levels of the enzyme were present in the mushrooms treated with 3 μM BL. The control mushrooms showed a slight increase in APX activity at day 4 and a constant decrease thereafter (Fig. 4c). APX activity was elevated in the mushrooms treated with 1 and 3 μM BL and was maintained at relatively high levels, which were 42 and 79 % higher, respectively, than those of the control at day 16. Likewise, Zhu et al. (2010) found that BL enhanced SOD activity and induced disease resistance in jujube fruit, and Wang et al. (2012) reported that BL increased the activities of CAT and APX and induced an antioxidant response to chill stress in pepper fruit. In this study, reduction of ROS by BL during cap browning might be due to BL-induced enhancement of the antioxidant defense system.
Conclusions
Reduced ROS accumulation and membrane oxidative damage induced by BL retards the reaction between PPO and phenolic compounds and leads to the alleviation of enzymatic browning in white button mushrooms. Compared with 1 μM BL, treatment with 3 μM BL is more effective in reducing discoloration on the mushroom caps. Thus, application of BL could be an important strategy in maintaining the color and extending the storage life of postharvest white button mushrooms.
References
Aebi, H. (1984). Catalase in vitro. Methods in Enzymology, 105, 121–126.
Aghdam, M. S., & Mohammadkhani, N. (2014). Enhancement of chilling stress tolerance of tomato fruit by postharvest brassinolide treatment. Food and Bioprocess Technology, 7(3), 909–914.
Anantheswaran, R. C., Roy, S., & Beelman, R. B. (1996). Modified atmosphere and modified humidity packaging of fresh mushrooms. Journal of Food Science, 61(2), 391–397.
Bajguz, A., & Hayat, S. (2009). Effects of brassinosteroids on the plant responses to environmental stresses. Plant Physiology and Biochemistry, 47(1), 1–8.
Bradford, M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, 72(1-2), 248–254.
Brennan, M. H., Le Port, G., & Gormley, R. (2000). Postharvest treatment with citric acid or hydrogen peroxide to extent the shelf life of fresh sliced mushrooms. LWT- Food Science and Technology, 33(4), 285–289.
Chomkitichai, W., Chumyam, A., Rachtanapun, P., Uthaibutra, J., & Saengnil, K. (2014). Reduction of reactive oxygen species production and membrane damage during storage of ‘Daw’ longan fruit by chlorine dioxide. Scientia Horticulturae, 170, 143–149.
Cliffe-Byrnes, V., & O’Beirne, D. (2008). Effects of washing treatment on microbial and sensory quality of modified atmosphere (MA) packaged fresh sliced mushroom (Agaricus bisporus L.). Postharvest Biology and Technology, 48, 283–294.
Dhindsa, R. S., Plumb-Dhindsa, P., & Thorpe, T. A. (1981). Leaf senescence: correlated with increased leaves of membrane permeability and lipid peroxidation and decreased levels of superoxide dismutase and catalase. Journal of Experimental Botany, 32(1), 93–101.
Ding, Z. S., Tian, S. P., Zheng, X. L., Zhou, Z. W., & Xu, Y. (2007). Responses of reactive oxygen metabolism and quality in mango fruit to exogenous oxalic acid or salicylic acid under chilling temperature stress. Physiologia Plantarum, 130(1), 112–121.
Gao, H., Kang, L. N., Liu, Q., Cheng, N., Wang, B. N., & Cao, W. (2015). Effect of 24-epibrassinolide treatment on the metabolism of eggplant fruits in relation to development of pulp browning under chilling stress. Journal of Food Science and Technology, 52(6), 3394–3401.
Gao, H., Zhang, Z. K., Lv, X. G., Cheng, N., Peng, B. Z., & Cao, W. (2016). Effect of 24-epibrassinolide on chilling injury of peach fruit in relation to phenolic and proline metabolisms. Postharvest Biology and Technology, 111, 390–397.
Gill, S. S., & Tuteja, N. (2010). Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiology and Biochemistry, 48(12), 909–930.
