Abstract
The emission of molecular iodine (I2) from the stipe, the meristematic area and the distal blade of the brown macroalga Laminaria digitata (Hudson) Lamouroux (Phaeophyceae) was monitored under low light and dark conditions. Photosynthetic parameters were determined to investigate both the extent of stress experienced by different thallus parts and the effects of emersion on photosynthesis. Immediately after air exposure, intense I2 emission was detectable from all thallus parts. I2 emission declined continuously over a period of 180 min following the initial burst, but was not affected by the light regime. The total number of mole of I2 emitted by stipes was approximately 10 times higher than those emitted from other thallus parts. Initial I2 emission rates (measured within 30 min of exposure to air) were highest for stipes (median values: 2,999 and 5,222 pmol g−1 dw min−1 in low light and dark, respectively) and lower, by one order of magnitude, for meristematic regions and distal blades. After exposure to air for between 60 and 180 min, I2 emission rates of all thallus parts were reduced by 70–80%. Air exposure resulted in a decrease of the maximum photosystem II (PSII) efficiency (F v/F m) by 3%, and in a 25–55% increase of the effective PSII quantum efficiency (\( \Updelta F/F^{\prime}_{\text{m}} \)); this was caused by a higher fraction of open reaction centres (qP), whereas the efficiency of the latter in capturing energy (\( F^{\prime}_{\text{v}} /F^{\prime}_{\text{m}} \)) remained constant. The results indicate the presence of an iodine pool which is easily volatilised and depleted due to air exposure, even under apparently low stress conditions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Kelp species of the genus Laminaria are large brown algae (Phaeophyceae) representing some of the most prominent constituents of Atlantic and Pacific rocky shores in temperate regions. Laminaria species form ecologically important, extensive seaweed beds which occur from the upper sublittoral fringe downwards (Lobban and Harrisson 1994). The vertical distribution of kelps within the sublittoral zone is determined by light penetration into water, the morphological capacity of the thallus to withstand mechanical stress caused by wave action, and its capacity to cope with periods of emersion (Lüning 1985). Laminaria digitata is particularly well adapted to the environmental conditions found in the upper sublittoral zone. The sporophyte of L. digitata consists of a holdfast which anchors the alga to hard substrata, a smooth and flexible stipe that prevents the alga from major mechanical damage during wave exposure, and a long and large blade, single for a plantlet and divided into several laminae for an older individual. The meristematic growth region is located at the base of the blade (van den Hoek et al. 1995). During low spring tides, L. digitata is exposed to the atmosphere for short periods. Emersion involves changes in the abiotic environment, and L. digitata is subjected to potential water loss, different carbon regimes, temperature fluctuations and increased irradiances. This requires an appropriate adjustment of the metabolism to prevent inhibition of photosynthesis (Lüning 1985). A combination of such stressors results in cumulative stress, i.e. higher stress levels experienced by the alga.
Similar to many other brown macroalgae, L. digitata accumulates high amounts of iodine (Saenko et al. 1978) from the surrounding seawater. With iodine concentrations in tissue more than 30,000 times those in seawater (Küpper et al. 1998; Verhaeghe et al. 2008), L. digitata is the most effective known iodine accumulator among living organisms (Ar Gall et al. 2004). Iodine uptake by L. digitata is mediated by an extracellular haloperoxidase (Küpper et al. 1998); within the thallus, iodine is retained as inorganic iodide (I−), probably associated not only in a non-covalent way with carbohydrates, polyphenols or proteins, in the apoplast but also in intracellular, non-specialised vesicles and physodes (Kylin 1929; Pedersén and Roomans 1983; Küpper et al. 2008; Verhaeghe et al. 2008). I− is a potent quencher of aqueous oxidants and ozone and, therefore, acts as an inorganic antioxidant in L. digitata (Küpper et al. 2008). Both exogenously induced and endogenous oxidative stress lead to the release of iodine from L. digitata into the surrounding seawater, which can be easily measured (Küpper et al. 2008), or into air in the form of molecular iodine (I2) and various volatile iodo-carbons (Laturnus et al. 2004; Palmer et al. 2005; Leblanc et al. 2006). The latter process is generally referred to as ‘iodovolatilisation’ (Küpper et al. 1998). Significant mixing ratios of I2 were measured from several air-exposed brown macroalgae under reasonably low stress conditions: the algae were temperature controlled in weakly oxidative atmospheres and studied in the dark or at low light levels to avoid cumulative effects of several abiotic stressors (Bale et al. 2008; Dixneuf et al. 2009; Ball et al. 2010).
Besides the role of iodine in algal stress responses (Laturnus et al. 2004; Küpper et al. 2008), atmospheric I2 has been identified as a vital actor in the photochemistry of the MBL (Carpenter 2003). The daylight photolysis of I2 leads to the formation of atomic iodine known to be one of the most important catalytic species of a complex chemistry which promotes the destruction of ozone and the formation of marine aerosols (O’Dowd et al. 2002; McFiggans et al. 2004; O’Dowd and Hoffmann 2005; McFiggans et al. 2010). These processes potentially affect the Earth’s radiation balance and therefore may have an impact on the global climate (Kolb 2002). During field campaigns on the Irish west coast, significant concentrations of tropospheric iodine oxide (IO) and I2 were observed at times of low tide, suggesting that I2 emitted by the air-exposed kelp beds could potentially play a more important role than the volatile iodo-carbons in the atmospheric photochemistry of coastal regions (McFiggans et al. 2004; Saiz-Lopez and Plane 2004; Bitter et al. 2005; Huang et al. 2010; Seitz et al. 2010). Furthermore, according to Palmer et al. (2005) and Küpper et al. (2008), the iodine identified in the particles formed above air-exposed L. digitata originated only from I2; the concomitant emission of volatile iodo-carbons did not contribute to particle production. Thus, the emission of I2 from kelp deserved a closer examination due to its key role in atmospheric chemistry processes (O’Dowd and Hoffmann 2005). Even though quantification techniques of biogenic iodine emissions are still being debated (Ball et al. 2010), only few I2 emission rates have been published for brown macroalgae (Palmer et al. 2005; Ball et al. 2010). These data suggest that I2 emission rates from macroalgae need to be considered in relation to several different parameters such as the corresponding species, biotic and abiotic environmental impacts and the physiological status of the algae (Laturnus et al. 2004).
In the present study, I2 emission from air-exposed L. digitata was quantified under controlled environmental conditions by applying incoherent broadband cavity-enhanced absorption spectroscopy (IBBCEAS) (Fiedler et al. 2003; Dixneuf et al. 2009). Relevant parts of the thallus, i.e. the stipe plus holdfast, the meristematic area and the distal blade, were considered individually so that their respective contribution to the total I2 emission from the alga could be estimated for the first time. Dividing the alga into different parts in the context of I2 emission provides useful information about the presumably multifunctional role of iodine in seaweeds.
