Abstract
The effects of 24-epibrassinolide (EBR) on chlorophyll fluorescence, leaf surface morphology and cellular ultrastructure of grape seedlings (Vitis vinifera L.) under water stress were investigated. The grape seedlings were subjected to 10 % (w/v) polyethylene glycol (PEG-6000) and treated with 0.05, 0.10 or 0.20 mg L−1 EBR, respectively. EBR application increased chlorophyll contents, the effective photochemical quantum yield of PSII, maximum photochemical efficiency of PSII, maximal fluorescence and non-photochemical quenching coefficient under water stress in each concentration. Compared with water stress control, higher stomatal density and stomatal length were observed in young leaves under EBR treatments, but not in mature leaves. In-depth analysis of the ultrastructure of leaves indicated that water stress induced disappearance of nucleus, chloroplast swelling, fractured mitochondrial cristae and disorder of thylakoid arrangement both in young leaves and mature leaves. However, EBR application counteracted the detrimental effects of water stress on the structure of the photosynthetic apparatus better in young leaves than in mature leaves. Compared to the other treatments, treatment of 0.10 mg L−1 EBR had best ameliorative effect against water stress. These results suggested that exogenous EBR could alleviate water stress-induced inhibition of photosynthesis on grape possibly through increasing chlorophyll content, lessening the stomatal and non-stomatal limitation of photosynthesis performance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Brassinosteroids (BRs) have been considered as the sixth phytohormone that plays a crucial role in plant development. In almost every organ of plants, BRs are ubiquitous as a group of plant polyhydroxysteroids. Similar to other plant hormones, BRs are involved in a range of fundamental processes, such as cell division and elongation, synthesis of DNA, RNA, and proteins, the growth and development of plant organs, senescence, and stress responses (Sasse 1999, 2003; Castle et al. 2003; Bajguz and Hayat 2009).
Brassinosteroids are present in free form and as conjugates bound to glucose and fatty acids. Since the discovery of BRs 35 years ago (Grove et al. 1979), seventy different types of BRs have been isolated and characterized from plants, suggesting that brassinolide (BR) and castasterone (CS) are two highly bioactive types of brassinosteroids. 24-epibrassinolide (EBR) and 28-homobrassinolide (HBR) are the synthetic BR exogenously applied to resist abiotic stress and biotic stress for the studies (Khripach et al. 2000).
In northwest grape-producing areas of China, arid and semi-arid climates lead to soil water stress, which influence sprout and seedling of grape in spring (Qi et al. 2006; Xi et al. 2007). Reducing the production and quality of grape by water stress could constrain the development of grape wine industry. Water stress can affect the regulation of photosynthesis via stomatal limitation and non-stomatal limitations (Zhou et al. 2013), including stomatal size, chlorophyll contents, and saturation of the photosynthetic apparatus. Numerous studies have shown that exogenous application of BRs can ameliorate drought-induced inhibition of photosynthesis to some extent (Yuan et al. 2010; Fariduddin et al. 2009; Li et al. 2012). However, this effect is largely determined by degrees of stress and concentrations or modes of BRs used.
To systematically confirm the cellular ultrastructure and photosynthetic characteristics caused by BRs when plants are subjected to abiotic stress, many studies used different concentrations and analogs of BRs application on a variety of plant development. Yu et al. (2004) reported that 0.10 mg L−1 EBR application increased the effective photochemical quantum yield of PSII (ΦPSII), probably due to a conspicuous increase in the photochemical quenching of cucumber (Cucumis sativus). Examining tomato (Lycopersicon esculentum), 0.10 mg L−1 EBR pretreatment alleviated the slight photoinhibition caused by heat stressed, as indicated by the reductions of ΦPSII. Specially, non-photochemical quenching coefficient (NPQ) was not affected by EBR pretreatment at a normal temperature whereas it was significantly increased more than 50 % by EBR treated at a high temperature (40/30 °C) (Ogweno et al. 2008). Under saline stress, exogenously applied EBR significantly increased F v/F m and chlorophyll contents of EBR salinity, but it was found non-effective in EBR control of wheat (Triticum aestivum L.) (Shahbaz et al. 2008). As observed in pepper, drought effectively decreased F v/F m, ΦPSII, and increased NPQ. However, EBR alleviated drought-induced photoinhibition extraordinarily by reducing NPQ, and it could use energy absorbed from excessive light to strengthen the pepper’s resistance (Hu et al. 2013).
