The mixing enthalpies of Al–Eu liquid alloys are measured by the calorimetric method at 1300 to 1473 K. The thermodynamic properties of Al–Eu melts are calculated in the entire composition range using the ideal associated solution model. The thermodynamic activities of melt components show negative deviations from ideal behavior and the mixing enthalpies show significant exothermic effects. The minimum mixing enthalpy of Al–Eu melts is –23.0 ± 2.2 kJ/mole at xEu = 0.39.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Alloys of aluminum with rare earth metals (REM) are used as deoxidizers, desulfurizers, and modifiers in the steel industry. To improve these processes, thermodynamic data for Al–REM alloys are needed, particularly for the Al–Eu system. The physicochemical properties of aluminum–europium alloys have been studied very insufficiently, and no Al–Eu phase diagram has been constructed. The literature only predicts the liquidus line and the existence of EuAl4, EuAl2, and EuAl intermetallic compounds; their lattice parameters and melting points have been determined [1]. Thus, EuAl2 melts congruently at 1573 K and is the most stable in this system. The standard enthalpy of EuAl2 formation has been determined by calorimetry and is –36.0 kJ/mole [2, 3].
The unique magnetic properties of EuAl2, in which europium is divalent, are known at low temperatures [4]. The valence of europium in aluminum melts at high temperatures is also to be further studied. Preliminary studies of REM melts with variable valence on the example of the Co–Ce system [5] have shown that thermodynamic data can be efficiently used to evaluate this effect, which is accompanied by a noticeable increase in the magnitude of partial mixing enthalpy of REMs in the melt.
The objective of this paper is to experimentally determine the mixing enthalpies of Al–Eu melts at 1300–1473 K and calculate the thermodynamic properties of the melt using the ideal associated solution model at 1573 K, which, according to the predicted phase diagram, corresponds to homogenous Al–Eu solutions in the entire composition range.
Experimental procedure
We employed isoperibolic calorimetry [5, 6] to determine the mixing enthalpies of Al–Eu melts. In the experiments, we determined the partial mixing enthalpies of components at 1300 K on the europium side (0 < xAl < 0.41) and at 1473 K on the aluminum side (0 < x Eu < 0.21). The experiments were performed in purged helium atmosphere at an excess pressure of 105 Pa to avoid evaporation of Eu (at 1300 K, the vapor pressure of liquid Eu is ~104 Pa [7]). The weight loss of the melt due to europium evaporation was no more than 5% and was taken into account in calculating the actual composition of melts. The experiments were performed in molybdenum and zirconium oxide crucibles. Molybdenum was used only in case of Eu-rich melts. The materials were of the following purity, %: 99.995 Al, 99.83 Eu, and 99.96 Mo.
The calorimeter was calibrated against a metal solvent (at the beginning of the experiment) or tungsten (at the end of the experiment) (99.96% pure), which hardly interacts with melt components at 1300–1600 K [6]. The initial weight of the metal in the crucible was 1–3 g and of the samples placed into a calorimetric bath 0.02–0.05 g.
The thermal effect of metal dissolution in the melt was calculated using thermal curves (dependences of melt temperature ΔT on time t) with numerical integration⎯finding the heat-exchange area: \( s = \int {\Delta T(t)dt} \).
The overall heat balance equation for metal dissolution in the melt is as follows:
where \( \Delta H_i^T \) is the enthalpy of heating one mole of the i-th metal from 298 K to the temperature T of the melt taken from [8]; \( \Delta {\bar{H}_i} \) is the unknown partial molar mixing enthalpy of the i-th component; t is the end time of the dissolution process; and k is the molar coefficient of heat exchange determined by calibration. The minus sign in the left-hand side of Eq. (1) shows that the dissolution processes in the Al–Eu system are exothermic. The partial mixing enthalpies of both components were calculated by Eq. (1).
According to the procedure [5, 6], a set of \( \Delta {\bar{H}_{\text{Eu}}} \) and \( \Delta {\bar{H}_{\text{Al}}} \) experimental values for further statistical analysis was represented as partial α-functions \( \left( {{\alpha_i} = \Delta {{\bar{H}}_i}{{\left( {1 - {x_i}} \right)}^{ - 2}}} \right) \). The calculational procedure that involves integration of the Gibbs–Duhem equation is used to obtain smoothed \( \Delta {\bar{H}_i} \) for both components and integral mixing enthalpies ΔH with confidence intervals equal to two root-mean-square deviations of the α-function. To determine consistent values of mixing enthalpies over the entire composition range, we employed the interpolation procedure using the integral α-function \( \left( {\alpha = \Delta H \cdot {x^{ - 1}} \cdot {{\left( {1 - x} \right)}^{ - 1}}} \right) \). Thus, two branches of this curve determined experimentally were jointly processed using a series of iterations for the integral α-function taking into account smoothed k(x) values for the entire composition range.
