Abstract
Malus hupehensis Rehd. var. pinyiensis Jiang (Pingyi Tiancha, PYTC) is a botanical variety of Malus (tea crabapple) originating from China. This species is characterized as apomictic, and it is highly capable of resisting water-logging, shade, cold, and various diseases. Mitogen-activated protein kinase (MAPK) cascades have been implicated in the regulation of stress and developmental signals in plants. In this study, an MAPK gene, MhMAPK, has been isolated from a PYTC complementary DNA (cDNA) library using rapid amplification of cDNA ends. The gene encodes a 373-amino-acid protein with high-sequence similarity to other previously reported plants MAPKs. MhMAPK contains all 11 MAPK conserved sub-domains and the phosphorylation motif TEY, and when fused to the green fluorescent protein, it is found to be localized in the nucleus of epidermal cells of onion. Transcripts of MhMAPK accumulate when PYTC is treated with 20% polyethylene glycol and 200 mM NaCl. These results indicated that MhMAPK may be functional within the nucleus by phosphorylating transcriptional factors. This, in turn, allows plants to rapidly respond to the environmental signals.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plants are continuously exposed to various environmental conditions, and they cannot evade environmental stress. In order to survive, plants must respond to various external environmental conditions and adapt to them. During this process, changes in the extracellular environment must be communicated in a specific manner from outside of the cell to the inside and ultimately to the nucleus where changes in gene expression may take place. Many of these mechanisms involve processes of protein phosphorylation by specific protein kinases and dephosphorylation by protein phosphatases (Hunter 1995; Hardie 1999). The MAP kinase cascade, one particular signal transduction mechanism, plays an important role in many different eukaryotic organisms (Jonak et al. 2002). Mitogen-activated protein kinases (MAPKs) are also known as external signal regulated protein kinases (ERKs) and belong to a class of conservative serine/threonine protein kinases (Hanks et al. 1988). Whether in animals, plants, or yeasts, all MAP kinases have common characteristics: Molecular weight is from 38 to 55 kDa; they contain 11 conservative protein kinase subregions; and there is a highly conservative TxY motif between VII and VIII subregions, but the x changes along with the biology category, for instance, x is usually E, P, or G in animals and yeasts, but E or D in plants (Machida et al. 1997).
In various organisms, the MAP kinase along with the MAP kinase cascade forms, including MAPKs, MAPK kinases (MAPKKs) and MAPKK kinases (MAPKKKs; Mizoguchi et al. 1996). MAPKs are activated when both tyrosine and threonine residues in the TxY motif are phosphorylated by dual-specificity kinases MAPKKs. MAPKKs are activated when serine and serine/threonine residues in the S/T–X3–5–S/T motif are phosphorylated by serine/threonine kinases MAPKKKs (Jonak et al. 1996). Phosphorylated MAP kinases may stay in the cytoplasm where they continue to phosphorylate other protein kinases or cytoskeleton, and in addition, they may go into nucleus to phosphorylate transcription factors and sequentially regulate the gene expression (Liu et al. 2000). As a result, external signals are transferred step by step until various physiological or biochemical responses occur in cells (Ligterink et al. 1997). Various stimuli are all able to activate the MAP kinase cascade such as environmental stress (Shoresh et al. 2006; Petersen et al. 2000), hormone and signal factor (Knetsch et al. 1996; Pagnussat et al. 2004). These stimuli can bring cell proliferation or differentiation and intracellular response of resistance (Jeong et al. 2006; Bogre et al. 1997).
Currently, about 20 MAPK genes have been isolated from plants, and based on their sequence homologies, these can be divided into four groups. The first group of MAPK genes is very different from all other groups, while a high sequence identity is observed between the second and third groups. In function, some members in the fourth group are related to cell periods, and other types of MAPKs mostly participate in the signal transduction of phytohormone or environmental stress (Liu et al. 2000). For instance, some MAPK genes in Arabidopsis thaliana (Mizoguchi et al. 1994), Oryza sativa L. (Seo et al. 1995), and Nicotiana tabacum (Zhang and Klessig 1997) have been cloned.