The alleviating effect of BRs on stress-induced inhibition of photosynthesis might be attributed to stomatal or non-stomatal factors (Ali et al. 2008). Water deficiency led to subcellular changes, such as closed stomata, and the rise of starch in the bundle sheath chloroplasts in sorghum leaves (Giles et al. 1976). As the crucial places of photosynthesis, cellular metabolism and reactive oxygen species generating in stressful environments, normal chloroplast and mitochondria play important roles in stabilizing plants (Liu et al. 2010; Xu et al. 2006).
Effects of BRs on chlorophyll fluorescence and cellular ultrastructure have been investigated very insufficiently. To date, few studies have reported the role of EBR on grape seedlings under water stress. In the present study, our study examined the effects of exogenous EBR on chlorophyll fluorescence parameters and cellular ultrastructure in water-stressed grape seedlings, and explored the mechanism of how EBR alleviated PEG-induced damage of photosynthetic system of grape.
Materials and methods
Plant materials and treatments
One-year old cuttings from V. vinifera L. cv. Riesling were collected from a vineyard at the Northwest A&F University, Shaanxi, China. They were raised in plastic containers (12 cm × 12 cm) with a mixture of garden soil, vermiculite, and humus (1:1:1 ratio) and sprouted with 70 % relative humidity at 28/18 °C (day/night) in a greenhouse for 8–10 weeks. Two hundred and forty young grapevines with 8 functional leaves were averagely transplanted into 10 black growth chambers (50 cm × 35 cm × 15 cm) filled with half-strength Hoagland nutrient solution in phytotron under the following controlled conditions: a 12-h photoperiod, 25/15 °C day–night temperature cycle, and photosynthetic photon flux density (PPFD) of 160 μmol m−2 s−1.
The 24-epibrassinolide (EBR, Sigma, USA) was dissolved in 1 mL 98 % ethanol and made to volume with distilled water, and then employed at 3 concentration levels, viz., 0.05, 0.10, and 0.20 mg L−1. In our preliminary experiment, we employed the wide range of concentrations of polyethylene glycol (PEG) and 10 % (w/v) PEG-6000 (moderate water stress) was chosen as the water stress intensity. EBR and PEG-6000 were mixed into Hoagland nutrient solution. Subjects were assigned into five different treatment groups: (1) 10 % PEG + 0.05 mg L−1 EBR, EBR0.05, (2) 10 % PEG + 0.10 mg L−1 EBR, EBR0.10, (3) 10 % PEG + 0.20 mg L−1 EBR, EBR0.20, (4)Hoagland nutrient solution + 10 % PEG (stressed control), and (5) Hoagland nutrient solution + DW (unstressed control). Each treatment group contained three replicates of 48 plants. On the 0, 3, 6, 9, 12th day of treatments, chlorophyll fluorescence parameters were measured. Samples of the third and eighth leaves were used for electron microscopy observation and others were used for chlorophyll estimation on the 9th day.
Chlorophyll contents determination
Chlorophyll contents were determined based on photosynthetic pigments absorption by the supernatant measured at 663 nm, 645 nm and 470 nm using the method by Gao (2006). 0.10 g of a sample leaf, 0.5 ml 100 % acetone and 15 ml 80 % acetone were added in a 25-ml scale test tubes. After 24 h in dark places (leaves changed to white), the reaction was stopped by adding 80 % acetone to the scale of 25 ml. The absorbance was recorded spectrophotometrically at 663, 645, and 470 nm, respectively. There were three replicates for each treatment. The chlorophyll contents were calculated using the formulas by Gao (2006).
