Abstract
The potential of algae to acclimate to environmental stress is commonly assessed using chlorophyll a fluorescence, with changes in parameters of photosynthesis versus irradiance (P/E) curves measured either as rapid light curves (RLC) or steady-state light curves (LC). Here, effects of emersion on primary photosynthesis of four brown macroalgae (Ascophyllum nodosum, Fucus serratus, Sargassum muticum, Laminaria digitata) were compared by applying both RLC and LC. When LC were used, photosynthetic performance was enhanced during emersion in A. nodosum and F. serratus as shown by increases in qP, rETRmax and E k. By contrast, emersion had no impact on photosynthetic parameters of S. muticum and L. digitata. Relative changes in the NPQ–rETR relationship were reduced in A. nodosum, F. serratus and S. muticum, but remained unaffected in L. digitata. As none of the species developed their potential NPQmax, corresponding values could not be determined from RLC. Using RLC, observed photosynthetic performance of F. serratus and L. digitata was reduced upon emersion, whilst values for NPQmax were enhanced. Only results derived from LC provide evidence for a potential physiological adaptation of brown macroalgae to their natural habitat; it is recommended using the LC protocol to detect environmental impacts on photosynthesis.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Temperate regions rocky shores are dominated by brown macroalgae which exhibit distinct vertical distribution patterns from the upper littoral to the sublittoral zone (Lobban and Harrison 1994). As a result of semi-diurnal tides on the Irish west coast, intertidal macroalgae are regularly (twice daily) subjected to contrasting environments, i.e. submersed and emersed conditions, imposing large oscillations in multiple abiotic factors. For example, as a result of emersion, algal physiology is influenced by dehydration, and changes in irradiance, temperature and/or the osmotic and ionic environment. These abiotic factors interact in a complex manner which depends on the intensity, duration and recurrence of each stressor (Wahl et al. 2011). It has been suggested that the capability of seaweeds to withstand, and recover from, extreme and prolonged environmental stress significantly contributes to their occurrence on shores, hence physiologically restricting their vertical distribution (Dring and Brown 1982). On the other hand, emersion results in changes in the carbon regime and may provide benefits for algae as CO2 is more readily available for photosynthesis (Johnston and Raven 1986; Giordano et al. 2005).
In Ireland, distinct belts of brown macroalgae are formed by the indigenous species Ascophyllum nodosum (upper to mid-littoral), Fucus serratus (lower littoral) and Laminaria digitata which inhabits the sublittoral fringe and upper subtidal. The non-native brown macroalga Sargassum muticum has successfully invaded marine Irish waters and locally inhabits rockpools and the mid- to low-littoral zone (Baer and Stengel 2010) and is therefore, similar to L. digitata, only rarely exposed to atmospheric conditions.
Pulse-amplitude modulated (PAM) chlorophyll (chl) a fluorescence is increasingly applied in ecophysiological research on macroalgae (Gómez et al. 2004), e.g. to examine photosystem II (PSII) activity, photoacclimative and photoprotective mechanisms or to estimate the extent of stress experienced by brown macroalgae upon changing irradiances or emersion in Pelvetia canaliculata (Harker et al. 1999), Saccharina latissima (Gevaert et al. 2002, 2003), Macrocystis pyrifera (Colombo-Pallotta et al. 2006), Nereocystis luetkeana (Poulson et al. 2011), Laminaria abyssalis (Rodrigues et al. 2002) and L. digitata (Nitschke et al. 2011). Using chl a fluorescence techniques, algal photosynthetic capacity, e.g. measured as photosynthesis versus irradiance (P/E) curves, can be determined using different protocols. Depending on the requirements of the experimental approach, e.g. in situ or laboratory studies, the duration of actinic light increments during the measurement of P/E curves is defined. Short light increments (commonly ≤30 s), i.e. so-called rapid light curves (RLC), are commonly applied to examine photo-physiological characteristics (e.g. Gevaert et al. 2003; Ralph and Gademann 2005; Colombo-Pallotta et al. 2006; Saroussi and Beer 2007a, b; Figueroa et al. 2009; Edwards and Kim 2010). However, when using RLC, the oxidation/reduction of the primary electron acceptor of PSII (QA) and the induction/reversal of the non-photochemical quenching (NPQ) of chl a fluorescence are most likely incomplete (Perkins et al. 2006, 2011). RLC are therefore defined as a measure of the operational photo-physiology (Perkins et al. 2011). By contrast, steady-state light curves (LC) determine potential photo-physiology since QA oxidation/reduction and NPQ induction/reversal reach completion during measurements (Perkins et al. 2011). The time required to reach steady-state conditions varies according to species, light history and the capability to activate photosynthetic downstream processes which can last up to minutes (White and Critchley 1999; Rascher et al. 2000; Ihnken et al. 2010).
Changes in some photosynthetic parameters, such as the photon-capturing efficiency of PSII (α), i.e. the initial slope of P/E curve, the maximum relative electron transport rate through PSII (rETRmax), the light saturation coefficient (E k), the photochemical quenching (qP) and NPQ, were calculated to characterise photo-physiological processes and to estimate stress or stress tolerance in macro- and microalgae (Harker et al. 1999; Jakob et al. 1999; Gevaert et al. 2002; Gómez et al. 2004; Blache et al. 2011).
In the present study, the impact of short-term emersion on different primary photosynthetic parameters of brown macroalgae was investigated under controlled conditions whilst avoiding interactions of multiple stressors to allow a comparison with previous data on photosynthetic changes during early stages of emersion (Johnson et al. 1974; Dring and Brown 1982; Johnston and Raven 1986; Gao et al. 1999). Main objectives of this research were, firstly, systematically compare results obtained from two commonly used fluorometry protocols, i.e. RLC or LC and, secondly, to assess physiological responses of species inhabiting different shore levels in a controlled environment using chl a fluorescence.
Materials and methods
Algal material and experimental conditions
The native brown macroalgae A. nodosum (Linnaeus) Le Jolis, F. serratus Linnaeus and L. digitata (Hudson) Lamouroux as well as the non-native and invasive species S. muticum (Yendo) Fensholt (Phaeophyceae) were collected at Spiddal (53°14.4′N, 9°18.7′W) on the west coast of Ireland during spring low tide in mid-January 2010 (daylength: 10 h light and 14 h dark), water temperature: 6.5–8.8 °C, irradiance E PAR (water surface): 3–466 μmol photons m−2 s−1 (400–700 nm), S. Ní Longphuirt, C. Eschmann and D. B. Stengel, unpublished data). All specimens of each species were similar in age and free from visible epiphytes and grazing marks. Algae were maintained (for 2–3 days prior to experiments) in filtered (0.45 μm, Whatman GmbH, Dassel, Germany) and tyndallised, aerated seawater (10 °C and E PAR of 50 μmol photons m−2 s−1; cool white fluorescent tubes, General Electric Company, Fairfield, USA); the daylength was 12 h:12 h (light:dark). E PAR as received by the algae was determined using a calibrated Li-Cor LI-1400 (Li-Cor Inc., Lincoln, USA) data logger equipped with a planar quantum sensor due to the overall flat morphology of algal thalli.