It is highly capable of resisting water-logging, shade, cold, and disease (Li 2001). Thus, it is often used as a rootstock for propagating apples. As Pingyi Tiancha (PYTC) is apogamic, plants are uniform, and little variation is observed among progeny. Moreover, it is amenable for genetic transformation and therefore ideally suited for genetic studies (Yang and Jie 1997).
In this paper, we report on the cloning and characterization of a new MAPK gene from PYTC, designated as MhMAPK. The deduced protein sequence of MhMAPK is highly identical to the N. tabacum NtMPK4. In addition, we have characterized the expression of MhMAPK under some particular environmental stimuli, including polyethylene glycol (PEG) and NaCl and investigated the subcellular localization of MhMAPK in epidermal cells of onion transformed with an MhMAPK-GFP fusion gene construct.
Materials and Methods
Plant Materials, Growth Condition, and Treatment
Full seeds of PYTC were selected and were surface-sterilized by soaking them for 10 min in saturated bleaching powder solution and then were thoroughly rinsed with sterile-distilled water and dipped in distilled water for one night and laminated at 4°C for about 40 days. After that, they were sown in plastic trays (diameter 12 cm and 18 cm high) filled with substratum, which contained peat, pearlite, and venmiculite (proportion, 3:1:1). When seedlings were six to eight leaves old, they were used for experiment. Before treatment, the seedlings were adaptive in distilled water for 24 h, and then displaced in treatment solution of 200 mM NaCl and 20% PEG-6000. Roots and leaves were harvested and placed immediately in liquid nitrogen and then stored at −70°C until use (1–2 weeks).
RNA Isolation and Reverse Transcription
Total RNA was extracted using the cetyltrimethylammonium bromide (CTAB) method (Cheng et al. 1993). Plant materials (about 2 g) were ground in liquid nitrogen, then suspended in 5 ml CTAB immediately, and then preheated in water at 65°C for 5 min. Sample was extracted with the equal volume of phenol H2O/chloroform/isoamyl alcohol (25:24:1, V/V) and repeated twice. RNA was precipitated overnight with one-fifth volume of 12 M LiCl at 4°C for about 12 h, and precipitate was suspended with SSTE buffer (contained 1 M NaCl, 0.5% sodium dodecyl sulfate and 10 mM ethylenediaminetetraacetic acid) and then extracted with equal volume of phenol H2O/chloroform/isoamyl alcohol (25:24:1, V/V) again and re-precipitated with double volumes of 100% ethanol at −70°C for 1 h, and centrifuged at 4°C for 12,000 rpm. The precipitate was then dissolved in ddH2O treated with diethylpyrocarbonate. RNA was treated with RNase-free DNase I in 40 mM Tris–HCl, pH 7.9, 10 mM NaCl, 6 mM MgCl2, and 1 mM CaCl2 for 30 min at 37°C. This was followed by a phenol/chloroform and chloroform extraction and a subsequent ethanolic precipitation. Reverse transcription was performed according to the TaKaRa RNA PCR Kit (AMV) Ver. 3.0, and the harvested complementary DNA (cDNA) was deposited at −20°C until use.
Cloning of the Middle Region of the MhMAPK
According to other plant MAPK sequences in NCBI, we analyzed their conservative regions by DNAman software, and then designed a pair of degenerate primers P1, GATCTBCAYCARATWATWCG and P2, GCYTCRTCAACTKTRATSCG (where R is A or G, Y is C or T, M is A or C, K is G or T, S is G or C, W is A or T, and B is G, T, or C). Then, the primers were used to polymerase chain reaction (PCR). The PCR reaction system was 25 μl, which contained 10× PCR buffer 2.5 μl, 25 mM MgCl2 2.5 μl, 10 mM deoxyribonucleotide triphosphates 1 μl, 10 μM P1 and P2 1 μl, respectively, cDNA 0.45 μl, ddH2O 16.4 μl, and 3 U Taq™ polymerase (TaKaRa). PCR reaction conditions were as follows: pre-denaturalization at 94°C for 5 min, denaturalization at 94°C for 45 s, annealing at 50°C for 45 s, extension at 72°C for 45 s, 35 cycles and at last extension at 72°C for 10 min. PCR product was purified by TaKaRa Agarose Gel DNA Purification Kit ver.2.0. The harvested fragment was cloned in PMD18-T vector (TaKaRa) and then sequenced (Invitrogen).