Chlorophyll fluorescence determination
Chlorophyll fluorescence parameters were measured with a pulse-amplitude modulated (PAM-2500) fluorometer (Walz, German). Measurement of chlorophyll fluorescence parameters was repeated once for each leaf, and three leaves of each treatment were chosen for dark adaptation for more than 20 min. After dark-adapted treatment, the minimal fluorescence (F o) and the maximal fluorescence (F m) were measured under a low modulated light over a 0.8-s period. The maximum fluorescence in the light-adapted state (F m′) was recorded after a second saturation pulse. Then, the actinic light (7,000 μmol m−2 s−1) turned off and the far-red light turned on for measuring the minimal fluorescence in a light-adapted state (\({F{^{\prime}}_ {\text{o}}}\)).The maximum photochemical quantum yield of PSII (F v/F m), the effective photochemical quantum yield of PSII (ΦPSII), and the non-photochemical quenching (NPQ) were calculated as (F m − F o)/F m, (F m′ − F′)/F m′ and F m/F m′ − 1, respectively (Kitajima and Butler 1975; Genty et al. 1989; Bilger and Björkman 1990).
Scanning electron microscopy
After 9 days, the third and eighth leaves of the five treatments were cut into approximately 5 mm × 5 mm segments and fixed in 4 % glutaraldehyde for 2 h at room temperature. There were three replicates for each treatment. Then, the samples were washed in phosphate buffer solution (0.10 M PBS, pH 6.8) four times with 10-min intervals between each washing. After repeated rinsing, the leaf samples were dehydrated in an ethanol series (30, 50, 70, 80 and 90 %) for 20 min in each different concentration fleetly. Then, for 30 min each time, they were washed by 100 % ethanol for three times and finally transferred into isoamyl acetate three times for 30 min. After being Hitachi HCP-2 critical point-dried (Tokyo, Japan) in CO2, the samples were sprayed with a thin layer of gold. Observation and photography were finished by scanning electron microscopy (SEM-2700, Hitachi, Tokyo, Japan).
Transmission electron microscopy
The pre-processing of samples was the same as scanning electron microscopy. Then, the samples were washed in phosphate buffer solution (0.10 M PBS pH 6.8) for five times every 15 min. At least 1 ml of 2 % osmium tetroxide was applied to each sample for post-fixation for approximately 2 h. After fixation, samples were dehydrated using an ethanol series (30, 50, 70, 80, 90 and 100 %) and finally dehydrated by 100 % acetone twice for 30 min each time. The samples were then infiltrated and embedded with epoxy resin. Ultrathin sections (75 nm) were made with a diamond knife on an ultramicrotome and mounted on copper grids for transmission electron microscopy observation.
Statistical analysis
Data were statistically analyzed with SPSS 18.0. One-way ANOVA and Duncan’s multiple range tests were used to determine the significance of the difference among the samples with a significance level of 0.05.
Results
Chlorophyll contents
Water stress control significantly decreased contents of chlorophyll a (Chl a) and chlorophyll b (Chl b) in grape leaves (Table 1). However, EBR application alleviated the loss of chlorophylls induced by water stress. Compared to stressed control, the Chl a contents of EBR0.05 and EBR0.10 were significantly increased by 23.37 and 28.97 %, respectively. There was no significant increase of Chl b between EBR treatments and stressed control. Meanwhile, the total chlorophyll contents were significantly increased by EBR0.05 and EBR0.10 in water-stressed grapes.
Chlorophyll fluorescence
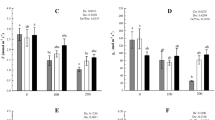
Under water stress, EBR treatments increased F v/F m, ΦPSII and NPQ, but reduced F o as compared to the stressed control (Fig. 1). Water deficiency significantly increased F o during the 12 days of water stress. Nevertheless, EBR treatments did not start to increase F o until the sixth day and were significantly different from stressed control. Compared to the stressed control, both of the EBR0.10 and EBR0.20 treatments significantly alleviated the decrease of F v/F m and NPQ. Water stress resulted in a decrease in the value of ΦPSII; however, supplementation of EBR to water stress treatments significantly improved ΦPSII value in grape leaves.