Furthermore, effects of air exposure on photosynthetic performance of different thallus parts of L. digitata were investigated. In vivo chlorophyll a fluorescence parameters are widely used as an indicator for “health” of photosynthetic active organisms (Maxwell and Johnson 2000) and changes can allow the quantification of potential stress experienced by the alga. Photosynthesis can be affected by air exposure due to desiccation and/or qualitative and quantitative changes in inorganic carbon supply (Dring and Brown 1982). Such effects potentially influence the efficiency of photosystem II (PSII), i.e. primary photosynthesis.
Materials and methods
Algal material and sampling strategy
Sporophytes of L. digitata (Hudson) Lamouroux were harvested on the west coast of Ireland at Finavarra (53°09′25″N, 09°06′58″W) during the spring low tide in early November 2009; the weather was overcast and the mean seawater temperature was ~8°C. All algae sampled were of similar age and 1.5 m long on average, and free from visible epiphytes, grazers or grazing marks. Only submersed specimens were collected and care was taken to keep the samples underwater at all times between harvesting and start of I2 emission measurements. The latter were conducted in the Finavarra field research station ca. 500 m from the collecting site. Thus, a potential loss of iodine caused by stressful conditions during the transport to the laboratory was avoided. Prior to experiments, seaweeds were kept for up to 8 days in a 200 L tank filled with seawater from their natural habitat and stored at low irradiances at ~8°C; the water was continuously aerated by diffusers and replaced every second day with fresh, local seawater.
Experimental design
Each L. digitata thallus was divided into three parts: (a) the ‘stipe’ (undivided and including the holdfast), (b) the ‘meristematic area’, from the top of the stipe to where the base of the blade divides into several laminae, and (c) the ‘distal blade’, i.e. one part of the remaining laminae (cf. inset in Fig. 2). The I2 emitted into the air was monitored by applying IBBCEAS (Fiedler et al. 2003). To investigate the effect of low irradiance on I2 emission, the air-exposed samples were studied for 180 min under two ambient light regimes: (a) low light, i.e. a PFD of 35 μmol photons m−2 s−1 PAR (400-700 nm) provided by a fluorescent tube (Lumilux cool daylight, Osram GmbH, Munich, Germany) or (b) dark conditions. Due to the absence of radiation below 400 nm when the light was switched on, the loss of I2 through UV photolysis inside the system was negligible. The experimental air temperature was controlled in the chamber at T in ~ 10.5°C.
Prior to measurements, the relevant thallus part was cut from the rest of the alga under water, as, given the size of the experimental setup (Fig. 1), it was not feasible to study an entire specimen. Algal samples were acclimated in natural seawater to the experimental light and temperature conditions for 30–60 min prior to air exposure and I2 emission measurements. Measurements generally started within 5 s after emersion of the sample. After 180 min, both dry weight (dw) and surface area (sa) of the sample were determined. Surface area of the meristematic area and distal blade was obtained by weighing a sample-shaped piece of paper whose surface area-to-weight ratio was known. Only one side of the meristematic area and distal blade was accounted for in the calculation of sa as only one face was in direct contact with air; the other was in contact with the wall of the flow tube. Approximating the stipe (and the main branches of the holdfast) by a cylinder with elliptical circumference, the surface area of these thallus parts was estimated. Dry weights were determined after freeze-drying. I2 emission rates are expressed as both pmol g−1 dw min−1 and pmol cm−2 min−1.
Schematic representation of the IBBCEAS setup for molecular iodine flux measurements. a M1, M2: high reflectivity plano-concave mirrors (separation 76 cm). The main carrying flow (3.9 L min−1) and the mirror purge flow (~0.2 L min−1 in total) were clean compressed air. The gas outlet was open to ambient air. Temperature in the sample compartment was T in ~ 10.5°C. b Details of the quartz flow tube: d 1 = 2.2 cm, d 2 = 9.3 cm, l 1 = 60 cm, l 2 = 25 cm, l 3 = 32 cm
In order to study the effect of air exposure on the photosynthetic performance of algal thallus parts, in vivo chlorophyll a fluorescence parameters were determined before and after I2 emission measurements; initial fluorescence values were taken on submersed samples. Water loss of individual thallus parts was also monitored: a tissue sample (~1 g fresh weight) was taken before and after I2 emission measurement and immediately frozen before freeze-drying; the water content was calculated as a reduction in weight before and after drying.
Determination of I2 emission rates using IBBCEAS
A schematic of the experimental setup for the detection of I2 is shown in Fig. 1. The optical cavity was formed by two plano-concave dielectric mirrors (2.5 cm diameter) with a radius of curvature of 200 cm and a separation of d = 76 cm. The spectrally filtered white light from a 75 W short arc Xe lamp was focused at the centre of the cavity; the light transmitted through the cavity was spectrally dispersed by a monochromator (Oriel MS125, 25 μm entrance slit, 1,200 lines mm−1 grating, LOT-Oriel, Darmstadt, Germany) and imaged onto a CCD detector (back illuminated DU401-BV, Andor Technology, Belfast, UK) cooled to −25°C. The spectral resolution was ~0.43 nm.
To achieve time-resolved flux measurements, the optical cavity was set up perpendicular to the direction of the I2 carrying flow by means of a four-way stainless steel cross-piece with an internal diameter of d 1 = 2.2 cm on all sides (Fig. 1a). The seaweed sample was placed in a quartz tube (diameter d 2 = 9.3 cm) at right angle to the cavity axis which was connected to the central cross-piece via a conical glass-to-metal seal (Fig. 1b); the other end of the tube was sealed with a plastic cap. In Fig. 1a, the open arrows represent the inlet of the main air flow (M c ~ 3.9 L min−1) through a 5 mm aperture, and the only gas outlet of the system at the cross-piece. The two thin black arrows indicate the purge gas inlets (total purge flow ~0.2 L min−1) located ~5 cm from the cavity mirrors. The purge flow was chosen so that the effect on the I2 carrying flow was minimal (see below).
Both the main flow and the purge flows were supplied by ambient air compressed through an oil-free air compressor (OF302-4MD2, Jun Air Ltd., Nørresundby, Denmark) equipped with a particle filter and an adsorption dryer. The purge air inlet line (10 m, 8 mm diameter) between the air compressor and the system also served as a scrubber for both I2 and O3 that may have been present in ambient air.
The quartz flow tube (i.e. the compartment where the seaweed was placed) was contained in a light-tight cylindrical copper chamber that was thermally stabilised using a closed cycle water cooler. Two thermocouples with an accuracy of ~0.5°C were used to monitor the temperature just above the seaweed (temperature T in ~ 10.5°C), and near the detection zone (temperature T ext ~ 13.5°C).
