The partial mixing enthalpy of aluminum and the integral mixing enthalpies of liquid alloys in the binary Al–Co system are studied by high-temperature calorimetry at 1870 ± 5 K in the composition range 0 < xCo < 0.25. The energies of forming alloys of aluminum with metals in the second half of the 3d series are compared.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The thermodynamic properties of liquid Al–Co alloys are of interest for studying the formation of hexagonal quasicrystals in this binary system and in multicomponent systems containing Al and Co [1, 2]. It should be pointed out that the literature offers very scarce information on the mixing enthalpies of liquid Al–Co alloys determined by direct calorimetry. There is only one experimental study [3] that employed high-temperature calorimetry at 1943 K to examine the mixing enthalpies ( ∆m H, “m” denoting mixing) over the entire composition range; the experimental data are presented only graphically, and the limiting partial enthalpies of components ( \( {\varDelta}_{\mathrm{m}}{\overline{H}}_{\mathrm{Me}}^{\infty } \) ) and extreme ∆m H are provided. There are data on the limiting partial mixing enthalpy of Al and Co ( \( {\varDelta}_{\mathrm{m}}{\overline{H}}_{\mathrm{Co}}^{\infty } \) ) in [4]. The paper [5] analyzes the thermodynamic data on liquid Al–Co alloys known at that time and provides their thermodynamic properties at 1873 K in a narrow composition range (0.05 < x Al < 0.35). They were calculated with use of the calorimetric data [3] and the activities of components determined by studying the distribution coefficient at 1873 K in the range 0 < x Al < 0.4 [6]. The mixing enthalpies of liquid alloys can also be found in [7], representing the entire range of thermodynamic properties for liquid Al–Co alloys at 2000 K, using the thermodynamic optimization provided in [8].
The enthalpies of dissolving solid cobalt in liquid aluminum ( \( {\varDelta}_{\mathrm{m}}{\overline{H}}_{\mathrm{Co}}^{\infty } \) ) at 1000–1100 K are determined in a series of experimental studies [9–12].
Table 1 summarizes all thermodynamic data on liquid Al–Co alloys available in the literature, and Table 2 provides enthalpies of dissolving solid Co in liquid Al. Table 2 also indicates enthalpies of mixing liquid supercooled Co with liquid Al, which we recalculated with use of the Co melting enthalpy (16.2 kJ · mol–1 [13]). We intentionally presented data in different tables since analysis of the literature revealed insufficiently clear differentiation between partial molar mixing and dissolution enthalpies. Most of the papers [9–12] do not indicate what quantity is indicated exactly (enthalpy of dissolving solid cobalt in liquid aluminum or enthalpy of mixing liquid supercooled cobalt with liquid aluminum), leading, for example, in [12] to incorrect comparison of partial dissolution [9–12] and mixing [3] enthalpies.
The analysis of data in Tables 1 and 2 shows that the only limiting partial mixing enthalpy of cobalt experimentally determined at 1943 K [3] substantially differs from more exothermic values recalculated from limiting partial dissolution enthalpies of Co determined at 1100 K [9–12]. It should be noted that the data reported in [9–12] seem to be used for the optimization in [7, 8]. Therefore, it is naturally to question the reliability of the data reported in [3] or the temperature dependence for the partial mixing enthalpy of cobalt in Al–Co melts.
Since the limiting partial mixing enthalpy is a very important quantity, reflecting the interaction between the central atom (Co in this case) and surrounding atoms of the other component (Al) and being widely used in model calculations of liquid alloy properties (for example, with the surrounded atom model), we decided to clarify \( {\varDelta}_{\mathrm{m}}{\overline{H}}_{\mathrm{Co}}^{\infty } \) by direct calorimetric measurement. This research is of interest for further study and simulation of the thermodynamic properties of ternary Al–Co–Me systems. In these studies, we measured the partial mixing enthalpies of cobalt in the Al–Co system in the limited composition range 0 < x Co < 0.25, being characterized by a lack of experimental data in the literature. The experiments were performed in purified helium under 105 Pa at 1620 and 1870 K employing a high-temperature dissolution calorimeter. The experimental setup and procedure were described previously in [14, 15]. Purity of the metals was 99.995% Co and 99.99% Al.
We measured the partial mixing enthalpies of cobalt during successive introduction of cobalt samples taken at 298 K (0.01–0.03 g in weight) into the calorimetric bath (molybdenum crucible with liquid aluminum). The initial metal weight in the bath was about 3 g. The metal content of the melt after introduction of each sample changed by no more than 1.5 mol.%; i.e., the partial molar enthalpies were determined with adequate accuracy. The partial and integral mixing enthalpies were measured with a relative error of ±5 and ±2%, respectively. The data were reproduced in each experiment within 3%.