Effects of 24-epibrassinolide on F o, F v/F m, NPQ and ΦPSII of leaves under water stress. (1) 10 % PEG + 0.05 mg L−1 EBR; EBR0.05, (2) 10 % PEG + 0.10 mg L−1 EBR; EBR0.10, (3) 10 % PEG + 0.20 mg L−1 EBR; EBR0.20, (4) Hoagland nutrient solution + 10 % PEG; stressed control, and (5) Hoagland nutrient solution + distilled water; unstressed control. Data represent the means of independent measurements of three replicates with standard deviations shown by vertical error bars (P ≤ 0.05)
Microscopic structure of leaf tissue
The stomatal length of young leaves in EBR0.05 and EBR0.20 increased to a much higher level than those in stressed control (Table 2; Fig. 2). However, in mature leaves, there was significant difference of stomatal length between stressed control and EBR0.20 (Table 3; Fig. 2). A higher stomatal width and stomatal opening degree were observed on unstressed control of mature leaves compared to other treatments, but no significant difference in young leaves. In young leaves, the stomatal density of EBR0.05, EBR0.10 and EBR0.20 increased by 43.7, 15.5 and 53.9 % compared with the stressed control, respectively, but no significant effect of the three treatments with EBR was found on the stomatal density of mature leaves under water stress.
Stomata and epidermal cells on the leaf epidermis of grape under EBR0.05, EBR0.10, EBR0.20, CK1, CK2. (1) 10 % PEG + 0.05 mg L−1 EBR; EBR0.05, YL (A, B), ML (c, d); (2)10 % PEG + 0.10 mg L−1 EBR; EBR0.10, YL (e, f) ML (g, h); (3) 10 % PEG + 0.20 mg L−1 EBR; EBR0.20, YL (i, j), ML (k, l); (4) Hoagland nutrient solution + 10 % PEG; stressed control, YL (m, n), ML (o, p); (5) Hoagland nutrient solution + distilled water; unstressed control, YL (q, r), ML (s, t). YL young leave, ML mature leave, Bar-10 μm (b, d, f, h, j, l, n, p, r, t), 100 μm (a, c, e, g, i, k, m, o, q, s)
Effects of 24-epibrassinolide on the ultrastructure of organelle in young and mature grape leaves under water stress for 9 days. (1) 10 % PEG + 0.05 mg L−1 EBR; EBR0.05, YL (a, b, c), ML (d, e, f); (2) 10 % PEG + 0.10 mg L−1 EBR; EBR0.10, YL (g, h, i) ML (j, k, l); (3) 10 % PEG + 0.20 mg L−1 EBR; EBR0.20, YL (m, n, o), ML (p, q, r); YL young leave, ML mature leave, Ch chloroplast, Chr chromatin, CW cell wall, Gt grana thylakoid, M mitochondrion, Nu nucleolus, Pg plastoglobule, SG starch grain. Bar-2 μm (a, b, c, e, g, h, i, j, q), 500 μm (d, f, k, l, m, n, o, p, r)
Ultrastructural changes of organelle
Transmission electron micrograph (TEM) observations showed photosynthetic mesophyll cells of the grape leaves with a delimited cell wall, containing chloroplasts with thylakoid stacking, mitochondria and nucleus. The unstressed control had integrated and clear cells. Chloroplasts were elongated ellipses that contained well-arranged granum and smooth thylakoid membranes along with numerous starch grains and plastoglobules. They exhibited a typical mitochondrion structure, with well displayed mitochondrial membranes organized in outer and inner membranes, tube-arranged cristae mitochondriales. A clear nucleolus and well-developed nuclear envelope were noticed in nucleus (Fig. 4). Under water stress, ultrastructural images of stressed control showed some noticeable changes of the organelles in Fig. 4. The chloroplast was nearly round and asymmetrically swelling, with an increased number of plastoglobules. Far away from cell wall, the chloroplast envelope was partially ruptured and the thylakoid membranes were loose and disrupted whereas the thylakoids were overly disorganized. Irregular swelling of mitochondrion and fractured arrangement of cristae were observed in stressed control. No cell nucleus was observed. After applying exogenous EBR, the organelles of mesophyll cells became ameliorative (Fig. 3) compared to the stressed control: (1) Observations showed that the shape of chloroplasts changed slightly from elongated ellipse to ellipse close to cell walls. Well-aligned internal lamellar system and less plastoglobules had been observed in young leaves compared to mature leaves. (2) Ultrastructural changes in mitochondria were inconspicuous. Only part of their cristae became dissolved both in young leaves and mature leaves. (3) In young leaves, cell nuclei were clear and apparent along with intact and notably nuclear membrane, nuclear scaffold and nucleolus. However, cell nuclei were blurred in mature leaves. In general, EBR pretreatments alleviated the effects of water stress because of the organelle integrity in grape leaves.
