Abstract
This study investigated the effects of different light spectral qualities and temperature on the photosynthesis and pigment content of a subtidal edible red alga, Meristotheca papulosa. Photosynthesis–irradiance (P–E) experiments were carried out under red (660 nm), blue (450 nm), green (525 nm, light-emitting diodes), and white light (visible light, metal halide lamp), and at 12, 20, and 28 °C, respectively. Maximum net photosynthetic rates (NPmax) were highest under green light. Other P–E parameter estimates were similar among algae under red, blue, and green light, including their lower initial slope (α) and higher saturation irradiances (Ek) as compared to those under white light. Additionally, NPmax and Ek under white light were highest at 28 °C, and lowest at 12 °C with characteristic photoinhibition at irradiances greater than 150 μmol photons m−2 s−1. Photosynthesis–temperature (P–T) experiment revealed that the maximum gross photosynthetic rate (GPmax) occurred at 22.1 °C, which was within the optimal temperature range of Fv/Fm (21.5–23.6 °C). Exposures to the different light qualities at 100 μmol photons m−2 s−1 for 7 days showed increased phycoerythrin (PE) concentration of algae under blue and green light, while chlorophyll-a and phycocyanin (PC) showed little variation in all light qualities. Therefore, considering future management prospects for M. papulosa mariculture, we suggest that green light could be utilized to enhance photosynthesis. Furthermore, if the aim is to achieve high PE content for an improved reddish-color fresh product, exposure to blue or green light could be a good alternative.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Meristotheca papulosa (Montagne) J. Agardh (Solieriaceae, Gigartinales) is a red alga that is widely distributed in the Indo-Pacific region, including the coastal waters of Madagascar (Silva et al. 1996), India (Rao and Gupta 2015), Australia (Watt et al. 2003), Philippines (Ang et al. 2014), Vietnam (Nguyen et al. 2013), China (Liu 2008), Korea (Lee 2008), and Japan (Yoshida et al. 2015). In Japan, this species can be found along the Pacific coast of central and southern Honshu, Shikoku, Kyushu, and Ryukyu islands, at depths of 3–30 m (Yoshida 1998; Faye et al. 2007; Lideman et al. 2012). Known as tosaka-nori in Japan, M. papulosa is a popular sea vegetable that is often used in seaweed salads and sashimi garnishes, although only natural populations have been exploited. This species has been harvested for commercial use and regional consumption in the prefectures of Kochi, Kumamoto, Miyazaki, and Kagoshima (Ohno 2004; Shinmura and Tanaka 2008); its annual production from Kagoshima alone reached 160–400 t of wet weight in 1995–2004 (approx. 2,000,000 US dollars; Shinmura and Tanaka 2008; Lideman et al. 2012). Because of the increasing demand for domestic tosaka-nori in Japan, there are concerns about overharvesting of this resource. For sustainable production of M. papulosa, appropriate management and the establishment of mariculture, based on an understanding of the ecophysiological characteristics of the species is essential. Initial field cultivation trials of this species were reported to be successful in Ehime Prefecture, Shikoku Island; however, commercial-scale cultivation remains elusive.
Indeed, several studies on M. papulosa have been conducted in Japan, concerning its vegetative and reproductive morphology (Faye et al. 2005), taxonomic features (Faye et al. 2007), callus induction, and thallus regeneration (Huang and Fujita 1997), as well as its phenology in growth and reproduction (Serisawa et al. 2000). Experimental tank culture studies (Ohno et al. 2002) and investigations related to photosynthesis and growth response of the species to ultraviolet radiation, irradiance, and temperature (Yokohama 1973; Murase et al. 1989; Maegawa et al. 1993; Lideman et al. 2011, 2012) have likewise been reported. For instance, photosynthesis experiments on M. papulosa using a pulse amplitude modulation (PAM)-chlorophyll fluorometer (Imaging-PAM) revealed its temperature optima for photosynthesis in the range of 18–28 °C (Lideman et al. 2012); such result was consistent with those obtained from oxygen evolution and growth experiments (Lideman et al. 2011). These studies have indeed contributed toward the elucidation of growth and photosynthesis characteristics of M. papulosa to establish effective techniques to conserve natural resources. However, there have been few reports of the effects of different light wavelengths on the photosynthetic efficiency, pigment content, and growth of this species (Murase et al. 2012).
Red, blue, and green light have special influence on regulating algae growth and photosynthetic pigments synthesis (Figueroa et al. 1995; Franklin et al. 2001; Tsekos et al. 2002; Godínez-Ortega et al. 2008; Wu 2016). Barufi et al. (2015) indicated red light as a possible reproductive inductor to produce tetraspores in the red alga Gracilaria birdiae Plastino et Oliveira (= Crassiphycus birdiae (Plastino et Oliveira) Gurgel, Norris et Fredericq; Gracilariaceae, Gracilariales), whereas green and blue light enhanced phycobiliprotein production. Phycobiliprotein is one important parameter to evaluate the quality of M. papulosa in the aspect of productivity and application. Aside from being accessory pigments that give the distinctive color and affect photosynthetic efficiency in red algae, phycobiliproteins have a variety of uses and offer interesting industrial perspectives (Sekar and Chandramohan 2008). Quality assessment of tosaka-nori is merely based on the appearance or color of the product, with a preference for dark-red thalli. Such alga growing subtidally at > 10 m depths was observed to have dark-red thalli, while those collected from shallow waters are yellowish-red, suggesting the influence of spectral composition on phycobiliprotein production. Understanding how the different light spectral qualities affect pigments synthesis of M. papulosa may assist in developing techniques to manipulate pigment composition of natural populations under controlled cultivation conditions.
The present study investigated the effects of different light spectral qualities and temperature on the photosynthetic performance, particularly on photosynthetic oxygen production and chlorophyll fluorescence of M. papulosa. Changes in the pigment content of the alga as subjected to the different light wavelengths were also examined.
