Abstract
Light is essential for all photosynthetic organisms while an excess of it can lead to damage mainly the photosystems of the thylakoid membrane. In this study, we have grown Chlamydomonas reinhardtii cells in different intensities of high light to understand the photosynthetic process with reference to thylakoid membrane organization during its acclimation process. We observed, the cells acclimatized to long-term response to high light intensities of 500 and 1000 µmol m−2 s−1 with faster growth and more biomass production when compared to cells at 50 µmol m−2 s−1 light intensity. The ratio of Chl a/b was marginally decreased from the mid-log phase of growth at the high light intensity. Increased level of zeaxanthin and LHCSR3 expression was also found which is known to play a key role in non-photochemical quenching (NPQ) mechanism for photoprotection. Changes in photosynthetic parameters were observed such as increased levels of NPQ, marginal change in electron transport rate, and many other changes which demonstrate that cells were acclimatized to high light which is an adaptive mechanism. Surprisingly, PSII core protein contents have marginally reduced when compared to peripherally arranged LHCII in high light-grown cells. Further, we also observed alterations in stromal subunits of PSI and low levels of PsaG, probably due to disruption of PSI assembly and also its association with LHCI. During the process of acclimation, changes in thylakoid organization occurred in high light intensities with reduction of PSII supercomplex formation. This change may be attributed to alteration of protein–pigment complexes which are in agreement with circular dichoism spectra of high light-acclimatized cells, where decrease in the magnitude of psi-type bands indicates changes in ordered arrays of PSII–LHCII supercomplexes. These results specify that acclimation to high light stress through NPQ mechanism by expression of LHCSR3 and also observed changes in thylakoid protein profile/supercomplex formation lead to low photochemical yield and more biomass production in high light condition.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Light energy is the major source for the photosynthetic process in which carbon fixation occurs for food production. Light-dependent reactions occur in thylakoid membrane that consists of multi-protein membrane complexes namely photosystem (PS) I and II that are connected by cytochrome (Cyt) b6/f complex. Both complexes are composed of core proteins that are responsible for charge separation and electron transport where an outer antenna increases the absorption capacity of photons. PSII and PSI complexes were functionally connected with each other and to their electron donor/acceptor and their capacity for absorbing photons increases by light-harvesting antenna complexes (LHCs) which binds with chlorophyll and carotenoid molecules. The LHCs have a conserved structure with three transmembrane helices and organized with a maximum of 14 chlorophylls (Chls) per monomer (5–9 Chl a and 4–6 Chl b). LHCs also have two carotenoid binding sites i.e., for lutein (Lut) and violaxanthin (Vio) while some LHCs have additionally two sites N1 for neoxanthin (Neo) and V1 for Vio/Lut located at the periphery of the complexes. It was reported that the xanthophylls in the V1 site are loosely bound and do not participate in excitation energy transfer and triplet quenching (Niyogi et al. 1997).
The light source is significant for photosynthesis while its excess exposure can lead to severe damage to the photosystems due to reactive oxygen species (ROS) production that inhibits PSII repair process. It was previously found that an increase in light intensities showed increased levels of both superoxides radical and hydrogen peroxide (H2O2) production by the photosynthetic electron transport chain (Ivanov et al. 2012). Moreover, the total increases in O2·− and H2O2 production take place within electron transport chain components of the thylakoid membrane rather than in stroma region (Mubarakshina et al. 2006). Photosynthetic organism employs various mechanisms to cope up with increasing light intensity based on their time scale of exposure to light conditions (Allorent et al. 2013). Under short-term responses to high light, various mechanisms take place that are sufficient for the rearrangement of chloroplast components without an influence on its biosynthesis or degradation. The major photoprotective mechanisms that triggers from seconds to few hours include thermal dissipation of excess light energy called as non-photochemical quenching (NPQ) (Elrad et al. 2002; Ruban et al. 2012; Suorsa et al. 2012) and state transitions that balance the light energy between two photosystems through LHCII phosphorylation (Pietrzykowska et al. 2014). The process of NPQ occurs mainly by the accumulation of protonated stress-related LHC protein (LHCSR3) which plays a major role in energy-dependent quenching capacity in Chlamydomonas reinhardtii (Peers et al. 2009). It was found that dimeric form of LHCSR3 predominantly binds to the antenna part of PSII supercomplex but not to the core (Semchonok et al. 2017). Generally, the long-term acclimation occurs from hours to weeks and involves selective synthesis and degradation of chloroplast components. The long-term response to high light includes adjustment of PSII antenna size, decrease of chlorophyll (Chl) a/b ratio (Ballottari et al. 2007; Wientjes et al. 2013a, b), and the density of PSII supercomplexes in the thylakoid membrane is modified (Kouřil et al. 2013). It is known that PSII is the major membrane complex involved in acclimation process among all other complexes involved in the light phase of photosynthesis. Its composition, functionality, and proportionality are dynamically adjusted in response to changes in light conditions (Ballottari et al. 2007; Betterle et al. 2009; Johnson et al. 2011; Belgio et al. 2014; Suorsa et al. 2015). Further, accumulation of zeaxanthin upon excess light from pre-existing violaxanthin increases NPQ (Dall’Osto et al. 2005) and also scavenging of ROS production (Dall’Osto et al. 2010). High light illumination would also lead to induce alternative pathways of electron transport, in particular, cyclic electron flow around PSI and the reduction of oxygen to water that is mediated by the plastid terminal oxidase PTOX (Diaz et al. 2007; Ibáñez et al. 2010). High light induced a decrease in LHCII and LHCI transcript levels and also the cellular amount of LHC protein which does not result in an altered LHCII/PSII stoichiometry or functional PSII antenna size (Durnford et al. 2003; Bonente et al. 2012; Mettler et al. 2014). It was also reported that fine-tuned LHC expression is required for the adjustment of photosystem antenna size and its composition that occur during the long-term response of high light (Wientjes et al. 2013a, b).