I2 was monitored in the spectral region between 530 and 553 nm. The retrieval of the molecular iodine number density (molecules cm−3), \( n_{{{\text{I}}_{2} }} \), has been described in Dixneuf et al. (2009). Using cavity-enhanced absorption techniques for quantitative purposes requires the knowledge of the effective absorption path length as a function of wavelength, L eff(λ) = L(1 − R(λ))−1, where R(λ) is the mirror reflectivity and L (cm) is the path length over which the target species in the cavity interacts with the light trapped inside the cavity. In the present study, L eff was established by flowing a known concentration of I2 into the system: a known, small flow of air was passed above iodine crystals contained in a U-shaped tube cooled to 0°C so that the iodine mixing ratio was deduced from the ratio of the vapour pressure of iodine at 0°C and atmospheric pressure. A maximum effective path length of ~30 ± 3 m at 542 nm was found.
The detection limit for I2 was ≈7 ± 2 nmol mol−1 for a 10 s average time. The systematic uncertainty on \( n_{{{\text{I}}_{2} }} \) (which is mostly due to the uncertainty of L eff(λ) and to the error of the I2 reference absorption cross-sections \( \sigma_{{{\text{I}}_{2} }} (\lambda ) \); Saiz-Lopez et al. 2004) was smaller than 20% (Dixneuf et al. 2009). Time-dependent emission rates \( E_{{{\text{I}}_{2} }} (t) \) were obtained as the product of \( n_{{{\text{I}}_{2} }} (t) \) and the constant volumetric flow rate M c:
Each emission profile \( E_{{{\text{I}}_{2} }} (t) \) was further characterised by averaging over two relevant time periods: (a) the interval 0–30 min where an initial I2 emission peak was observed, and (b) the interval 60–180 min where the I2 emission was found to gradually reach the detection limit. For each sample, both the mean initial and the mean long term emission rates, \( \langle E_{{{\text{I}}_{2} }} \rangle \), were thus obtained.
Note that adsorption/desorption of I2 on/from the walls of the flow cell affected the temporal emission profile. Under the present flow conditions passivating the walls with iodine was not possible. For this reason, the mean initial emission rates presented here are in principle lower limits (due to potential adsorption of I2), while the emission rates observed during the interval 60–180 min are potential upper limits (due to the potential desorption of I2). The I2 wall adsorption/desorption can be accounted for when integrating the temporal emission profile \( E_{{{\text{I}}_{2} }} (t) \) until the detection limit is reached, in the present case usually after 180 min (corresponding amounts of I2 emitted in total, \( N_{{{\text{I}}_{2} }} \), are given in Table 1). Averaging the emission profile over the periods 0–30 and 60–180 min remains a relevant way of discussing relative emission changes as a function of time.
Photosynthetic performance
A pulse-amplitude modulated fluorometer (PAM-2000, Heinz Walz GmbH, Effeltrich, Germany) was used for measurements of in vivo chlorophyll a fluorescence parameters which were determined directly before (in natural seawater) and after 180 min (in air) I2 emission studies. The measuring principle was based on the technique by Schreiber et al. (1986). The maximum PSII efficiency, F v/F m, the effective PSII quantum efficiency under low light exposure (35 μmol photons m−2 s−1 PAR), \( \Updelta F/F^{\prime}_{\text{m}} \), the photochemical quenching, which is the fraction of open PSII reaction centres, qP, and the efficiency of the latter in capturing energy, \( F^{\prime}_{\text{v}} /F^{\prime}_{\text{m}} \), were determined for each thallus part. The nomenclature used in this study is according to Kromkamp and Forster (2003). F 0 was determined with a 1.6 kHz pulsed red measuring light (~0.2 μmol photons m−2 s−1, 650 nm) and F m and \( F^{\prime}_{\text{m}} \) were measured with a 600 ms completely saturating white light pulse (20 kHz, ~9,200 μmol photons m−2 s−1). The thallus parts were kept in the dark for 30 min and, in the case of the low light experiment, additionally for 30 min at 35 μmol photons m−2 s−1 PAR, prior to measuring the aforementioned photosynthesis parameters. F v/F m was calculated as follows:
where F v is the variable chlorophyll a fluorescence, F m is the maximal chlorophyll a fluorescence after dark acclimation, and F 0 is the minimal level chlorophyll a fluorescence.
\( \Updelta F/F^{\prime}_{\text{m}} \) was calculated according to the following equation:
where \( F^{\prime}_{\text{m}} \) is the maximal chlorophyll a fluorescence after acclimation to light and F is the steady-state fluorescence of chlorophyll a in light (Genty et al. 1989; Kromkamp and Forster 2003).
The parameter qP was computed as follows:
where \( F^{\prime}_{0} \) and \( F^{\prime}_{\text{m}} \) are, respectively, the minimal and maximal chlorophyll a fluorescence after acclimation to light (Kromkamp and Forster 2003).
Finally, \( F^{\prime}_{\text{v}} /F^{\prime}_{\text{m}} \) was calculated as:
Statistical analysis
I2 emission experiments were carried out using three different thallus parts under two ambient light regimes (low light and dark conditions) and each treatment was replicated five times (n = 5). Due to a high variability in the obtained emission data, the I2 emission rates \( \langle E_{{{\text{I}}_{2} }} \rangle \) are presented as median and as minimum and maximum showing the data range. Results of the photosynthetic performance (F v/F m, \( \Updelta F/F^{\prime}_{\text{m}} \), qP and \( F^{\prime}_{\text{v}} /F^{\prime}_{\text{m}} \)) of the different thallus parts are presented as mean and one standard deviation.
The effects of the fixed factors “thallus part” (i.e. stipe, meristematic area and distal blade), “time” (i.e. 0–30 and 60–180 min) and “light regime” (i.e. low light and dark conditions) on the variables “\( \langle E_{{{\text{I}}_{2} }} \rangle \)” and “F v /F m” were analysed by applying three-way analyses of variance (ANOVAs). Effects of the fixed factors “thallus part” and “time” on the variables “\( \Updelta F/F^{\prime}_{\text{m}} \)”, “qP” and “\( F^{\prime}_{\text{v}} /F^{\prime}_{\text{m}} \)” were analysed using two-way ANOVAs. The main effects were computed. The Tukey test was used to find a posteriori homogeneous sub-groups that differed significantly (level of significance: P < 0.05). Regarding I2 emission rates \( \langle E_{{{\text{I}}_{2} }} \rangle \), the data were logarithmically transformed prior to statistical analyses (Sokal and Rohlf 1995). All data (or, regarding \( \langle E_{{{\text{I}}_{2} }} \rangle \), the transformed data) were normally distributed (Kolmogorov–Smirnov test) and the variances were homogeneous (Levene test).