Materials and methods
Specimen collection and maintenance
Individuals of M. papulosa (> 20 on each date) were collected by SCUBA from offshore of Cape Sata, Kagoshima Prefecture (31°0′ 11.5″ N, 130° 40′08″ E) at depths of 7–10 m on 13 June 2018 for oxygen evolution and chlorophyll fluorescence measurements, and from offshore of Nagashima, Kagoshima (32° 13′ 06″ N, 130° 10′ 59″ E) at same depths on 20 July 2018 for pigment analysis. Collected algae were transported to the laboratory of Kagoshima University in buckets filled with seawater at ambient temperature (c. 20 °C). Samples were held for 3 days prior to experiments in an aquarium tank (400 L) with a salinity of 33, pH of 8.0, seawater temperature of 20 °C and an irradiance of 200 μmol photons m−2 s−1 (14:10 light/dark cycle). Water motion was provided by aeration (c. 2.0 L min−1). Only intact and healthy thalli were selected for the subsequent experimental treatments.
Underwater irradiance
Underwater irradiance was measured near the collection site, off the coast of Cape Sata (31° 30′ N, 130° 38′ E) between 13:34 and 13:48 on 14 July 2018 aboard the training vessel T/S Nansei-Maru, Kagoshima University. Measurements of downwelling irradiance below the seawater surface (1 m) and at depths of 3, 5, 10, 15, 20, 30, 40, and 50 m were taken using a 2π quantum data-logger (DEFI-L, JFE Advantech Co. Ltd., Hyogo, Japan) that was attached to a CTD. Irradiance was measured at 1 Hz, and the sampling duration was 1 min for each depth. Irradiance measurements were used to determine the extinction coefficient (Ku m−1), according to Beer–Lambert equation:
where E(Z) is irradiance at the depth (Z) in meters, E(0) is the surface irradiance and Ku is the extinction coefficient (Cosgrove and Borowitzka 2011).
Photosynthesis–irradiance (P–E) experiments under different light spectral qualities
P–E experiments on M. papulosa were conducted under red, blue, green, and white light. The different light qualities were obtained using light-emitting diode (LED) arrays (CCS Inc., Kyoto, Japan) for red (646–664 nm [peak: 660 nm]; ISL-150X150-H4RR-SN), blue (444–467 nm [peak; 450 nm]; ISL-150X150-BB45), and green (510–543 nm [peak: 525 nm]; ISL-150X150-HGG) light, and a metal halide lamp (MHN-150D-S, Nichido Ind. Co. Ltd., Osaka, Japan) for white light. The wavelength ranges emitted by each LED array were measured with an illuminance spectrophotometer (CL-500A, Konica Minolta, Inc., Japan). Net photosynthesis (NP) rates were determined at 0, 30, 60, 100, 150, 200, 250, 500, and 1000 (800 in green light) μmol photons m−2 s−1 (n = 5 per irradiance level), which were obtained by appropriately layering neutral density screens between the reaction vessel and the light source. A spherical (4π) submersible quantum sensor (LI-192, LI-250A, LI-COR, USA) was used to measure irradiance. Water temperature in the reaction vessel was fixed at 20 °C using a recirculating water bath (Coolnit CL-600R, Taitec, Inc., Japan). Dissolved oxygen (DO) was measured using DO meters equipped with optical dissolved oxygen sensors (ProODO-BOD, YSI Inc., USA).
Thallus segments (c. 2–3 cm long, 1–2 cm wide) of M. papulosa were initially incubated overnight (12 h) with sterilized natural seawater at 20 °C in the dark. On the day of the experiment, four to five explants (0.51 ± 0.02 g SD fresh weight, gfw) were randomly selected and placed in 100-mL biological oxygen demand (BOD) bottles (n = 5) containing sterilized natural seawater. The samples were acclimated to each experimental irradiance for 30 min before DO concentration (mg L−1) readings were made every 5 min over a 30-min interval. Seawater medium was continuously stirred throughout the measurement to avoid boundary layer effects and was renewed after each irradiance exposure and measurement to avoid any effects that can be attributed to nutrient and dissolved carbon dioxide depletion.
The slope of the linear regression of the DO concentrations over a 30-min period was determined to estimate net photosynthesis and dark respiration rates, expressed in μg O2 gfw−1 min−1. All rates were normalized to water volume of each BOD bottle and fresh weight of the sample.
P–E experiments at three different temperatures
P–E experiments were conducted at 12, 20, and 28 °C under white light. The three temperature treatments were assigned based on the mean seawater temperatures near the collection site (southern Kyushu–Shikoku islands) in mid-winter (February), spring or autumn (April–July/October–December), and summer (August–September; Terada et al. 2016). Meristotheca papulosa explants were acclimated overnight (12 h) in an incubator at each temperature treatment prior to the start of the experiment. Methods for P–E experiments were the same as previously described (Borlongan et al. 2017, 2018, 2019).
Photosynthesis–temperature (P–T) experiment
Net photosynthesis and dark respiration rates of M. papulosa were determined at 8 temperature treatments (i.e., 8, 12, 16, 20, 24, 28, 32, and 36 °C), with methods similar to those described in the P–E experiments.
Four to five thallus segments that were approximately 0.51 gfw (SD ± 0.03) were placed into each BOD bottles (n = 5). They were acclimated to each temperature treatment under a constant saturating irradiance of 200 μmol photons m−2 s−1 for 30 min, followed by DO concentration measurements every 5 min for 30 min. Measurement of respiration rates (i.e., “post-illumination respiration”; Colombo-Pallotta et al. 2010; Vásquez-Elizondo and Enríquez 2016) were carried out after wrapping the BOD bottles with aluminum foil. Seawater was also renewed between temperature treatments.
Maximum quantum yield–temperature (Fv/Fm–T) experiment
The maximum quantum yields (maximum photochemical efficiency of photosystem II, Fv/Fm; Cosgrove and Borowitzka 2011) of M. papulosa were measured from 8 to 32 °C in 4 °C increments, with a Mini Imaging-PAM (Heinz Walz GmbH, Germany), as described in our recent articles (Terada et al. 2018, 2020). Sections of the thalli (c. 2–3 cm long, 1–2 cm wide) were previously incubated overnight (12 h) at 20 °C in the dark. Ten sections, which served as replicates, were then randomly assigned to each temperature treatment. Fv/Fm at 0 μmol photons m−2 s−1 was measured to provide initial values. Samples were subsequently placed in separate 500-mL flasks wrapped with aluminum foil, and were incubated in the dark at each temperature treatment for 72 h (EYELA MTI-201B, Tokyo Rikakikai Co., LTD., Japan). Fv/Fm (n = 10) at each temperature was measured every 24 h.
