Abstract
A trace element, iron (Fe) plays a pivotal role in photosynthesis process which in turn mediates the plant growth and productivity. Here, we have focused majorly on the photochemistry of photosystem (PS) II, abundance of proteins, and organization of supercomplexes of thylakoids from Fe-depleted cells in Chlamydomonas reinhardtii. Confocal pictures show that the cell's size has been reduced and formed rosette-shaped palmelloids; however, there is no cell death. Further, the PSII photochemistry was reduced remarkably. Further, the photosynthetic efficiency analyzer data revealed that both donor and acceptor side of PSII were equally damaged. Additionally, the room-temperature emission spectra showed the fluorescence emission maxima increased due to impaired energy transfer from PSII to PSI. Furthermore, the protein data reveal that most of the proteins of reaction center and light-harvesting antenna were reduced in Fe-depleted cells. Additionally, the supercomplexes of PSI and PSII were destabilized from thylakoids under Fe-deficient condition showing that Fe is an important element in photosynthesis mechanism.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Iron is a relatively abundant micronutrient in the earth’s crust, which often chronically limits to photosynthesis in the ocean and on land also. Iron is present predominantly in the poor form of insoluble complexes, such as Fe(III) ferric oxides in oxygen-rich surface water and neutral to alkaline soil. The soluble form of Fe(II) is very limited in soil, and the low bioavailability of iron complexes creates a major obstacle for photosynthetic organisms (Glaesener et al. 2013). Fe deficiency in photosynthetic organisms is evident by the development of chlorosis (loss of chlorophyll), which is accompanied by loss of photosynthetic machinery and inhibition of photosynthetic electron transport reactions (Spiller and Terry 1980).
It is well known that Fe is a cofactor in photosystem (PS) II, PSI, the cytochrome (Cyt) b6/f complex, and Cyt c6 (Yadavalli et al. 2011, 2012a). Since PSI is the prime target because of its relatively high Fe content (12 Fe per PSI) and for instance, changes from 4:1 ratio of PSI:PSII to 1:1 under Fe deficiency in cyanobacteria (Straus 2004). Further, Fe deficiency causes a decrease in the number of PSI complex, resulting in a bottleneck in the photosynthetic electron flow (Strzepek and Harrison 2004). Most of the cyanobacteria species express the auxiliary light-harvesting proteins which increase the cross section of PSI to balance a similar level of electron throughput with a smaller Fe investment to avoid the oxidative stress in the cell (Chauhan et al. 2011). In cyanobacteria, the alteration in the pigment–protein complex and appearance of an additional pigment–protein complex around PSII (IdiA: Iron deficiency induced protein) protect PSII at the acceptor side against damage (Michel et al. 1996; Michel and Pistorius 2004) and formation of an iron stress-induced protein A (IsiA) (Boekema et al. 2001; Chauhan et al. 2011). Fe deficiency induces a large drop in PSI/PSII unit ratio by expression of isiA gene product CP43′ that forms 18 subunits around the trimeric PSI core (Andaluz et al. 2006).
Fe deprivation in the green alga Chlamydomonas (C.) reinhardtii results in remodeling of PSI, changes in the LHCI polypeptide composition, and decreased the efficiency of energy transfer between the light-harvesting and reaction center (RC) (Moseley et al. 2002; Naumann et al. 2005). We have also reported that Fe deficiency induces rapid reduction of the levels of photosynthetic pigments due to a decrease in chlorophyll synthesis. Chlorophyll is important not only as a light-harvesting pigment but also has a structural role, particularly in the pigment-rich light-harvesting complex (LHC)I subunits. The reduced level of chlorophyll molecules inhibits the formation of large PSI–LHCI supercomplexes, further decreasing the photosynthetic efficiency in C. reinhardtii (Yadavalli et al. 2012a). Furthermore, in Dunaliella salina, a photoautotrophic green alga, a homolog protein is induced by Fe deficiency. In which Fe deprivation induced increased cell size and formation of Tidi-LHCI antenna system, coupled to the reaction center of PSI (Varsano et al. 2006).
In lettuce plants, the effect of different degrees of iron deficiency in leaf disks, the photooxidation/reduction kinetics of P700, and the chlorophyll content was severely affected. Besides a strong decline in active P700, measurements of the dark rereduction of oxidized P700 (P700+) after the termination of FR-light have shown that the different path-ways of cyclic electron flow around PSI were largely modified under iron-deficient conditions (Msilini et al. 2013a, b). During Fe deprivation in rice, the PSI core proteins PsaC and PsaD decreased by about 50 %, and PsaE was completely degraded. Among the LHCI subunits, Lhca1 and Lhca2 decreased by 40 and 50 %, respectively, whereas Lhca3 and Lhca4 were completely degraded. The dissociation of LHCI subunits may be due to increased levels of reactive oxygen species, which is suggested by the increased activity of superoxide dismutase (Yadavalli et al. 2012a, b). Also, in higher plants, Fe deficiency leads to decrease in the abundance of photosynthetic proteins, electron transport chain (Andaluz et al. 2006), PSI level (Timperio et al. 2007), and PSII quantum yield (Msilini et al. 2011). Further, in C. reinhardtii, Fe deprivation associated with antenna proteins in the two photosystems is differentially affected, although LHCII overall abundance and composition remain fairly stable (Naumann et al. 2005).