3′RACE of MhMAPK
The first-strand cDNA was synthesized according to the manufacture’s guidelines of TaKaRa RNA PCR Kit (AMV) Ver. 3.0 by using the oligo dT-adaptor primer provided in the kit. Then, according to the middle region and 5′end Oligo dT of cDNA, we designed P3, CCAAACAGGCGCATCACAG, as specific forward primer and B26, GACTCGAGTCGACATCGATTTTTTTTTTTTTTTTT as reverse primer. PCR was carried out by pre-denaturing the cDNA at 94°C for 5 min followed by 35 cycles of amplification (94°C for45 s, 55°C for 45 s, and 72°C for 45 s) and by a final extension at 72°C for 10 min. PCR product was purified by TaKaRa Agarose Gel DNA Purification Kit ver.2.0. The harvested fragment was cloned in PMD18-T vector (TaKaRa) and then sequenced (Invitrogen).
5′RACE and Full-Length cDNA Cloning of MhMAPK
Poly-C was added at the 3′end of cDNA with terminal deoxynucleotidyl transferase (TdT) and the reaction system was as follows: cDNA 5 μg, 5× TdT buffer 10 μl, 0.1% bovine serum albumin 5 μl, 10 mM 2′-deoxycytidine 5′-triphosphate 2.5 μl, TdT 15 U, and then reacted at 37°C for 30 min. Sample was extracted with the equal volume of chloroform/isoamyl alcohol (1:1, V/V) and then precipitated with double volumes of 100% ethanol at −20°C for 2 h and centrifuged at 4°C for 12,000 rpm. Precipitate was dissolved in ddH2O and deposited at −20°C until use. According to the 3′end poly-C of cDNA and middle fragment, we designed AAP, GGCCACGCGTCGACTA GTACGGGGGGGGGGGGGGGG as specific forward primer, and P10, GCTGTCAACGCTGGGGCTCG TGAG as reverse primer. PCR was performed as follows: pre-denaturalization at 94°C for 5 min followed by 35 cycles of amplification (94°C for 45 s, 59°C for 45 s, 72°C for 45 s) and final extension at 72°C for 10 min. PCR product was purified by TaKaRa Agarose Gel DNA Purification Kit ver.2.0. The harvested fragment was cloned in PMD18-T vector (TaKaRa) and then sequenced (Invitrogen).
By comparing and aligning the sequences of middle region, 3′RACE, and the 5′RACE products, the full-length cDNA sequence of MhMAPK was obtained, which then was amplified via PCR using a pair of primers P17 (CCATGGACTCGAGCTCTGCT) and P12 (CGAATGTGTGAAAGGAGTCAATAGC) and then sequenced. The full-length MhMAPK was subsequently analyzed for molecular characterization such as the conserved motifs, sequence homology, secondary structure, hydropathicity analysis, and subcellular localization. The primers’ positions labeled by the base-pair sequence are shown in Table 1.
The Preparation of GFP-MhMAPK Fusion and its Expression
To observe the subcellular localization of MhMAPK, we prepared MhMAPK-mGFP5 fusion constructs. The full-length cDNA of MhMAPK was cloned over again using the P17 as forward primer and GFP1, GCTGGATCCCTTGAAATGGAT, as the reverse primer, which was added to a linker (GGTTCC), which could be recognized by BamHI. Then, the full-length cDNA was cloned in PMD18-T vector. PMD18-T-MhMAPK and PBIN-mGFP5 were digested with BamHI and linked digested PBIN fragment and MhMAPK gene fragment with T4-ligase, and then, the fusion construct of MhMAPK-mGFP5 was obtained. MhMAPK-mGFP5 was transformed into agrobacterium EHA105 and then infected onion inner-epidermis, followed by observance under laser scanning confocal microscope.