Photosynthetic pigment determination
To test the effect of the different light qualities on pigment content, M. papulosa explants were exposed to red, blue, green, and white light, respectively, for 7 days. Twenty to thirty thallus sections (c. 2–3 cm long, 1–2 cm wide) were placed in four separate 1000-mL beakers, which represent the four spectral light treatments. Irradiance was set at 100 μmol photons m−2 s−1, while temperature was fixed at 20 °C in a water bath. Water in the beakers was continuously stirred throughout the experimental period. Temperature and irradiance controls used were the same as previously described.
Pigment analysis was conducted upon completion of the experimental period. Ten replicates were derived from the samples exposed to each light treatment; five replicates were used for chlorophyll-a (chl-a) and phycobiliprotein measurements, respectively. Chl-a in the samples (0.49 ± 0.21 gfw) were extracted under dark refrigeration using 10 mL of N,N-dimethylformamide (DMF) for 24 h. Phycobiliprotein pigments [phycoerythrin (PE) and phycocyanin (PC)] in samples (0.44 ± 0.16 gfw) were likewise extracted under dark refrigeration using 10 mL extraction buffers (0.1 M phosphate buffer, pH = 6.8) for 24 h. Absorbance of the extracts was measured by UV-VIS spectrophotometer (DR5000, Hach Company, USA). Chl-a (mg gfw−1) was calculated according to Porra et al. (1989):
PE and PC (mg gfw−1) were calculated according to Beer and Eshel (1985) and Ding et al. (2013):
Modeling the photosynthetic response to irradiance and temperature
Photosynthetic responses of M. papulosa under the different light qualities and temperatures were determined in the form of P–E curves. Calculations for photosynthetic parameters were made by fitting the data to an exponential equation (Webb et al. 1974; Jassby and Platt 1976; Platt et al. 1980; Henley 1993) that included a respiration term and a term to model the effects of photoinhibition:
where NP is the net photosynthetic rate, NPmax is the maximum net photosynthetic rate, α is the initial slope of the P–E curve, Rd is the respiration rate, and E is the incident irradiance. β is a parameter to model the effects of photoinhibition and is set to 0 under conditions of no photoinhibition. Estimates for saturation irradiance (Ek) and compensation irradiance (Ec) were derived from the parameters, where Ek = NPmax/α; and Ec = \( N{P}_{max}\mathit{\ln}\left(\frac{N{P}_{max}}{\left({NP}_{max}-{R}_d\right)}\right)/\alpha \) when β is 0.
The responses of gross photosynthesis and Fv/Fm to temperature were analyzed by fitting the data to a peaked-Arrhenius model (Alexandrov and Yamagata 2007; Eq. 6), which had the form:
where y is the response variable, which can either be the gross photosynthetic rate or Fv/Fm, and K is temperature in Kelvin. There are four parameters in this model: ymax is a scaling parameter to fit the model to the range of y; Kopt is the absolute temperature where y is maximized; Ha is the activation energy in kJ mol−1; and Hd is the deactivation energy in kJ mol−1. R in this model is the ideal gas constant, with a value of 8.314 J mol−1. The optimal value of yopt at Kopt can be determined by substituting Kopt into the equation. The gross photosynthesis rate, which is assumed as a hidden state revealed by the model (Eq. 6), was estimated by simultaneously fitting the measured dark respiration rates to the Arrhenius equation (Eq. 7), and the observed net photosynthesis rates to the difference in Eq. 6 and Eq. 7. Gross photosynthesis rates were estimated by assuming that respiration rates after light pre-incubation were a good proxy for “kinetically limited” mitochondrial respiration, wherein oxygen is not a limiting factor in the process (Colombo-Pallotta et al. 2010). Rm is the respiration rate at the mean temperature (i.e., 22 °C; Km = 295.15) and Ea is the activation energy. Simultaneous model fittings and determination of model parameters for Eq. 6 and 7 were carried out using a Bayesian approach, with appropriate prior probability distributions assigned to the parameters.
In the case of Fv/Fm, the expected values were assumed to be beta distributed, given that Fv/Fm is a ratio that is bounded by 0 and 1.
Statistical analysis
Statistical analyses were done using RStudio version 3.6.0 (R Development Core Team 2019), and Bayesian model fittings using RStan version 2.18.9 (Stan Development Team 2019). RStan primarily uses a variant of a Hamiltonian Monte Carlo sampler to construct the posterior distributions of the parameters; and four chains of at least 500,000 samples/ chain were generated and assessed for convergence, which provided at least 1000 samples of each of the parameters of interest.
One-way ANOVA and Tukey’s HSD test were used to analyze differences among spectral light treatments for each photosynthetic pigment, and the significance level was set at 0.05.
Results
Underwater irradiance in the habitat
On 2018 July 14, underwater irradiance measured offshore of Cape Sata ranged from 1382 ± 261 μmol photons m−2 s−1 (mean ± SD) at 1 m to 21 ± 1 μmol photons m−2 s−1 at 50 m (Supplementary Fig. 1). Meanwhile, irradiance at the seawater surface was 2046 ± 16 μmol photons m−2 s−1. The skies were clear at the study site during measurement. The Beer–Lambert equation was fitted to the irradiance data using a linear regression on the log-transformed irradiances; the extinction coefficient (Ku m−1) was 0.082 m−1. Underwater irradiance at depths where M. papulosa samples are generally found were estimated based on these parameters. Estimated maximum irradiance in the habitat of this species ranged from 175 (at 30 m) to 1600 μmol photons m−2 s−1 (at 3 m).
P–E curves of M. papulosa under the different light spectral qualities
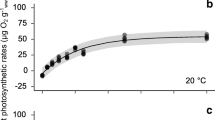
P–E curves under red, blue, green, and white light showed a typical hyperbolic shape with increases in NP rates at low irradiance until saturation at higher levels (Fig. 1). Photoinhibition was not observed on all light treatments; neither a decline in the convexity of the P–E curves nor a decrease in quantum yields of oxygen evolution (β) was found up to 1000 μmol photons m−2 s−1. At 20 °C, NP rates of M. papulosa under red light rose from − 0.26 ± .016 μg O2 gfw−1 min−1 (mean ± SD) at 0 μmol photons m−2 s−1 to 6.58 ± 1.57 μg O2 gfw−1 min−1 at 1000 μmol photons m−2 s−1 (Fig. 1a), whereas M. papulosa under blue light had NP rates ranging from − 0.37 ± 0.15 to 5.77 ± 1.30 μg O2 gfw−1 min−1 (Fig. 1b). Highest NP rates were recorded for samples under green light, which reached 7.77 ± 0.46 μg O2 gfw−1 min−1 at 800 μmol photons m−2 s−1 (Fig. 1c). As for samples under white light, their NP rates increased from − 0.34 ± 0.06 at 0 μmol photons m−2 s−1 to 5.92 ± 0.62 μg O2 gfw−1 min−1 at 1000 μmol photons m−2 s−1 (Fig. 1d).