It should be noted that there is a refractory intermetallic, CoAl, congruently melting at 1918 K in this system according to the phase diagram [16]. Therefore, the liquid-phase experiment requires quite high temperatures, which is complicated by the volatility of aluminum in these conditions. For this reason, the experiment performed at 1870 K may have a higher error. The experiment conducted at 1620 K was characterized by better reproducibility of the results, but the melt could be examined only in a limited composition range (0 << x Co < 0.12). The data obtained at this temperature were recalculated for standard state, liquid supercooled cobalt.
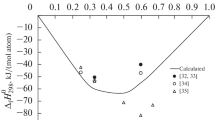
The experimental data are presented in Fig. 1. The partial molar mixing enthalpies of cobalt at different temperatures seem to be in satisfactory agreement in our two experiments, which allowed their use as a single data array.
The \( {\varDelta}_{\mathrm{m}}{\overline{H}}_{\mathrm{Co}}^{\infty } \) values experimentally obtained with the procedure reported in [17] were fitted to power polynomials to calculate the partial mixing enthalpy of cobalt and the integral mixing enthalpies at rounded contents for the range 0 < x Co < 0.25 (Table 3, the confidence interval is equal to two standard deviations of the approximation function). To produce the composition dependence for ∆m H over the entire range, we also used data on the cobalt side at 1873 K reported in [5]. Two branches of the ∆m H values were processed commonly; the selfconsistent mixing enthalpies of Al–Co melts are shown in Table 3 and Fig. 1 (solid line).
The data in Table 3 testify that liquid Al–Co alloys are formed with substantial heat release. The extreme integral mixing enthalpy (–35.3 kJ mol–1) falls at the composition corresponding to a cobalt molar fraction of 0.4.
According to [16], this system has several aluminum-rich compounds (Co2Al9, CoAl3, Co4Al13, and Co2Al5) that form peritectically and a refractory congruently melting intermetallic (CoAl). For the intermetallic, the papers [18–20] indicate formation enthalpies ∆f H o determined by different methods and varying from –53 to –66 kJ ∙ mol–1, which agrees well with the mixing enthalpies of liquid alloys, considering measurement temperature.
There is good agreement between our results and the data reported in [3] and substantial difference between the \( {\varDelta}_{\mathrm{m}}{\overline{H}}_{\mathrm{Co}}^{\infty } \) values that we found experimentally and the optimized data reported in [7, 8]. Taking this into account and considering that \( {\varDelta}_{\mathrm{m}}{\overline{H}}_{\mathrm{Co}}^{\infty } \) taken from [7, 8] is similar to the values recalculated from the results [9–12] obtained at 1100 K (Table 2), it can be concluded that there is a temperature dependence for \( {\varDelta}_{\mathrm{m}}{\overline{H}}_{\mathrm{Co}}^{\infty } \) that does not seem to be properly accounted for in [7, 8].
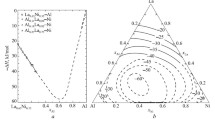
We compared our \( {\varDelta}_{\mathrm{m}}{\overline{H}}_{\mathrm{Co}}^{\infty } \) values with the experimental partial mixing enthalpies of components in Al alloys for infinite dilution with other metals from the second half of the 3d series, such as Mn, Fe, Co, Ni, and Cu. The data provided in Fig. 2 a and Table 4 show that the exothermicity of mixing enthalpies, both integral and partial, monotonically increases from Mn–Al to Ni–Al systems and further decreases for Cu–Al. This can be reasonably attributed to greater difference in the electronegativities or in the relative positions of Fermi levels of the components. We used the latter characteristic, which can be a measure of the electron work function for respective metals. The electron work functions and metallic radii of elements are provided in Table 4 and the delta electron work functions (∆W e = ∆W e(3dMe) – ∆W e(Al)) for components in the relevant systems are shown in Fig. 2 b.