It is clear that Fe deprivation leads to pronounced changes in PSI but has less affect on PSII. Thus, in this study, we have focused mainly on Fe deficiency in PSII from C. reinhardtii. The present study focuses on changes in the morphology, functional aspects, thylakoid organization, and protein profile of the PSII core and LHCII complexes.
Materials and methods
Growth and culture conditions
Cells of wild-type C. reinhardtii strain CC125 (obtained from the Chlamydomonas resource center, University of Minnesota, USA) were grown following the standard procedures (Subramanyam et al. 2006; Subramanyam et al. 2010). An initial culture of C. reinhardtii cells was harvested and washed twice with Fe-free TAP medium and then inoculated into Fe-free TAP media of 1.0 L culture flask. Cells were grown under both control (Fe sufficient) and Fe-deficient conditions with continuous illumination (30–40 µmol m−2 s−1). Thylakoid membranes were isolated from lysed cells according to Fischer et al. (1997) and resuspended in buffer containing 200 mM sorbitol, 5 mM Tris-HCL (pH 7.5), and 5 mM CaCl2.
Confocal microscopy
Chlamydomonas reinhardtii cells from both iron-sufficient and iron-deficient media were subsequently immobilized with molten agar at room temperature as described earlier (Neelam and Subramanyam 2013). All the cells were captured using (Leica Microsystems, Heidelberg) with an equal magnification of 10 µm.
Fluorescence measurements
The room-temperature fluorescence emission spectra were measured as reported previously by Msilini et al. (2013a). The fluorescence spectra were measured with a Perkin-Elmer LS55 spectrofluorimeter. The excitation and emission spectral widths were fixed at 6 nm, respectively. Chl fluorescence was excited at 436 nm. The Chl content of the samples was adjusted to 10 µg mL−1.
The fast OJIP fluorescence transient measurements
Chlorophyll fluorescence transients were measured with the Plant Efficiency Analyzer (PEA) at room temperature. In the Handy PEA fluorescence instrument (Hansatech Instruments Ltd., UK), activating light is provided by an array of three high-intensity light-emitting diodes, which are focused onto the cell surface to provide uniform illumination. The diodes provide red light with a peak wavelength of 650 nm and an intensity of 3000 μmol photons m−2 s−1, which is readily absorbed by the chloroplasts. 1 ml of actively growing cells was taken from both control (+Fe) and iron-deficient (−Fe) cells. Then each cell suspension was kept in dark for 30 min at room temperature. 10 µM of Diuron (DCMU) was mixed with iron-sufficient (+Fe) and iron-deficient (−Fe) cells, respectively. The fluorescence measurements were taken after 30 min of dark incubation as described in (Antal et al. 2006; Kodru et al. 2015). The fluorescence transient at 20 μs is designated F 0, and the maximum fluorescence yield (F m) occurs around 200 ms. The difference between F 0 and F m is known as variable fluorescence (F v). The ratios F v/F 0 and F v/F m are used to assess photosynthetic efficiency. O-J-I-P kinetics were visualized using Biolyzer HP 3 software (for calculating JIP test parameter that quantifies energy flow through PSII) developed by Bioenergetics Laboratory, University of Geneva, Switzerland.
Blue Native gel electrophoresis
Thylakoid membranes were isolated from control (Fe sufficient), Fe-deficient cells, and repelled as mentioned above. First dimension of blue native gel separation was carried out in 4–12 % gradient gel by solubilization of thylakoids in 1 % n-dodecylβ-d-maltoside (DM) along with the protease inhibitors 1 mM Aminocaproic acid (ACA), 1 mM benzamidine hydrochloride, and 1 mM PMSF; the gel with 50 mM ACA was run at 4 °C with increasing voltage 35, 55, 75, 120, 150, and 200 V as described in references (Schägger and von Jagow 1991; Madireddi et al. 2014).
Immunoblots
Thylakoid membranes isolated from both iron-sufficient and iron-deficient cells were separated on SDS-PAGE. Electrophoresis was performed on 12 % resolving and 5 % stacking gel of polyacrylamide. An equal amount of chlorophyll content was loaded on to each lane. Proteins were separated by electrophoresis and transferred to PVDF membrane, incubated with primary antibodies, and detected, as described by Towbin et al. (1979) for identification and quantification of polypeptides contained in the PSII–LHCII supercomplex. Primary antibodies dilutions were PsbA, 1:5000; PsbB, 1:5000; PsbO, 1:5000; PsbP, 1:5000; and LHCII complex proteins Lhcb1, 1:5000; Lhcb2, 1:5000; CP29, 1:10,000; CP26, 1:5000; and Lhcbm5, 1:10,000. All antibodies were purchased from Agrisera. Subsequently, membrane was incubated with the HRP-linked secondary antibody with dilution of 1:10,000, purchased from Cell signaling technology and for D1 antibody, the antichicken secondary antibody was used. The images were then detected by chemiluminescence.