Gormley, R. (1975). Chill storage of mushrooms. Journal of the Science of Food and Agriculture, 26, 401–411.
Jahangir, M. M., Jiang, T. J., Jiang, Z. H., Amjad, M., & Ying, T. J. (2011). Methyl jasmonate enhances postharvest physicochemical and microbial quality of button mushroom (Agaricus bisporus). Journal of Food, Agriculture and Environment, 9(2), 91–95.
Jiang, L., Hou, T., Yuan, X., Jiang, J., & Yu, Z. (2010). Effect of storage temperature and packaging method on the decay and physiology of fresh leaves of Gynura bicolor D.C. Journal of Food Processing and Preservation, 34(5), 858–871.
Khan, Z. U., Aisikaer, G., Khan, R. U., Bu, J. W., Jiang, Z. H., Ni, Z. D., et al. (2014). Effects of composite chemical pretreatment on maintaining quality in button mushrooms (Agaricus bisporus) during postharvest storage. Postharvest Biology and Technology, 95, 36–41.
Koorapati, A., Foley, D., Pilling, R., & Prakash, A. (2004). Electron-beam irradiation preserves the quality of white button mushroom (Agaricus bisporus) slices. Journal of Food Science, 69(1), 25–29.
Lagnika, C., Zhang, M., Nsor-Atindana, J., & Bashari, M. (2014). Effects of ultrasound and chemical treatments on white mushroom (Agaricus bisporus) prior to modified atmosphere packaging in extending shelf-life. Journal of Food Science and Technology, 51(12), 3749–3757.
Lei, B. L., Liu, J. Y., & Yao, X. J. (2015). Unveiling the molecular mechanism of brassinosteroids: Insights from structure-based molecular modeling studies. Steroids, 104, 111–117.
Li, B. Q., Zhang, C. F., Cao, B. H., Qin, G. Z., Wang, W. H., & Tian, S. P. (2012). Brassinolide enhances cold stress tolerance of fruit by regulating plasma membrane proteins and lipids. Amino Acids, 43(6), 2469–2480.
Maalekuu, K., Elkind, Y., Leikin-Frenkel, A., Lurie, S., & Fallik, E. (2006). The relationship between water loss, lipid content, membrane integrity and LOX activity in ripe pepper fruit after storage. Postharvest Biology and Technology, 42, 248–255.
Meng, D. M., Song, T. Z., Shen, L., Zhang, X. H., & Sheng, J. P. (2012). Postharvest application of methyl jasmonate for improving quality retention of Agaricus bisporus fruit bodies. Journal of Agricultural and Food Chemistry, 60(23), 6056–6062.
Miklus, M. B., & Beelman, R. B. (1996). CaCl2 treated irrigation water applied to mushroom crops (Agaricus bisporus) increases Ca concentration and improves postharvest quality and shelf life. Mycologia, 88, 403–409.
Mohapatra, D., Frias, J. M., Oliveira, F. A. R., Bira, Z. M., & Kerry, J. (2008). Development and validation of a model to predict enzymatic activity during storage of cultivated mushrooms (Agaricus bisporus spp.). Journal of Food Engineering, 86(1), 39–48.
Nakano, Y., & Asada, K. (1981). Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplast. Plant and Cell Physiology, 22(5), 867–880.
Patterson, B. D., Macrae, E. A., & Ferguson, I. B. (1984). Estimation of hydrogen peroxide in plant extracts using titanium (IV). Analytical Biochemistry, 139(2), 487–492.
Qin, Y. Y., Liu, D., Wu, Y., Yuan, M. L., Li, L., & Yang, J. Y. (2015). Effect of PLA/PCL/cinnamaldehyde antimicrobial packaging on physicochemical and microbial quality of button mushroom (Agaricus bisporus). Postharvest Biology and Technology, 99, 73–79.
Sapers, G. M., Miller, R. L., Miller, F. C., Cooke, P. H., & Choi, S.-W. (1994). Enzymatic browning control in minimally processed mushrooms. Journal of Food Science, 59, 1042–1047.