Results
Temporal profile of I2 emission
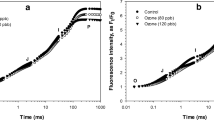
Two examples of the I2 emission profiles, \( E_{{{\text{I}}_{2} }} (t) \), obtained from each of the three parts of the strongest I2 emitting specimens of L. digitata are presented in Fig. 2 with a time resolution of 10 s. Immediately after t = 0 when the thallus was first exposed to air, an intense initial burst of I2 was detectable from all thallus parts, lasting about 30 min (note that initially the thallus was still surrounded by a thin water film). The median HWHM of the initial I2 emission burst was 21 min for the stipe, 15 min for the meristematic area and 11 min for the distal blade. The strongest initial burst was observed from the stipe with a maximum \( E_{{{\text{I}}_{2} }} \) about 5 to 10 times higher than bursts from the meristematic area and the distal blade, respectively. I2 emission from each part of the alga decreased continuously over time to about 30% of the initial emission rate; the decay did not follow a single exponential decay. During exposure between 60 and 180 min, emission rates continued to decrease gradually and dropped to the detection limit after 180 min. For the two specimens exemplified in Fig. 2, additional short and intense I2 emission bursts were later observed from the stipe and the meristematic area. Similar subsequent bursts were frequently detectable from these two thallus parts, at both low light and dark conditions, but were only once observed from the distal blade. These intense, additional bursts were neither periodic nor did they follow any specific pattern; they occurred at random times t after exposure to air. The median HWHM of the additional I2 emission bursts (on the local I2 emission level at the time of the burst) was approximately 10 min for the stipes and only ca. 1 min for the meristematic areas and the (one) distal blade. The peak height of the short bursts ranged from 0.2 to 6 times the height of the initial peak (data not shown). Furthermore, the light regime (i.e. low light or dark conditions) did not seem to have any impact on the general emission pattern.
The strongest I2 emitting specimens of air-exposed Laminaria digitata as examples of an I2 emission rate, \( E_{{{\text{I}}_{2} }} (t) \) (pmol g−1 dw min−1) as a function of time, with respect to different parts of the thallus: ‘stipe’, ‘meristematic area’, and ‘distal blade’. Emission into air at ~10.5°C was determined over 180 min. a Low light condition (35 μmol photons m−2 s−1 PAR); inset shows a schematic of a L. digitata thallus and relevant parts. b In darkness; the ‘distal blade’ measurement was terminated after 92 min
I2 emission rates from different thallus parts
The total amounts of I2 released by different thallus parts of the algae, \( N_{{{\text{I}}_{2} }} \), are presented in Table 1 relative to the dry weight and surface area. Under either light regime, the largest amount of I2 was emitted by the stipe, followed by the meristematic area and the distal blade, i.e. \( N_{{{\text{I}}_{2} }} ({\text{stipe}}) > N_{{{\text{I}}_{2} }} ({\text{meristematic}}\,{\text{area}}) > N_{{{\text{I}}_{2} }} ({\text{distal}}\,{\text{blade}}) \). The difference in the amounts of I2 emitted by the stipe and the other two thallus parts was roughly an order of magnitude. For further characterisation, the temporal emission profiles were divided into two distinct phases. Table 2 shows the I2 emission rates \( \langle E_{{{\text{I}}_{2} }} \rangle \) of different thallus parts, averaged over the first 30 min of the experiment (initial I2 emission) and over the period 60–180 min.
At low light conditions, the median of the initial I2 emission rate of the stipe was 2,999 pmol g−1 dw min−1, and therefore 3.6 and 17 times higher than that of the meristematic area and the distal blade, respectively (Table 2). Between 60 and 180 min after the initial air exposure, the I2 emission rates of the stipes were reduced by 75% and the corresponding median was found to be 758 pmol g−1 dw min−1. Emission rates of the meristematic area and the distal blade also decreased by 70%; they were 5 and 14 times lower than those of stipes, respectively (P < 0.001, supplemental Table S1).
Similar results were observed under dark conditions (Table 2). The median of the initial I2 emission rate of the stipe amounted to 5,222 pmol g−1 dw min−1 and was thus about 13 times higher than that of the meristematic area and the distal blade. Between 60 and 180 min of exposure to air, median values of I2 emission rates of all thallus parts had decreased by 85%. Overall, I2 emission rates were highly variable (Tables 1, 2).
I2 emission rates differed significantly between the three thallus parts (\( E_{{{\text{I}}_{2} }} ({\text{stipe}}) > E_{{{\text{I}}_{2} }} ({\text{meristematic}}\,{\text{area}}) > E_{{{\text{I}}_{2} }} ({\text{distal}}\,{\text{blade}}) \), P < 0.001, supplemental Table S1), but were independent of the experimental light regime (P = 0.362).
When considering the emission rates relative to the surface area, a high variability was also observed. As shown in Table 2, emission rates of the stipe were still the highest. A median emission rate of 253 pmol cm−2 min−1 occurred within the first 30 min of exposure to air, in darkness; this value was 36 times higher than the median values for the meristematic area and for the distal blade. Initial I2 emission rates of the meristematic area and the distal blade were in a similar range, between 1 and 15 pmol cm−2 min−1, but a high rate was observed for one distal blade (310 pmol cm−2 min−1). After exposure to air for more than 60 min, the stipe exhibited emission rates of up to 118 pmol cm−2 min−1, i.e. 100 times higher than the other L. digitata thallus parts. Similar results were observed under low light conditions (Table 2).
Photosynthetic performance and water content
As shown in Fig. 3a and b, the values for the maximum PSII efficiency (F v/F m) were generally high before exposure to air; mean F v/F m values ranged from 0.751 ± 0.010 to 0.777 ± 0.028 for all thallus parts. F v/F m was dependent on the thallus part (P = 0.041, supplemental Table S1), with the lowest values for the meristematic area and the highest for the distal blade. F v/F m was significantly affected by the emersion time (P < 0.001, supplemental Table S1) and reduced by 3% after 180 min of exposure to air in each thallus part of L. digitata. No significant difference was observed between F v/F m obtained for the different thallus parts investigated at two light regimes (P = 0.057, supplemental Table S1).