To investigate photo-responses during early stages of emersion, chl a fluorescence parameters were measured when algae were either submersed in natural seawater or emersed 45 min after initial exposure to ambient air at 10 °C and 99 % relative humidity (RH) (thermohygrometer 8501 H, Delta Ohm S.r.L., Padova, Italy). For comparability, photosynthetic performance was determined in the relevant meristematic area of each species. Due to the short emersion period and high RH used during experiments, water loss was negligible and thus the likelihood of potential impacts of changes in the ionic/osmotic environment was limited. The experimental temperature was kept constant at 10 °C. Two common protocols for measuring photosynthetic performance as P/E curves, i.e. RLC and LC, were applied, and qP and NPQ as well as changes in parameters of P/E curves (α, rETRmax and E k) upon air exposure were determined.
Measurement of variable chl a fluorescence
In vivo chl a fluorescence parameters were measured using a PAM-2000 fluorometer (Heinz Walz GmbH, Effeltrich, Germany) after Schreiber et al. (1986), it follows the fluorescence terminology according to Kromkamp and Forster (2003).
Prior to start of fluorescence readings, all algae were dark-acclimated either in seawater for 30 min or with regards to measurements on air-exposed algae, samples were exposed to air at 50 μmol photons m−2 s−1 for 15 min and subsequently dark-acclimated for 30 min. Although electron transport might be active during dark acclimation due to chlororespiration (Jakob et al. 1999), periods of 20–30 min were often used to study photosynthetic characteristics Heterokontophyta (e.g. Jakob et al. 1999; Mouget and Tremblin 2002; Gómez et al. 2004; Nielsen and Nielsen 2008; Serôdio and Lavaud 2011; Poulson et al. 2011). Initial F 0 was determined with 1.6 kHz pulsed measuring light (650 nm, ~0.3 μmol photons m−2 s−1), whereby a 600 ms, completely saturating, white light pulse (20 kHz, ~9,200 μmol photons m−2 s−1) was applied to determine F m and F′ m. Subsequently, algae were exposed to ten increasing E PAR levels between 0 and 1,160 μmol photons m−2 s−1 which were provided by the internal halogen lamp of the PAM-2000. Strong illumination generated by the halogen lamp can potentially result in decreasing intensities. Thus, E PAR of each light increment was recalibrated and corrected prior to experiments using the above-mentioned Li-Cor LI-1400 data logger.
When using the RLC protocol, each light level was applied for 30 s; for details, see White and Critchley (1999) and Ralph and Gademann (2005). Preliminary measurements revealed that, depending on the species, steady-state fluorescence in light (F) was reached after 2.5–3 min exposure to actinic light. Accordingly, when using LC, each light level was applied for 3 min. After each light increment, a saturating pulse was given to determine the effective PSII quantum efficiency (ΔF/F′ m), qP and NPQ followed by a far-red pulse for 3 s (735 nm, ~1 μmol photons m−2 s−1) to re-oxidise the electron transport chain (Ihnken et al. 2010). In a small number of measurements, F′m was marginally higher than F m indicating that minor quenching occurred in the dark-acclimated state; in these instances, NPQ was estimated by replacing F m with F′ m,m which is the maximum F′ m value measured during RLC or LC (Serôdio et al. 2005; Lefebvre et al. 2011):
In general, no distinctive bi-phasic pattern with NPQ decreasing from initially high values (measured in the dark) to minimum values under low irradiances as described previously for F. serratus (Mouget and Tremblin 2002) was observed.
Electron transport rate (ETR) through PSII was calculated according to Genty et al. (1989). Since optical properties of the algae were not estimated, and both the built-in absorption coefficients and the photosystem stoichiometry were not known, ETR is expressed as rETR (Saroussi and Beer 2007a, b; Nielsen and Nielsen 2008; Perkins et al. 2011). It was therefore not possible, or intended, to interpret rETR as an estimation of primary production and rETR of different species could not be compared. However, relative changes in the photosynthetic performance of one species with treatment allowed an estimation of changes in its photo-response capacity (Ihnken et al. 2010; Perkins et al. 2011), i.e. submersion versus 45 min emersion and RLC versus LC, respectively.
P/E curves with rETR as a function of E PAR were fitted after Walsby (1997) due to the occurrence of photoinhibition (β), i.e. suppression of rETR at high E PAR:
Maximum rETR values as revealed by the model (rETRmax,Walsby) were overestimated when β was high. Thus, rETRmax was determined by calculation of the analytical maximum of the model:
The photon-capturing efficiency of PSII in the light limited range (α) was estimated by calculating the slope of the first three to four data points of P/E curves using linear regression (Serôdio et al. 2006) since the Walsby model overestimated the initial slope (αWalsby) by the factor 1.52 ± 0.32 (P < 0.001, Student’s t test, using all data) independent of species, duration of actinic light increments and treatment. The light saturation coefficient of P/E curves (E k) was calculated as a quotient of rETRmax and α (Henley 1993).
For a quantitative description of NPQ as a function of E PAR, NPQ versus E PAR curves were fitted after Serôdio and Lavaud (2011) as all requirements were met:
where NPQfit is the corrected NPQ value, NPQmax is the maximum NPQ value of the NPQ versus E PAR curve, E 50 is the irradiance at which NPQ attains 50 % of NPQmax and n represents the Hill coefficient characterising the sigmoidicity of the curve. Computed data from this model allowed the direct comparison of parameters from P/E and NPQ versus E PAR curves such as E k and E 50 (Serôdio and Lavaud 2011). NPQmax and E 50 as presented in this study were calculated by using Eq. 4.
Relative changes in short-term photoacclimation, which are indicated by changes in NPQ as a function of rETR (Küster et al. 2005; Eggert et al. 2007), were computed for the first six data points of P/E curves, i.e. just before rETRmax was reached. The relationship between rETR and NPQ was consistently parabolic, but only when LC were measured, and respective NPQ versus rETR curves were fitted to a polynomial second-order equation (Y = A + B × X + C × X 2, where Y = NPQ and X = rETR; Eggert et al. 2007) with coefficients of determination r 2 > 0.90 for most datasets. The coefficient C of the fit represents the slope of the respective curves.