The parameter estimates which describe the significant features of each P–E curve are shown in Table 1. Maximum net photosynthetic rate (NPmax) was highest under green light (8.36 μg O2 gfw−1 min−1). Other P–E parameter estimates were similar among algae under red, blue, and green light; their initial slopes (α) were 0.03, 0.02, and 0.04 μg O2 gfw−1 min−1 (μmol photons m−2 s−1) –1, respectively. As for M. papulosa under white light, α was slightly higher at 0.07 μg O2 gfw−1 min−1 (μmol photons m−2 s−1) –1. Thus, its saturation irradiance (Ek = 97 μmol photons m−2 s−1) was lower compared to other spectral light treatments (208–316 μmol photons m−2 s−1).
P–E curves of M. papulosa at three different temperatures
NP rates of M. papulosa at 12 °C were − 0.40 ± 0.10 μg O2 gfw−1 min−1 (mean ± SD) at 0 μmol photons m−2 s−1 and 1.80 ± 0.16 μg O2 gfw−1 min−1 at 150 μmol photons m−2 s−1. Rates declined thereafter to 0.67 ± 0.15 μg O2 gfw−1 min−1 at 1000 μmol photons m−2 s−1 (Fig. 2a). On one hand, NP rates were highest at 28 °C, which ranged from − 1.18 ± 0.13 μg O2 gfw−1 min−1 at 0 μmol photons m−2 s−1 to 8.27 ± 1.54 μg O2 gfw−1 min−1 at 1000 μmol photons m−2 s−1 (Fig. 2c).
P–E curves of Meristotheca papulosa at a 12, b 20, and c 28 °C. See the caption in Fig. 1 for the details
The P–E curves indicated that the lowest NPmax was observed at 12 °C (2.43 μg O2 gfw−1 min−1), and the highest at 28 °C (9.58 μg O2 gfw−1 min−1). P–E curve at 12 °C also depicted photoinhibition (β = − 0.002), which occurred at irradiances greater than 150 μmol photons m−2 s−1. α did not differ greatly among the three temperature treatments at 0.05–0.09 μg O2 gfw−1 min−1 (μmol photons m−2 s−1) –1. Ek levels increased with rise in temperature treatments, from 28 μmol photons m−2 s−1 at 12 °C to 212 μmol photons m−2 s−1 at 28 °C. Other model parameter estimates are presented in Table 2.
Effect of temperature on photosynthesis and dark respiration rates
Photosynthetic response of M. papulosa to temperature gradients depicted a characteristic bell-shaped curve (Fig. 3a); measured NP rates were low at 8 °C (0.61 ± 0.12 μg O2 gfw−1 min−1), peaked at 20 °C (5.90 ± 1.69 μg O2 gfw−1 min−1), and then declined with rising temperature to as low as − 2.11 ± 0.85 μg O2 gfw−1 min−1 at 36 °C.
Oxygenic photosynthesis and dark respiration responses of Meristotheca papulosa to temperature. a Net photosynthetic rates determined at 200 μmol photons m−2 s−1. b Modeled gross photosynthetic rates at 200 μmol photons m−2 s−1. Data were derived from the model curve of net photosynthesis and dark respiration. c Dark respiration rates determined at 0 μmol photons m−2 s−1. See the caption in Fig. 1 for the details
Maximum gross photosynthetic rate (GPmax) was estimated to be 7.05 μg O2 gfw−1 min−1 [6.40–7.75 μg O2 gfw−1 min−1, 95% Bayesian prediction interval (BPI)] at 22.1 °C (GPTopt; Fig. 3b). The activation (Ha) and deactivation (Hd) energies were 107 (74–150, 95% BPI) and 279 kJ mol−1 (235–336, 95% BPI), respectively.
Dark respiration rates increased from 0.35 ± 0.19 μg O2 gfw−1 min−1 at 8 °C to 1.09 ± 0.13 μg O2 gfw−1 min−1 at 36 °C (Fig. 3c). The respiration rate at mean temperature of 22 °C (R22) was 0.77 μg O2 gfw−1 min−1 (0.66–0.89, 95% BPI), with an activation energy (Ea) of 44 kJ mol−1 (33–56, 95% BPI).
Effect of temperature on Fv/Fm
Measured Fv/Fm of M. papulosa were relatively low (< 0.5) and variable across temperature gradients (8–32 °C) throughout the 72-h exposure period. Nevertheless, its response pattern appears to be represented by a bell-shaped curve (Fig. 4), similar to net photosynthesis. Parameter estimates of the Fv/Fm–T model indicated the maximum Fv/Fm of 0.32 at 23.6 °C (Topt), activation energy of 78 kJ mol−1, and deactivation energy of 231 kJ mol−1 in samples exposed for 72 h. Model parameter estimates in M. papulosa after 24- and 48-h temperature exposures are presented in Table 3.
Effect of different light qualities on pigment content of M. papulosa
Pigment contents of M. papulosa after exposure to the different spectral light treatments are summarized in Fig. 5. Results of the ANOVA for photosynthetic pigments are presented in Table 4. Higher PE contents were observed in thalli exposed to blue (0.048 ± 0.027 mg gfw−1) and green (0.049 ± 0.021 mg gfw−1) light. PE levels under red and white light were 0.003 ± 0.002 and 0.010 ± 0.003 mg gfw−1, respectively. Chl-a contents did not greatly differ among samples exposed to the four light treatments, with mean values ranging from 0.012 to 0.016 mg gfw−1. Slight increase in PC level was observed in blue (0.005 ± 0.002 mg gfw−1) and green (0.004 ± 0.001 mg gfw−1) light, when compared to red (0.002 ± 0.001 mg gfw−1) and white (0.002 ± 0.001 mg gfw−1) light.