Effects of stressed control and unstressed control on the ultrastructure of organelle in young and mature grape leaves for 9 days. (4) Hoagland nutrient solution + 10 % PEG; stressed control, YL (s, t), ML (u, v); (5) Hoagland nutrient solution + distilled water; unstressed control, YL (w, x), ML (y, z). YL young leave, ML mature leave, Ch chloroplast, Chr chromatin, CW cell wall, Gt grana thylakoid, M mitochondrion, Nu nucleolus, Pg plastoglobule, SG starch grain. Bar-500 μm (s, t, v, w, y, z), 100 nm (u, x)
Discussion
In the present study, we found that application of EBR enhanced chlorophyll contents of grape seedling under water stress. The result was similar to the observations on papaya (Gomes et al. 2013), chickpea (Ali et al. 2007), eggplant seedlings (Wu et al. 2014), Brassica juncea (Hayat et al. 2007), and geranium (Swamy and Rao 2008). As main light absorbing and transmitting pigments (antenna pigments), Chl a and Chl b had an effect on increasing light capture efficiency for enhancing net photosynthesis rate (Melkozernov 2006). Our results suggested that the application of exogenous EBR prevented the loss of photosynthetic efficiency in water deficit stress-induced grape seedlings, probably because EBR-treated water-stressed grape seedlings showed higher contents than non-EBR-treated plants.
Chlorophyll fluorescence is a subtle reflection of primary reactions of photosynthesis. It has been widely used in describing photosynthesis mechanism and photosynthetic physiology under environmental stress (Sayed 2003). Our results showed that EBR treatments significantly increased maximum photochemical efficiency of PSII (F v/F m) and the effective photochemical quantum yield of PSII (ΦPSII) in the plants exposed to water stress (Fig. 1). Water stress induced inhibition of PSII electron transport. The limitation of electron transfers from the reaction center of PSII to the primary acceptor plastoquinone (Q A) and the secondary acceptor plastoquinone (Q B) inhibits transfer of excitation energy from the light-harvesting complex (LHC) to PSII (Qi 2006). LHC as the most abundant protein complexes on thylakoid membrane, 50 % of which are composed of Chl a and Chl b. Therefore, Chlorophyll contents and the integrity of thylakoid play an important role in electron transport of PSII. The value of NPQ implies high photo-protective ability of thermal energy dissipation through high de-epoxidation level of xanthophyll cycle (Adams and Adams 1996). In this study, we found that EBR treatment significantly increased NPQ during the initial 6 days of water stress treatment. It is possible that the increase in NPQ by EBR could have provided protection against damage by excessive energy. From 7 to 12 days, the decrease of NPQ may show the loss of excessive energy caused by non-radioactive energy path. The results are in agreement with previous studies on pepper (Hu et al. 2013) and Amur grape (Vitisamur ensis Rupr.) (Qin et al. 2013).
The regulation of stomatal movement plays an important role in controlling gas exchange and balancing water requirement. We found in young grape leaves, EBR-treated leaves had longer stomatal length and more stomata than those of stressed control, but stomatal width and degree of stomatal opening did not increase. However, in mature leaves, there was no obvious difference in stomatal density, stomatal length or stomatal width between EBR-treated grapes and stressed control. Compared to unstressed control, mature leaves in EBR treatments and stressed control all had less stomatal opening degree. One explanation of the results is that water had been forced redistributed in different organs or tissues according to different water potentials caused by water stress. To maintain normal growth of plants, young leaves robbed water from mature leaves, reducing total photosynthesis leaf area. These results showed that EBR alleviated water stress-induced inhibition of photosynthesis which was caused by stomatal limitation.