This study aims to evaluate the impact of high light stress on acclimation and photosynthetic process under long-term exposure/growth of C. reinhardtii. The microalga C. reinhardtii was chosen as the model organism due to its rapid growth rate, its ease of use in laboratory conditions. It is a unicellular green alga and its genome has been sequenced with several molecular and genomic tools available (Merchant et al. 2007) and the complete biology has been described in detail, making this microalga extremely useful to study adaptive responses at cellular, physiological, biochemical, and molecular levels upon exposure to high light conditions. Moreover, stoichiometry proportions of PSI and PSII are equal in C. reinhardtii which is different in higher plants. The thylakoid membrane of C. reinhardtii harbors at least six LHCII trimers per monomeric PSII core complex (Drop et al. 2014). The supercomplexes comprise different antenna sizes that are LhcbM1, LhcbM2/7, and LhcbM3 which are the major components of the trimer in the PSII supercomplex while LhcbM5 is a part of the extra LHCII pool not directly associated with the supercomplex. In higher plants, LHCII is well characterized and composed of three gene products (Lhcb1–3) organized as heterotrimer. Each LHCII apoprotein binds eight Chl a, six Chl b, and four carotenoids. The other Chlorophyll a/b-binding proteins—Lhcb4, Lhcb5, and Lhcb6, also known as CP29, CP26, and CP24, respectively—exist as monomers and have different pigment composition, whereas CP24 is absent in C. reinhardtii (Passarini et al. 2009). However, the LHCI complexes consist of nine subunits arranged as two double layers around PSI core in C. reinhardtii (Yadavalli et al. 2011a). Thus, the composition of photosystems and energy transfer process in higher plant slightly differs from C. reinhardtii. Recently, major progress was made on the characterization of short- and long-term acclimation mechanisms which adjust the light-harvesting capacity in plants and microalgae to ever-changing environmental conditions. Previous studies in microalgae reported, short-term acclimation to high light results in insignificant damage of LHC’s, lack of efficient energy transfer to the reaction center deciphering the structural, and functional changes to the photosynthetic cells to regulate the light-harvesting capacity in excess light condition (Nama et al. 2015). There are no reports on how C. reinhardtii cells behave to long-term adaptation/acclimation to high light conditions in terms of photosynthetic organization. Thus, in the present study, we focused on acclimation process to high light stress from C. reinhardtii in terms of various photosynthetic parameters, changes in protein content of photosystems, and overall organization of thylakoid protein complexes.
Materials and methods
Growth conditions of C. reinhardtii
Chlamydomonas reinhardtii wild-type strain CC-125 was grown in batch culture at 25 °C with continuous shaking at 115 rpm. Light intensities used for the growth of cultures were defined as low light intensities (50 µmol m−2 s−1) and high light intensities 1 and 2, respectively 500 and 1000 µmol m−2 s−1. A light intensity of 50 µmol m−2 s−1 is optimum light for the growth of C. reinhardtii. Cells were grown in photoheterotrophic condition (Tris-acetate phosphate medium) and measured the optical density at 750 nm at different time interval followed by cell counting using haemocytometer. The chlorophyll content was estimated by using the standard protocol (Porra et al. 1989). Further experiments were carried out from cells collected at the mid-log phase of growth.
Pigment content determination by HPLC analysis
Cells of C. reinhardtii were collected at the mid-log phase of growth (3 × 106 cells mL−1) and lyophilized to obtain the dry weight of cells. By taking the equal dry weight of the treated samples, chlorophyll content was determined by resuspending in 1 mL of 100% methanol. The cells were centrifuged and the chlorophyll content of the supernatant was measured according to the optical absorbance at 652 and 665 nm using UV-visible spectrophotometer. Pigments were extracted with 100% methanol from the cells with equal dry weight by vortexing at maximum speed for 1 min. The extract was centrifuged at 14,000 rpm and the supernatant was filtered through a 0.45 µm filter which was subjected to HPLC. The separation and chromatographic analysis of pigments were performed by HPLC (Shimadzu) on a C-18 column (250 × 4.6 mm, 5 µm; Phenomenex) using a 60-min isocratic gradient of methanol:acetonitrile (90:10, v/v) plus triethylamine (9 µM) as the mobile phase. Further, carotenoids were identified and quantified by using standards, retention time, and peak areas of each compound. Detection of pigments was carried out at 455 nm using a UV–Vis detector (SPD 20 A; Shimadzu).
Chl a fluorescence transient
Chl a fluorescence fast induction curves were carried out by Chl fluorimeter (PEA, plant efficiency analyzer, Hansatech, King’s Lynn, and Norfolk, UK) and measured for one second with excitation light wavelength at 650 nm. A light intensity of 3000 µmol m−2 s−1 was employed to generate maximal fluorescence (Fm) in the instrument. Fluorescence was detected by a PIN-photodiode after passing through a long-pass filter (50% transmission at 720 nm).
Dual PAM measurements
All fluorescence measurements were performed using Dual PAM 100 Chlorophyll fluorometer comprising a computer-operated PAM control unit and a detector unit. The actinic and saturating light was provided by LED lamp having an extremely wide range of measuring light frequencies (1–400 Hz). The culture was taken in a cuvette connected to the magnetic stirrer and a temperature controller. The treated algal cells were illuminated to successively increasing actinic light levels for measuring the photosynthetic parameters in the form of light-response curves with the help of programmed pre-installed Dual PAM software for fluorometer.
Oxygen evolution measurements
The oxygen-evolving activity of cells was measured by taking an equal concentration of chlorophyll 20 µg mL−1, supplemented with the artificial electron acceptor phenyl-p-benzoquinone (PBQ) and ammonium chloride at a final concentration of 1 mM. Oxygen evolution rates were measured at 25 °C under saturating light using a Clark-type electrode (Hansatech Instruments, UK).
Isolation of thylakoids
After the light treatment, the thylakoid membranes were isolated as described earlier (Fischer et al. 1997; Subramanyam et al. 2006, 2010). The final pellet was resuspended in 2.0 mL of thylakoid resuspension buffer containing 5 mM Tris–HCl (pH 7.5), 0.2 M Sorbitol, 5 mM CaCl2, and stored at − 20 °C.
Circular dichroism measurements
Circular dichroism (CD) spectral measurements were recorded in JASCO815 Spectropolarimeter. The readings were recorded within the wavelength range of 400–750 nm and a scan speed of 100 nm min−1. An optical pathlength of 1 cm, a bandwidth of 2 nm, and data pitch of 0.5 nm were used. Chlorophyll concentration of 25 µg mL−1 was obtained by suspending the cells in buffer containing 0.3 M sorbitol and the same buffer was used as a blank.
SDS-PAGE and immunoblot analysis
Thylakoids isolated from high light-treated cells were separated by 12% SDS-PAGE with an equal amount of protein content (5 µg) in each lane. To identify and quantify the polypeptides contained in the thylakoid membranes, immunoblotting was performed. Electrophoretic transfer of proteins to PVDF membranes that were incubated with polyclonal antibodies (primary antibodies) developed in rabbits was performed. Primary antibodies against LHCII, PSII, and PSI complex proteins were purchased from Agrisera. Peptide tag antibodies of LHCI complexes were developed in our laboratory (Yadavalli et al. 2012). The primary antibodies were used with dilutions as follows: D1 and D2 (1:5000), Cp43 and Cp47 (1:2000), PsbO (1:5000), Lhcb1–Lhcb5 (1:5000), Lhcbm5 (1:5000), PsaA, PsaC and PsaD (1:1000), PsaH and PsaG (1:10,000), Lhca1 (1:5000), Lhca3 (1:5000), Lhca4 (1:250), Lhca6 (1:250), Lhca7 (1:250), and Lhca8 (1:250). Subsequently, the secondary antibody ligated to horse radish peroxidase (1:10,000 dilution) was applied. Chemi-luminescence reagents obtained from the Thermo Scientific™ brand were used to develop the signal on the PVDF membrane. The images were recorded on a Bio-Rad’s New Chemi Doc™ Touch Imaging System.