Chlorophyll-a (chl-a), phycoerythrin (PE), and phycocyanin (PC) contents of Meristotheca papulosa after exposure to the different spectral light treatments for 7 days. Data are expressed as mean ± SD (n = 5). Error bars are standard deviations. Different letters indicate statistical significance between spectral light treatments (P < 0.05)
Discussion
In this study, it is shown that M. papulosa were able to adjust their photosynthetic performance to accommodate various light spectra. P–E responses of this alga varied among the four light qualities; however, we interpreted these results with caution, as oxygen evolution experiments in the present study were conducted under short-term exposures (c. 1 h). Results revealed that a “stimulatory” effect was observed under green light, as shown by the higher NP rates compared to other light qualities. This response could have resulted from the absorption of green light by phycobiliproteins in phycobilisomes associated with PSII, which in turn transfer the collected energy to chlorophyll reaction centers for photosynthesis (Dumay et al. 2014). Although the minimum irradiance requirements (Ec) did not greatly differ among the spectral light treatments, α (quantum yield of oxygen evolution) was slightly lower under red, blue, and green light, resulting in higher saturation irradiances (Ek) when compared to white light. Such distinct light responses suggest the acclimation potential of the alga to spectral irradiance, corresponding to the spectrum distribution occurring in deep coastal waters. Indeed, M. papulosa occurs in the upper and middle subtidal zones between 3 and 30-m depths (Lideman et al. 2012), where blue and green light prevails. Photoacclimation of M. papulosa to green light may also be related to a change in the structure of its phycobilisomes. Ohki and Fujita (1992) reported that the phycobilisomes of a cyanobacterium, Phormidium sp. (Oscillatoriales) acclimated to green light were twice the size as those cells acclimated to red light; these changes in the phycobilisome structure occurred during chromatic adaptation of this marine cyanophyte. Other ways in which macroalgae can achieve acclimation include adjustment of the photosynthetic apparatus itself via changes of the reaction center ratio, or changes in the relative content of light protective pigments; all of these mechanisms influence primarily pigment ratios of the algae (Marquardt et al. 2010). On one hand, the higher growth rates of a red alga, Pyropia haitanensis (Chang et Zheng) Kikuch et Miyata (Bangiaceae, Bangiales), under blue, green, and white light were associated with increase in the photosynthetic efficiency of the alga, as evidenced by their higher rETRmax (photochemical electron transport rate), qP (photochemical quenching), Fv/Fm, and α (Wu 2016). Studies on longer time-scales along with chlorophyll fluorescence measurements must be carried out for a more realistic assessment of how M. papulosa achieve “physiological” acclimation to the light climate (including irradiance and light quality) they are subjected to in nature. Implications of their physiological versus morphological photoacclimation strategies could provide insights for future management of mariculture of this species.
Nevertheless, the effects of the different light qualities on PE content of M. papulosa presented higher effect in blue and green light treatments, which was consistent with the results in P–E experiments, particularly under green light. Such increase in PE could be ascribed to acclimation in blue and green light spectra, as well as to improved energy absorption to make up for the absence of other light qualities that are usually absorbed by chlorophyll. Higher PE (and PC) contents were likewise found in several red algae under blue and green light [e.g., Chondrus crispus Stackhouse (Gigartinaceae, Gigartinales), Corallina elongata Ellis et Solander (Corallinaceae, Corallinales), Plocamium cartilagineum (Linnaeus) Dixon (Plocamiaceae, Plocamiales), Porphyra umbilicalis Kützing, Pyropia leucosticta (Thuret) Neefus et Brodie, P. haitanensis (Bangiaceae, Bangiales), and G. birdiae] (López-Figueroa and Niell 1989; Figueroa et al. 1995; Franklin et al. 2001; Tsekos et al. 2002; Barufi et al. 2015; Wu 2016). However, chl-a and PC contents of M. papulosa in the present study showed little variation in all light qualities. Our results differed from those of Kim et al. (2015) that showed increased chlorophyll concentration, hence superior growth rates in green and red + blue light-grown Gracilaria tikvahiae McLachlan (Gracilariaceae, Gracilariales); phycobiliprotein synthesis also seemed to be stimulated in parallel with chlorophyll synthesis. Higher chl-a were observed in P. haitanensis grown under blue and white light, but chl-a content decreased when the thalli were grown under red light; these results matched with their relative growth rates (Wu 2016). Nonetheless, the manifestation of the various types of photopigments, and their arrangement in the thylakoid membranes (Gantt 1981; Dumay et al. 2014) are responsible for the different photosynthetic efficiencies at various light spectra, which affect the overall photosynthetic performance of macroalgae. The high PE and PC contents in M. papulosa suggest enhanced production in pigment proteins by blue and green light. This response could also be considered as a photoprotection mechanism related to energy dissipation, similar to the elevated content of carotenoids and xanthophyll pigments in red algae G. birdiae (Ursi et al. 2003) and Gracilaria tenuistipitata var. liui Zhang et Xia (= Agarophyton tenuistipitatum (Chang et Xia) Gurgel, Norris et Fredericq; Gracilariaceae, Gracilariales) (Barufi et al. 2011). The sublittoral kelp Laminaria saccharina (Linnaeus) Lamouroux (= Saccharina latissima (Linnaeus) Lane, Mayes, Druehl et Saunders; Laminariaceae, Laminariales) was also shown to hold large amounts xanthophyll pigments to minimize photoinhibition during the high irradiance periods of spring (Gevaert et al. 2002). Several red algae including M. papulosa were found to have seasonal changes in thallus color, with “yellowing” being primarily dependent on nitrogen level as a material base of phycoerythrin, and secondarily on light condition in the sunlit bottom (Kobayashi and Fujita 2014). Pigment synthesis is driven by light, and is regulated by various photoreceptors for absorption of different light qualities (Rüdiger and López-Figueroa 1992). Green light effects on chlorophyll synthesis in red algae C. crispus, Halymenia floresii (Clemente) C. Agardh (Halymeniaceae, Halymeniales), and the brown alga Desmarestia aculeata (Linnaeus) Lamouroux (Desmarestiaceae, Desmarestiales), as well as on protein synthesis in the green alga Ulva rigida C. Agardh (Ulvaceae, Ulvales) were pointed to a possible coaction between a green photoreceptor and phytochrome (López-Figueroa 1991a, b; Rüdiger and López-Figueroa 1992; Godínez-Ortega et al. 2008). While blue and green light stimulate nitrogen assimilation and the accumulation of phycobiliproteins (Godínez-Ortega et al. 2008), red light blocks chlorophyll biosynthesis, which results in a decrease in the function of the light-harvesting system (Senger et al. 2002). Further investigations are required to elucidate how the different light spectral qualities regulate the biosynthesis process.