Mechanical damage in cell is an important cause of plant death under water stress. Maintaining organelles integrity including chloroplast, mitochondrion and nucleus is essential in energy conversion and electron transfer for photosynthesis. Gunning and Steer (1996) found some different protein complexes embedded in the thylakoid membrane of the chloroplast were important components involved in PSI and PSII. Water stress (Pääkkönen et al. 1998; Giles et al. 1974), heavy metals stress (Ali et al. 2013, 2014; Basile et al. 2013), and high temperature stress (Zhang et al. 2009) induced collapse of organelle structure, inhibiting photosynthesis. Under water stress, thylakoids became swollen with distorted stroma and grana lamella of chloroplast similar to Giles et al. (1976) findings. In addition, mitochondria swelled irregularly, and nucleus was disaggregated. At the ultrastructural level, we observed that the cellular structure of leaves in EBR treatment groups remained intact with orderliness of chloroplast and flattened stacks of thylakoids, but complete nucleus only in young leaves (Fig. 3). However, mitochondrial cristae of mesophyll cell were mildly disorganized in EBR treatment groups. In plant cells, chloroplasts, mitochondria, and nucleus have pivotal roles in photosynthesis, ATP production, and the expression of stress genes. These results demonstrated that EBR regulated water stress responses, possibly through alleviating the inhibition of photosynthesis caused by non-stomatal limitation and inducing protein and gene expression.
Our results showed that exogenous EBR alleviated water stress-induced inhibition of photosynthesis in grape, most likely through increasing chlorophyll content, reducing stomatal and non-stomatal limitations of photosynthetic performance. EBR applied at 0.10 mg L−1 was the most effective concentration in this study. However, further research is needed to explore the relationship between EBR and photosynthesis using advanced physiological and molecular biological technology.
Author contribution statement
Conceived and designed the experiments: Zhu-mei Xi. Performed the experiments and analyzed the data: Zhi-zhen Wang. Contributed reagents/materials/ analysis tools: Peng Zheng, Jiang-fei Meng. Wrote the paper: Zhi-zhen Wang.
Abbreviations
- BRs:
-
Brassinosteroids
- EBR:
-
24-Epibrassinolide
- PEG-6000:
-
Polyethylene glycol-6000
- Chl:
-
Chlorophyll
- PSII:
-
Photosystem II
- F o :
-
Minimal fluorescence
- F v/F m :
-
Maximum photochemical quantum yield of PSII
- ΦPSII:
-
Effective photochemical quantum yield of PSII
- NPQ:
-
Non-photochemical quenching coefficient
- LHC:
-
Light-harvesting complex
- Chr:
-
Chromatin
- CW:
-
Cell wall
- Gt:
-
Grana thylakoid
- M:
-
Mitochondrion
- Nu:
-
Nucleolus
- Pg:
-
Plastoglobule
- SG:
-
Starch grain
References
Adams DB, Adams WW (1996) The role of xanthophylls cycle carotenoids in the protection of photosynthesis. Trends Plant Sci 1:21–26
Ali B, Hayat S, Ahmad A (2007) 28-Homobrassinolide ameliorates the saline stress in chickpea (Cicer arietinum L.). Environ Exp Bot 59:217–223
Ali Q, Athar HR, Ashraf M (2008) Modulation of growth, photosynthetic capacity and water relations in salt stressed wheat plants by exogenously applied 24-epibrassinolide. Plant Growth Regul 56:107–116
Ali S, Farooq MA, Yasmeen T, Hussain S, Arif MS, Abbas F, Bharwana SA, Zhang G (2013) The influence of silicon on barley growth, photosynthesis and ultra-structure under chromium stress. Ecotox Environ Safe 89:66–72
Ali B, Qian P, Jin R, Ali S, Khan M, Aziz R, Tian T, Zhou W (2014) Physiological and ultra-structural changes in brassica napus seedlings induced by cadmium stress. Biol Plantarum 58:131–138