Results
Growth curve and morphological changes observed from confocal images (Fig. 1a) show control (+Fe) and iron-deficient (−Fe) cells grown under optimal growth conditions for 6 days. The control (+Fe) cells exhibited optimal growth, whereas iron-deficient (−Fe) cells showed slow growth pattern. Control (+Fe) cells were found to be active and high optical density in log phase but it exhibited slow growth rate in iron (−Fe)-deficient cells. It is evident from the results that iron (−Fe)-deficient cells showed reduced growth when compared to control (+Fe) cells (Fig. 1a). The confocal images were obtained from autofluorescence generated from chlorophyll in C. reinhardtii cells. The chlorophyll was excited at 488 nm, and the emission range was in between 600–700 nm. The control (wt) cells reveal normal shape. However, the cells were appearing in rosette shape (Fig. 1b) called as palmelloids in Fe-deficient conditions. This indicates a characteristic feature of algae when grown under adverse conditions (Neelam and Subramanyam 2013). In control, the size of the cells is measured as 10–12 µm, whereas in Fe-deficient the cell size is measured as 4–6 µm which indicates the size of the cells decreased under Fe-deficient condition. The cells were in a dividing state which was grouping together. These cells grown in Fe stress resemble the electrical double layers.
a Growth curve of C. reinhardtii (wt) cells grown under control (+Fe) and iron-starved (−Fe) conditions for 6 days O.D at 750 nm. b Confocal microscopy images of C. reinhardtii showing altered cell size and cell morphology as forming palmelloid when grown under control (+Fe) and Fe-starved conditions. All images were taken equal magnification of 10 µm
Analysis of fluorescence emission spectra
The RT fluorescence emission spectra recorded from isolated thylakoids of wt cells showed the typical peak at 683 nm and a broad shoulder at 710–740 nm which corresponds with the previous report (Ferroni et al. 2011). The 683 nm peak originated mainly from PSII, while the long wavelength emission is assigned to PSI–LHCI with the contribution of vibrational satellites of PSII (Ferroni et al. 2011). The RT fluorescence emission spectra of thylakoids from four-day grown cells in Fe-deficient medium showed a significant increase of maximum fluorescence at 683 nm than control (without Fe). This indicates the energy transfer from PSII to PSI is impaired. Fe was supplemented to the Fe-deficient cells after four days of growth (equal to the Fe concentration in +Fe medium) and further the cells were grown for 32 h and the RT fluorescence emission spectrum was measured from isolated thylakoids. The fluorescence emission almost reached the control (wt) level indicating that photosynthesis (excitation electron transport) was restored like in control (Fig. 2).
Room-temperature fluorescence emission spectra of the C. reinhardtii cells grown under control (+Fe), iron deprivation (−Fe), and repelled (+Fe) conditions. Measurements were performed with equal Chl concentration of 10 µg/ml. Each curve is the average of 3 different measurements from three independent samples and obtained identical spectra
Analysis of transient Chla fluorescence
The transient Chla fluorescence is sensitive to various abiotic stresses, which can be analyzed by the JIP test. When dark-adapted photosynthetic cells were exposed to a 1-s pulse of strong saturating light, the transient Chla fluorescence shows complex kinetics referred to as the OJIP transient. The first step was the rise in fluorescence from the O (F o) to the J level in about 2 ms. The second step consists of an increase in Chla fluorescence intensity to the I level in about 30 ms; this was followed by a slow increase in fluorescence to the P peak (F m). It is generally accepted that the OJ phase reflects the reduction of \( Q_{\text{A}}^{ - } \) while JI could represent a further closure of PSII reaction centers with their accumulation in \( Q\;Q_{\text{B}}^{2 - } \) stage (Kodru et al. 2015). The IP phase reflects a further reduction of the PQ pool due to a decrease in its reoxidation, as the electron carriers beyond Cytb 6 /f become reduced. Schansker et al. (2005) used methylviologen and dibromothymoquinone treatments in pea leaves to show that the IP phase is related to PSI activity.