Statistical analysis
RLC and LC measurements during submersion/emersion were replicated five times for A. nodosum, F. serratus and L. digitata and four times for S. muticum. Photosynthetic characteristics (qP, NPQ, NPQmax, E 50, α, rETRmax, E k) are presented as means with standard deviation. The E 50:E k ratio pooled for all species is shown as box whisker plots with median, 25th and 75th percentiles, 10th and 90th percentiles, and 5th and 95th percentiles.
Effects of emersion on α, rETRmax, E k, NPQmax and E 50 as obtained from both RLC and LC, and coefficient C of NPQ versus rETR parabola (calculated from LC) were analysed by using Student’s t test. rETRmax and E k values were logarithmically transformed prior to analysis. All data were normally distributed (Kolmogorov–Smirnov test). The E 50:E k ratios between RLC and LC were analysed using non-parametric Mann–Whitney U test.
Results
Effects of short-term emersion on chl a fluorescence responses in the four brown macroalgae were dependent on species and the protocol used, i.e. RLC or LC.
Parameters of P/E curves (α, rETRmax and E k)
No impact of emersion on α was detected for A. nodosum when applying either RLC or LC, but α determined from RLC was ~23 % lower than that determined from LC (Table 1). When LC were used, rETRmax of A. nodosum increased from 75.2 to 90.8 (P = 0.031) during emersion, but remained constant at 80.8 during exposure to air when the RLC were applied. Similarly, E k was not influenced by emersion when measured by RLC (~170 μmol photons m−2 s−1), but an increase of 28 % (P = 0.005) upon air exposure was obtained from LC (Table 1).
When LC were applied to F. serratus, α was reduced by 9 % after emersion (P = 0.017), but it was similar under submersion and emersion when using RLC (Table 1). α obtained from RLC was 38 % (submersed) and 26 % (emersed) lower than those obtained from LC. When rETRmax was determined from RLC, it was reduced in emersed F. serratus (P = 0.022), but increased (by a factor of 1.3; P = 0.015) when applying LC. Similarly, E k was reduced from 156 to 102 μmol photons m−2 s−1 (P < 0.001) when using RLC, but a 38 % increase was revealed by LC (P < 0.001).
α of S. muticum were reduced by 47 % (submersed) and 18 % (emersed) when determined by RLC compared to LC. RLC revealed a 1.6-fold, increase in α during emersion (P = 0.004), which was not found when using LC (Table 1). Similarly, using RLC, rETRmax was enhanced by 55 % upon exposure to air (P = 0.024); again, this result could not be repeated by LC where rETRmax remained nearly constant at submersion and emersion. In comparison, rETRmax obtained from RLC was up to 50 % lower than that obtained from LC. Emersion did not affect E k when applying either RLC or LC, but E k appeared to be 24 % higher when LC were used.
α of L. digitata was not influenced by air exposure regardless of the protocol applied. Overall values of α obtained from RLC were ~33 % lower than values obtained from LC (Table 1). A 24 % decrease in rETRmax (P = 0.013) upon emersion was only found when RLC were used; according to LC, rETRmax remained constant at 38.0 during emersion. The RLC also indicated a decrease in E k by 29 μmol photons m−2 s−1 due to emersion (P < 0.001) which was not found using LC. E k obtained from RLC was higher than that from LC.
Photochemical quenching (qP)
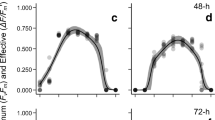
The duration of light increments affected qP at each level of E PAR (Fig. 1). For all species, qP was higher when using LC at submersion and emersion. During RLC, qP measured after dark incubation exhibited a decrease by 25 % in A. nodosum, 30 % in F. serratus and up to 40 % in S. muticum and L. digitata, regardless of treatment. Such reduction was not observed during LC (Fig. 1). Effects of emersion on qP versus E PAR were only observed in A. nodosum and F. serratus, and only when LC were applied (Fig. 1a, b), i.e. a clear enhancement of qP between 200 and 1,160 μmol photons m−2 s−1. qP of S. muticum and L. digitata was not affected by emersion during either protocol (Fig. 1c, d).
Effect of short-term emersion on photochemical quenching (qP) as a function of irradiance (E PAR) in a A. nodosum, b F. serratus, c S. muticum and d L. digitata as obtained from applying RLC (30 s light increments, circles) and steady-state LC (3 min light increments, triangles); Submersed (filled symbols), emersed (open symbols). Data represent means with standard deviations; n = 5, for S. muticum n = 4
Non-photochemical quenching (NPQ)
NPQ of A. nodosum, F. serratus and S. muticum increased with increasing E PAR during submersion and emersion without reaching saturation when using either protocol (Fig. 2). L. digitata developed no substantial NPQ during submersion when using RLC (Fig. 2d), but during emersion this species exhibited an up-regulation of NPQ with increasing E PAR during RLC.
Effect of short-term emersion on NPQ as a function of irradiance (E PAR) in a A. nodosum, b F. serratus, c S. muticum and d L. digitata as obtained from applying RLC (30 s light increments, circles) and steady-state LC (3 min light increments, triangles); Submersed (filled symbols), emersed (open symbols). Data represent means with standard deviations; n = 5, for S. muticum n = 4. NPQ versus E PAR curves were fitted to Eq. 4
None of the species developed their potential NPQmax when using RLC (Fig. 2; Table 2). Compared to NPQmax obtained from LC, NPQmax determined via RLC was 65 and 67 % lower for A. nodosum, 79 and 57 % lower for F. serratus, 82 and 74 % lower for S. muticum and 95 and 51 % lower for L. digitata under submersion or emersion, respectively (Table 2). When RLC were used, NPQmax of A. nodosum was not influenced by emersion, but it was significantly higher when LC were applied. For F. serratus, RLC revealed a significant enhancement of NPQmax by a factor of 2.5 upon emersion, but when LC were measured, NPQmax was independent of air exposure. Exposure to air had no significant effect on NPQmax of S. muticum, but it was significantly higher in emersed than in submersed L. digitata when either protocol was used (Table 2).
E 50 ranged between 41.5 and 236.9 μmol photons m−2 s−1 for the four species when using RLC, but for F. serratus and L. digitata E 50 was significantly higher upon emersion. Values determined by LC were consistently higher than those from RLC and independent from air exposure (Table 2).
Comparison of parameters from NPQ versus E PAR and P/E curves
When data for all species were pooled, E 50 obtained from RLC decreased slightly with increasing E k (r 2 = 0.042, P = 0.218, Fig. 3a). When LC were used, a highly significant increase in E 50 increased with increasing E k (r 2 = 0.595, P < 0.0001, Fig. 3b).