Mixing enthalpies of Al–Mn (Fe, Co, Ni, Cu) melts (a) and delta electron work function for components in these systems according to [22] (b)
It should be noted that \( {\varDelta}_{\mathrm{m}}{\overline{H}}_{\mathrm{A}1}^{\infty } \) values are less exothermic than \( {\varDelta}_{\mathrm{m}}{\overline{H}}_{3d\mathrm{Me}}^{\infty } \) for the systems with Fe and Co and much more exothermic for the systems with Ni and Cu (these quantities differ little for the manganesecontaining system) (Table 4, Fig. 2 a). This feature is of special interest. For example, when \( {\varDelta}_{\mathrm{m}}{\overline{H}}_{\mathrm{A}1}^{\infty } \) is more exothermic, the phase diagrams of respective systems (Al–Ni, Al–Cu) [16] show a greater number of intermetallics in the region rich in the 3d metal (or show higher thermal stability and wider homogeneity ranges, significantly shifted toward the 3d metal, for these intermetallics) than in the aluminum-rich one. On the contrary, where dissolution of the 3d metal in pure aluminum is accompanied by greater heat release, a higher number of aluminumrich phases (Al–Fe, Al–Co) is observed.
There are also structural data [24, 25] that show the correlation between ordering in liquid alloys of specific compositions and higher exothermic partial mixing enthalpies of one of the components in the same composition range. Based on X-ray diffraction of liquid Al–Co alloys, it is concluded in [24] that the parameters characterizing the ordering of atoms at near and far distances at 85–74 at.% Al, with a peak close to the 76.5 at.% Al melt approximately corresponding to Co4Al13 intermetallic, have nonmonotonic dependences. This agrees well with the fact that the first partial enthalpies of cobalt are more exothermic than the first partial enthalpies of aluminum. At the same time, structurally ordered atoms of various types belonging to specific intermetallic compounds are present in the Al–Ni system even with 10 at.% Al [25].
To explain the varying ratio between the limiting partial mixing enthalpies of components in the 3dMe–Al systems, one should bear in mind that the partial molar mixing enthalpy of a component, such as Me1, is a conventional sum of mainly two thermal effects: (i) endothermic break of bonds between atom Me1 with z 1 adjacent atoms Me1 (z 1 is the average number of the first coordination sphere in the melt) and (ii) exothermic formation of bonds between atom Me1 with z 2 adjacent atoms Me2 to create a comparatively stable cluster of the central atom surrounded by atoms of the other kind (both contributions are calculated per mole of atoms Me1). The greater the difference between the Fermi levels and the smaller the size difference between the components, the higher the latter contribution. It should be noted that the size mismatch is virtually the same for all systems in question (Table 4), allowing us to analyze more accurately the influence of metallic chemical factors on the energy of alloy formation.
Considering the atomic sizes of 3d metals and Al (Table 4) and the calculated number [26, 27] of spheres of the same arbitrary radii that may touch the sphere of a given radius, we can reasonably assume that one atom of a 3d metal may be surrounded by approximately nine coarser Al atoms and one Al atom by approximately thirteen atoms of the 3d transition metal. For each pure metal, the number of neighboring atoms of the first coordination sphere is ~10–11 on the average. As for the atomic bond strength, it is much higher for transition metals than for aluminum (as evidenced by at least their melting points).
It is assumed that the limiting mixing enthalpies \( {\varDelta}_{\mathrm{m}}{\overline{H}}_{3d\mathrm{Me}}^{\infty } \) for 3d metals should be less exothermic than \( {\varDelta}_{\mathrm{m}}{\overline{H}}_{\mathrm{A}1}^{\infty } \) since 3d metals are characterized by much higher positive endothermic contribution from break of stronger bonds with their neighbors. This is observed exactly in the Al–Ni and Al–Cu systems.
In case of the A–Fe and Al–Co systems, the partial mixing enthalpies of the components in infinite dilution show the inverse ratio: \( {\varDelta}_{\mathrm{m}}{\overline{H}}_{\mathrm{Fe}}^{\infty } \) and \( {\varDelta}_{\mathrm{m}}{\overline{H}}_{\mathrm{Co}}^{\infty } \) are more exothermic than \( {\varDelta}_{\mathrm{m}}{\overline{H}}_{\mathrm{A}1}^{\infty } \) (Fig. 2 a).