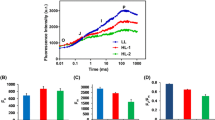
The fluorescence data show significant differences during the time course, and at the three phases of OJIP transients between the control and Fe-deficient cells (Fig. 3a, b). In control cells, the three phases of OJIP are very clear and have attained maximum fluorescence indicating the efficient electron transport in the Z-scheme, which constitutes PSII, Cytb 6 /f, and PSI in a sequential order. The initial fluorescence level F o decreased at 24 h under iron deficiency (data not shown), however after 72 h of growth, it recovered to the control level (Supplementary Fig. 1). A decline in F o has also been reported in lettuce under iron-deficient condition (Msilini et al. 2011). The onset of Fe deficiency caused the F v/F o and F v/F m values to increase, followed by a decrease after prolonged Fe deficiency (Fig. 3). Initially, after 24 h under iron deficiency PSII acceptor side limitation, incomplete \( Q_{\text{A}}^{ - } \) reduction at OJ phase, very little accumulation of \( Q_{\text{A}}^{ - } \;Q_{\text{B}}^{2 - } \) and PQ pool reduction at JI phase, and limitation in further reduction of PQ pool and impairment of PSI activity were found. The electron transport beyond QA can be blocked by DCMU. DCMU-treated control cells (+Fe) depict that the P level was close to the Fm value of untreated cells (Fig. 3a). However, in the DCMU-treated Fe-deficient cells, the P values were reduced. This indicates that in Fe-deficient cells (in the absence of DCMU) at the maximum of the peak in the 0.2–2 s time range (P) not all PSII reaction centers were closed, and thus the F v/F m and F v/F o were low (Fig. 3c, d). This, however, does not necessarily mean that the maximum quantum yield of PSII was very much reduced in Fe deficiency.
a Chla fluorescence transients (OJIP) curves from (wt) control (+Fe), control with DCMU (3-(3,4-dichlorophenyl)-1,4-dimethyl urea) for 30 min in darkness, with 10 µM concentration. Logarithmic time scale of C. reinhardtii cells with and without Fe; b the same on a linear scale; c F v/F m; d F v/F o. Excitation of samples at 3000 µmol photons m−2 s−1 650 nm light. The experiments were repeated three times from different cultures
In addition to damage to PSII, reduced electron flow through PSII may occur as a consequence of the retardation of the transfer of electrons from the reaction center to the PQ pool. This is due to the immediate onset of stress conditions and thereby the cells were unable to cope with the Fe deficiency. However, the three phases of OJIP transient can be seen with a slight reduction in I peak (Fig. 3a). The kinetics of OJIP transient after 48 h show that cells adapted to the Fe-deficient conditions and I peak almost absent, but J and P peaks are still clear which indicates a limitation of PQ pool reduction (data not shown). After 72 h under iron-deficient conditions, the phases of the curve were no longer distinguishable; complete loss of I and P peaks indicates lower concentrations of \( Q_{\text{A}}^{ - } \;Q_{\text{B}}^{2 - } \) , and PQH2, as well as impairment of PSI and PSII under Fe-deficient conditions.
Characterization of supercomplexes from thylakoid membrane
Blue native polyacrylamide gel electrophoresis (BN-PAGE) is one of the efficient tools to analyze the membrane supercomplexes. Hence, BN-PAGE was used for the separation of thylakoid membrane complexes from wt (containing Fe) and iron deficiency of C. reinhardtii grown under optimal growth conditions. By solubilization with a mild detergent like β-DM, we could separate the supercomplexes of C. reinhardtii as reported earlier (Madireddi et al. 2014). The results obtained from wt were similar to that of our previous observation (Madireddi et al. 2014) where the PSI–LHCI, PSII–LHCII, PSII RCC dimer, ATP synthase, Rubisco, Cyt b6f, PSI monomer, PSII RCC, LHC trimers, and LHC monomers were separated. However, there were significant changes in abundance of supercomplexes in isolated thylakoids from Fe deficiency (Fig. 4). The major supercomplexes PSII–LHCII and PSI–LHCI were completely destabilized (Fig. 4 lane 2) in Fe-deprived conditions. Also, PSII reaction center dimer, ATP synthase, RUBISCO, and Cyt b6f complexes were not assembled in Fe-deficient cells. Interestingly, the PSI and PSII monomers significantly increased. There are 2 LHCII trimers separated in wt; surprisingly, one of the trimer (LHC 3a) content has been increased, whereas, LHC 3b content has been decreased. The recovery by the addition of Fe to cells induced supercomplexes assembly similar to the control cells, which indicates that Fe is very important for the stabilization of supercomplexes and for the efficient performance of photosynthesis.
Blue native PAGE of thylakoid membranes isolated from control (+Fe), iron-deprived (−Fe), and iron-replenished cells after deprivation (+Fe) were solubilized with 1 % β-DM and separated by BN-PAGE, complexes are identified according to previous literature. Equal amount of chlorophyll (20 µg) was loaded into each lane
Further, the BN-PAGE gels were placed on second dimension (SDS-PAGE) to separate the individual proteins. The wild-type supercomplexes were separated into individual proteins of phenotypic in nature (data not shown). Surprisingly, both PSII–LHCII and PSI–LHCI protein accumulation were decreased under Fe-deficient conditions. Interestingly, the protein content of LHC monomers is not much affected. Though the LHCs are present, they were not forming supercomplexes with PSI and PSII. Hence, the excited electron transfer is impaired.