Relationship between E 50 (μmol photons m−2 s−1) of NPQ versus E PAR curves, i.e. the irradiance at which 50 % of NPQmax was attained, and the light saturation coefficient E k (μmol photons m−2 s−1) of P/E curves which were measured as a RLC (30 s light increments) and b steady-state LC (3 min light increments). NPQ versus E PAR curves were obtained from such P/E curves and fitted to Eq. 4. RLC and LC were applied on four species of temperate brown macroalgae: A. nodosum (circles), F. serratus (triangles), S. muticum (squares), and L. digitata (diamonds) under submersed (filled symbols) and emersed (open symbols) conditions. Lines represent linear regression of pooled data for RLC or LC; respective equations, r 2 and P values are given
As presented in Fig. 4, the E 50:E k ratio differed between RLC and LC (P < 0.001) when data were pooled. Median values were 1.05 (data range: 0.26–3.68) and 1.89 (data range: 1.08–3.66) for RLC and LC, respectively.
Comparison of E 50:E k ratios as obtained from RLC (30 s light increments) and steady-state LC (3 min light increments). E 50 values (μmol photons m−2 s−1) were computed from NPQ versus E PAR curves, which were fitted to Eq. 4, and represent the irradiance at which 50 % of NPQmax was attained. E k (μmol photons m−2 s−1) is the light saturation coefficient of P/E curves. RLC and LC were applied to four temperate brown macroalgae (A. nodosum, F. serratus, S. muticum and L. digitata) under submersed and emersed conditions. Data of all algae and treatments were pooled and are shown as box whisker plots with median (line), 25th and 75th percentiles (box), 10th and 90th percentiles (whisker) and 5th and 95th percentiles (circles). Difference in E 50:E k ratios between RLC and LC was analysed by using non-parametric Mann–Whitney U test; the P value is given
NPQ as a function of rETR
During LC, NPQ of all species increased with increasing rETR in a parabolic manner before rETRmax was reached (Fig. 5). During emersion, both higher NPQ at low E PAR and increased rETR at higher E PAR resulted in the reduction of slopes of rETR versus NPQ parabolas (represented by the coefficient C, Table 3), i.e. during emersion, NPQ did not increase with rETR as fast as under submersion. In L. digitata, the rETR–NPQ relationship was saturated before rETRmax was reached, suggesting that higher rETR did not result in higher NPQ as found for the other three species (Fig. 5). Accordingly, the slope C of the NPQ versus rETR parabola was not affected in emersed L. digitata (P = 0.420, Table 3), but values for C were highest, indicating that NPQ in this species increased fastest. Submersed F. serratus exhibited similar values for C, but its emersion resulted in a >50 % reduction of C (Table 3) which was comparable to results calculated for A. nodosum and S. muticum.
Effect of short-term emersion on relative changes in NPQ as a function of the relative electron transport rate (rETR) in the light limited range of primary photosynthesis in a A. nodosum b F. serratus c S. muticum and d L. digitata as obtained from steady-state LC. Submersed (filled triangles), emersed (open triangles). Data represent means with standard deviations; n = 5, for S. muticum n = 4. Curves were fitted to a polynomial second-order equation (Y = A + B × X + C × X 2, where Y = NPQ and X = rETR)
The relationship between NPQ and rETR was inconsistent and species-dependent when using RLC (Fig. 6). NPQ increased with rETR in a non-linear manner in A. nodosum, and no effect of emersion on this relationship was found. In F. serratus, air exposure altered the shape of rETR versus NPQ curves. There was no apparent relationship between rETR and NPQ for S. muticum, or for submersed L. digitata (Fig. 6c, d). During exposure to air, NPQ increased with rETR in a parabolic manner in the latter species.
Effect of short-term emersion on relative changes in NPQ as a function of the relative electron transport rate (rETR) in the light limited range of primary photosynthesis in a A. nodosum b F. serratus c S. muticum and d L. digitata as obtained from RLC. Submersed (filled circles), emersed (open circles). Data represent means with standard deviations; n = 5, for S. muticum n = 4
Discussion
Photosynthetic responses differed greatly between the four macroalgal species and were affected by the duration of light increments during P/E curve measurements.
Comparison of RLC and LC
Chl a fluorescence measurements are based on fluorescence yield modulation by the re-oxidation of the primary electron acceptor of PSII QA, as well as QB and the plastoquinone pool (Krause and Weis 1991; Lefebvre et al. 2011). During light increments of RLC, the redox state of QA was imbalanced as shown by discontinuous reduction in the first three to four qP versus E PAR data points. This had implications for electron transport, generation of the ΔpH, NPQ, and enzyme activation and, thus, for derived photosynthetic parameters. ETR is affected by the redox state of the plastoquinone pool which depends on (1) equilibrated QA redox state and (2) the influence of alternative electron flows such as cyclic electron transfer. A slow re-oxidation of QA results in an imbalanced redox state of the PSI electron acceptor site; this has feedback impacts on the redox state of the plastoquinone pool (Schansker et al. 2006; Joly and Carpentier 2007; Joly et al. 2010). Activation processes on the PSI electron acceptor site have exhibited short half-times of a few seconds (Carrillo and Vallejos 1983). However, as shown for Ulva sp., only a certain proportion of PSI was potentially involved in cyclic electron transfer under low-stress conditions (Gao et al. 2011), here qP and rETR obtained during RLC were most likely affected by an incomplete establishment of electron transport (reflected by consistently lower qP values) with implications for the ΔpH. Fluorescence responses were also dependent on chloroplast ATPase activation kinetics. These are species-dependent and the required activation time can vary from seconds (Kramer et al. 1990) to several minutes (Noctor and Mills 1987). Thus, photosynthetic performance was probably influenced by a delay in chloroplast ATPase activation. Moreover, it was shown for vascular plants that photosynthesis required RuBisCO activation which requires ΔpH (Eichelmann et al. 2009). The latter authors also showed that the ribulose 1,5-bisphosphate carboxylation/oxygenation capacity of RuBisCO was regulated in balance with electron transport.
After a dark–light transition, photosynthetic downstream processes described above need to be established, whilst the activation time required may vary according to species. However, particularly during the first light increments of RLC, these processes were imbalanced. Photosynthetic characteristics revealed by RLC were also likely to be affected by consequential, additive effects between light increments (Perkins et al. 2006), as well as incomplete NPQ induction, which had previously been reported (Perkins et al. 2006; Lefebvre et al. 2011). Since a range of processes interact in a complex manner, the exact extent of their impacts on photo-responses could not be estimated, but the results indicate that impacts were likely to be greater during RLC than LC.