This may be due to different capacities of the d levels in transition metals (or due to the population of the d level in respective metals for infinite dilution). Note that the number of d electrons per atom in metallic state in transition elements differs from the one corresponding to the configuration of electron shells of isolated atoms, which is peculiar to the metallic state. The paper [28] indicates the following numbers of d electrons per atom: 6.94 Fe, 7.86 Co, 8.97 Ni, and 9.91 Cu. Hence, the d level in copper is virtually filled. When a copper atom is surrounded by more electropositive aluminum atoms, it can hardly decrease efficiently the energy of valence electrons of the surrounding aluminum atoms, resulting in very small exothermic \( {\varDelta}_{\mathrm{m}}{\overline{H}}_{\mathrm{Cu}}^{\infty } \) . An aluminum atom surrounded by a greater number of more electronegative Cu atoms is much more capable of reducing the energy of its valence electrons. Thus, \( {\varDelta}_{\mathrm{m}}{\overline{H}}_{\mathrm{A}1}^{\infty } \) is much more exothermic. This is also the case with Ni, in which the d level can accommodate ~9 d electrons per atom. A Ni atom surrounded by nine more electropositive Al atoms needs only one electron to acquire a stable, filled d level. It easily gets one electron and can accommodate no more. That is why \( {\varDelta}_{\mathrm{m}}{\overline{H}}_{\mathrm{A}1}^{\infty } \) is more exothermic than \( {\varDelta}_{\mathrm{m}}{\overline{H}}_{\mathrm{Ni}}^{\infty } \) in this case as well, but the difference between these two values is much less than in the Al–Cu system.
Although the difference between the Fermi levels of components in the Al–Co system is somewhat lower than in the Al–Ni system, the twice as great capacity of the unoccupied part in the d level of cobalt leads to almost the same \( {\varDelta}_{\mathrm{m}}{\overline{H}}_{\mathrm{Ni}}^{\infty } \) and \( {\varDelta}_{\mathrm{m}}{\overline{H}}_{\mathrm{Co}}^{\infty } \) . More exothermic \( {\varDelta}_{\mathrm{m}}{\overline{H}}_{\mathrm{Co}}^{\infty } \) and \( {\varDelta}_{\mathrm{m}}{\overline{H}}_{\mathrm{Fe}}^{\infty } \) compared to \( {\varDelta}_{\mathrm{m}}{\overline{H}}_{\mathrm{A}1}^{\infty } \) can also be explained by greater capacity of the unoccupied parts in d levels of cobalt and iron than that of nickel. Note that the latter quantity ( \( {\varDelta}_{\mathrm{m}}{\overline{H}}_{\mathrm{A}1}^{\infty } \) ) is influenced more by the difference in Fermi levels of the components than by the capacity of electron shells of a more electronegative component. Indeed, when one aluminum atom is surrounded by approximately thirteen atoms of a transition metal, the aluminum atom will have almost the same ability to efficiently reduce the energy of its three valence electrons, no matter whether the d level in the second metal can accommodate one, two, or three electrons per atom; the most important for this capacity is to be greater than conditional 3/13 electrons per atom. In this case, the difference between the Fermi levels of Al and 3dMe influences the exothermic effect.
Therefore, the limiting partial mixing enthalpies of the components are important parameters, directly associated with structural ordering in liquid alloys. The analysis of different metallic chemical factors allows explaining the regular variation in the limiting partial and integral mixing enthalpies in the Al–3d-Me systems.
References
B. Grushko, D. Holland-Moritz, and K. Bickmann, “Decagonal quasicrystals in Al–Co and ternary alloys containing Cu and Ni,” J. Alloys Compd., 236, No. 1–2, 243–252 (1996).
B. Grushko and D. Holland-Moritz, “Decagonal quasicrystals in Al–Co, Al–Ni and in their ternary alloys,” Mater. Sci. Eng. A, 226–228, 999–1003 (1997).
P. V. Geld, Yu. O. Esin, M. S. Petrushevskii, and V. M. Sandakov, “Partial and integral enthalpies for forming liquid alloys of nickel and cobalt with aluminum,” in: Proc. 5th All-Union Conf. Calorimetry [in Russian], Mosk. Gos. Univ., Moscow (1971), pp. 42–44.
V. G. Dyubanov, A. Ya. Stomakhin, and A. F. Filippov, “Studying the formation enthalpies of diluted iron, cobalt, and nickel solutions,” Izv. Vuz. Chern. Metall., No. 3, 69–71 (1972).
G. I. Batalin, E. A. Beloborodova, and V. P. Kazimirov, Thermodynamics and Constitution of Aluminum-Based Liquid Alloys [in Russian], Metallurgy, Moscow (1983), p. 160.
F. Vachet, F. Desre, and E. Bonnier, “On the thermodynamics of liquid systems (Fe, Al, O); (Co, Al, O); (Ni, Al, O) in the region rich in iron, cobalt, or nickel a 1600°C,” C. R. Acad. Sci. Paris, 260, No. 6, 453–456 (1965).
“Al–Co (aluminum–cobalt),” in: Landolt-Börnstein New Series. Group IV Physical Chemistry, Vol. 19B1, Springer-Verlag, Berlin, Heidelberg (2002), pp. 129–133.