Separation of supercomplexes from BN-PAGE
Thylakoid proteins were isolated from cells treated with high light intensities as explained above. The first dimension of blue native gel separation was performed by solubilization of thylakoids in 1% n-dodecyl β-d-maltoside (β-DM) (Sigma) along with the protease inhibitors of 1 mM 6-aminocaproic acid (ACA), 1 mM benzamidine hydrochloride, and 1 mM phenyl methane sulfonyl fluoride (PMSF), and the gel with 50 mM ACA was run at 4 °C with increasing voltage (Madireddi et al. 2014). For second dimension, BN gel strips were treated with solubilization buffer; Laemmli buffer: 138 mM Tris–HCl (pH 6.8), 6 M urea, 22.2% (v/v) glycerol, 4.3% (w/v) SDS, and 5% (v/v) 2-β mercaptoethanol. The second dimension of the BN gel was run in 12.5% SDS gels. The protein spots on second dimension gels were visualized by colloidal Coomassie staining method.
Protein spot identification by MALDI-TOF
The 2D-SDS gels were washed twice with water. For this study, six spots were selected for identification of proteins. The spots were picked manually with a sterile blade and transferred into sterile 1.5 mL vials. Further, the spots were digested with trypsin and the peptide fragments obtained were separated by using MALDI-TOF mass spectrometry as described earlier from our laboratory (Yadavalli et al. 2011b). The monoisotopic masses gained were blasted using the MASCOT server.
Results and discussion
Change in the growth pattern of C. reinhardtii under high light conditions
In order to evaluate the acclimation process of C. reinhardtii, the cells were grown in acetate medium (TAP) under high light conditions. High light-grown C. reinhardtii cells showed contrast difference in growth pattern (Fig. S1). The cells were grown under photoheterotrophic condition for 48 h at different light intensities of 50 µmol m−2 s−1 (LL), 500 µmol m−2 s−1 (HL-1), and 1000 µmol m−2 s−1 (HL-2). Cells at high light intensities (HL-1 and HL-2) showed faster growth when compared to LL cells. This result suggests that the cells were acclimated at all intensities but acclimation to HL leads to changes in pigment content and many other biochemical changes that have been reported in further experiments. For a better understanding of growth pattern, we reported growth curve of LL, HL-1, and HL-2 cells (Fig. S1A) from photoheterotrophic condition. For different light conditions, the cell count was evaluated by hemocytometer at different time intervals, resulting in higher values for high light stress samples, although the values were substantially lower for LL cells (Fig. S1B). We also estimated the chlorophyll content for different time points, which show clearly that Chl content was almost double in HL as compared to low light condition (Fig. S1C). These data indicate that high light intensities show influence at both phenotypically and physiologically level. Further, the cells were also grown in HSM (high saline medium) which creates a photoautotrophic condition with moderate growth, whereas faster growth has been observed in high light conditions like in acetate medium (data not shown). The growth and Chl accumulation of cells in HSM and acetate medium show almost similar pattern. This result demonstrated that cells were acclimatizing to high light stress condition with an increase in biomass yield (Fig. S2). Similar reports have been observed: with an increase in the light intensity, the C. reinhardtii biomass increased (Perozeni et al. 2018), this increase bioss mas could be due to the expression of LHCSR3. This indicates that the NPQ rate has been increased as a measure of protection against high light intensity, this was further discussed in our results.
Chl a fluorescence analysis
Chl a fluorescence induction kinetics studies articulate about the photochemical activity of PSII of oxygen-evolving organisms in a dark-adapted state (Govindjee 1971; Papageorgiou 1975). This induction curve shows characteristics that vary on time i.e., two transient phases which have been represented as fast wave (up to hundreds of milliseconds) known as OJIP and a slow wave (seconds to tens of minutes) named as PSMT (Strasser and Srivastava 1995; Kodru et al. 2015). The OJIP transients were measured which are represented as follows: O, initial fluorescence (Fo); O–J phase that reflects reduction of QA to QA−; J–I and I–P phases involved in the reduction of PQ pool as well as electron acceptor side of PSI; at maximum fluorescence level (Fm), reduced electron carriers in between the PSII reaction center and NADP (Kodru et al. 2015). In the present study, the OJIP transient curves for all the high light-treated samples have a cell density of 3 × 106 cells mL−1 that showed an increase of Fo for HL-1- and HL-2-treated samples when compared to LL-treated samples (Fig. 1A). The initial fluorescence (Fo) increased in high light condition as represented in Fig. 1B. Probably, this could be due to detachment of core antenna from reaction center (Stirbet et al. 2014) (Fig. 1). After O–J phase, reduction of fluorescence intensity has been observed indicating over-reduction of PQ pool and effect on PSI electron acceptor side. The decrease of maximal fluorescence (Fm) determines low photochemical yield under high light stress condition (Fig. 1C). The decline in Fv/Fm values in HL-1 and HL-2 shows a marginal change in photochemical yield (Fig. 1D). From this induction kinetics, we demonstrate that even at low photochemical yield, the cells acclimatized to stress through various adaptive mechanisms.
A Chlorophyll a fluorescence induction curve (OJIP) was measured by using handy PEA. Cells subjected to different light intensities (LL, HL-1 and HL-2), BFo; initial fluorescence, CFm; maximum fluorescence, DFv/Fm; photochemical yield and the experiment was carried out for C. reinhardtii cells grown for 48 h in high light. Three biological replicates were measured and mean graphs were presented
Analysis of pigment content
Acclimation to different light conditions is well known to induce variation in pigment content and relative composition in higher plants. The pigments of C. reinhardtii were extracted in 100% methanol and analyzed by using HPLC. The mid-log phase of cells showed a marginal change in Chla/b ratio in high light condition (Fig. S2). From HPLC analysis, carotenoids were identified and quantified using standards with retention time and peak areas. The resultant violaxanthin (Vio) and neoxanthin (Neo) were reduced to 50% after 2 days of growth in high light (HL-1 and HL-2) while lutein and β-carotene showed different values in HL-1 and HL-2 as compared to LL grown cells (Table 1). The previous report stated that epoxidation of violaxanthin under high light leads to the formation of zeaxanthin which plays an essential role in non-photochemical quenching in the photoprotection mechanism (Jahns et al. 2009). However, we did not report zeaxanthin content in HPLC analysis, but with reference to many articles, we assume that reduction of violaxanthin might have converted to zeaxanthin. Our results are in agreement with the previous report that under abiotic stress, the violaxanthin could convert to zeaxanthin (Jahns et al. 2009). This demonstrates that cells were adapted by zeaxanthin-dependent NPQ mechanism and the reduced levels of neoxanthin in high light results in lack of stability of supercomplex.