Model Calculations
To determine the mixing enthalpies of melts in the entire composition range and calculate the thermodynamic activity, Gibbs energy ΔG, and mixing entropy ΔS, we used the ideal associated solution (IAS) model according to the procedure [9]. The IAS model relates negative deviations of thermodynamic properties of the melt from ideal solutions to A i B j associates of unlike atoms formed in the melt. The A–B solution is regarded as a mixture of A 1 , B 1 monomers and A i B j associates formed from them. Its equilibrium composition is characterized by N reactions of the following type:
which are solved by a system of N equations according to the mass action law:
where K n is the equilibrium constant in the reaction of formation of the n-th associate; x n is the molar fraction of the n-th associate; and \( {x_A}_{_1} \) and \( {x_B}_{_1} \) are the molar fractions of monomers in the melt.
The temperature dependence of the equilibrium constant in the association reaction is related to the thermodynamic properties of the associate as follows:
where ΔS n and ΔH n are the entropy and enthalpy of formation of the n-th associate; T is temperature.
In this case, the model of thermodynamic properties of the melt represents a system of nonlinear equations whose parameters are the number and composition of associates, ΔS n , and ΔH n :
When N associates form in the melt, the model parameters are found by solving a system of N + 4 equations. Note that the minimum necessary number L of reference points (mixing enthalpies) should be equal to N. In this instance, the model parameters are calculated by solving a system of L(N + 4) equations. The number of reference points used obeys condition N ≤ L ≤ 3 N.
In solving the system of model equations, additional restrictions on interaction between components in melts need to be considered: 0 < a B < 1, 0 < < a A < 1, 0 < x π < 1, ΔH < 0, ΔG < 0, and ΔS < 0. In calculations, we took into account the existence of EuAl4, EuAl2, and EuAl associates and data on the liquidus [1] and formation enthalpies of solid compounds [2, 3]. As a result, we determined the mixing enthalpy, activities of components and associates, Gibbs energies, and mixing entropies for the entire composition range.
To confirm the existence of such associates in the melt, we subjected the cast alloys obtained in calorimetric experiments to x-ray diffraction. Reflections of x-rays from Al, Eu, three above-mentioned intermetallides were revealed for powders of all alloys. This testifies that there are liquid associates whose composition is close to the relevant intermetallides.
Experimental Results
The integral mixing enthalpy of the melts fits to the equation derived in the iteration procedure in the entire composition range:
where x = x Eu and 0 ≤ x ≤ 1.
The consistent values of both partial energies of components with confidence intervals were calculated using the Gibbs–Duhem equation through the α-function of either component. Hence, the interpolation polynomial for the α-function of europium becomes (x = x Eu)
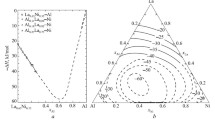
The mixing enthalpies of Al–Eu melts at 1573 K (for standard state of pure liquid metals) are summarized in Table 1 and Fig. 1 along with experimental partial molar enthalpies of components. The mixing enthalpies of Al– Eu melts are high negative values; the extreme value of integral mixing enthalpy is –23.0 ± 2.2 kJ/mole at x Eu = 0.39.
The thermodynamic parameters of associates for liquid and solid alloys are summarized in Table 2. We have calculated the thermodynamic activities (Fig. 2) and excess Gibbs energies (Fig. 3) of aluminum, europium, and AlEu, Al2Eu, and Al4Eu associates in melts of this system at 1573 K. The ΔH and ΔG values for the Al–Eu melts have been used to calculate the excess mixing entropies by the formula: TΔS ex = ΔH – ΔG ex. They are negative, the minimum value being about –9 J/(mole · K) at x Eu = 0.39. The boundary value of the partial excess Gibbs energy of Eu is –45 kJ/mole, which agrees (within the experimental error) with the value determined in [10] with the electromotive force method (–41.4 kJ/mole at 1000 K).
Discussion of Results
The mixing enthalpies of Al–Eu liquid alloys show great exothermic effects associated with quite a high difference in electronegativities of aluminum and europium, which is 0.46 eV [11]. However, the magnitude of the Al–Eu mixing enthalpy is much smaller than the enthalpies of most alloys of aluminum with trivalent REMs (minimum integral enthalpy in such systems is close to –40 kJ/mole [12, 13]). This is also the case for the standard formation enthalpies of solid compounds, particularly for the most stable phases of Al2Ln type. Thus, Δƒ H is –36 kJ/mole for Al2Eu, though it is from –50 to –60 kJ/mole for other compounds of aluminum with REMs [2, 3, 14]. We can assume that europium has valence +2 in melts with aluminum. The same assumption was made previously for the Al2Eu phase following the study of magnetic properties [4].
In general, great exothermal effects in the formation of Al–REM alloys are determined by the transfer of electrons from REM (only two 6 s 2 electrons in case of Eu) to aluminum. Note that the mixing enthalpies of Al–Eu melts are close to those in melts of aluminum with alkaline-earth metals, which are uniquely divalent. The experimental data reported in [15] show that minimum ΔH is –21.5 kJ/mole at 1125 K in the Al–Ca system and is –20.1 kJ/mole at 1130 K in the Al–Sr system, being very close to our ΔH for Al–Eu melts (Table 1).