Scandalios, J. G. (1993). Oxygen stress and superoxide dismutases. Plant Physiology, 101(1), 7–12.
Sevillano, L., Sánchez-Ballesta, M. T., Romojaro, F., & Flores, F. B. (2009). Physiological, hormonal and molecular mechanisms regulating chilling injury in horticultural species. Postharvest technologies applied to reduce its impact. Journal of the Science of Food and Agriculture, 89(4), 555–573.
Sharma, P., Jha, A. B., Dubey, R. S., & Pessarakli, M. (2012). Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. Journal of Botany, 2012, 1–26.
Simón, A., González-Fandos, E., & Vázquez, M. (2010). Effect of washing with citric acid and packaging in modified atmosphere on the sensory and microbiological quality of sliced mushrooms (Agaricus bisporus L.). Food Control, 21, 851–856.
Singleton, V. L., & Rossi, J. A., Jr. (1965). Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. American Journal of Enology and Viticulture, 16(3), 144–158.
Thompson, J. E., Legge, R. L., & Barber, R. F. (1987). The role of free radicals in senescence and wounding. New Phytologist, 105(3), 317–344.
Todd, J. F., Paliyath, G., & Thompson, J. E. (1990). Characteristics of a membrane associated lipoxygenase in tomato fruit. Plant Physiology, 94(3), 1225–1232.
Wang, Q., Ding, T., Gao, L., Pang, J., & Yang, N. (2012). Effect of brassinolide on chilling injury of green bell pepper in storage. Scientia Horticulturae, 144, 195–200.
Yang, E., Lu, W., Qu, H., Lin, H., Wu, F., Yang, S., Chen, Y., & Jiang, Y. M. (2009). Altered energy status in pericarp browning of lychee fruit during storage. Pakistan Journal of Botany, 41(5), 2271–2279.
Ye, J. J., Li, J. R., Han, X. X., Zhang, L., Jiang, T. J., & Xia, M. (2012). Effects of active modified atmosphere packaging on postharvest quality of shiitake mushrooms (Lentinula edodes) stored at cold storage. Journal of Integrative Agriculture, 11(3), 474–482.
Yingsanga, P., Srilaong, V., Kanlayanarat, S., Noichinda, S., & Mcglasson, W. B. (2008). Relationship between browning and related enzymes (PAL, PPO and POD) in rambutan fruit (Nephelium lappaceum Linn.) cvs. Rongrien and See-chompoo. Postharvest Biology and Technology, 50(2-3), 164–168.
Zhao, R. R., Sheng, J. P., Lv, S. N., Zheng, Y., Zhang, J., Yu, M. M., & Shen, L. (2011). Nitric oxide participates in the regulation of LeCBF1 gene expression and improves cold tolerance in harvested tomato fruit. Postharvest Biology and Technology, 62(2), 121–126.
Zhu, L. Q., Zhou, J., Zhu, S. H., & Guo, L. H. (2009). Inhibition of browning on the surface of peach slices by short-term exposure to nitric oxide and ascorbic acid. Food Chemistry, 114(1), 174–179.
Zhu, Z., Zhang, Z. Q., Qin, G. Z., & Tian, S. P. (2010). Effects of brassinosteroids on postharvest disease and senescence of jujube fruit in storage. Postharvest Biology and Technology, 56(1), 50–55.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 31401551), the Special Fund for Agro-scientific Research in the Public Interest (No. 201303080), the Fundamental Research Funds for the Central Research Institutes (No. 0032015017), and the Agricultural Science and Technology Innovation Program (ASTIP) from the Chinese Central Government.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Ding, Y., Zhu, Z., Zhao, J. et al. Effects of Postharvest Brassinolide Treatment on the Metabolism of White Button Mushroom (Agaricus bisporus) in Relation to Development of Browning During Storage. Food Bioprocess Technol 9, 1327–1334 (2016). https://doi.org/10.1007/s11947-016-1722-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-016-1722-1