BajguzA HayatS (2009) Effect of brassinosteroids on the plant responses to environmental stresses. Plant Physiol Biochem 47:1–8
Basile A, Sorbo S, Conte B, Cardi M, Esposito S (2013) Ultrastructural changes and heat shock proteins 70 induced by atmospheric pollution are similar to the effects observed under in vitro heavy metals stress in Conocephalum conicum (Marchantiales-Bryophyta). Environ Pollut 182:209–216
Bilger W, Björkman O (1990) Role of the xanthophyll cycle in photoprotection elucidated by measurements of light-induced absorbance changes, fluorescence and photosynthesis in leaves of Hedera canariensis. Photosynth Res 25:173–185
Castle J, Montoya T, Bishop GJ (2003) Selected physiological responses of brassinosteroids: a historical approach. In: Hayat S, Ahmad A (eds) Brassinosteroids: Bioactivity and Crop Productivity. Kluwer Academic Publishers, Dordrecht, pp 45–68
Fariduddin Q, Khanam S, Hasan SA, Ali B, Hayat S, Ahmad A (2009) Effect of 28-homobrassinolide on the drought stress-induced changes in photosynthesis and antioxidant system of Brassica juncea L. Acta Physiol Plant 31:889–897
Gao JF (2006) Experimental guide for Plant Physiology. Higher Education Press, Beijing
Genty B, Briantais JM, Baker NR (1989) The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim Biophys Acta 990:87–92
Giles KL, Beardsell MF, Cohen D (1974) Cellular and ultrastructural changes in mesophyll and bundle sheath cells of maize in response to water stress. Plant Physiol 54:208–212
Giles KL, Cohen D, Beardsell MF (1976) Effects of water stress on the ultrastructure of leaf cells of Sorghum bicolor. Plant Physiol 57:11–14
Gomes MMA, Netto AT, Campostrini E, Smith RB, Zullo MAT, Ferraz TM, Siqueira LN, Leal NR, VÁZquez MN (2013) Brassinosteroid analogue affects the senescence in two papaya genotypes submitted to drought stress. Theor Exp Plant Physiol 25:186–195
Grove MD, Spencer GF, Rohwedder WK, Mandava N, Worley JF, Warthen JD, Steffens GL, Flippen-Anderson JL, Cook JC (1979) Brassinolide, a plant growth-promoting steroid isolated from Brassica napus pollen. Nature 281:216–217
Gunning BES, Steer MW (1996) Plant Cell Biology: structure and function. Jones and Bartlett Publishers, Sudbury
Hayat S, Ali B, Hasan SA, Ahmad A (2007) Brassinosteroid enhanced the level of antioxidants under cadmium stress in Brassica juncea. Environ Exp Bot 60:33–41
Hu WH, Yan XH, Xiao YA, Zeng JJ, Qi HJ, Ogweno JO (2013) 24-Epibrassinosteroid alleviate drought-induced inhibition of photosynthesis in Capsicum annuum. Sci Hortic 150:232–237
Khripach V, Zhabinskii V, De Groot A (2000) Twenty years of brassinosteroids: steroidal plant hormones warrant better crops for the XXI century. Ann Bot 86:441–447
Kitajima M, Butler WL (1975) Excitation spectra for photosystem I and photosystem II in chloroplasts and the spectral characteristics of the distributions of quanta between the two photosystems. Biochimicaet Biophysica Acta 408:297–305
Li YH, Liu YJ, Xu XL, Jin M, An LZ, Zhang H (2012) Effect of 24-epibrassinolide on drought stress-induced changes in Chorisporabungeana. BiolPlantarum 56:192–196
Liu ZJ, Guo YK, Bai JG (2010) Exogenous hydrogen peroxide changes antioxidant enzyme activity and protects ultrastructure in leaves of two cucumber ecotypes under osmotic stress. J Plant Growth Regul 29:171–183
Melkozernov AN (2006) Photosynthetic functions of chlorophylls. In: Blankenship RE, Grimm B, Porra RJ, Rüdiger W, Scheer H (eds) Chlorophylls and bacteriochlorophylls: biochemistry, biophysics, functions and applications. Springer, Dordrecht, pp 397–412
Ogweno JO, Song XS, Shi K, Hu WH, Mao WH, Zhou YH, You JQ (2008) Nogués S.Brassinosteroids alleviate heat-induced inhibition of photosynthesis by increasing carboxylation efficiency and enhancing antioxidant systems in Lycopersicon esculentum. J Plant Growth Regul 27:49–57