Reaction center protein profile of PSII changes in Fe deficiency
Since there are remarkable changes in supercomplex arrangements, one would expect their protein content to be altered. We separated the thylakoid proteins of both wt and Fe-deficient thylakoids on SDS-gels and transferred those proteins onto PVDF membrane. These blots were probed with different antibodies of PSII core and LHCII. Figure 5a, depicts the core proteins of PSII core antenna, CP43 and CP47 are significantly diminished their content. Further, reaction center proteins of D1 and D2 content also decreased. Among the water oxidation complex subunits, PsbO content has been reduced significantly. However, PsbP is not changed in Fe-deficient conditions (Fig. 5a).
Immunoblots of PSII and LHC proteins content from control (+Fe) and Iron-deficient (Fe) cells. Equal chlorophyll was loaded with different dilutions (25, 50 and 100 %) as a positive control and was separated by SDS gel electrophoresis. a PSII core proteins D1, D2, OEC (PsbO, PsbP), and Cyt b6; b major LHCII proteins LhcB1, LHcb2, CP29, CP26, and Lhcbm5 are detected. The adjacent bar diagram were developed by Image J software to show the quantity of protein present
Light-harvesting complexes protein profile change in PSII
LHCII is the most abundant membrane protein complex on earth. It is well known that it participates in the efficient harvesting of sunlight and transferring excitation energy to the core complex in the photosynthesis process. Here, we have checked the abundance of LHCII proteins under Fe-deficient condition to understand the level of harvesting energy. Interestingly, the major LHCII trimer subunits (Lhcb1 and Lhcb2) were reduced, whereas the minor subunits CP26 and LhcBm5 were not significantly changed. However, CP29 and other minor subunits have remarkably changed under Fe deficiency (Fig. 5b). These results indicate that changes in LHCII protein level would hamper the efficiency of light-harvesting capacity and reduce the transfer of light energy to the reaction center.
Discussion
Iron is very well known as an essential element for plant growth and nutrition, but the effect of Fe deficiency at the photosynthetic level, particularly PSII is not well understood. Previously, we have shown that Fe deficiency induces severe damage to the PSI which leads to decreased electron transport in C.reinhardtii and rice (Yadavalli et al. 2012a, b). Iron is an essential element for Chl biosynthesis, pigment–protein complexes, and hence these are the first target toward iron deficiency. When the cells were grown under Fe-deficient conditions, the cells were observed as clumps which have a peculiar characteristic feature as cells assemble into a rosette-shaped structure (Fig. 1b). This kind of phenomenon is known as palmelloid which we have also observed in salt stress (Neelam and Subramanyam 2013). It is known that C.reinhardtii is a unicellular algae with an average diameter of 10 µm which has been decreased in size of palmelloids in Fe-deficient condition. The previous reports suggested the palmelloid formation in C. reinhardtii is either due to flagellar loss/malfunction or progressive membrane gelatinization after its interaction with the environment (Lürling and Beekman 2006). In C. applanata, under low pH of 3.4 excess mucilage production enables sticking of palmelloid colonies together that results in large clumps (Visviki and Santikul 2000). Our results indicate that the palmelloid formation in Fe deficiency may be due to cell-wall abnormality.
Interestingly, the cell viability is the same as in control indicates even after four days of growth (Fig. 1a) but the cell size has been decreased. These data are an agreement with the palmelloid formation; at this stage the cell size has been reduced which indicates the photosynthetic activity was reduced. After being grown for 72 h under Fe-deficient conditions, the first stage of inhibitory action was detected as a relative increase of the amplitude of the OJ phase of the Chl fluorescence transient with a corresponding decline of JI, which agrees well with the treatment of pea leaves with dibromothymoquinone (DBMIB). DBMIB is an artificial quinone which inhibits the reoxidation of plastoquinol by binding to the Cytb 6 /f complex, causing the IP phase to disappear (Schansker and Strasser 2005). The JI phase was previously shown to be complementary to O–J, the sum of the amplitudes of OJ and JI representing the complete reduction of Q A (Kodru et al. 2015). The OJ phase is related to the initial reduction of Q A (Kodru et al. 2015) and its enhancement was shown to be associated with a slower rate of electron transfer between Q A and Q B. JI phase declined in Fe deficiency as compared to control, and similar results have been obtained when C. reinhardtii cultures were deprived of sulfur (Antal et al. 2006), and limitation of Fe in lettuce (Msilini et al. 2011) suggesting that Fe and S deprivation might function similarly, through their effects on the Fe–S clusters of the electron transport chain. This is also confirmed by the addition of DCMU to cells which showed a decline of JIP phases under Fe deficiency. Also, C. reinhardtii belongs to the C3 type and the contribution of PSI activity to F o was about 30 %; the deviation in F o, which is observed in Fig. 3, is most likely due to both PSI