Effects of air exposure on the photosynthetic performance of different thallus parts of Laminaria digitata. Maximum PSII efficiency (F v/F m), effective PSII quantum efficiency (\( \Updelta F/F^{\prime}_{\text{m}} \)), photochemical quenching (qP) and efficiency of open reaction centres in capturing energy (\( F^{\prime}_{\text{v}} /F^{\prime}_{\text{m}} \)) were determined in stipes (black bars), meristematic areas (unfilled bars) and distal blades (hatched bars) prior to air exposure, i.e. when thallus parts were submersed in natural seawater (before the time 0 of the experiment), and after 180 min in air. a F v/F m obtained in the low light experiment. b F v/F m obtained in the dark experiment. c \( \Updelta F/F^{\prime}_{\text{m}} \), d qP and e \( F^{\prime}_{\text{v}} /F^{\prime}_{\text{m}} \) obtained in the low light experiment. The experimental temperature was ~10.5°C. Data are presented as mean and standard deviation (n = 5). Levels of significance of the fixed factors “thallus part”, “time” and “light regime” on F v/F m were calculated by three-way ANOVA (“thallus part” P = 0.041, “time” P < 0.001, “light regime” P = 0.057; note that data shown in a and b were analysed together). Levels of significance of the fixed factors “thallus part” and “time” on \( \Updelta F/F^{\prime}_{\text{m}} \), qP and \( F^{\prime}_{\text{v}} /F^{\prime}_{\text{m}} \) were calculated by two-way ANOVAs (\( \Updelta F/F^{\prime}_{\text{m}} \): “thallus part” P < 0.001, “time” P < 0.001; qP: “thallus part” P = 0.031, “time” P < 0.001; \( F^{\prime}_{\text{v}} /F^{\prime}_{\text{m}} \): “thallus part” P = 0.016, “time” P = 0.249). Different uppercase letters are used to indicate significant differences within variables as revealed by Tukey’s post hoc test (see supplemental Table S1)
The low light experiment allowed the determination of the effective PSII quantum efficiency (\( \Updelta F/F^{\prime}_{\text{m}} \)), the photochemical quenching (qP), as well as the capacity to capture energy (\( F^{\prime}_{\text{v}} /F^{\prime}_{\text{m}} \)) at 35 μmol photons m−2 s−1 PAR (Fig. 3c–e). \( \Updelta F/F^{\prime}_{\text{m}} \) was dependent on thallus part (P < 0.001, supplemental Table S1). Prior to air exposure, mean values for the stipe (0.345 ± 0.071) were significantly lower than values for the meristematic area (0.469 ± 0.051) and the distal blade (0.458 ± 0.058), respectively. \( \Updelta F/F^{\prime}_{\text{m}} \) in the meristematic area and the distal blade was 34% higher than in the stipe and did not differ significantly from each other. After exposure to air for 180 min, \( \Updelta F/F^{\prime}_{\text{m}} \) had increased by 25–55% in all thallus parts (P < 0.001), but remained lower in the stipe (Fig. 3c). Prior to exposure to air, the lowest qP values were again observed from the stipe (0.560 ± 0.103) and reached 74% of qP of the meristematic area (0.753 ± 0.052) and 81% of qP of the distal blade (0.692 ± 0.102). As illustrated in Fig. 3d, air exposure resulted in an increase of qP for all thallus parts of L. digitata (P < 0.001, supplemental Table S1), i.e. by 46% for the stipe, 16% for the meristematic area and 22% for the distal blade. The efficiency of open reaction centres in capturing energy (\( F^{\prime}_{\text{v}} /F^{\prime}_{\text{m}} \)) was significantly lower in the stipe than in the distal blade but that of other thallus parts were similar (Fig. 3e; supplemental Table S1). Furthermore, \( F^{\prime}_{\text{v}} /F^{\prime}_{\text{m}} \) remained nearly constant during the experiment (P = 0.249, supplemental Table S1; Fig. 3e).
The average water content of L. digitata was 83%. The water loss during exposure to air for 180 min was similar for all thallus parts, about 2%, irrespective of the light regime.
Discussion
In this study, I2 emission rates of different thallus parts of L. digitata sporophytes were monitored at low light (35 μmol photons m−2 s−1 PAR) and dark conditions at ~10.5°C applying a highly sensitive cavity-enhanced absorption spectroscopy technique (Fiedler et al. 2003; Dixneuf et al. 2009). To estimate the extent of stress experienced by each thallus part over the course of the experiment, photosynthetic performance was determined by measuring parameters of primary photosynthesis using chlorophyll a fluorescence.
For all thallus parts, I2 emission systematically showed a strong initial emission peak lasting about 30 min, which was followed by a gradual decrease. The temporal profiles are, therefore, consistent with those recently observed (Bale et al. 2008; Dixneuf et al. 2009; Ball et al. 2010). For a complete L. digitata thallus, Ball et al. (2010) observed a decrease of the maximum I2 emission rate to half its value in 5 min. The HWHM observed in this study was different for different thallus parts and ranged from 11 to 21 min. During incubation experiments, an intense initial I2 emission peak from entire thalli of L. hyperborea and Saccharina latissima (Laminariales) was also observed (Ball et al. 2010) immediately after first exposure to air, followed by an exponential decay over a period of 30–40 min. Thus, for the species of the order Laminariales investigated so far, exposure to air resulted in an immediate I2 emission. It has been shown that iodine is mostly retained as I− in external mucilage of epidermal cell layers (Küpper et al. 2008; Verhaeghe et al. 2008). The apoplastic stock of I− is believed to be associated with charged polymers for a rapid remobilisation and (bio)availability (Verhaeghe et al. 2008). Temporal I2 emission profiles from L. digitata and other Laminariales (Bale et al. 2008; Dixneuf et al. 2009; Ball et al. 2010; present study) indicate the occurrence of either a rapidly mobilised or a directly iodovolatilisable iodine pool, that is being depleted during emersion.
Besides the strong initial I2 emissions, additional short, intense and sporadic I2 emission bursts were detectable during the extended period of exposure to air. These additional bursts had been previously observed for the first time from an entire L. digitata thallus in a static chamber (Dixneuf et al. 2009). In the present study, such bursts were frequently observed from the stipe and the meristematic area, but observed only once from the distal blade. Iodine has been found in intracellular, vesicle-like structures, which are mainly located in meristodermic cells of the stipe and, much lower in density, in the blade (Pedersén and Roomans 1983; Verhaeghe et al. 2008). It has been suggested that the outer cells of the stipe have a distinctive ability to store iodine and that the content of such vesicle-like structures can probably easily be leached (Verhaeghe et al. 2008). A sporadic release of iodine by intracellular vesicles (Evans and Holligan 1972) might have been a physiological origin for the short additional I2 bursts that were mainly detected from the stipe.
As I2 emission rates were found to be not affected by the experimental light regime (low light and darkness), the suggested depletion of an easily mobilised iodine pool indicates a physiologically multifunctional role of iodine in L. digitata. The physiological significance for the higher I2 emission rates observed from the stipe and the meristematic area remains, however, speculative.
At the beginning of the measurement, L. digitata thalli were surrounded by a thin seawater layer. Iodine exists in coastal waters mainly as iodate (IO3 −, up to 0.35 μmol L−1), iodide (I−, up to 0.20 μmol L−1), and to a smaller extent as organic-bound iodine (Hou et al. 2009), dependent on the local water chemistry. Therefore, iodine is generally considered a trace element in seawater, in particular when compared to the concentrations found in living marine organisms (Hou et al. 2009). Thus, in the present study, the remarkable amounts of I2 detected in the gaseous phase originated mainly from labile iodine made available by the thallus parts; the contribution of I2 elusion from the thin layer of seawater to the total amount of I2 detected was most likely negligible.
In response to environmental stress and as part of chemical defence mechanisms, the release of iodine from macroalgae is generally linked with the quenching of hydrogen peroxide and other reactive oxygen species (ROS) (Palmer et al. 2005; Küpper et al. 2001, 2008). Emission of inorganic and organic iodine species was previously observed when algae were exposed to aqueous oxidants, ozone and ultraviolet radiation (Laturnus et al. 2004; Palmer et al. 2005; Küpper et al. 2008). The mechanisms and chemical reactions leading to the retransmission of iodine to the gaseous environment, mainly in its molecular form (I2), are still subject of ongoing research (e.g. Leblanc et al. 2006). Since the ozone mixing ratio in the main carrying flow was not monitored in the present study and no information on the composition of the aqueous surface layer surrounding the seaweed was available, the immediate strong I2 emission cannot be unambiguously attributed to a specific oxidising process.