Real-Time Fluorescent Quantitative PCR
The real-time fluorescent quantitative PCR was analyzed by Roch LightCycler1.5. The size of amplified fragments was 89 bp, and the annealing temperature of all primers was 60°C. Sequences of primers used were MhMAPK, forward, GGCGCATTACAGTTGATGAGG, reverse, GAAAGGCATTGGGCAGACA; 18S, forward, AAACGGCTACCACATCCA, reverse, CACCAGACTTGCCCTCCA (RaKaRa designed). The specificity of the primers to the genes for which they were designed was tested by using melting curve analysis of the PCR reaction, as well as sequence analysis of the PCR product amplified. PCR was carried out in quartzose capillaries as the guidelines of SYBR PrimeScript™ RT-PCR Kit (Perfect Real Time, TaKaRa), and the reaction system contained SYBR Premix Ex Taq (2×) 10 μl, PCR forward primer (10 μM) 0.4 μl, PCR reverse primer (10 μM) 0.4 μl, DNA templet 2.0 μl, and ddH2O 7.2 μl. The reaction protocol was stage 1, pre-denaturalization at 95°C for 10s, 20°C/s, 1 cycle; stage 2, PCR reaction, 95°C for 5 s, 20°C/s, 60°C for 20 s, 20°C/s, 40 cycles; stage 3, melting curve analysis, 95°C for 0 s, 20°C/s, 65°C for 15 s, 20°C/s, 95°C for 0 s, 0.1°C/s. After the process, the result was analyzed by relative quantitative analysis of Roch software.
Results
Cloning of the Full-Length cDNA of MhMAPK
According to the conservative region of cDNA sequences in other plants, primers P1 and P2 were designed for the amplification of the middle region of MAPK-like cDNA from PYTC. A fragment about 560 bp, showing high homology to the NtMAPK4, was obtained (Fig. 1a). Then, based on the middle region sequence, a forward specific primer P3 and B26 were designed for 3′RACE and a fragment about 685 bp was obtained, in which a 3′-untranslated region (UTR) of 492 bp was found downstream from the stop codon (Fig. 1b). A reverse specific primer P10 and AAP were designed for 5′RACE, and a fragment about 440 bp was obtained and a 5′-UTR of 248 bp was found upstream of the start codon (Fig. 1c). According to the middle region, 3′RACE and 5′RACE products, the full-length of the cDNA was amplified using a pair of primers P17 and P12 (Fig. 1d). The cloned full-length cDNA of MAPK gene from PYTC was 1,862 bp, which contained a 1,122-bp open reading frame encoding a protein of 373 amino acids with a molecular weight about 42.95 kDa and a pI of about 5.65. The protein exhibited a high homology to the NtMAPK4 of N. tabacum, and the Gen-Bank accession number is EF427897.
Sequence Analysis of MhMAPK
The MhMAPK contains all 11 conservative sub-domains of protein kinases with serine/threonine specificity. Between VII and VIII sub-domains, the TEY motif, which includes the threonine and tyrosine residues whose phosphorylation is necessary for MAP kinase activity, is a the feature of MAP kinase and is also conservative in the MhMAPK protein sequence (Fig. 2). A putative polyadenylation signal AATAAA is located at nucleotide position 1816–1821 (Fig. 2). An alignment of the predicted amino acid sequence of MhMAPK with the cloned MAPKs from other plants was performed by using DNAman software, and the sequence was homologous at the conservative region (Fig. 3).
Analysis of the Predicted Protein of MhMAPK
The secondary structure of the putative MhMAPK protein was analyzed by DNAman software, and the result showed that the putative MhMAPK peptide contained 39.5% alpha helix, 13% extended strand, and 47.5% random coil (Fig. 4a). The alpha helix and random coil constituted interlaced domination of the main part of the secondary structure.