and PSII activity. By 72 h of Fe deficiency, the level of I peak of Fe-stressed cells approaches 90 % of F m. There is also some deviation of F m from the control value, possibly due to the cumulative effects of Fe deficiency on the entire photosynthetic electron transport chain. The decline in F v/F m together with the amplitudes of the O–J and I–P phases of the curves indicates that the OEC failed to provide electrons for PSII to reduce adequately the quinone acceptors of the photosystem in the absence of Fe, thus decreasing the maximal fluorescence yield. Also, the P phase was remarkably altered even in DCMU-treated cells indicating that acceptor side of PSII was also damaged. Thus, these results could suggest that the effects of Fe deficiency cause impairment of PSII, both acceptor and donor side of PSII. Also, these results were in agreement with the RT fluorescence emission data that increase in fluorescence maximum at 683 nm, indicating the energy transfer block from Chl a to PSII (Fig. 2). If there is a block in excitation energy transfer from Chl a to the reaction center, obviously the PSII fluorescence will increase. This was well supported by the previously published work on UV-B effect on cyanobacteria Spirulina platensis, considering this increase is due to uncoupling of energy transfer from Chl a to PSII which induced enhanced emission (Kolli et al. 1998). However, the addition of Fe to control cells shows the restoration of photosynthetic function within 32 h. This is confirmed by fluorescence emission, blue native gel, and immunoblot analysis of PSII core and LHCII subunits.
Further, supercomplexes of PSI and PSII from thylakoids have been equally disorganized. The monomeric forms of PSI, PSII, and LHC’s were existing but to some extent the content of supercomplexes has been increased (Fig. 4). Because of Fe deficiency, an organization of supercomplexes was not formed besides addition of Fe; it has been restored after 32 h. Thus, Fe plays an important role in the formation or organization of supercomplexes. Under Fe-deficient conditions the Chl content was reduced. However, it is well known that iron limitation induces leaf chlorosis due to the decrease of Chl content. Subsequently, a pronounced iron deficiency resulted in a reduction of light-harvesting complexes content in both PSI and PSII (Timperio et al. 2007; Yadavalli et al. 2012a, b). We also reported that the detergent-solubilized thylakoids were separated on a sucrose gradient, which showed that PSI supercomplexes were subverted. The destabilization of supercomplexes in Fe deficiency may be due to less Chl and protein biosynthesis. It is clear that if the Chl biosynthesis or protein synthesis is slowed down or retarded, it may obviously cause disorganization of supercomplexes of PSI and PSII which attributes to hampered photochemistry.
Further, the protein accumulations of PSII were characterized to see the abundance of proteins in Fe deficiency. In which most of the reaction center proteins of PSII were impaired except the PsbP (Fig. 5a). It is expected that excitation energy transfer from PSII to PSI is hampered due to less accumulation of reaction center proteins. Additionally, the LHCII proteins (Lhcb1, Lhcb2, CP26, CP29, and LhcBm5) were significantly reduced in Fe-depleted cells which indicate that light-harvesting capacity was minimized (Fig. 5b). It is well known that accumulation of PSI–LHCI proteins was declined due to lack of required amount of Fe. Plants change their antenna size depending on the light environment to optimize light-harvesting capacity (Anderson and Andersson 1988). PSI appears to be the first target of iron deficiency (Moseley et al. 2002), perhaps because of its high Fe content as the electron transfer involves 12 iron atoms in three Fe–S centers. As a consequence, PSI can be less tolerant than PSII in case of iron stress because any iron deficiency may have a site of action on the acceptor side of PSI where the majority of Fe–S centers were localized. Photoheterotrophic Chlamydomonas (CC424 mt-) to iron starvation showed decreased photosynthetic proteins, while respiratory proteins were increased suggests that the prioritization of respiration over photosynthesis in iron-deficient condition (Naumann et al. 2007). Also, when the C. reinhardtii cells grown under Fe deficiency in both photoheterotrophically (acetate) and photoautotrophically (CO2), the accumulation of proteins was different. From these results, we demonstrate that photoheterotrophically grown cells show less accumulation of proteins, but there is no significant change in proteins while cells were grown photoautotrophically. In the presence of acetate, iron-limited Chlamydomonas cells maintain high growth rates by suppressing photosynthesis and increasing the respiration, while phototrophic cells maintain efficient photosynthetic systems, but still lose overall photosynthetic activity at the beginning of iron deficiency, which is delayed in phototrophic cells may be due to the increased iron content (Terauchi et al. 2010). Hence, the above data are very well in agreement with our studies that under photoheterotrophic conditions the photosynthesis is vulnerable to Fe deficiency. In our case, the PSII also damaged remarkably apart from PSI when Fe is depleted. Hence, the PSII photochemistry is impaired.