In S. latissima, iodine has been demonstrated to be translocated from the distal blade towards the meristematic area (Amat and Srivastava 1985). In L. digitata, previous observations of higher average iodine contents in the stipe than in the blade (Küpper et al. 1998; Verhaeghe et al. 2008) correlate to the observed I2 emission rate differences for different thallus parts observed here (i.e. dw- and sa-normalised emission of stipe > meristematic area > distal blade). Ar Gall et al. (2004) have shown that haloperoxidase activity and iodine uptake rates were highest in distal parts of the blade and lower in the meristematic area. The gradual increase of haloperoxidase activity, which accompanies increasing iodine uptake rates, from meristem towards distal blade parts is consistent with the fact that lowest I2 emission rates were observed for the distal blade in the present study.
Different I2 emission rates have been reported for thalli of L. digitata from different European populations under presumed low stress conditions (i.e. low light levels and/or controlled temperature). Average emissions of 3.7 pmol g−1 fresh weight (fw) min−1 (Palmer et al. 2005) and 3.0 pmol g−1 fw min−1 (Ball et al. 2010) were measured for a towel-dried strip of L. digitata from the west coast of Scotland and for an entire alga from French Brittany, respectively. Bale et al. (2008) have reported I2 mixing ratios comparable to the ones found in this investigation, i.e. a maximum of 300 ppbv for a whole L. digitata thallus (50 g fw), also from the west coast of Ireland, that corresponds to a peak emission rate of ca. 2,500 pmol g−1 fw min−1 for a 10 L min−1 carrying air flow. These data cannot easily be compared with the results presented here as emission rates presented in this study were normalised by the dry weight or surface area, and median values of I2 emission rates from three separate thallus parts were calculated.
Seasonal variation in the iodine content in L. digitata has also been documented; e.g. Ar Gall et al. (2004) found higher iodine levels in various European populations in autumn and winter and lower contents during spring and summer months. Similarly, in a Norwegian L. digitata population, the maximum level of iodine concentration was observed in February and the minimum in summer (Haug and Jensen 1954). Since the present study was performed in early November, the observed high I2 emission may have been related to a high (autumn) initial iodine content. However, further investigations are required to elucidate aspects of potential seasonal variability of biogenic I2 emission.
Exposure to air imposes a number of constraints or benefits on algal physiology in terms of inorganic carbon supply, irradiance, temperature changes and cellular water loss (Johnson et al. 1974; Harker et al. 1999; Gómez et al. 2004; Hunt and Denny 2008). Upper distribution limits of intertidal algae are notably determined by their capacity to tolerate extended periods of emersion (Schonbeck and Norton 1978; Wiltens et al. 1978). To assess the physiological performance of algae during a tidal cycle, many studies have focused on photosynthesis using chlorophyll a fluorescence-based techniques due to the potential sensitivity of parameters of primary photosynthesis (e.g. F v/F m, \( \Updelta F/F^{\prime}_{\text{m}} \)) to environmental changes (Harker et al. 1999; Gevaert et al. 2002; Gómez et al. 2004). In the present study, F v/F m values in different thallus parts of the subtidal alga L. digitata ranged from 0.751 to 0.777 at either submersed conditions and even after 180 min of emersion. Such values are typical for unstressed brown macroalgae (Büchel and Wilhelm 1993). Algal physiology is significantly affected by a combination of numerous abiotic and biotic factors (Dethier and Williams 2009); it seems that the ambient experimental conditions during the emersion were not stressful enough to substantially inhibit photosynthesis.
The results of primary photosynthesis presented here were based on overall chlorophyll a fluorescence signals measured from several cell layers (depending on the thickness of the alga); they, therefore, indicate an integrated status of the photosynthetic performance of the thallus part investigated, without allowing for a distinction between surface and subsurface cells. However, since desiccation proceeded most likely from the thallus surface inwards, photosynthesis of the surface cells may have been more influenced by the emersion than photosynthesis of the subsurface cells. Given that the inner cells of blades of L. digitata were shown to have generally lower F v/F m values than surface cells (Garbary and Kim 2005), it appeared difficult to quantify the stress in L. digitata by measuring F v/F m only.
In contrast to F v/F m, \( \Updelta F/F^{\prime}_{\text{m}} \) is the proportion of absorbed energy which is used in photochemistry and relates to achieved photosynthetic efficiency. As such it can give an indication of the overall photosynthesis (Maxwell and Johnson 2000). Unexpectedly, in the present study, \( \Updelta F/F^{\prime}_{\text{m}} \) was increased significantly in all thallus parts of L. digitata after 180 min of emersion, suggesting an overall increase in photosynthesis. An enhanced photosynthetic activity was also found by Dring and Brown (1982) in Fucus serratus and L. digitata during emersion; in that instance, the increase lasted between 30 and 40 min and was followed by a continuous decline, which only occurred if thalli were allowed to dry out (Dring and Brown 1982). However, under the present experimental conditions, intracellular water was mainly retained by L. digitata. Hence, a critical water status for severe inhibition of overall photosynthesis was probably not achieved, but photosynthetic activity most likely depended on the distance of the cells to the thallus surface. The response of \( \Updelta F/F^{\prime}_{\text{m}} \) is defined by either, the fraction of open reaction centres (qP), their efficiency in capturing energy (\( F^{\prime}_{\text{v}} /F^{\prime}_{\text{m}} \)) or a combination of both (Krause and Weis 1991; Linger and Brüggemann 1999). Here, exposure to air had pronounced positive effects on qP in all thallus parts of L. digitata, whereas \( F^{\prime}_{\text{v}} /F^{\prime}_{\text{m}} \) remained unchanged. An increase in \( \Updelta F/F^{\prime}_{\text{m}} \) is, thus, primarily caused by a higher fraction of open reaction centres suggesting an enhanced performance of the photosynthetic dark reactions (Linger and Brüggemann 1999). The efficiency of energy capture (\( F^{\prime}_{\text{v}} /F^{\prime}_{\text{m}} \)) depends on the thermal energy dissipation in the antennae and interrelates with F v/F m and the NPQ of chlorophyll a fluorescence. As \( F^{\prime}_{\text{v}} /F^{\prime}_{\text{m}} \) was not affected, the observed slight reduction in F v/F m had to be based on an altered efficiency in NPQ. In conclusion, air exposure of L. digitata at low light conditions (35 μmol photons m−2 s−1) and a moderate temperature (~10.5°C) resulted most likely in changes in the NPQ efficiency, causing, in turn, a minimal decline in F v/F m which was yet sufficient to maintain a high fraction of open reaction centres (qP). Moreover, the efficiency of primary photosynthesis (\( \Updelta F/F^{\prime}_{\text{m}} \)) was more pronounced in aerial than in submersed conditions, due to an increase in open reaction centres, whereas energy capture efficiency in the antennae of open reaction centres (\( F^{\prime}_{\text{v}} /F^{\prime}_{\text{m}} \)) was unaffected. This pronounced performance may have been caused by an increase in the capacity of photosynthetic dark reactions caused by qualitative and/or quantitative changes in inorganic carbon supply (e.g. CO2).