Under the high light condition, the differential and strong accumulation of lutein have been reported (Matsubara et al. 2009), this is in agreement with the proposed function of lutein as an efficient light-harvesting xanthophyll (Table 1). It is known to play an essential role in LHCII trimerization and also the stability of antenna proteins (Dall’Osto et al. 2006; Wehner et al. 2006). The ultimate conversion from violaxanthin to zeaxanthin and lutein (to a lesser extend) may be involved in dissipating excess light energy in C. reinhardtii. This explanation clearly emphasizes that lutein and zeaxanthin under high light condition play a key role in the stable organization of photosystems which might lead to faster acclimation and growth. Thus, our results are in correlation with the recent report of a rice plant that high light induces greater zeaxanthin content leading to an increase in NPQ which, in turn, protects the plants against high light (Zhao et al. 2017).
Determination of rate of oxygen evolved from PSII
In order to measure oxygen evolution, the cells which were grown in high light were used and to these cells artificial electron acceptor 2-phenyl-1,4-benzoquinone (PBQ) and ammonium chloride (NH4Cl) were added. A slight change in oxygen evolution has been observed in cells grown in HL-1 and HL-2 when compared to LL cells indicating that the impact of strong light was not drastic to damage PSII and showing the effect on the rate of oxygen evolution. The study demonstrates that the cells are acclimating when they are grown under high light (Fig. 2). Further, as we correlate this result with biochemical changes, the oxygen-evolving complex subunit PsbO slightly decreased in amount was shown in further experiment. This result suggests that cells are showing an adjustment to high light stress which is an indication of the adaptive mechanism. The oxygen evolution results are in agreement with the pigment and biochemical changes.
Analysis of photosynthetic parameters
Light-response curves have been widely used to estimate the photosynthetic parameters that provide information on the photosynthetic activity, the photo-acclimation state of photoautotrophic organisms, and their driven processes. Light curves basically measure the steady-state rates of photosynthesis under a range of relevant irradiance levels which reveal photosynthetic activity as a function of environmental cues. This curve mainly provides detailed information on electron transport capacity and limitations of the two photosystems by simultaneous measurement of fluorescence and P700 responses that reflect the interplay of the light reactions of PSII and PSI that are connected via electron transport chain. Here, we reported photosynthetic parameters for high light-grown cells (HL-1 and HL-2) along with low light-grown cells (LL) (Fig. 3). Photo-acclimation to high light condition resulted in distinct light-response patterns such as Y(II) (effective quantum yield of PSII), ETR(II) (electron transport rate of PSII), NPQ (non-photochemical quenching), qN (coefficient of non-photochemical quenching), Y(NO), and Y(NPQ) (non-regulatory and regulatory quantum yield of energy dissipation, respectively) (Fig. 3A–F). NPQ is a process in which excess excitation energy reaching PSII is not only used for photochemistry but also dissipating as heat non-radiatively (Derks et al. 2015). This de-excitation depends on a large trans-thylakoid proton gradient that could establish in the excessive light.
Effect of different light intensities on CC-125 wild-type cells in photosynthetic parameters in terms of A ETR(II) (electron transport rate of PSII), B Y(II) (effective quantum yield of PSII), C NPQ (non-photochemical quenching), D Y(NPQ) and E Y(NO) (regulatory and non-regulatory quantum yield of energy dissipation, respectively), F qN (coefficient of non-photochemical quenching). Values correspond to the mean ± SD
Cells grown under high light intensities have reported a decrease of ETR(II) with gradual rise of light intensities mainly for HL-2 acclimatized cells (Fig. 3A). These results, in turn, reflect in lower values of Y(II) known to be a fraction of energy that is photo-chemically used in PSII (Fig. 3B). The remaining fraction of energy loss has been split into two components; Y(NO) and Y(NPQ), reflecting energy that is passively dissipated in the form of heat and fluorescence. NPQ was also reported in reduced levels that details the overall quenching process which the system acquired for photoprotection against stress condition (Fig. 3C). The increase of quantum yield of energy dissipation in a regulatory pathway [Y(NPQ)] was observed for HL-treated cells (Fig. 3D), whereas, in the non-regulatory pathway [Y(NO)], the yield decreased gradually (Fig. 3E). Generally, the high values of Y(NO) reflect the inability of a plant to protect itself against damage by excess illumination (Klughammer and Schreiber 2008; Zhao et al. 2017), in contrast to this, lower Y(NO) values were observed in HL-2 treated cells. This result indicates that the system utilized most of the light energy to withstand stress and acclimatize to the high light. This clearly emphasizes that high light conditions cause the system to adapt to different mechanisms, reduction of electron transport rate, and manage to adjust high light stress by transfer of energy through NPQ mechanism. The coefficient of non-photochemical quenching denoted as qN showed increased levels in HL-2 (Fig. 3F). Additionally, relatively different values were noticed in HL-1 as comparison with HL-2 in light-response curves. This difference might be due to the use of photoheterotrophic growth condition. The increase of Y(NPQ) is mainly by virtue of down-regulated functional light-harvesting antenna. Carotenoids are known to play an important functional role in several mechanisms such as NPQ depends on decreased or increased level of carotenoids under high light stress (van Oort et al. 2018). Our data show that due to a marginal change in Chl and a probable increase in zeaxanthin is in agreement with enhanced NPQ adaptation. The above results interpret that the increase in cell number, change in pigment content, and photosynthesis performance induces the cells to harvest more light through NPQ mechanism leading to expression of LHCSR3 protein under high light condition due to down-regulation of the light-harvesting functions.
Protein–pigment interaction studies and changes in macro-organization of supercomplexes
Circular dichroism is a non-invasive technique used to monitor the structural change of pigment–pigment interactions along with the macro domain organization of supercomplexes. The spectra were measured in the visible region that is sensitive to the coupling between pigments and used in determining the differences in pigment organization. The CD spectra of cells revealed the features called psi-type CD bands. The positive band at around 690 nm and the negative band at around 674 nm originated from Chl molecules associated with macro-domains of photosynthetic proteins (Nagy et al. 2014). The blue region psi-type band at around 506 nm is attributed to carotenoids found in a long-range ordered structure and the excitonic CD bands characteristic for LHCII trimers, at around 650 nm negative band (Garab and van Amerongen 2009). Our results show that the amplitude of (+) 690 nm and (−) 674 nm bands reduced drastically in high light-grown cells determining the alteration of the macro-organization of supercomplexes containing mainly Chl molecules (Fig. 4). These results most probably reflect the altered macro-aggregation due to ordered structural changes of PSII–LHCII supercomplexes. The positive band at 506 nm in the sort region originates from the β-carotene of the core complex (Tóth et al. 2016), exhibits no or weak signal that might be due to a decrease in protein content of core complex in cells subjected to high light stress (Fig. 4). It is clear that though there is acclimation, the LHCs to reaction center assemble is changed which describes that the LHCs are detached from the reaction center. The data are in agreement with our blue native gels that the supercomplexes and mega-complexes have been disturbed. This is one of the reasons for dramatic change in CD signal in the red region. However, the trimer content is not altered or slightly increased which was shown from blue native gels and this could be the main adaptive mechanism to high light conditions.