In conclusion, the cell viability is similar in both control and Fe-deficient cells. However, the cell size has been reduced. Further, the complete depletion of Fe affects the photochemistry of PSII, which indicates that the electron transport from Q A to Q B is impaired. Also, it suggests that both acceptor and donor side of PSII has been damaged, which is concomitant with the decreased abundance of both reaction center antenna and core proteins. Also, the differential degradation or damage of LHCII proteins observed may be due to loss of chlorophyll biosynthesis. Further, the organization of supercomplexes was disturbed due to lack of Fe and hence, Fe may be required for the organization of the thylakoid supercomplexes.
References
Andaluz S, Lopez-Millan AF, De las Rivas J, Aro EM, Abadia J, Abadia A (2006) Proteomic profiles of thylakoid membranes and changes in response to iron deficiency. Photosynth Res 89:141–155
Anderson JM, Andersson B (1988) The dynamic photosynthetic membrane and regulation of solar energy conversion. Trends Biochem Sci 13:351–355
Antal TK, Volgusheva AA, Kukarskih GP, Bulychev AA, Krendeleva TE, Rubin AB (2006) Effects of sulfur limitation on photosystem II functioning in Chlamydomonas reinhardtii as probed by chlorophyll a fluorescence. Physiol Plant 128:360–367
Boekema E, Hifney A, Yakushevska AE, Piotrowski M, Keegstra W, Berry S, Michel KP, Pistorius EK, Kruip J (2001) A giant chlorophyll–protein complex induced by iron deficiency in cyanobacteria. Nature 412:745–748
Chauhan D et al (2011) A novel photosynthetic strategy for adaptation to low-iron aquatic environments. Biochemistry 50:686–692
Ferroni L, Baldisserotto C, Giovanardi M, Pantaleoni L, Morosinotto T, Pancaldi S (2011) Revised assignment of room-temperature chlorophyll fluorescence emission bands in single living cells of Chlamydomonas reinhardtii. J Bioener Biomem 43:163–173
Fischer N, Sétif P, Rochaix J-D (1997) Targeted mutations in the psaC gene of Chlamydomonas reinhardtii: preferential reduction of FB at low temperature is not accompanied by altered electron flow from photosystem I to ferredoxin. Biochemistry 36:93–102
Glaesener AG, Merchant SS, Blaby-Haas CE (2013) Iron economy in Chlamydomonas reinhardtii. Front Plant Sci 4:337
Kodru S, Malavath T, Devadasu E, Nellaepalli S, Stirbet A, Subramanyam R, Govindjee (2015) The slow S to M rise of chlorophyll a fluorescence reflects transition from state 2 to state 1 in the green alga Chlamydomonas reinhardtii. Photosynth Res 125:219–231
Kolli BK, Tiwari S, Mohanty P (1998) Ultraviolet-B induced damage to photosystem II in intact filaments of Spirulina platensis. Zeitschrift fuer Naturforschung. C, A J Biosci 53:369–377
Lürling M, Beekman W (2006) Growth of Daphnia magna males and females fed with the cyanobacterium Microcystis aeruginosa and the green alga Scenedesmus obliquus in different proportions. Acta Hydrochim Hydrobiol 34:375–382
Madireddi SK, Nama S, Devadasu ER, Subramanyam R (2014) Photosynthetic membrane organization and role of state transition in cyt, cpII, stt7 and npq mutants of Chlamydomonas reinhardtii. J Photochem Photobiol B 137:77–83
Michel KP, Pistorius EK (2004) Adaptation of the photosynthetic electron transport chain in cyanobacteria to iron deficiency: the function of IdiA and IsiA. Physiol Plant 120:36–50
Michel K-P, Thole HH, Pistorius EK (1996) IdiA, a 34 kDa protein in the cyanobacteria Synechococcus sp. strains PCC 6301 and PCC 7942, is required for growth under iron and manganese limitations. Microbiol 142:2635–2645
Moseley JL, Allinger T, Herzog S, Hoerth P, Wehinger E, Merchant S, Hippler M (2002) Adaptation to Fe-deficiency requires remodeling of the photosynthetic apparatus. EMBO J 21:6709–6720
Msilini N, Zaghdoudi M, Govindachary S, Lachaal M, Ouerghi Z, Carpentier R (2011) Inhibition of photosynthetic oxygen evolution and electron transfer from the quinone acceptor Q −A to Q B by iron deficiency. Photosynth Res 107:247–256
Msilini N, Essemine J, Zaghdoudi M, Harnois J, Lachaal M, Ouerghi Z, Carpentier R (2013a) How does iron deficiency disrupt the electron flow in photosystem I of lettuce leaves? J Plant Physiol 170:1400–1406
Msilini N, Oueslati S, Amdouni T, Chebbi M, Ksouri R, Lachaâl M, Ouerghi Z (2013b) Variability of phenolic content and antioxidant activity of two lettuce varieties under Fe deficiency. J Sci Food Agri 93:2016–2021