N. Dupin and I. Ansara, “Thermodynamic evaluation of the system Al–Co,” Revue de Métallurgie-Cahiers D Informations Techniques, 95, 1121–1129 (1998).
E. Th. Henig, H. L. Lukas, and G. Z. Petzow, “Enthalpy of formation and description of the defect structure of the ordered β-phase in Co–Al,” Metallkd., 71, 389–402 (1980).
M. Jeymond, D. Landaud, M. Legardeur, and A. Pasturel, “Microcomputer controlled experimentation in calorimetry. Application to the determination of the partial enthalpies of 3d transition metals in liquid aluminum,” Thermochim. Acta, 55, No. 3, 301–306 (1982).
P. A. Gomozov, Yu. V. Zasypalov, and B. M. Mogutnov, “Formation enthalpies for intermetallic compounds with structure CsCl (CoTi, CoZr, CoAl, NiTi),” Zh. Fiz. Khim., 60, No. 8, 1865–1867 (1986).
A. A. Zubkov, “Dissolution enthalpies of 3d transition metals in liquid aluminum,” J. Chem. Thermodyn., 26, No. 12, 1267–1274 (1994).
A. T. Dinsdale, “SGTE data for pure elements,” Calphad, 15, No. 4, 317–425 (1991).
N. I. Usenko, M. I. Ivanov, V. M. Petiuh, and V. T. Witusiewicz, “Thermochemistry of binary liquid alloys of copper with barium and lanthanide metals (europium, dysprosium and ytterbium),” J. Alloys Compd., 190, 149–155 (1993).
N. I. Usenko, M. I. Ivanov, and V. V. Berezutski, “Mixing enthalpies of liquid Co–Ce and Co–Sm alloys,” J. Alloys Compd., 346, L7–L10 (2002).
H. Okamoto, “Al–Co (aluminum–cobalt),” J. Phase Equilib., 17, No. 4, 367 (1996).
I. V. Nikolaenko and G. I. Batalin, “On application of orthogonal functions for representing data in the thermodynamics of binary alloys,” Teor. Éxp. Khim., 23, No. 2, 198–203 (1987).
S. V. Meschel and O. J. Kleppa, “Thermochemistry of alloys of transition metals and lanthanide metals with some IIIB and IVB elements in the periodic table,” J. Alloys Compd., 321, 183–200 (2001).
G. Borzone, R. Raggio, and R. Ferro, “Comments on intermetallic thermochemistry,” J. Min. Metall., 38, No. 3–4, 249–272 (2002).
R. Hu and Ph. Nash, “Review: Experimental enthalpies of formation of compounds in Al–Ni–X systems,” J. Mater. Sci., 41, 631–641 (2006).
V. S. Sudavtsova, N. V. Tkachenko, N. V. Kotova, and T. N. Zinevich, “Thermodynamic properties of Ni–Al melts,” Zh. Fiz. Khim., 78, No. 9, 1720–1722 (2004).
David R. Lide, Handbook of Chemistry and Physics, 76th ed., CRC Press, New York (1995).
S. S. Batsanov, Structural Chemistry. Facts and Relations [in Russian], Dialog-MGU, Moscow (2002), p. 292.
A. S. Roik, A. V. Samsonnikov, V. P. Kazimirov, and V. É. Sokolskii, “X-ray diffraction of Al–Co alloys,” Zh. Struk. Khim., 47, 171–176 (2006).
V. P. Kazimirov, A. S. Roik, A. V. Samsonnikov, et al., “Ordering of atoms in the melt and surface properties of systems with intermetallic compounds,” Sverkhtverd. Mater., No. 4, 40–54 (2009).
S. L. Vasilenko, Golden Domes in the Problem of Cone Packing in the Euclidean Space, http://www.artmatlab.ru, 2011.
R. H. Hardin, N. J. A. Sloane, W. D. Smith, et al., “Spherical codes. Nice arrangements of points on a sphere in various dimensions,” in: Information Sciences Research. AT&T Shannon Lab, http://www2.research.att.com/~njas/packings/ (1994).
D. A. Papaconstantopoulos, Handbook of Band Structure of Elemental Solids, Plenum Press, New York (1986).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Poroshkovaya Metallurgiya, Vol. 54, No. 5–6 (503), pp. 92–101, 2015.
Rights and permissions
About this article
Cite this article
Usenko, N.I., Shevchenko, M.A., Kotova, N.V. et al. Mixing Enthalpies of Al–Co Melts. Powder Metall Met Ceram 54, 324–330 (2015). https://doi.org/10.1007/s11106-015-9717-4
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11106-015-9717-4