The secondary structure (a), specifically functional sites (b), and hydropathy plot (c) of the predicted polypeptide of MhMAPK. a The helix, strands, and coil were indicated, respectively, with black, blue, and red lines. b a From 40 to 326 amino acids was protein kinase domain; b from 46 to 70 amino acids was protein kinases ATP-binding region signature; c from 75 to 178 amino acids was MAP kinase signature; d from 162-174aa was serine/threonine protein kinases active-site signature. c Amino acid residues were numbered from left to right on the horizontal axis, and the vertical axis indicates the average hydropathicity. The real line indicated that the amino acid had a transmembrane preference from inside to outside and the broken line indicated that the amino acid had a transmembrane preference from outside to inside. The black arrow showed the strong transmembrane helix. The hydrophobic and hydrophilic positions were plotted above and below the ordinate, respectively
The specifically functional sites of predicted protein were analyzed by PROSITE (http://au.expasy.org/), and the results showed that the region from 40 to 326 amino acids was protein kinase domain, where the domain from 46 to 70 amino acids was protein kinases adenosine triphosphate-binding region signature, the domain from 75 to 178 amino acid was MAP kinase signature, and the domain from 162 to 174 amino acid was serine/threonine protein kinases active-site signature (Fig. 4b).
Hydropathicity analysis by TMpred program showed that MhMAPK protein was a hydrophobic protein, and there was a strong transmembrane helix between 200 and 250 amino acids (Fig. 4c).
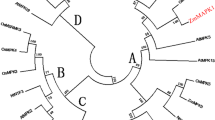
A phylogenetic tree based on the genetic distance of the protein sequences was constructed by the Clustal method using DANStar software. Based on the relationship tree of cloned plant MAPKs, MhMAPK can be grouped into subgroup C (Fig. 5). Comparison of the predicted protein sequences of the MhMAPK with MAP kinases of other plants in NCBI showed that MhMAPK was most homologous to the Malus micromalus MAPK (94%), Petroselinum crispum MAPK4 (88%), Medicago sativa MMK2 (85%), N. tabacum MAPK (84%), and A. thaliana MPK4(84%).
The phylogenetic relationship between the PYTC MhMAPK and other MAPKs from plant species. A phylogenetic tree based on the genetic distance of the protein sequences was constructed by the Clustal method using DNAStar software. The protein sequences of the MAPKs used for construction of the tree are listed in the GenBank database under the following accession numbers: NtMAPK4 (BAE46985), PcMAPK4 (AAN65180), MsMMK2 (CAA57719), MmMAPK (AF435805), ATMPK4 (NP_192046), BnMAPK4 (ABB69023), AtMPK11 (Q9LMM5), ATMPK12 (NP_182131), ZmosMAPK1 (ABD77415), StMPK3 (BAB93531), AtMPK5 (Q39025), NtNRK1 (BAB32406), MsMAPK (CAB37188), ATMPK13 (NP_001030990), CaMAPK1 (AAF81419), SpMAPK3 (ABW34945), NtWIPK (BAA09600), AtMPK3 (NP190150), CbMAPK3 (AAV68711), PsMAPK3 (AAF73236), AsMAPK (CAA56314), ZmMAPK4 (BAA74733), OsBIMK1 (AAK01710), CaMAPK2 (AAF81420), NtNtf4-1 (ABB16417), NtSIPK (AAB58396), EeMAPK (AAF65766), AtMPK6 (NP181907), ZmMAPK5 (BAA74734), and AtMPK10 (NP_191538)
Intracellular Localization of MhMAPK-GFP Fusion Protein
From the above sequence analyses, MhMAPK was found to have many common characteristics to MAPKs in other plants, and family C members tended to share highly similar sequences.
To determine the intracellular localization of the MhMAPK protein, a chimeric gene comprised of the coding regions of MhMAPK and mGFP-5 under control of a CaMV 35S promoter was made, and this construction was introduced into onion inner-epidermal cells by using agrobacterium. In cells where the empty GFP vector was introduced alone (Fig. 6a), fluorescence was visualized throughout the cell with no clear preference for localization. However, in cells expressing the MhMAPK-GFP construction, fluorescence was found to be clearly localized to the nucleus (Fig. 6b,c). The position of the nuclei was determined under laser scanning confocal microscope.