Naumann B, Stauber EJ, Busch A, Sommer F, Hippler M (2005) N-terminal processing of Lhca3 Is a key step in remodeling of the photosystem I-light-harvesting complex under iron deficiency in Chlamydomonas reinhardtii. J Biol Chem 280:20431–20441
Naumann B, Busch A, Allmer J, Ostendorf E, Zeller M, Kirchhoff H, Hippler M (2007) Comparative quantitative proteomics to investigate the remodeling of bioenergetic pathways under iron deficiency in Chlamydomonas reinhardtii. Proteomics 7:3964–3979
Neelam S, Subramanyam R (2013) Alteration of photochemistry and protein degradation of photosystem II from Chlamydomonas reinhardtii under high salt grown cells. J Photochem Photobiol B 124:63–70
Schägger H, von Jagow G (1991) Blue native electrophoresis for isolation of membrane protein complexes in enzymatically active form. Anal Biochem 199:223–231
Schansker G, Strasser RJ (2005) Quantification of non-Q B-reducing centers in leaves using a far-red pre-illumination. Photosynth Res 84:145–151
Schansker G, Tóth SZ, Strasser RJ (2005) Methylviologen and dibromothymoquinone treatments of pea leaves reveal the role of photosystem I in the Chl a fluorescence rise OJIP. Bioch Bioph Acta Bioeng 1706:250–261
Spiller S, Terry N (1980) Limiting Factors in Photosynthesis: II. iron stress diminishes photochemical capacity by reducing the number of photosynthetic units. Plant Physiol 65:121–125
Straus N (2004) Iron deprivation: physiology and gene regulation. In: Bryant D (ed) The molecular biology of cyanobacteria, vol 1., Advances in photosynthesis and respirationSpringer Netherlands, Berlin, pp 731–750
Strzepek RF, Harrison PJ (2004) Photosynthetic architecture differs in coastal and oceanic diatoms. Nature 431:689–692
Subramanyam R, Jolley C, Brune DC, Fromme P, Webber AN (2006) Characterization of a novel Photosystem I-LHCI supercomplex isolated from Chlamydomonas reinhardtii under anaerobic (State II) conditions. FEBS Lett 580:233–238
Subramanyam R, Jolley C, Thangaraj B, Nellaepalli S, Webber A, Fromme P (2010) Structural and functional changes of PSI–LHCI supercomplexes of Chlamydomonas reinhardtii cells grown under high salt conditions. Planta 231:913–922
Terauchi AM, Peers G, Kobayashi MC, Niyogi KK, Merchant SS (2010) Trophic status of Chlamydomonas reinhardtii influences the impact of iron deficiency on photosynthesis. Photosynth Res 105:39–49
Timperio AM, D’Amici GM, Barta C, Loreto F, Zolla L (2007) Proteomics, pigment composition, and organization of thylakoid membranes in iron-deficient spinach leaves. J Exp Bot 58:3695–3710
Towbin H, Staehelin T, Gordon J (1979) Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA 76:4350–4354
Varsano T, Wolf SG, Pick U (2006) A chlorophyll a/b-binding protein homolog that is induced by iron deficiency is associated with enlarged photosystem I units in the eucaryotic alga Dunaliella salina. J Biol chem 281:10305–10315
Visviki I, Santikul D (2000) The pH tolerance of Chlamydomonas applanata (Volvocales, Chlorophyta). Arch environ cont toxicol 38:147–151
Yadavalli V, Malleda C, Subramanyam R (2011) Protein-protein interactions by molecular modeling and biochemical characterization of PSI–LHCI supercomplexes from Chlamydomonas reinhardtii. Mol BioSyst 7:3143–3151
Yadavalli V, Jolley CC, Malleda C, Thangaraj B, Fromme P, Subramanyam R (2012a) Alteration of proteins and pigments influence the function of photosystem I under iron deficiency from Chlamydomonas reinhardtii. PLOS One 7:e35084
Yadavalli V, Neelam S, Rao ASVC, Reddy AR, Subramanyam R (2012b) Differential degradation of photosystem I subunits under iron deficiency in rice. J Plant Physiol 169:753–759
Acknowledgments
R.S was supported by the Council of Scientific and Industrial Research (Nos. 38(1279)/11/EMR-II and 38 (1381)/14/EMR-II), Department of Biotechnology (BT-BRB-TF-1-2012), Department of Science and Technology (DST/INT/JSPS/P-159/2013), and DST-FIST, Govt. of India, for financial support. E.R.D thank RGNF, UGC, India for Junior Research Fellowship.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Devadasu, E.R., Madireddi, S.K., Nama, S. et al. Iron deficiency cause changes in photochemistry, thylakoid organization, and accumulation of photosystem II proteins in Chlamydomonas reinhardtii . Photosynth Res 130, 469–478 (2016). https://doi.org/10.1007/s11120-016-0284-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11120-016-0284-4