Pääkkönen E, Vahala J, Pohjola M, Holopainen T, Kärenlampi L (1998) Physiological, stomatal and ultrastructural ozone responses in birch (Betula pendula Roth.) are modified by water stress. Plant Cell Environ 21:671–684
Qi W, Tan H, Zhai H (2006) Photosynthetic characters and fluorescence parameters of different grape stocks under water stress. Chin J Appl Ecol 17:835–838
Qin HY, Ai J, Xu PL, Wang ZX, Zhao Y, Yang YM, Fan ST, Shen YJ (2013) Chlorophyll fluorescence parameters and ultrastructure in amur grape (Vitis amurensis Rupr.) under salt stress. Acta Bot Boreal 33:1159–1164
Sasse J (1999) Physiological actions of brassinosteroids. In: Sakurai A, Yokota T, Clouse SD (eds) Brassinosteroids: steroidal plant hormones. Springer Verla gGmbh, Tokyo, pp 137–161
Sasse JM (2003) Physiological actions of brassinosteroids: an update. J Plant Growth Regul 22:276–288
Sayed OH (2003) Chlorophyll fluorescence as a tool in cereal crop research. Photosynthetica 41:321–330
Shahbaz M, Ashraf M, Athar HR (2008) Does exogenous application of 24-epibrassinolide ameliorate salt induced growth inhibition in wheat (Triticuma estivumL.)? Plant Growth Regul 55:51–61
Swamy KN, Rao SSR (2008) Influence of 28-homobrassinolide on growth, photosynthesis metabolite and essential oil content of geranium (Pelargonium graveolens (L.) Herit). Amer J Plant Physiol 3:173–179
Wu XX, Zhu ZW, Yao XF, Zhang H, Chen JL, Zha DS (2014) Brassinosteroids protect photosynthesis and antioxidant system of eggplant seedlings from high-temperature stress. Acta Physiol Plant 36:251–261
Xi ZM, Sun WJ, Zhang ZW (2007) Effect of exogenous Ca2+ on drought resistance physiological indexes of wine grape cultivar Pinot Noir under water stress. J Northwest A&F Univ 35:137–146
Xu S, Li JL, Zhang XQ, Wei H, Cui LJ (2006) Effects of heat acclimation pretreatment on changes of membrane lipid peroxidation, antioxidant metabolites, and ultrastructure of chloroplasts in two cool-season turfgrass species under heat stress. Environ Exp Bot 56:274–285
Yu JQ, Huang LF, Hu WH, Zhou YH, Mao WH, Ye SF, Nogués S (2004) A role for brassinosteroids in the regulation of photosynthesis in Cucumis sativus. J Exp Bot 55:1135–1143
Yuan GF, Jia CG, Li Z, Sun B, Zhang LP, Liu N, Wang QM (2010) Effect of brassinosteroids on drought resistance and abscisic acid concentration in tomato under water stress. Sci Hortic 126:103–108
Zhang GL, Chen LY, Zhang ST, Zheng H, Liu GH (2009) Effects of high temperature stress on microscopic and ultrastructural characteristics of mesophyll cells in flag leaves of rice. Rice Sci 16:65–71
Zhou SX, Duursma RA, Medlyn BE, Kelly Jeff WG, Prentice IC (2013) How should we model plant responses to drought? An analysis of stomatal and non-stomatal responses to water stress. Agr Forest Meteorol 182–183:204–214
Acknowledgments
This study was supported by the National Technology System for Grape Industry (CARS-30-zp-9), the Natural Science Foundation of Shaanxi Province (2011JM3004). Thanks for the Key Laboratory of Horticultural Plant Biology and Germplasm Innovation in Northwest, Ministry of Agriculture of China. The authors are obliged to Tong Lu, M.S (Texas Christian University), who provided some useful comments on an earlier draft of this paper.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by P.K. Nagar.
Rights and permissions
About this article
Cite this article
Wang, Z., Zheng, P., Meng, J. et al. Effect of exogenous 24-epibrassinolide on chlorophyll fluorescence, leaf surface morphology and cellular ultrastructure of grape seedlings (Vitis vinifera L.) under water stress. Acta Physiol Plant 37, 1729 (2015). https://doi.org/10.1007/s11738-014-1729-z
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11738-014-1729-z