Partial re-oxidation of QA affects rETRmax and α (Perkins et al. 2006; Lefebvre et al. 2011). Both parameters also depend on absorption characteristics which are related to morphology, pigment packaging, environmental conditions and season (Saroussi and Beer 2007a, b; Nielsen and Nielsen 2008), as well as light history and duration of dark incubation prior to chl a fluorescence readings (Perkins et al. 2006; Ihnken et al. 2010). A dependency of α and rETRmax on cellular absorptivity has been demonstrated for diatoms (Blache et al. 2011). A comparison of absolute rETRmax and α values obtained for different species is therefore difficult, but relative changes upon changing environments can indicate, however, stress (tolerance), assuming that absorption features have not been altered significantly. Experimental conditions used here were unlikely to result in major modifications of algal absorption properties, even though an operating xanthophyll cycle, energy-dependent quenching (qE) or state transition could have contributed to the adjustment of the PSII absorption cross-section within tens of minutes (Falkowski and Raven 1997; Müller et al. 2001). Evidence that state transitions occurs in brown algae is still missing (Fork et al. 1991). In addition, Gevaert et al. (2002) showed that the composition of photosynthetically active pigments of the brown macroalga S. latissima did not change over a simulated tidal cycle.
The photon-capturing efficiency α denotes the maximal quantum yield under the condition at which P/E curves were measured (Gómez et al. 2004; Saroussi and Beer 2007a), and here α obtained from RLC was consistently lower than from LC. α generally relies on absorption and QA re-oxidation capabilities (Saroussi and Beer 2007a; Perkins et al. 2006; Ihnken et al. 2010). Since optical characteristics were unlikely to have changed significantly here, the imbalanced QA redox state during RLC may resulted in an underestimation of α as shown for microphytobenthos (Lefebvre et al. 2011) and diatoms (Perkins et al. 2006). ‘Short’ RLC (10 s light increments) also revealed lower values for α than ‘long’ RLC (90 s light increments) in the brown macroalga Lessonia variegata, but not in Ecklonia radiata (Ihnken et al. 2010). This suggests a species- or genus-dependent impact of the duration of light increments on the activation of photosynthetic processes with implications for α.
Thallus morphology also may influence light-harvesting capacities (e.g. Lüning and Dring 1985), and light absorption may increase with chl a concentration and thallus thickness (Enríquez et al. 1994; Häder and Figueroa 1997). Particularly during high E PAR exposure of the last light increments of LC, irradiance may be received by deeper cell layers with possible implications on chl a fluorescence responses. Whilst in sub-surface cells, PSII performance was potentially increased and cells on the thallus surface could have suffered from photoinhibition. Although, Gómez et al. (2004) showed that morphology of green, brown and red macroalgae had no impact on photosynthetic parameters derived from chl a fluorometry.
Beyond E k, photosynthesis is limited by electron transport and/or carbon procurement (Henley 1993). E k directly relates to rETRmax and is independent of units used to express photosynthesis and thus should be preferred when comparing individuals (Henley 1993; Blache et al. 2011). However, an increase in α and rETRmax is potentially not represented by E k, as shown for e.g. L. variegata (Ihnken et al. 2010).
Thermal dissipation of excess excitation energy, denominated as qE (Müller et al. 2001), is a major component of total NPQ in Phaeophyceae (Goss and Jakob 2010). Changes in qE have been shown to coincide with modulations in algal pigment composition, underlying the important role of the violaxanthin cycle in photoprotective mechanisms of brown macroalgae (Gevaert et al. 2002, 2003; García-Mendoza and Colombo-Pallotta 2007). Full activation of qE depends on the presence of specific PSII antenna proteins such as PsbS in vascular plants (Li et al. 2000), LHCSR in green microalgae (Peers et al. 2009), LHCX in diatoms (Bailleul et al. 2010) and ΔpH (Müller et al. 2001). Diatoms and the brown macroalga Ectocarpus siliculosus lack the PsbS subunit (Müller et al. 2001; Cock et al. 2010). However, since ΔpH was unlikely to be fully established during RLC, ΔpH-triggered NPQ activation was limited. This incomplete induction of NPQ in addition to insufficient activation of photosynthetic processes (as indicated by E 50:E k ratio) affected derived photosynthesis parameters, as reported from diatoms (Perkins et al. 2006), microphytobenthos (Lefebvre et al. 2011), and L. variegata and E. radiata (Ihnken et al. 2010); these parameters were therefore difficult to interpret, particularly when they were examined with regard to environmental effects (in this study: emersion) without taking into account methodological considerations.
NPQ mechanisms in Phaeophyceae based on the violaxanthin cycle, i.e. zeaxanthin-related qE, differ significantly from those observed in green algae (Goss and Jakob 2010). For M. pyrifera, zeaxanthin-dependent NPQ did not rely on ΔpH (García-Mendoza and Colombo-Pallotta 2007) which was also found for diatoms (Lavaud et al. 2002). It was assumed that enhanced NPQ in the absence of ΔpH was caused by antenna structure of Heterokontophyta (Goss and Jakob 2010). However, the exact biophysical mechanisms of qE are not fully understood (Müller et al. 2001; Jahns and Holzwarth 2012). In vascular plants, qE potentially comprises of (1) ΔpH as trigger, (2) antenna as site, (3) antenna dynamics as mechanics and (4) quencher(s) (Ruban et al. 2012), although mechanistic models are currently debated (Demmig-Adams et al. 2012). Under light-limiting conditions, PSII antenna exhibit an effective energy transfer and therefore qE should not be induced, i.e. NPQ mechanisms are up-regulated at irradiances higher than E k (Falkowski and Raven 1997). Considering E 50 as threshold for a substantial enhancement of NPQ, NPQ increased below E k (i.e. E 50 < E k) in A. nodosum, F. serratus and L. digitata during both experimental conditions when using RLC. When measuring LC, E 50 values were consistently higher than those of E k for all species, indicating that substantial photo-protection was initiated after the saturation of photochemistry. Only the latter data are in accordance with Falkowski and Raven (1997) and recent findings in the diatom Nitzschia palea (Serôdio and Lavaud 2011).
E 50 obtained from RLC was generally lower than that from LC. The correct determination of NPQmax is an essential requirement for an accurate quantification of E 50. Here, it was possible that during RLC the xanthophyll convertible pool was insufficiently ‘activated’, as indicated by the limited induction of NPQ (Fig. 2), which resulted in lower NPQmax and, thus, in an underestimation of E 50. As a consequence, the expected increase in E 50 with increasing E k was only obtained from LC. Such an increase was also found for the vascular plant Arabidopsis thaliana and N. palea when grown under different irradiances (Serôdio and Lavaud 2011).
The E 50:E k ratio computed from RLC (median: 1.05) was significantly lower than that from LC (median: 1.89). In organisms exhibiting the violaxanthin cycle (such as Phaeophyceae), this ratio varies between 1.9 and 5 (Serôdio and Lavaud 2011) which is only confirmed by LC. A further comparison of NPQ versus rETR data showed coherent parabolic relations when using LC. A parabolic increase of NPQ with increasing rETR has previously been reported (Eggert et al. 2007). However, according to data obtained from RLC, this relationship was highly inconsistent.