Protein complexes separation and protein spots identification by MALDI-TOF
The changes in photosynthetic parameters and their thylakoid organization through CD spectral studies could result either by a lack of assembly or disruption in complex formation. We analyzed the profiles of photosynthetic supercomplexes in the thylakoid membranes of C. reinhardtii subjected to different light intensities i.e., LL, HL-1, and HL-2 (Fig. 5). Based on previous reports, the protein complex of thylakoid membrane separated from Chlamydomonas was identified (Nama et al. 2015). Thylakoids isolated from high light grown cells displayed severely reduced levels of PSII supercomplexes. PSII dimer supercomplexes probably are formed by the attachment of LHCII trimers to PSII dimers which is in agreement with previous reports (Minagawa and Takahashi 2004; Nelson and Yocum 2006). Our results show that absence of PSII supercomplex in the high light which could be due to disruption in the association of PSII with LHCII complex (Fig. 5A). Similarly, the changes in the macro-organization of supercomplex were detected in protein–pigment interaction studies (Fig. 4). We found a higher level of LHCII assembly in high light-grown cells (HL-1 and HL-2) when compared to low light-grown cells (LL) which could be due to an adaptive/acclimation mechanism. This result indicates that although formation of supercomplex was reduced, assembly of LHCII might be established in high light condition to avoid stress and also harvest light to its maximum as reported in earlier studies (Ballottari et al. 2012). We also performed the second dimension of BN gel strips to separate the protein subunits from respective complexes on SDS-PAGE to identify the complexes (Fig. 5B). Further, the proteins spots were picked up from the stained gel and identified by MALDI-TOF (Fig. S3). MASCOT search was carried out for monoisotopic masses and the proteins were identified as shown in Table 2. Spots identified were mainly from PSII core complex proteins i.e., PsbA (D1), PsaD (D2), PsaB (CP47), PsbC (CP43) and also PSI core subunits (PsaA and PsaF). Under low light (LL) condition, PSII core subunits D1, D2, CP43, and CP47 were associated with PSII supercomplexes, while in high light (HL-1 and HL-2) PSII subunits were negligible. Thus, these subunits might have present in low molecular weight form, including PSII dimer and PSII monomers (Fig. 5B).
A Blue native PAGE of thylakoid membranes isolated from cells subjected to different light intensities. B The 2nd dimension of BN-PAGE labeled as SC (supercomplexes), I (PSI monomer), II (PSIIRCC monomer), LA (LHC assembly), L3 (LHC trimers), L (LHC monomers). SDS-PAGE of BN gel strips protein spots were identified by MALDI-TOF
Analysis of protein profile from immunoblots
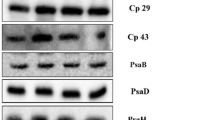
In order to identify the protein level changes, the isolated thylakoid membranes from cells grown at high light conditions i.e., HL-1 and HL-2 samples were subjected to SDS-PAGE and further it was transferred to nitrocellulose membrane which was probed with thylakoid membrane complex protein subunits (Figs. 6, 7). The results demonstrate that the cells grown in HL-2 (1000 µmol m−2 s−1) showed marginally decreased protein content of core D1 and D2 along with antenna proteins CP43 and CP47 (Fig. 6), while in the short-term exposure of high light (2000 µmol m−2 s−1) to cells showed changes in reaction center or antenna complexes (Nama et al. 2015). In high light-grown cells, the continuous repair mechanism occurred for D1 and D2 proteins, hence we could observe the marginal change in their abundance. The heterodimer of D1 and D2 proteins serve as the reaction center-binding proteins for PSII that carry out essential redox components for charge separation. The closely associated with these core protein are core antennae chlorophyll-binding proteins such as CP43 and CP47. From de novo synthesis pathway, studies reveal that the photodamage of PSII repair was inhibited by ROS production (Murata et al. 2007) and the translational initiation of PsbA transcripts activated through redox signal associated with electron transport in PSI as well as reduction of the plastoquinone pool (Trebitsh and Danon 2001). Since PSII core protein synthesis and its photodamage repair depend on many factors that could affect the process of their acclimatization resulting in minimal change in its content. Expression of LHCSR3 protein has been observed under HL as expected (Fig. 6). Its association with PSII supercomplex, transfer’s excitation energy from light-harvesting complexes to the chlorophyll-binding protein CP43 is selectively inhibited compared to CP47, preventing excess excitation energy from overloading reaction center as explained in the recent report that can result in a change in the content of core antenna proteins (Kim et al. 2017). In LHCII trimer, Lhcb1 and Lhcb3 which are peripherally arranged depicted a drastic change in HL-2, while Lhcb2 that is near to exposed PSII core showed no change in protein content. This result indicates that high light affects mostly LHCs that are arranged peripherally in the supercomplexes (Fig. 6) as they are the primary light harvesting units. When we look at the LHCs monomers, Lhcb5 show decay in content while there was no change in Lhcbm5 indicating that light stress mainly on outermost arranged light-harvesting antennas. Light-harvesting complex proteins Lhcb3 and Lhcb5 were reported to be more tightly associated with PSII core than Lhcb1 and 2 (Iwai et al. 2008). This might be the reason for inhibition of outer antennae proteins. An isoform of Lhcb4 connects trimeric LHCII with PSII core and stabilizes the PSII–LHCII dimer that binds to the monomeric core with the help of LHCII suggesting its primary role in the regulation of the excitation energy flow and non-photochemical quenching process (Caffarri et al. 2009). Interestingly, we found that Lhcb4 has no significant change; thus, Lhcb4 might increase the rate of non-photochemical quenching that is generally more at HL conditions. In C. reinhardtii, qE depends on the light-induced accumulation of the LHCSR proteins, particularly LHCSR3 (Peers et al. 2009). It was reported that LHCSR3 is attributed to a pigment binding member of the LHC family which is activated via protonation of acidic amino acid residues upon acidification of the lumen, allowing for the reversible switch from a light-harvesting to dissipative state which can be observed from our results through the increase of NPQ rate and expression of LHCSR3.