A common feature of REM–p-metal melts is the effect of short-range ordering related to the existence of associates. The composition dependence of enthalpy and excess Gibbs energy in Al–Eu melts (Fig. 3) correlates with the data reported in [1], testifying that there are aluminum-rich intermetallides. The mixing enthalpies of liquid alloys in Al–REM binary systems may be thought to be determined by strong interaction between unlike components with predominant ionic-covalent bonding. Hence, the IAS model is appropriate and, given limited thermodynamic data, may be an efficient tool to identify and predict thermodynamic properties of melts over a wide range of compositions.
Therefore, experimental data on mixing enthalpies have been obtained for the first time and basic thermodynamic properties of Al–Eu melts have been calculated to allow the phase diagram to be refined. The variation in mixing enthalpies of Al–REM binary melts along the entire lanthanide raw is of interest and requires systematic experimental study. It is especially the case for alloys of aluminum with heavy lanthanides, which are still to be studied and should become an interesting subject of further experiments.
Conclusions
The mixing enthalpies of Al–Eu liquid alloys have been determined for the first time with calorimetry over the entire composition range.
The thermodynamic activities, Gibbs energies, and mixing entropies of Al–Eu melts have been calculated using the ideal associated solution model.
The thermodynamic functions of formation of Al–Eu melts are characterized by significant exothermic values and short-range ordering effects.
References
K. A. Gschneidner and F. W. Calderwood, “The Al–Eu (aluminum–europium) system,” Bull. Alloys Phase Diagrams, 9, No. 6, 679 (1988).
S. V. Meschel and O. J. Kleppa, “Thermochemistry of alloys of transition metals and lanthanide metals with some IIIB and IVB elements in the periodic table,” J. Alloys Compd., 321, No. 1, 183–200 (2001).
C. Colinet, “The thermodynamic properties of rare earth metallic systems,” J. Alloys Compd., 225, No. 2, 409–422 (1995).
K. A. Gschneidner and L. Eyring (eds.), Handbook on the Physics and Chemistry of Rare Earths, North- Holland Publishing Co., Amsterdam (1979).
N. I. Usenko, M. I. Ivanov, and V. V. Beresutski, “Mixing enthalpies of liquid Co–Ce and Co–Sm alloys,” J. Alloys Compd., 346, No. 1, L7–L10 (2002).
V. V. Berezutskii and M. I. Ivanov, “Mixing enthalpies in samarium–transition metal melts,” Powder Metall. Met. Ceram., 48, No. 7–8, 454–461 (2009).
A. N. Nesmeyanov, Steam Pressure of Chemical Elements [in Russian], Izd. AN SSSR, Moscow (1961), 396.
A. T. Dinsdale, “SGTE data for pure elements,” Calphad, 15, No. 4, 319–427 (1991).
M. A. Turchanin, I. V. Belokonenko, and P. G. Agraval, “Use of the ideal associated solution theory for assessment of the temperature–composition dependence of the thermodynamic properties of binary melts,” Rasplavy, No. 1, 58–69 (2001).
V. A. Lebedev, V. I. Kober, and L. F. Yamshchikov, Thermochemistry of Rare-Earth and Actinoid Alloys [in Russian], Metallurgiya, Chelyabinsk (1989), p. 336.
M. C. Day and J. Selbin, Theoretical Inorganic Chemistry, Reinhold, New York (1962).
Yu. O. Esin, S. P. Kolesnikov, V. M. Baev, et al., “Enthalpies of formation of liquid binary alloys of aluminum and tin with lanthanum,” Zh. Fiz. Khim., 55, No. 6, 1587–1588 (1981).
Yu. O. Esin, G. M. Ryss, and P. V. Geld, “Enthalpies of formation of liquid alloys of cerium wiht aluminum,” Zh. Fiz. Khim., 53, No. 9, 2380–2381 (1979).
G. Cacciamani and R. Ferro, “Thermodynamic modeling of some aluminum–rare earth binary systems: Al– La, Al–Ce, Al–Nd,” Calphad, 25, No. 4, 583–597 (2001).
F. Sommer, J. J. Lee, and B. Predel, “Thermodynamische Untersuchung flussiger Aluminium–Calcium, Aluminium–Strontium, Nickel − Magnesium und Nickel − Calcium Legierungen,” Z. Metallkunde, 74, No. 2, 100–104 (1983).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Poroshkovaya Metallurgiya, Vol. 50, No. 7–8 (480), pp. 189–196, 2011.
Rights and permissions
About this article
Cite this article
Ivanov, M.I., Shevchenko, M.O., Berezutskii, V.V. et al. Thermodynamic properties of Al–Eu liquid alloys. Powder Metall Met Ceram 50, 538–543 (2011). https://doi.org/10.1007/s11106-011-9356-3
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11106-011-9356-3