The limited NPQ induction and the direct comparison parameters derived from P/E and NPQ versus E PAR curves strongly suggest that the status of photoacclimation was imbalanced during RLC which may have implications for the interpretation of photosynthetic parameters calculated (Ralph and Gademann 2005).
Photo-response patterns of brown macroalgae during early stages of emersion
As shores in Ireland experience semi-diurnal tides, algae are subjected to regular submersion or emersion regimes. It has been reported repeatedly that photosynthetic gas exchange increases during early stages of emersion in intertidal macroalgae (e.g. Johnson et al. 1974; Dring and Brown 1982; Johnston and Raven 1986; Gao et al. 1999).
The duration of light increments affected relative changes in rETRmax upon early stages of emersion, as revealed by the two different measuring protocols. In addition to the previously described differences between species to tolerate (and recover from) stress during prolonged emersion (e.g. Dring and Brown 1982), the capacity of species from different shore levels to maintain photosynthesis during emersion was of interest here. As obtained from LC, rETRmax increased in A. nodosum (upper to mid-littoral) and F. serratus (lower littoral) and remained unaffected in S. muticum (rockpools in the lower littoral) and L. digitata (sublittoral) after exposure to air. Using RLC, rETRmax was unchanged upon emersion in A. nodosum, decreased in F. serratus and L. digitata and increased in S. muticum. Previously, the extent of carbon assimilation was higher for F. serratus than for L. digitata in which it also remained nearly stable during 1 h exposure to air at 34 % RH (also measured under laboratory conditions at 10 °C; Dring and Brown 1982), suggesting that this was related to the natural shore level of the species. This was supported only by fluorescence data obtained here by LC, but not by RLC. In addition, PSII performance of L. digitata was increased within 3 h of emersion under similar conditions, which was caused by enhanced qP (Nitschke et al. 2011).
Except for F. serratus, the impact of emersion on E k reported here was based on changes in rETRmax but not on modifications in α. An enhancement of rETRmax and E k found for A. nodosum and F. serratus using LC is in agreement with Dring and Brown (1982) and Johnston and Raven (1986). Moreover, air exposure resulted in an enhanced qP in A. nodosum and F. serratus at E PAR higher than 200 μmol photons m−2 s−1, but again only when LC were applied. These results, together with increased rETRmax during emersion (LC), also correspond to previous CO2 exchange measurements at (saturating) irradiances ≥500 μmol photons m−2 s−1 (Dring and Brown 1982; Johnston and Raven 1986).
Brown macroalgae exhibit high photo-protection capacities which are expressed via high NPQ, e.g. up to ten from the high-shore species P. canaliculata (Harker et al. 1999) and of about six from the low-shore S. latissima (Gevaert et al. 2002). Here, NPQmax derived from LC during submersion was ~4.4 for A. nodosum, ~3.4 for F. serratus and 2.5 and 2.2 for S. muticum and L. digitata, respectively, potentially suggesting habitat-specific capabilities in algal photo-protection as shown for diatoms (Goss and Jakob 2010).
During LC, NPQ in the light limited range increased fastest in F. serratus and L. digitata (as shown by highest slopes C of rETR versus NPQ curves), indicating their capacity for rapid PSII protection. This probably explains why emersion resulted in pronounced NPQ in these species even during RLC. The PSII protection capacity decreased in A. nodosum, F. serratus and S. muticum after exposure to air, but NPQ regulation (as derived from LC) remained nearly unchanged. In L. digitata, the PSII protection capacity was not affected which accompanied with increased NPQmax.
These observations suggest that photosynthetic performance of mid- and low-intertidal macroalgae under different environmental regimes, including short- and long-term emersion, is related to conditions in their natural habitat. The previously suggested physiological basis of algal vertical distribution (Johnson et al. 1974; Dring and Brown 1982; Harker et al. 1999; Rodrigues et al. 2002; Gómez et al. 2004) was only supported when LC protocols were applied.
Conclusion
The duration of light increments applied when using different measuring protocols influenced photosynthetic parameters of the four brown macroalgae upon emersion. During RLC, re-oxidation of QA was imbalanced and NPQ was not fully induced, resulting in an underestimation of α and NPQmax, with further implications for the determination of rETR, E k and E 50. The results of this study suggest that LC should be applied in ecophysiological studies on macroalgae where the experimental approach allows an extended duration of P/E curve measurements.
References
Baer J, Stengel DB (2010) Variability in growth, development and reproduction of the non-native seaweed Sargassum muticum (Phaeophyceae) on the Irish west coast. Estuar Coast Shelf Sci 90:185–194
Bailleul B, Rogato A, Martino A, Coesel S, Cardol P, Bowler C, Falciatore A, Finazzi G (2010) An atypical member of the light harvesting complex stress-related protein family modulates diatom responses to light. Proc Natl Acad Sci USA 107:18214–18219
Blache U, Jakob T, Su W, Wilhelm C (2011) The impact of cell-specific absorption properties on the correlation of electron transport rates measured by chlorophyll fluorescence and photosynthetic oxygen production in planktonic algae. Plant Physiol Biochem 49:801–808
Carrillo N, Vallejos RH (1983) The light-dependent modulation of photosynthetic electron transport. Trends Biochem Sci 8:52–56