Subcellular localization of MhMAPK fused to GFP after transient transformation of onion epidermal cells and visualized using laser scanning confocal microscope. a Onion cell transformed with empty GFP vector alone; b fluorescence from onion cells transformed with MhMAPK-GFP fusion construct under 10 objective and c fluorescence from onion cells transformed with MhMAPK–GFP fusion construct under 20 objective
In addition, we also used ProtComp Version 6.1 (www.softberry.com) to analyze the subcellular localization, and the results showed that the integral prediction of protein localized in nucleus (Table 2).
Expression of MhMAPK Under Different Environmental Stress
In order to investigate MhMAPK expression pattern in different tissues of PYTC under stress, total RNA was isolated from leaves and roots, and subjected to real-time fluorescent quantitative PCR analysis.
As shown in Fig. 7, MhMAPK expressed after treatment with 200 mM NaCl for different time periods. Compared with control, the transcripts of MhMAPK in leaves changed little during 1 h and only went to a high level at 1.5 h after treatment, but in roots, there was a sharp increase of the relative level of MhMAPK mRNA at 40 min after treatment. The results showed that the induction of MhMAPK was earlier in roots than that in leaves.
Under the treatment of 20% PEG, the relative level of MhMAPK mRNA in leaves increased at 40 min and reached at the highest level at 1 h after treatment (Fig. 8). In roots, the highest relative level of MhMAPK mRNA was found at 1 h (Fig. 8), but comparing two levels of mRNA in roots and leaves, the relative level of MhMAPK mRNA was higher in roots than that in leaves.
Discussion
Though many MAPK genes in plants have been isolated so far, identified MAPKs in woody plants are still little. PYTC is one kind of special woody plant used as apple rootstock and one kind of apogamic plant whose gene can be transmitted stably through apomixes. The isolation and cloning of full-length cDNA of MhMAPK from PYTC would redound to the breeding of apple rootstock.
MAPKs are thought to play key roles in integrating multiple intracellular signals transmitted by various second messengers. In tobacco, salinity stress could activate a 42-kDa MAP kinase (Mikolajczyk et al. 2000), and a MAP kinase, AtMPK3, in A. thaliana also could be activated by the drought and high salinity (Liu et al. 1998). It has become clear that MAPK-signaling pathways are involved in plant resistance (Cheong et al. 2003; Zhang et al. 2006). In our results, MhMAPK mRNA could be induced by drought (PEG-6000) and high salt (NaCl) in leaves and roots (Figs. 7 and 8). These results suggested that MhMAPK also could be regulated at mRNA level not only at protein phosphorylation level by drought (PEG-6000) and high salt (NaCl) in woody plant. And MAPK might function in signal transduction pathways under dehydration and salt stress conditions wherever in roots and leaves of PYTC.
Besides, the analysis of predicted polypeptide showed that there was a transmembrane structure at the C-end of MhMAPK (Fig. 4c). This was analogous with the mitogen-activated protein kinase ERK2 in mammals. In mammals, the T-loop conformation of phosphorylated ERK2 changed, then the ERK2 combined with the transmembrane structure called L16 of the C-end of another ERK2 and the formed homologous dimer had an ability of going into nucleus (Dong et al. 2005). This implied that MhMAPK might localize in nucleus at last, and this deduction was accordant with our results of MhMAPK-GFP fusion localization (Fig. 6). MAPKs can regulate the expression of many genes through the phosphorylation of transcription factors. In A. thaliana, the first integral MAPK cascade AtMEKK1-AtMEK4/5-AtMPK3/6 could activate the transcription factor AtWRKY22/29 and start up the expression of some defensive genes (Asai et al. 2002); analogously in O. sativa, a MAP kinase OsBWMK1 could interact with a analogous transcription factor OsEREBP1 and regulate the expression of defensive genes and arouse responses to pathogeny (Cheong et al. 2003). As a result, MhMAPK might also play a role in nucleus via phosphorylation of the transcription factors and sequentially regulate some resistance genes expression.