Net photosynthesis, dark respiration and chlorophyll fluorescence results of M. papulosa likewise revealed the sensitivity of the alga to temperature gradients. Their P–E curves and parameter estimates at 12, 20, and 28 °C were distinct from each other. NP rates were lowest at 12 °C, in parallel to the low NP rates and Fv/Fm in the temperature exposure experiments. A characteristic photoinhibition (i.e., decline in NP rates and quantum yield of oxygen evolution) was also observed at this critical temperature, thereby restricting the growth of this alga. Under these circumstances, low winter temperature could explain the absence of natural populations of M. papulosa in the cold-temperate regions of Hokkaido and northern Honshu islands. Perhaps, incident irradiance of more than 200 μmol photons m−2 s−1 at or below 12 °C in winter in the natural state would cause low temperature-induced photodamage to this species (Fukumoto et al. 2018). At the boundary of northern distribution of this alga (in northern Kyushu or in the Pacific coast of central Honshu), winter temperature could sometimes drop to 12 °C (Terada et al. 2016); however, low-temperature induced photodamage will probably not occur due to the low PAR environment at the subtidal habitat of this species at 3–30 m depths.
In contrast, NPmax was highest at 28 °C and so was Ek, allowing M. papulosa to utilize more light in photosynthesis. Such adjustment in photosynthetic response suggests efficient cultivation of the seaweed also in shallow waters during summer. While no indication of photoinhibition was detected after an hour of exposure to 1000 μmol photons m−2 s−1 at this temperature, the possibility of PSII inactivation under variable field conditions, particularly under circumstances in which temporarily supersaturating irradiances may hit the algae still needs to be tested. Indeed, a declining trend in dark-acclimated Fv/Fm (and GP rates) was already observed at 28 °C; photodamage to PSII in the alga will likely accelerate upon exposure to excessive irradiances (Terada et al. 2018; Borlongan et al. 2018, 2019). Maximum irradiance range in the depth distribution of this species was 175–1600 μmol photons m−2 s−1. On one hand, Fv/Fm–T responses of M. papulosa showed peak values at temperature range of 21.5–23.6 °C; the temperature at which GPmax occurred (GPTopt = 22.1 °C) was also within this range. These temperature optima for photosynthesis were in the same range as that for growth of M. papulosa (20–24 °C; Lideman et al., 2012). Dark respiration rates gradually increased from 8 to 28 °C; a temperature threshold in respiration was found above 28 °C, minimizing the impact of carbon and/or oxygen limitation at elevated temperatures (Vásquez-Elizondo and Enríquez 2016; Borlongan et al. 2017). Meristotheca papulosa is widely distributed along the Pacific coasts of southern Japan (Yoshida 1998), with seawater temperature range of 12–29 °C (Borlongan et al. 2017; Terada et al. 2016, 2018). They are abundant during spring and early summer (April–July), when temperatures rise to 18–26 °C (Shinmura 1974; Serisawa et al. 2000; Lideman et al. 2012; Kobayashi and Fujita 2014).
From the results obtained, the following conclusions can be drawn: green light has advantageous effects on the photosynthesis of M. papulosa. Blue or green light treatments in mariculture of this red alga can be used to stimulate phycobiliprotein production, thereby improving thallus color and product quality. Indeed, Japanese consumers generally prefer reddish-color product for this species. Exposure to blue or green light of yellow-colored individuals harvested from relatively shallow waters for a few days may be done prior to selling or shipment to buying centers. The ability of M. papulosa to adjust its P–E responses at different temperatures suggests its efficient cultivation under natural seawater temperatures within its natural distribution in Japan. Such information could provide significant contributions toward the advancement in cultivation of this edible resource, in addition to expanding upon existing scientific knowledge.
References
Alexandrov GA, Yamagata Y (2007) A peaked function for modeling temperature dependence of plant productivity. Ecol Model 200:189–192
Ang PO, Leung SM, Choi MM (2014) A verification of reports of marine algal species from the Philippines. Philipp J Sci 142:5–49
Barufi JB, Korbee N, Oliveira MC, Figueroa FL (2011) Effects of N supply on the accumulation of photosynthetic pigments and photoprotectors in Gracilaria tenuistipitata (Rhodophyta) cultured under UV radiation. J Appl Phycol 23:457–466
Barufi JB, Figueroa FL, Plastino EM (2015) Effects of light quality on reproduction, growth and pigment content of Gracilaria birdiae (Rhodophyta: Gracilariales). Sci Mar 79:15–24
Beer S, Eshel A (1985) Determining phycoerythrin and phycocyanin concentrations in aqueous crude extracts of red algae. Aust J Mar Freshw Res 36:785–792
Borlongan IA, Nishihara GN, Shimada S, Terada R (2017) Photosynthetic performance of the red alga Solieria pacifica (Solieriaceae) from two different depths in the sublittoral waters of Kagoshima, Japan. J Appl Phycol 29:3077–3088
Borlongan IA, Matsumoto K, Nakazaki Y, Shimada N, Kozono J, Nishihara GN, Shimada S, Watanabe Y, Terada R (2018) Photosynthetic activity of two life history stages of Costaria costata (Laminariales, Phaeophyceae) in response to PAR and temperature gradient. Phycologia 57:159–168
Borlongan IA, Nishihara GN, Shimada S, Terada R (2019) Assessment of photosynthetic performance in the two life history stages of Alaria crassifolia (Laminariales, Phaeophyceae). Phycol Res 67:28–38