Cock JM, Sterck L, Rouzé P, Scornet D, Allen AE, Amoutzias G, Anthouard V, Artiguenave F, Aury JM, Badger JH, Beszteri B, Billiau K, Bonnet E, Bothwell JH, Bowler C, Boyen C, Brownlee C, Carrano CJ, Charrier B, Cho GY, Coelho SM, Collén J, Corre E, Da Silva C, Delage L, Delaroque N, Dittami SM, Doulbeau S, Elias M, Farnham G, Gachon CMM, Gschloessl B, Heesch S, Jabbari K, Jubin C, Kawai H, Kimura K, Kloareg B, Küpper FC, Lang D, Le Bail A, Leblanc C, Lerouge P, Lohr M, Lopez PJ, Martens C, Maumus F, Michel G, Miranda-Saavedra D, Morales J, Moreau H, Motomura T, Nagasato C, Napoli CA, Nelson DR, Nyvall-Collen P, Peters AF, Pommier C, Potin P, Poulain J, Quesneville H, Read B, Rensing SA, Ritter A, Rousvoal S, Samanta M, Samson G, Schroeder DC, Ségurens B, Strittmatter M, Tonon T, Tregear JW, Valentin K, von Dassow P, Yamagishi T, Van de Peer Y, Wincker P (2010) The Ectocarpus genome and the independent evolution of multicellularity in brown algae. Nature 465:617–621
Colombo-Pallotta MF, García-Mendoza E, Ladah LB (2006) Photosynthetic performance, light absorption, and pigment composition of Macrocystis pyrifera (Laminariales, Phaeophyceae) blades from different depths. J Phycol 42:1225–1334
Demmig-Adams B, Cohu CM, Muller O, Adams WW III (2012) Modulation of photosynthetic energy conversion efficiency in nature: from seconds to seasons. Photosynth Res. doi:10.1007/s11120-012-9761-6
Dring MJ, Brown FA (1982) Photosynthesis of intertidal brown algae during and after periods of emersion: a renewed search for physiological causes of zonation. Mar Ecol Prog Ser 8:301–308
Edwards MS, Kim KY (2010) Diurnal variation in relative photosynthetic performance in giant kelp Macrocystis pyrifera (Phaeophyceae, Laminariales) at different depths as estimated using PAM fluorometry. Aquat Bot 92:119–128
Eggert A, Nitschke U, West JA, Michalik D, Karsten U (2007) Acclimation of the intertidal red alga Bangiopsis subsimplex (Stylonematophyceae) to salinity changes. J Exp Mar Biol Ecol 343:176–186
Eichelmann H, Talts E, Oja V, Padu E, Laisk A (2009) Rubisco in planta k cat is regulated in balance with photosynthetic electron transport. J Exp Bot 60:4077–4088
Enríquez S, Agustí S, Duarte CM (1994) Light absorption by marine macrophytes. Oecologia 98:121–129
Falkowski PG, Raven JA (1997) Aquatic photosynthesis. Blackwell, Oxford
Figueroa FL, Israel A, Neori A, Martínez B, Malta E-J, Ang P Jr, Ihnken S, Marquardt R, Korbee N (2009) Effects of nutrient supply on photosynthesis and pigmentation in Ulva lactuca (Chlorophyta): responses to short-term stress. Aquat Biol 7:173–183
Fork DC, Herbert SK, Malkin S (1991) Light energy distribution in the brown alga Macrocystis pyrifera (giant kelp). Plant Physiol 95:731–739
Gao K, Ji Y, Aruga Y (1999) Relationship of CO2 concentrations to photosynthesis of intertidal macroalgae during emersion. Hydrobiologia 398(399):355–359
Gao S, Shen S, Wang G, Niu J, Lin A, Pan G (2011) PSI-driven cyclic electron flow allows intertidal macro-algae Ulva sp. (Chlorophyta) to survive in desiccated conditions. Plant Cell Physiol 52:885–893
García-Mendoza E, Colombo-Pallotta MF (2007) The giant kelp Macrocystis pyrifera presents a different nonphotochemical quenching control than higher plants. New Phytol 173:526–536
Genty B, Briantais JM, Baker NR (1989) The relationship between the quantum yield of photosynthetic electron-transport and quenching of chlorophyll fluorescence. Biochim Biophys Acta 990:87–92
Gevaert F, Créach A, Davoult D, Holl AC, Seuront L, Lemoine Y (2002) Photo-inhibition and seasonal photosynthetic performance of the seaweed Laminaria saccharina during a simulated tidal cycle: chlorophyll fluorescence measurements and pigment analysis. Plant Cell Environ 25:859–872
Gevaert F, Créach A, Davoult D, Migné A, Levavasseur G, Arzel P, Holl A-C, Lemoine Y (2003) Laminaria saccharina photosynthesis measured in situ: photoinhibition and xanthophyll cycle during a tidal cycle. Mar Ecol Prog Ser 247:43–50
Giordano M, Beardall J, Raven JA (2005) CO2 concentrating mechanisms in algae: mechanisms, environmental modulation, and evolution. Annu Rev Plant Biol 56:99–131
Gómez I, Figueroa FL, Ulloa N, Morales V, Lovengreen C, Huovinen P, Hess S (2004) Patterns of photosynthesis in 18 species of intertidal macroalgae from southern Chile. Mar Ecol Prog Ser 270:103–116
Goss R, Jakob T (2010) Regulation and function of xanthophyll cycle-dependent photoprotection in algae. Photosynth Res 106:103–122
Häder D-P, Figueroa FL (1997) Photophysiology of marine macroalgae. Photochem Photobiol 66:1–14
Harker M, Berkaloff C, Lemoine Y, Britton G, Young A, Duval JC, Rmiki N-E, Rousseau B (1999) Effects of high light and desiccation on the operation of the xanthophyll cycle in two marine brown algae. Eur J Phycol 34:35–42
Henley WJ (1993) Measurement and interpretation of photosynthetic light-response curves in algae in the context of photoinhibition and diel changes. J Phycol 29:729–739
Ihnken S, Eggert A, Beardall J (2010) Exposure times in rapid light curves affect photosynthetic parameters in algae. Aquat Bot 93:185–194
Jahns P, Holzwarth AR (2012) The role of the xanthophyll cycle and of lutein in photoprotection of photosystem II. Biochim Biophys Acta 1817:182–193
Jakob T, Goss R, Wilhelm C (1999) Activation of diadinoxanthin de-epoxidase due to a chlororespiratory proton gradient in the dark in the diatom Phaeodactylum tricornutum. Plant Biol 1:76–82
Johnson WS, Gigon A, Gulmon SL, Mooney HA (1974) Comparative photosynthetic capacities of intertidal algae under exposed and submerged conditions. Ecology 55:450–453
Johnston AM, Raven JA (1986) The analysis of photosynthesis in air and water of Ascophyllum nodosum (L.) Le Jol. Oecologia 69:288–295
Joly D, Carpentier R (2007) Regulation of energy dissipation in photosystem I by the redox state of the plastoquinone pool. Biochemistry 46:5534–5541
Joly D, Jemâa E, Carpentier R (2010) Redox state of the photosynthetic electron transport chain in wild-type and mutant leaves of Arabidopsis thaliana: impact on photosystem II fluorescence. J Photochem Photobiol B 98:180–187
Kramer DM, Wise RR, Frederick JR, Alm DM, Hesketh JD, Ort DR, Crofts AR (1990) Regulation of coupling factor in field-grown sunflower: a redox model relating coupling factor activity to the activities of other thioredoxin-dependent chloroplast enzymes. Photosynth Res 26:213–222
Krause GH, Weis E (1991) Chlorophyll fluorescence and photosynthesis—the basics. Annu Rev Plant Physiol Plant Mol Biol 42:313–349
Kromkamp JC, Forster RM (2003) The use of variable fluorescence measurements in aquatic ecosystems: differences between multiple and single turnover measuring protocols and suggested terminology. Eur J Phycol 38:103–112