In conclusion, the sporophyte of L. digitata released remarkable amounts of I2 even under presumed low-stress conditions. The stipe, which exhibited a reduced photosynthetic performance, emitted highest amounts of I2, whereas emission rates observed from the meristematic area and the distal blade were lower by one order of magnitude. The temporal profile of I2 emission indicates the occurrence of an iodine pool which is easily mobilised and becomes depleted upon air exposure. Since iodine is mainly retained as apoplastic I− in L. digitata (Küpper et al. 2008), a respective chemical oxidation is required to generate I2. According to Küpper et al. (1998), the intracellular production of hydrogen peroxide seems to be not the major source for the oxidation of apoplastic I−. In the present study, exogenous oxidative sources, e.g. gaseous ozone or oxidative compounds in the thin water film surrounding the alga particularly during early stages of emersion, were not investigated. Besides the high I2 emission, air exposure enhanced primary photosynthesis of all investigated thallus parts under the experimental conditions. Since chlorophyll a fluorescence did not detect any potential stress experienced by the alga, it has so far not been possible to establish a direct relationship between I2 emission and physiological stress. ROS that would cause the retransmission of iodine to the gaseous phase remained below a stressful level at which the efficiency of the PSII would have been inhibited.
Abbreviations
- IBBCEAS:
-
Incoherent broadband cavity-enhanced absorption spectroscopy
- HWHM:
-
Half-width at half-maximum
- MBL:
-
Marine boundary layer
- NPQ:
-
Non-photochemical quenching
- PFD:
-
Photon flux density
- PAR:
-
Photosynthetic active radiation
- PS:
-
Photosystem
References
Amat MA, Srivastava LM (1985) Translocation of iodine in Laminaria saccharina (Phaeophyta). J Phycol 21:330–333
Ar Gall E, Küpper FC, Kloareg B (2004) A survey of iodine content in Laminaria digitata. Bot Mar 47:30–37
Bale CSE, Ingham T, Commane R, Heard DE, Bloss WJ (2008) Novel measurements of atmospheric iodine species by resonance fluorescence. J Atmos Chem 60:51–70
Ball SM, Hollingsworth SA, Humbles J, Leblanc C, Potin P, McFiggans G (2010) Spectroscopic studies of molecular iodine emitted into the gas phase by seaweed. Atmos Chem Phys 10:6237–6254
Bitter M, Ball SM, Povey IM, Jones RL (2005) A broadband cavity ringdown spectrometer for in situ measurements of atmospheric trace gases. Atmos Chem Phys 5:2547–2560
Büchel C, Wilhelm C (1993) In vivo analysis of slow fluorescence induction kinetics in algae: progress, problems and perspectives. J Photochem Photobiol 58:137–148
Carpenter LJ (2003) Iodine in the marine boundary layer. Chem Rev 103:4953–4962
Dethier MN, Williams SL (2009) Seasonal stresses shift optimal intertidal algal habitats. Mar Biol 156:555–567
Dixneuf S, Ruth AA, Vaughan S, Varma RM, Orphal J (2009) The time dependence of molecular iodine emission from Laminaria digitata. Atmos Chem Phys 9:823–829
Dring MJ, Brown FA (1982) Photosynthesis of intertidal brown algae during and after periods of emersion: a renewed search for physiological causes of zonation. Mar Ecol Prog Ser 8:301–308
Evans LV, Holligan MS (1972) Correlated light and electron microscope studies on brown algae. II. Physode production in Dictyota. New Phytol 71:1173–1180
Fiedler SE, Hese A, Ruth AA (2003) Incoherent broad-band cavity-enhanced absorption spectroscopy. Chem Phys Lett 371:284–294
Garbary DJ, Kim KY (2005) Anatomical differentiation and photosynthetic adaptation in brown algae. Algae 20:233–238
Genty B, Briantais JM, Baker NR (1989) The relationship between the quantum yield of photosynthetic electron-transport and quenching of chlorophyll fluorescence. Biochem Biophys Acta 990:87–92
Gevaert F, Creach A, Davoult D, Holl AC, Seuront L, Lemoine Y (2002) Photo-inhibition and seasonal photosynthetic performance of the seaweed Laminaria saccharina during a simulated tidal cycle: chlorophyll fluorescence measurements and pigment analysis. Plant Cell Environ 25:859–872
Gómez I, López-Figueroa F, Ulloa N, Morales V, Lovengreen C, Huovinen P, Hess S (2004) Patterns of photosynthesis in 18 species of intertidal macroalgae from southern Chile. Mar Ecol Prog Ser 270:103–116
Harker M, Berkaloff C, Lemoine Y, Britton G, Young A, Duval JC, Rmiki N-E, Rousseau B (1999) Effects of high light and desiccation on the operation of the xanthophyll cycle in two marine brown algae. Eur J Phycol 34:35–42
Haug A, Jensen A (1954) Seasonal variations in the chemical composition of Alaria esculenta, Laminaria saccharina, Laminaria hyperborea and Laminaria digitata from northern Norway. Norwegian Institute of Seaweed Research, Report no. 84, p 34
Hou X, Hansena V, Aldahanb A, Possnert G, Lindd OC, Lujaniene G (2009) A review on speciation of iodine-129 in the environmental and biological samples. Anal Chim Acta 632:181–196
Huang RJ, Seitz K, Buxmann J, Pöhler D, Hornsby KE, Carpenter LJ, Platt U, Hoffmann T (2010) In situ measurements of molecular iodine in the marine boundary layer: the link to macroalgae and the implications for O3, IO, OIO and NO x . Atmos Chem Phys 10:4823–4833
Hunt LJH, Denny MW (2008) Desiccation protection and disruption: a trade-off for an intertidal marine alga. J Phycol 44:1164–1170
Johnson WS, Gigon A, Gulmon SL, Mooney HA (1974) Comparative photosynthetic capacities of intertidal algae under exposed and submerged conditions. Ecology 55:450–453
Kolb CE (2002) Iodine’s air of importance. Nature 417:597–598
Krause GH, Weis E (1991) Chlorophyll fluorescence and photosynthesis: the basics. Annu Rev Plant Physiol Plant Mol Biol 42:313–349
Kromkamp JC, Forster RM (2003) The use of variable fluorescence measurements in aquatic ecosystems: differences between multiple and single turnover measuring protocols and suggested terminology. Eur J Phycol 38:103–112
Küpper FC, Schweigert N, Ar Gall E, Legendre JM, Vilter H, Kloareg B (1998) Iodine uptake in Laminariales involves extracellular, haloperoxidase-mediated oxidation of iodide. Planta 207:163–171