Immunoblots for PSII and LHCII complex proteins that are isolated from cells at high light intensities, i.e., 500 (HL-1) and 1000 (HL-2) µmol m−2 s−1 along with control (LL), the expressed LHCSR3 protein in high light condition, and the amount of respective protein quantified by using ImageJ software is shown in right panel
PsaA and PsaB are core proteins of PSI that binds to all cofactors required for electron transfer chain except the last FA and FB cluster (Jordan et al. 2001). Under HL stress condition, PsaA show reduced protein content that might be due to the changes in chlorophyll and carotenoid molecules around the protein which coordinates the cofactors to transfer electrons to ferredoxin (Fig. 7). The associated stromal subunits PsaC, PsaD, and PsaE act as a binding site for ferredoxin, which leads to efficient electron transport chain (Fischer et al. 1998). Reduction of stromal subunits (PsaC and PsaD) can distract the assembly of PSI (Fig. 7). Further, PsaH is known to be a docking site for LHCII during state transitions (Lunde et al. 2000). Our results elucidate that there is no change in protein expression and also the energy balance through PSII to PSI is not hampered under high light condition resulting in acclimation. Further, we observed sensitivity of PsaG to high light conditions indicating that assembly of PSI core to LHCI may be disturbed (Ozawa et al. 2010). Then, we examined the content of few light-harvesting complex proteins of PSI that are arranged in two layers towards the stromal side of PSI core. The outer half ring of the supercomplex includes Lhca4, Lhca5, and Lhca6, whereas the inner half ring of the supercomplex is composed of Lhca1, Lhca3, Lhca7, and/or Lhca8 with Lhca3 located near to PsaK, based on previous reports (Drop et al. 2011; Yadavalli et al. 2011a, b). Interestingly, the light-harvesting complexes of PSI are stable in high light conditions (Fig. 7), whereas in short exposure to high light affected the LHCI proteins (Nama et al. 2015). Several reports have postulated that oxidative mechanisms are the basis of PSI photodamage in intact leaves (Tjus et al. 1999), isolated chloroplasts (Inoue et al. 1986), or isolated PSI fragments (Rajagopal et al. 2005). So far, the photoinhibition happens more to the PSII. However, in C. reinhardtii, both PSI and PSII are equally affected by shorter exposure to high light (Nama et al. 2015). The results also suggest that shorter exposure (48 h) of high light (500 and 1000 µmol m−2 s−1) and longer duration (72 h) under photoheterotrophic growth condition induced a similar effect on both PSI and PSII (Fig. S4). The change in protein expression could be affecting the synthesis of the respective proteins due to mild oxidative damage. Interestingly, there was a marginal increase of reactive oxygen species in the isolated thylakoids when the cells are grown in high light conditions (data not shown) indicating that the cells were acclimatized. In general, reactive oxygen species damage the photosynthetic complexes, however, in our study, the damage was minimal in terms of photosynthetic efficiency and protein content of both PSI and PSII. Thus, the higher NPQ rate leads to increased expression of LHCSR3 in high light conditions which possibly reduce the reactive oxygen generation as a protective measure. However, the stoichiometry of the PSI and PSII are maintained, indicating that C. reinhardtii is acclimating to the high light condition when grown for the longer time period.
Conclusion
Chlamydomonas reinhardtii acclimatized to high light intensities when the cells are grown for longer durations exhibiting more biomass. This acclimation is mainly due to the dissipation of excess light through NPQ which is one of the photoprotective mechanisms. High light-grown cells showed pronounced expression of LHCSR3 that is known to play a key role in NPQ mechanism. NPQ attributed by reduced levels of violaxanthin which in turn could have been converted to zeaxanthin in high light-grown cells. Additionally, most of the peripherally arranged light-harvesting complexes of PSII showed damage due to strong light. This could be a protective mechanism for PSII core proteins resulting in acclimatizing the cells. Further, changes in thylakoid organization in terms of reduced PSII supercomplex and increased LHCII assembly might be due to excess light-harvesting capacity to its saturation levels. Furthermore, the stoichiometry of PSI and PSII were maintained despite some proteins were down-regulated when the cells were grown in high light condition. Hence, this study showed that the cells were acclimatized to high light through NPQ-dependent process.
References
Allorent G, Tokutsu R, Roach V, Peers G, Cardol P, Girard-Bascou J, Seigneurin-Berny D, Petroutsos D, Kuntz M, Breyton C (2013) A dual strategy to cope with high light in Chlamydomonas reinhardtii. Plant Cell 25:545–557
Ballottari M, Dall’Osto L, Morosinotto T, Bassi R (2007) Contrasting behavior of higher plant photosystem I and II antenna systems during acclimation. J Biol Chem 282:8947–8958
Ballottari M, Girardon J, Dall’Osto L, Bassi R (2012) Evolution and functional properties of photosystem II light harvesting complexes in eukaryotes. Biochim Biophys Acta 1817:143–157
Belgio E, Kapitonova E, Chmeliov J, Duffy CD, Ungerer P, Valkunas L, Ruban AV (2014) Economic photoprotection in photosystem II that retains a complete light-harvesting system with slow energy traps. Nat Commun 5:4433
Betterle N, Ballottari M, Zorzan S, De Bianchi S, Cazzaniga S, Dall’Osto L, Morosinotto T, Bassi R (2009) Light-induced dissociation of an antenna hetero-oligomer is needed for non-photochemical quenching induction. J Biol Chem 284:15255–15266
Bonente G, Pippa S, Castellano S, Bassi R, Ballottari M (2012) Acclimation of Chlamydomonas reinhardtii to different growth irradiances. J Biol Chem 287:5833–5847
Caffarri S, Kouřil R, Kereïche S, Boekema EJ, Croce R (2009) Functional architecture of higher plant photosystem II supercomplexes. EMBO J 28:3052–3063
Dall’Osto L, Caffarri S, Bassi R (2005) A mechanism of nonphotochemical energy dissipation, independent from PsbS, revealed by a conformational change in the antenna protein CP26. Plant Cell 17:1217–1232
Dall’Osto L, Lico C, Alric J, Giuliano G, Havaux M, Bassi R (2006) Lutein is needed for efficient chlorophyll triplet quenching in the major LHCII antenna complex of higher plants and effective photoprotection in vivo under strong light. BMC Plant Biol 6:32