Küster A, Schaible R, Schubert H (2005) Sex-specific light acclimation of Chara canescens Charophyta. Aquat Bot 83:129–140
Lavaud J, Rousseau B, Etienne A-L (2002) In diatoms, a transthylakoid proton gradient alone is not sufficient to induce a non-photochemical fluorescence quenching. FEBS Lett 523:163–166
Lefebvre S, Mouget J-L, Lavaud J (2011) Duration of rapid light curves for determining the photosynthetic activity of microphytobenthos biofilm in situ. Aquat Bot 95:1–8
Li X-P, Björkman O, Shih C, Grossman AR, Rosenquist M, Jansson S, Niyogi KK (2000) A pigment-binding protein essential for regulation of photosynthetic light harvesting. Nature 403:391–395
Lobban CS, Harrison PJ (1994) Seaweed ecology and physiology. Cambridge University Press, Cambridge
Lüning K, Dring MJ (1985) Action spectra and spectral quantum yield of photosynthesis in marine macroalgae with thin and thick thalli. Mar Biol 87:119–129
Mouget J-L, Tremblin G (2002) Suitability of the fluorescence monitoring system (FMS, Hansatech) for measurement of photosynthetic characteristics in algae. Aquat Bot 74:219–231
Müller P, Li X-P, Niyogi KK (2001) Non-photochemical quenching. A response to excess light energy. Plant Physiol 125:1558–1566
Nielsen HD, Nielsen SL (2008) Evaluation of imaging and conventional PAM as a measure of photosynthesis in thin- and thick-leaved marine macroalgae. Aquat Biol 3:121–131
Nitschke U, Ruth AA, Dixneuf S, Stengel DB (2011) Molecular iodine emission rates and photosynthetic performance of different thallus parts of Laminaria digitata (Phaeophyceae) during emersion. Planta 233:737–748
Noctor G, Mills JD (1987) Control of CO2 fixation during the induction period: the role of thiol-mediated enzyme activation in the alga, Dunaliella. Biochim Biophys Acta 894:295–303
Peers G, Truong TB, Ostendor E, Busch A, Elrad D, Grossman AR, Hippler M, Niyogi KK (2009) An ancient light-harvesting protein is critical for the regulation of algal photosynthesis. Nature 462:518–522
Perkins RG, Mouget J-L, Lefebvre S, Lavaud J (2006) Light response curve methodology and possible implications in the application of chlorophyll fluorescence to benthic diatoms. Mar Biol 149:703–712
Perkins RG, Kromkamp JC, Serôdio J, Lavaud J, Jesus B, Mouget J-L, Lefebvre S, Forster RM (2011) The application of variable chlorophyll fluorescence to microphytobenthic biofilms. In: Suggett DJ, Prášil O, Borowitzka MA (eds) Chlorophyll a fluorescence in aquatic sciences: methods and applications. Springer, New York, pp 237–275
Poulson ME, McNeil AJ, Donahue RA (2011) Photosynthetic response of Nereocystis luetkeana (Phaeophyta) to high light. Phycol Res 59:156–165
Ralph PJ, Gademann R (2005) Rapid light curves: a powerful tool to assess photosynthetic activity. Aquat Bot 82:222–237
Rascher U, Liebig M, Lüttge U (2000) Evaluation of instant light-response curves of chlorophyll fluorescence parameters obtained with a portable chlorophyll fluorometer on site in the field. Plant Cell Environ 23:1397–1405
Rodrigues MA, dos Santos CP, Young AJ, Strbac D, Hall DO (2002) A smaller and impaired xanthophyll cycle makes the deep sea macroalgae Laminaria abyssalis (Phaeophyceae) highly sensitive to daylight when compared with shallow water Laminaria digitata. J Phycol 38:939–947
Ruban AV, Johnson MP, Duffy CDP (2012) The photoprotective molecular switch in the photosystem II antenna. Biochim Biophys Acta 1817:167–181
Saroussi S, Beer S (2007a) Alpha and quantum yield of aquatic plants derived from PAM fluorometry: uses and misuses. Aquat Bot 86:89–92
Saroussi S, Beer S (2007b) Acclimation of macroalgae as reflected in photosynthetic parameters derived from PAM fluorometry, and possible implications for abundance patterns. Mar Ecol 28:377–383
Schansker G, Tóth SZ, Strasser RJ (2006) Dark recovery of the chl a fluorescence transient (OJIP) after light adaptation: the qT-component of non-photochemical quenching is related to an activated photosystem I acceptor side. Biochim Biophys Acta 1757:787–797
Schreiber U, Schliwa U, Bilger W (1986) Continuous recording of photochemical and nonphotochemical chlorophyll fluorescence quenching with a new type of modulation fluorometer. Photosynth Res 10:51–62
Serôdio J, Lavaud J (2011) A model for describing the light response of the nonphotochemical quenching of chlorophyll fluorescence. Photosynth Res 108:61–76
Serôdio J, Cruz S, Vieira S, Brotas V (2005) Non-photochemical quenching of chlorophyll fluorescence and operation of the xanthophyll cycle in estuarine microphytobenthos. J Exp Mar Biol Ecol 326:157–169
Serôdio J, Vieira S, Cruz S, Coelho H (2006) Rapid light-response curves of chlorophyll fluorescence in microalgae: relationship to steady-state light curves and non-photochemical quenching in benthic diatom-dominated assemblages. Photosynth Res 90:29–43
Wahl M, Jormalainen V, Eriksson BK, Coyer JA, Molis M, Schubert H, Dethier M, Karez R, Kruse I, Lenz M, Pearson G, Rohde S, Wikström SA, Olsen JL (2011) Stress ecology in Fucus: abiotic, biotic and genetic interactions. Adv Mar Biol 59:37–105
Walsby A (1997) Modelling the daily integral of photosynthesis by phytoplankton: its dependence on the mean depth of the population. Hydrobiologia 349:65–74
White AJ, Critchley C (1999) Rapid light curves: a new fluorescence method to assess the state of the photosynthetic apparatus. Photosynth Res 59:63–72
Acknowledgments
The authors thank Dr. Sorcha Ní Longphuirt (Botany and Plant Science and Ryan Institute, NUI Galway) for valuable comments on the manuscript. UN gratefully appreciates financial support through the Irish Research Council for Science, Engineering and Technology (IRCSET ‘Embark Initiative’).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nitschke, U., Connan, S. & Stengel, D.B. Chlorophyll a fluorescence responses of temperate Phaeophyceae under submersion and emersion regimes: a comparison of rapid and steady-state light curves. Photosynth Res 114, 29–42 (2012). https://doi.org/10.1007/s11120-012-9776-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11120-012-9776-z