Colombo-Pallotta MF, Rodríguez-Román A, Iglesias-Prieto R (2010) Calcification in bleached and unbleached Montastraea faveolata: evaluating the role of oxygen and glycerol. Coral Reefs 29:899–907
Cosgrove J, Borowitzka MA (2011) Chlorophyll fluorescence terminology: an introduction. In: Suggett DJ, Prášil O, Borowitzka MA (eds) Chlorophyll a fluorescence in aquatic sciences: Methods and developments. Springer, Dordrecht, pp 1–17
Ding L, Ma Y, Huang B, Chen S (2013) Effects of seawater salinity and temperature on growth and pigment contents in Hypnea cervicornis J. Agardh (Gigartinales, Rhodophyta). BioMed res Int 2013: 594308. https://doi.org/10.1155/2013/594308
Dumay J, Morançais M, Munier M, Guillard CL, Fleurence J (2014) Phycoerythrins: valuable proteinic pigments in red seaweeds. Adv Bot Res 71:321–343
Faye EJ, Shimada S, Kawaguchi S, Masuda M (2005) Characterization of the edible red alga Meristotheca papulosa (Solieriaceae, Gigartinales) from Japan. Phycol Res 53:234–245
Faye EJ, Kogame K, Shimada S, Kawaguchi S, Masuda M (2007) Taxonomic features of the red alga Meristotheca coacta (Solieriaceae, Gigartinales) from Japan. Phycol Res 55:150–158
Figueroa FL, Aguilera J, Niell FX (1995) Red and blue light regulation of growth and photosynthetic metabolism in Porphyra umbilicalis (L.) Kützing (Bangiales, Rhodophyta). Eur J Phycol 30:11–18
Franklin LA, Kräbs G, Kuhlenkamp R (2001) Blue light and UV-A radiation control the synthesis of mycosporine-like amino acids in Chondrus crispus (Florideophyceae). J Phycol 37:257–270
Fukumoto R, Borlongan IA, Nishihara GN, Endo H, Terada R (2018) The photosynthetic responses to PAR and temperature including chilling-light stress on the heteromorphic life history stages of a brown alga, Cladosiphon okamuranus (Chordariaceae) from Ryukyu Islands, Japan. Phycol Res 66:209–217
Gantt E (1981) Phycobilisomes. Annu Rev Plant Physiol 32:327–347
Gevaert F, Creach A, Davoult D, Holl AC, Seuront L, Lemoine Y (2002) Photoinhibition and seasonal photosynthetic performance of the seaweed Laminaria saccharina during a simulated tidal cycle: chlorophyll fluorescence measurements and pigment analysis. Plant Cell Environ 25:859–872
Godínez-Ortega JL, Snoeijs P, Robledo D, Freile-Pelegrín Y, Pedersén M (2008) Growth and pigment composition in the red alga Halymenia floresii cultured under different light qualities. J Appl Phycol 20:253–260
Henley WJ (1993) Measurement and interpretation of photosynthetic light-response curves in algae in the context of photoinhibition and diel changes. J Phycol 29:729–739
Huang W, Fujita Y (1997) Callus induction and thallus regeneration of the red alga Meristotheca papulosa (Rhodophyta, Gigartinales). Bot Mar 40:55–61
Jassby AD, Platt T (1976) Mathematical formulation of the relationship between photosynthesis and light for phytoplankton. Limnol Oceanogr 21:540–547
Kim JK, Mao Y, Kraemer G, Yarish C (2015) Growth and pigment content of Gracilaria tikvahiae McLachlan under fluorescent and LED lighting. Aquaculture 436:52–57
Kobayashi M, Fujita D (2014) Can thallus color of red algae be used as an environmental indicator in shallow waters? J Appl Phycol 26:1123–1131
Lee YP (2008) Marine algae of Jeju. Academy Press, Seoul, p 477
Lideman, Nishihara GN, Noro T, Terada R (2011) In vitro growth and photosynthesis of three edible seaweeds, Betaphycus gelatinus, Eucheuma serra and Meristotheca papulosa (Solieriaceae, Rhodophyta). Aquaculture Sci 59: 563–571.
Lideman NGN, Noro T, Terada R (2012) Effect of temperature and light on the photosynthetic performance of two edible seaweeds: Meristotheca coacta and Meristotheca papulosa. Aquaculture Sci 60:377–388
Liu RY (2008) Checklist of marine biota of China seas. China Science Press, Beijing, p 1267
López-Figueroa F (1991a) Control by light quality of chlorophyll synthesis in the brown alga Desmarestia aculeata. Z Naturforsch 46:542–548
López-Figueroa F (1991b) Red, blue and green light photoreceptors controlling chlorophyll, biliprotein and total protein synthesis in the red alga Chondrus crispus. Br Phycol J 26:383–393
López-Figueroa F, Niell FX (1989) Red-light and blue-light photoreceptors controlling chlorophyll a synthesis in the red alga Porphyra umbilicalis and in the green alga Ulva rigida. Physiol Plantarum 76:391–397
Maegawa M, Kunieda M, Kida W (1993) The influence of ultraviolet radiation on the photosynthetic activity of several red algae from different depth. Jpn J Phycol 41:207–214
Marquardt R, Schubert H, Varela DA, Huovinen P, Henríquez L, Buschmann AH (2010) Light acclimation strategies of three commercially important red algal species. Aquaculture 299:140–148
Murase N, Maegawa M, Kida W (1989) Photosynthetic characteristics of several species of Rhodophyceae from different depth in the coastal area of Shima peninsula, Central Japan. Jpn J Phycol 37:213–220
Murase N, Takada J, Abe M, Noda M, Suda Y (2012) Growth and photosynthesis of Meristotheca papulosa under different light quality from light emitting diodes (LEDs). Algal Resour 5:61–69 (in Japanese with English abstract)
Nguyen TV, Le NH, Lin SM, Steen F, De Clerck O (2013) Checklist of the marine macroalgae of Vietnam. Bot Mar 56:207–227
Ohki K, Fujita Y (1992) Photoregulation of phycobilisome structure during complementary chromatic adaptation in the marine cyanophyte Phormidium sp. C86. J Phycol 28:803–808
Ohno M (2004) Edible local seaweeds. In: Ohno M (ed) Biology and Technology of Economic Seaweeds. Uchida Rokakuho Publishing Co. Ltd., Tokyo, pp 283–296
Ohno M, Yano M, Hiraoka M, Oka N, Taniguchi M (2002) Tank culture of Eucheuma serra and Meristotheca papulosa using with deep sea water. Bull Mar Sci Fish Kochi Univ 20:35–40 (in Japanese)