Dall’Osto L, Cazzaniga S, Havaux M, Bassi R (2010) Enhanced photoprotection by protein-bound vs free xanthophyll pools: a comparative analysis of chlorophyll b and xanthophyll biosynthesis mutants. Mol Plant 3:576–593
Derks A, Schaven K, Bruce D (2015) Diverse mechanisms for photoprotection in photosynthesis. Dynamic regulation of photosystem II excitation in response to rapid environmental change. Biochim Biophys Acta 1847:468–485
Diaz M, de Haro V, Munoz R, Quiles MJ (2007) Chlororespiration is involved in the adaptation of Brassica plants to heat and high light intensity. Plant Cell Environ 30:1578–1585
Drop B, Webber-Birungi M, Fusetti F, Kouril R, Redding KE, Boekema EJ, Croce R (2011) Photosystem I of Chlamydomonas reinhardtii contains nine light-harvesting complexes (Lhca) located on one side of the core. J Biol Chem 286:44878–44887
Drop B, Yadav K, Boekema EJ, Croce R (2014) Consequences of state transitions on the structural and functional organization of photosystem I in the green alga Chlamydomonas reinhardtii. Plant J 78:181–191
Durnford DG, Price JA, McKim SM, Sarchfield ML (2003) Light-harvesting complex gene expression is controlled by both transcriptional and post-transcriptional mechanisms during photoacclimation in Chlamydomonas reinhardtii. Physiol Plant 118:193–205
Elrad D, Niyogi KK, Grossman AR (2002) A major light-harvesting polypeptide of photosystem II functions in thermal dissipation. Plant Cell 14:1801–1816
Fischer N, Sétif P, Rochaix JD (1997) Targeted mutations in the psaC gene of Chlamydomonas reinhardtii: preferential reduction of FB at low temperature is not accompanied by altered electron flow from photosystem I to ferredoxin. Biochemistry 36:93–102
Fischer N, Hippler M, Sétif P, Jacquot JP, Rochaix JD (1998) The PsaC subunit of photosystem I provides an essential lysine residue for fast electron transfer to ferredoxin. EMBO J 17:849–858
Garab G, Van Amerongen H (2009) Linear dichroism and circular dichroism in photosynthesis research. Photosynth Res 101:135–146
Govindjee PG (1971) Chlorophyll fluorescence and photosynthesis: fluorescence transients. Photophysiology 6:1–46
Ibáñez H, Ballester A, Muñoz R, Quiles MJ (2010) Chlororespiration and tolerance to drought, heat and high illumination. J Plant Physiol 167:732–738
Inoue K, Sakurai H, Hiyama T (1986) Photoinactivation sites of photosystem I in isolated chloroplasts. Plant Cell physiol 27:961–968
Ivanov B, Kozuleva M, Mubarakshina M (2012) Oxygen metabolism in chloroplast. Cell metabolism-cell homeostasis and stress response, InTech 39–72
Iwai M, Takahashi Y, Minagawa J (2008) Molecular remodeling of photosystem II during state transitions in Chlamydomonas reinhardtii. Plant Cell 20:2177–2189
Jahns P, Latowski D, Strzalka K (2009) Mechanism and regulation of the violaxanthin cycle: the role of antenna proteins and membrane lipids. Biochim Biophys Acta 1787:3–14
Johnson MP, Goral TK, Duffy CD, Brain AP, Mullineaux CW, Ruban AV (2011) Photoprotective energy dissipation involves the reorganization of photosystem II light-harvesting complexes in the grana membranes of spinach chloroplasts. Plant Cell 23:1468–1479
Jordan P, Fromme P, Witt HT, Klukas O, Saenger W, Krauß N (2001) Three-dimensional structure of cyanobacterial photosystem I at 2.5 Å resolution. Nature 411:909–917
Kim E, Akimoto S, Tokutsu R, Yokono M, Minagawa J (2017) Fluorescence lifetime analyses reveal how the high light-responsive protein LHCSR3 transforms PSII light-harvesting complexes into an energy-dissipative state. J Biol Chem 292:18951–18960
Klughammer C, Schreiber U (2008) Complementary PS II quantum yields calculated from simple fluorescence parameters measured by PAM fluorometry and the Saturation Pulse method. PAM Appl Notes 1:201–247
Kodru S, Malavath T, Devadasu E, Nellaepalli S, Stirbet A, Subramanyam R, Govindjee (2015) The slow S to M rise of chlorophyll a fluorescence reflects transition from state 2 to state 1 in the green alga Chlamydomonas reinhardtii. Photosynth Res 125:219–231
Kouril R, Wientjes E, Bultema JB, Croce R, Boekema EJ (2013) High-light vs. low-light: effect of light acclimation on photosystem II composition and organization in Arabidopsis thaliana. Biochim Biophys Acta 1827:411–419
Lunde C, Jensen PE, Haldrup A, Knoetzel J, Scheller HV (2000) The PSI-H subunit of photosystem I is essential for state transitions in plant photosynthesis. Nature 408:613–615
Madireddi SK, Nama S, Devadasu ER, Subramanyam R (2014) Photosynthetic membrane organization and role of state transition in cyt, cpII, stt7 and npq mutants of Chlamydomonas reinhardtii. J Photochem Photobiol B 137:77–83
Matsubara S, Krause GH, Aranda J, Virgo A, Beisel KG, Jahns P, Winter K (2009) Sun-shade patterns of leaf carotenoid composition in 86 species of neotropical forest plants. Funct Plant Biol 36:20–36
Merchant SS, Prochnik SE, Vallon O, Harris EH, Karpowicz SJ, Witman GB, Terry A, Salamov A, Fritz-Laylin LK, Maréchal-Drouard L (2007) The Chlamydomonas genome reveals the evolution of key animal and plant functions. Science 318:245–250
Mettler T, Muhlhaus T, Hemme D, Schottler MA, Rupprecht J, Idoine A, Veyel D, Pal SK, Yaneva-Roder L, Winck FV (2014) Systems analysis of the response of photosynthesis, metabolism, and growth to an increase in irradiance in the photosynthetic model organism Chlamydomonas reinhardtii. Plant Cell 26:2310–2350
Minagawa J, Takahashi Y (2004) Structure, function and assembly of photosystem II and its light-harvesting proteins. Photosynth Res 82:241–263
Mubarakshina M, Khorobrykh S, Ivanov B (2006) Oxygen reduction in chloroplast thylakoids results in production of hydrogen peroxide inside the membrane. Biochim Biophys Acta 1757:1496–1503
Murata N, Takahashi S, Nishiyama Y, Allakhverdiev SI (2007) Photoinhibition of photosystem II under environmental stress. Biochim Biophys Acta 1767:414–421
Nagy G, Unnep R, Zsiros O, Tokutsu R, Takizawa K, Porcar L, Moyet L, Petroutsos D, Garab G, Finazzi G (2014) Chloroplast remodeling during state transitions in Chlamydomonas reinhardtii as revealed by noninvasive techniques in vivo. Proc Natl Acad Sci USA 111:5042–5047
Nama S, Madireddi SK, Devadasu ER, Subramanyam R (2015) High light induced changes in organization, protein profile and function of photosynthetic machinery in Chlamydomonas reinhardtii. J Photochem Photobiol B 152:367–376