Küpper FC, Kloareg B, Guern J, Potin P (2001) Oligoguluronates elicit an oxidative burst in the brown algal kelp Laminaria digitata. Plant Physiol 125:278–291
Küpper FC, Carpenter LJ, McFiggans GB, Palmer CJ, Waite TJ, Boneberg E-M, Woitsch S, Weiller M, Abela R, Grolimund D, Potin P, Butler A, Luther GW, Kroneck PMH, Meyer-Klaucke W, Feiters MC (2008) Iodide accumulation provides kelp with an inorganic antioxidant impacting atmospheric chemistry. Proc Natl Acad Sci USA 105:6954–6958
Kylin H (1929) Über das Vorkommen von Jodiden, Bromiden und Jodidoxidasen bei den Meeresalgen. Hoppe-Seylor’s Z Physiol Chem 186:50–84
Laturnus F, Svensson T, Wiencke C, Öberg G (2004) Ultraviolet radiation affects emission of ozone-depleting substances by marine macroalgae: results from a laboratory incubation study. Environ Sci Technol 38:6605–6609
Leblanc C, Colin C, Cosse A, Delage L, La Barre S, Morin P, Fiévet B, Voiseux C, Ambroise Y, Verhaeghe E, Amouroux D, Donard O, Tessier E, Potin P (2006) Iodine transfers in the coastal marine environment: the key role of brown algae and of their vanadium-dependent haloperoxidases. Biochimie 88:1773–1785
Linger P, Brüggemann W (1999) Correlations between chlorophyll fluorescence quenching parameters and photosynthesis in a segregating Lycopersicon esculentum × L. peruvianum population as measured under constant conditions. Photosynth Res 61:145–156
Lobban CS, Harrisson PJ (1994) Seaweed ecology and physiology. Cambridge University Press, Cambridge
Lüning K (1985) Meeresbotanik. Gustav Thieme Verlag, Stuttgart
Maxwell K, Johnson GN (2000) Chlorophyll fluorescence − a practical guide. J Exp Bot 51:659–668
McFiggans G, Coe H, Burgess R, Allan J, Cubison M, Alfarra MR, Saunders R, Saiz-Lopez A, Plane JMC, Wevill DJ, Carpenter LJ, Rickard AR, Monks PS (2004) Direct evidence for coastal iodine particles from Laminaria macroalgae – linkage to emissions of molecular iodine. Atmos Chem Phys 4:701–713
McFiggans G, Bale CSE, Ball SM, Beames JM, Bloss WJ, Carpenter LJ, Dorsey J, Dunk R, Flynn MJ, Furneaux KL, Gallagher MW, Heard DE, Hollingsworth AM, Hornsby K, Ingham T, Jones CE, Jones RL, Kramer LJ, Langridge JM, Leblanc C, LeCrane JP, Lee JD, Leigh RJ, Longley I, Mahajan AS, Monks PS, Oetjen H, Orr-Ewing AJ, Plane JMC, Potin P, Shillings AJL, Thomas F, von Glasow R, Wada R, Whalley LK, Whitehead JD (2010) Iodine-mediated coastal particle formation: an overview of the Reactive Halogens in the Marine Boundary Layer (RHaMBLe) Roscoff coastal study. Atmos Chem Phys 10:2975–2999
O’Dowd CD, Hoffmann T (2005) Coastal new particle formation: a review of the current state-of-the-art. Environ Chem 2:245–255
O’Dowd CD, Jimenez JL, Bahreini R, Flagan RC, Seinfeld JH, Hameri K, Pirjola L, Kulmala M, Jennings SG, Hoffmann T (2002) Marine aerosol formation from biogenic iodine emissions. Nature 417:632–636
Palmer CJ, Anders TL, Carpenter LJ, Küpper FC, McFiggans GB (2005) Iodine and halocarbon response of Laminaria digitata to oxidative stress and links to atmospheric new particle production. Environ Chem 2:282–290
Pedersén M, Roomans GM (1983) Ultrastructural localization of bromine and iodine in the stipes of Laminaria digitata (Huds.) Lamour., Laminaria saccharina (L.) Lamour. and Laminaria hyperborea (Gunn.) Fosl. Bot Mar 26:113–118
Saenko GN, Kravtsova YY, Ivanenko VV, Sheludko SI (1978) Concentration of iodine and bromine by plants in seas of Japan and Okhotsk. Mar Biol 47:243–250
Saiz-Lopez A, Plane JMC (2004) Novel iodine chemistry in the marine boundary layer. Geophys Res Lett 31:L04112
Saiz-Lopez A, Saunders RW, Joseph DM, Ashworth SH, Plane JMC (2004) Absolute absorption cross-section and photolysis rate of I2. Atmos Chem Phys 4:1443–1450
Schonbeck M, Norton TA (1978) Factors controlling upper limits of fucoid algae on shore. J Exp Mar Biol Ecol 31:303–313
Schreiber U, Schliwa U, Bilger W (1986) Continuous recording of photochemical and nonphotochemical chlorophyll fluorescence quenching with a new type of modulation fluorometer. Photosynth Res 10:51–62
Seitz K, Buxmann J, Pöhler D, Sommer T, Tschritter J, Neary T, O’Dowd C, Platt U (2010) The spatial distribution of the reactive iodine species IO from simultaneous active and passive DOAS observations. Atmos Chem Phys 10:2117–2128
Sokal RR, Rohlf FJ (1995) Biometry: the principles and practice of statistics in biological research. Freeman, New York
van den Hoek C, Mann DG, Jahns HM (1995) Algae: an introduction to phycology. Cambridge University Press, Cambridge
Verhaeghe E, Fraysse A, Guerquin-Kern J-L, Wu T-D, Devès G, Mioskowski C, Leblanc C, Ortega R, Ambroise Y, Potin P (2008) Microchemical imaging of iodine distribution in the brown alga Laminaria digitata suggests a new mechanism for its accumulation. J Biol Inorg Chem 13:257–269
Wiltens J, Schreiber U, Vidaver W (1978) Chlorophyll fluorescence induction – indicator of photosynthetic activity in marine algae undergoing desiccation. Can J Bot Rev Can Bot 56:2787–2794
Acknowledgments
The authors thank Professor Colin O’Dowd (School of Physics and Ryan Institute, NUI Galway), Dr. Solène Connan (Botany and Plant Science and Ryan Institute, NUI Galway) and Dr. Stewart Vaughan (School of Chemistry, University of Leeds, UK) for comments on the experimental design and Dr. Jerome Sheahane (Statistics and Applied Mathematics, School of Mathematics, NUI Galway) for his help with statistical analyses. UN and SD gratefully appreciate financial support through the Irish Research Council for Science, Engineering and Technology (IRCSET ‘Embark Initiative’).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Nitschke, U., Ruth, A.A., Dixneuf, S. et al. Molecular iodine emission rates and photosynthetic performance of different thallus parts of Laminaria digitata (Phaeophyceae) during emersion. Planta 233, 737–748 (2011). https://doi.org/10.1007/s00425-010-1334-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-010-1334-3