Platt T, Gallegos CL, Harrison WG (1980) Photoinhibition of photosynthesis in natural assemblages of marine phytoplankton. J Mar Res 38:687–701
Porra RJ, Thompson WA, Kriedemann PE (1989) Determination of accurate extinction coefficients and simultaneous for assaying chlorophylls a and b extracted with four different solvents: verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochim Biophys Acta 975:384–394
R Development Core Team (2019) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org
Rao PSN, Gupta RK (2015) Algae of India. In: A checklist of Indian marine algae (excluding diatoms and dinoflagellates), vol 3. Botanical Survey of India, Kolkata, p 93
Rüdiger W, López-Figueroa F (1992) Photoreceptors in algae. Ann Rev Photochem Photobiol 55:949–954
Sekar S, Chandramohan M (2008) Phycobiliproteins as a commodity: trends in applied research, patents and commercialization. J Appl Phycol 20:113–136
Senger H, Humbeck K, Schiller H (2002) Light adaptation of the photosynthetic apparatus of green algae. In: Werner D, Heldmaier G (eds) Environmental signal processing and adaptation. Springer, Berlin, pp 71–86
Serisawa Y, Taino S, Ohno M (2000) Ecological study of utilized red alga, Meristotheca papulosa (Solieriaceae, Gigartinales) population off Susaki, Tosa Bay, Southern Japan. Suisazoshoku 48:597–601 (in Japanese with English abstract)
Shinmura I (1974) The seasonal variation of growth and the period of spore-liberation in Meristotheca papulosa. Bull Jap Soc Phycol 22:124–129 (in Japanese with English abstract)
Shinmura I, Tanaka T (2008) Useful algae in Kagoshima prefecture III: Rhodophyceae. Jap J Phycol 56:123–128 (in Japanese)
Silva PC, Basson PW, Moe RL (1996) Catalogue of the benthic marine algae of the Indian Ocean. Univ Calif Publ Bot 79:1–1259
Team SD (2019) RStan: the R interface to Stan. R package version 2:9 http://mc-stan.org
Terada R, Shikada S, Watanabe Y, Nakazaki Y, Matsumoto K, Kozono J, Saino N, Nishihara GN (2016) Effect of PAR and temperature on the photosynthesis of Japanese alga, Ecklonia radicosa (Laminariales), based on field and laboratory measurements. Phycologia 55:178–186
Terada R, Matsumoto K, Borlongan IA, Watanabe Y, Nishihara GN, Endo H, Shimada S (2018) The combined effects of PAR and temperature including the chilling-light stress on the photosynthesis of a temperate brown alga, Sargassum patens (Fucales), based on field and laboratory measurements. J Appl Phycol 30:1893–1904
Terada R, Nakashima Y, Borlongan IA, Shimabukuro H, Kozono J, Endo H, Shimada S, Nishihara GN (2020) Photosynthetic activity including the thermal- and chilling-light sensitivities of a temperate Japanese brown alga Sargassum macrocarpum. Phycol Res 68: 70–79
Tsekos I, Niell FX, Aguilera J, Figueroa FL, Delivopoulos SG (2002) Ultrastructure of the vegetative gametophytic cells of Porphyra leucosticta (Rhodophyta) grown in red, blue and green light. Phycol Res 50:251–264
Ursi S, Pedersén M, Plastino EM, Snoeijs P (2003) Intraspecific variation of photosynthesis, respiration and photoprotective carotenoids in Gracilaria birdiae (Gracilariales, Rhodophyta). Mar Biol 142:997–1007
Vásquez-Elizondo RM, Enríquez S (2016) Coralline algal physiology is more adversely affected by elevated temperature than reduced pH. Sci Rep 6:19030
Watt NJ, Chiovitti A, Craik DJ, Kraft GT (2003) The cell wall galactans from Australian representatives of the genus Meristotheca (Solieriaceae, Rhodophyta). Phycologia 42:572–581
Webb WL, Newton M, Starr D (1974) Carbon dioxide exchange of Alnus rubra: a mathematical model. Oecologia 17:281–291
Wu H (2016) Effect of different light qualities on growth, pigment content, chlorophyll fluorescence, and antioxidant enzyme activity in the red alga Pyropia haitanensis (Bangiales, Rhodophyta). Biomed Res Int 2016:7383918
Yokohama Y (1973) Photosynthetic properties of marine benthic red algae from different depth in the coastal area. Bull Jap Soc Phycol 21:119–124 (in Japanese)
Yoshida T (1998) Marine algae of Japan. Uchida Rokakuho Publishing, Tokyo, p 1222 (in Japanese)
Yoshida T, Suzuki M, Yoshinaga K (2015) Checklist of marine algae of Japan (revised in 2015). Jpn J Phycol 63:129–189 (in Japanese)
Acknowledgements
We thank Captain Akimasa Habano and the crew of T/S Nansei-Maru, Faculty of Fisheries, Kagoshima University, for their kind assistance in the measurement of underwater PAR. We also thank Professor Kazuhiko Anraku, Faculty of Fisheries, Kagoshima University for his help in the measurement of light wavelength. All authors have provided consent.
Funding
This research was supported in part by the Grant-in-Aid for Scientific Research (#16H02939) from the Japanese Ministry of Education, Culture, Sport and Technology and the Japan Society for the Promotion of Science (JSPS).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary Fig. 1
Vertical distribution of the underwater irradiance near the study site, Cape Sata (31°30’N, 130°38′E), Kagoshima, Japan between 13:34 to 13:48 on 14 July 2018. Measurement was taken just below the seawater surface (1 m), and at depths of 3, 5, 10, 20, 30, 40, and 50 m. Irradiance was measured at 1 Hz, and the sampling duration was 1 min for each depth. Irradiance measurements were used to determine the extinction coefficient (Ku m−1), according to Beer–Lambert equation. (JPG 176 kb)
Rights and permissions
About this article
Cite this article
Borlongan, I.A., Suzuki, S., Nishihara, G.N. et al. Effects of light quality and temperature on the photosynthesis and pigment content of a subtidal edible red alga Meristotheca papulosa (Solieriaceae, Gigartinales) from Japan. J Appl Phycol 32, 1329–1340 (2020). https://doi.org/10.1007/s10811-020-02045-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-020-02045-z