Nelson N, Yocum CF (2006) Structure and function of photosystems I and II. Annu Rev Plant Biol 57:521–565
Niyogi KK, Bjorkman O, Grossman AR (1997) The roles of specific xanthophylls in photoprotection. Proc Natl Acad Sci USA 94:14162–14167
Ozawa S, Onishi T, Takahashi Y (2010) Identification and characterization of an assembly intermediate subcomplex of photosystem I in the green alga Chlamydomonas reinhardtii. J Biol Chem 285:20072–20079
Papageorgiou G (1975) Chlorophyll fluorescence: an intrinsic probe of photosynthesis. In: Govindjee (ed) Bioenergetics of photosynthesis. Academic Press, New York, pp 320–366
Passarini F, Wientjes E, Hienerwadel R, Croce R (2009) Molecular basis of light harvesting and photoprotection in CP24 unique features of the most recent antenna complex. J Biol Chem 284:29536–29546
Peers G, Truong TB, Ostendorf E, Busch A, Elrad D, Grossman AR, Hippler M, Niyogi KK (2009) An ancient light-harvesting protein is critical for the regulation of algal photosynthesis. Nature 462:518
Perozeni F, Stella GR, Ballottari M (2018) LHCSR expression under HSP70/RBCS2 promoter as a strategy to increase productivity in microalgae. Int J Mol Sci 19:155
Pietrzykowska M, Suorsa M, Semchonok DA, Tikkanen M, Boekema EJ, Aro EM, Jansson S (2014) The light-harvesting chlorophyll a/b binding proteins Lhcb1 and Lhcb2 play complementary roles during state transitions in Arabidopsis. Plant Cell 26:3646–3660
Porra R, Thompson W, Kriedemann P (1989) Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochim Biophys Acta 975:384–394
Rajagopal S, Joly D, Gauthier A, Beauregard M, Carpentier R (2005) Protective effect of active oxygen scavengers on protein degradation and photochemical function in photosystem I submembrane fractions during light stress. FEBS J 272:892–902
Ruban AV, Johnson MP, Duffy CD (2012) The photoprotective molecular switch in the photosystem II antenna. Biochim Biophys Acta 1817:167–181
Semchonok DA, Yadav KS, Xu P, Drop B, Croce R, Boekema EJ (2017) Interaction between the photoprotective protein LHCSR3 and C2S2 photosystem II supercomplex in Chlamydomonas reinhardtii. Biochim Biophys Acta 1858:379–385
Stirbet A, Riznichenko GY, Rubin A (2014) Modeling chlorophyll a fluorescence transient: relation to photosynthesis. Biochemistry 79:291–323
Strasser RJ, Srivastava A (1995) Polyphasic chlorophyll a fluorescence transient in plants and cyanobacteria. Photochem Photobiol 61:32–42
Subramanyam R, Jolley C, Brune DC, Fromme P, Webber AN (2006) Characterization of a novel photosystem I–LHCI supercomplex isolated from Chlamydomonas reinhardtii under anaerobic (state II) conditions. FEBS lett 580:233–238
Subramanyam R, Jolley C, Thangaraj B, Nellaepalli S, Webber AN, Fromme P (2010) Structural and functional changes of PSI-LHCI supercomplexes of Chlamydomonas reinhardtii cells grown under high salt conditions. Planta 231:913–922
Suorsa M, Jarvi S, Grieco M, Nurmi M, Pietrzykowska M, Rantala M, Kangasjarvi S, Paakkarinen V, Tikkanen M, Jansson S (2012) PROTON GRADIENT REGULATION5 is essential for proper acclimation of Arabidopsis photosystem I to naturally and artificially fluctuating light conditions. Plant Cell 24:2934–2948
Suorsa M, Rantala M, Mamedov F, Lespinasse M, Trotta A, Grieco M, Vuorio E, Tikkanen M, Jarvi S, Aro EM (2015) Light acclimation involves dynamic re-organization of the pigment–protein megacomplexes in non-appressed thylakoid domains. Plant J 84:360–373
Tjus SE, Moller BL, Scheller HV (1999) Photoinhibition of photosystem I damages both reaction centre proteins PSI-A and PSI-B and acceptor-side located small photosystem I polypeptides. Photosynth Res 60:75–86
Tóth TN, Rai N, Solymosi K, Zsiros O, Schröder WP, Garab G, Van Amerongen H, Horton P, Kovács L (2016) Fingerprinting the macro-organisation of pigment–protein complexes in plant thylakoid membranes in vivo by circular-dichroism spectroscopy. Biochim Biophys Acta 1857:1479–1489
Trebitsh T, Danon A (2001) Translation of chloroplast psbA mRNA is regulated by signals initiated by both photosystems II and I. Proc Natl Acad Sci USA 98:12289–12294
Van Oort B, Roy LM, Xu P, Lu Y, Karcher D, Bock R, Croce R (2018) Revisiting the role of xanthophylls in non-photochemical quenching. J Phys Chem Lett 9:346–352
Wehner A, Graßes T, Jahns P (2006) De-epoxidation of violaxanthin in the minor antenna proteins of photosystem II, LHCB4, LHCB5, and LHCB6. J Biol Chem 281:21924–21933
Wientjes E, Van Amerongen H, Croce R (2013a) LHCII is an antenna of both photosystems after long-term acclimation. Biochim Biophys Acta 1827:420–426
Wientjes E, Van Amerongen H, Croce R (2013b) Quantum yield of charge separation in photosystem II: functional effect of changes in the antenna size upon light acclimation. J Phys Chem B 117:11200–11208
Yadavalli V, Malleda C, Subramanyam R (2011a) Protein–protein interactions by molecular modeling and biochemical characterization of PSI-LHCI supercomplexes from Chlamydomonas reinhardtii. Mol Bio Syst 7:3143–3151
Yadavalli V, Nellaepalli S, Subramanyam R (2011b) Proteomic analysis of thylakoid membranes. Methods Mol Biol 684:159–170
Yadavalli V, Jolley CC, Malleda C, Thangaraj B, Fromme P, Subramanyam R (2012) Alteration of proteins and pigments influence the function of photosystem I under iron deficiency from Chlamydomonas reinhardtii. PLoS ONE 7:e35084
Zhao X, Chen T, Feng B, Zhang C, Peng S, Zhang X, Fu G, Tao L (2017) Non-photochemical quenching plays a key role in light acclimation of rice plants differing in leaf color. Front Plant Sci 7:1968
Acknowledgements
R.S was supported by the Department of Biotechnology (BT/PR14964/BPA/118/137/2015), Council of Scientific and Industrial Research (No. 38 (1381)/14/EMR-II) and DST-FIST, UGC-SAP, Govt. of India, for financial support. SN acknowledged CSIR for fellowship (JRF/SRF). We thank Dr. Kizu Zamir, Department of Plant Sciences, University of Hyderabad for critical reading of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Nama, S., Madireddi, S.K., Yadav, R.M. et al. Non-photochemical quenching-dependent acclimation and thylakoid organization of Chlamydomonas reinhardtii to high light stress. Photosynth Res 139, 387–400 (2019). https://doi.org/10.1007/s11120-018-0551-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11120-018-0551-7