In general, plants respond to the external stress by the receptor in the membrane, and the receptor can be a receptor-like protein kinase, which then transferred the external signals by interacting with other proteins. A receptor-like protein kinase, RPK1, identified in A. thaliana, could receive the signals about drought, high salinity, and low temperature (Hong et al. 1997) and could phosphorylate a MAPK cascade AtMEKK1-MEK1-AtMPK4, which also transmitted the signals of drought, high salinity, and low temperature (Mizoguchi et al. 1998). Then, the phosphorylated MAPK could interact with some transcription factors, such as WRKY, and phosphorylated WRKY could combine the W-box [(T) (T) TGAC (C/T)] of numerous defense genes and brought various physiological and biochemical responses. WRKY transcription factors phosphorylated by SIPK/WIPK and WRKY1 by SIPK (Kim and Zhang 2004; Menke et al. 2005), NtWIF transcription factor phosphorylated by WIPK in tobacco (Yap et al. 2005; Waller et al. 2006), and senescence-related WRKY53 transcription factor directly phosphorylated by Arabidopsis MEKK1 (Miao et al. 2007) could all result in regulating the expression of many defense genes.
Drought and high salinity could change the metabolism conditions of cells directly, but before these changes happened, drought and salinity signals must be recognized and transmitted by protein kinases in membrane or cytoplasm. Our results showed that the MhMAPK localized in nucleus (Fig. 6). This implied that, when the PYTC encountered the drought or high-salinity stress, these signals might be transmitted by phosphorylating MhMAPK in cytoplasm, and then the phosphorylatd MhMAPK goes to the nucleus to regulate some defensive genes expression by phosphorylating the transcript factors; on the other hand, drought or high salinity stress might activate the MhMAPK by some ways in nucleus directly and then cells could respond to them more quickly. However, it still needs further researches whether in cytoplasm or in nucleus.
Abbreviations
- MAPK:
-
mitogen-activated protein kinase
- PYTC:
-
Pingyi Tiancha (Chinese name of Malus hupehensis Rehd. var. pinyiensis Jiang)
- PCR:
-
polymerase chain reaction
- RACE:
-
rapid amplification of cDNA ends
References
Asai T, Tena G, Plotnikova J, Willmann MR, Chiu WL, Gomez Gomez L, et al. MAP kinase signalling cascade in Arabidopsis innate immunity. Nature 2002;415:977–83. doi:10.1038/415977a.
Bogre L, Ligterink W, Meskiene I, Barker PJ, Heberle-Bors E, Huskisson NS, et al. Wounding induces the rapid and transient activation of a specific MAP kinase pathways. Plant Cell 1997;9:75–83.
Cheng S, Puryear J, Cairney J. A simple and efficient method for isolating RNA from pine trees. Plant Mol Biol Rep 1993;11:113–6. doi:10.1007/BF02670468.
Cheong YH, Moon BC, Kim JK, Kim CY, Kim MC, Kim IH, et al. BWMK1, a rice mitogen-activated protein kinase, locates in the nucleus and mediates pathogenesis-related gene expression by activation of a transcription factor. Plant Physiol 2003;132:1961–72. doi:10.1104/pp.103.023176.
Dong CY, Liu TF, Qi JP. Expression of TGF-β1, ERK1, ERK2 and c-jun in human pancreatic cancer tissue and clinical significance. Chin J Pancreatol 2005;5:28–32.
Hanks SK, Quinn AM, Hunter T. The protein kinase family: conserved features and deduced phylogeny of the catalytic domains. Science 1988;241:42–52. doi:10.1126/science.3291115.
Hardie DG. Plant protein serine threonine kinases: classification and functions. Annu Rev Plant Physiol Plant Mol Biol 1999;50:97–131. doi:10.1146/annurev.arplant.50.1.97.
Hong SW, Jon JH, Kwak JM, Nam HG. Identification of a receptor-like protein kinase gene rapidly induced by abscisic acid, dehydration, high salt, and cold treatment in Arabidopsis thaliana. Plant Physiol 1997;113:1203–12. doi:10.1104/pp.113.4.1203.
Hunter T. Protein kinases and phosphatases: the ying and yang of protein phosphorylation and signalling. Cell 1995;80:225–36. doi:10.1016/0092-8674(95)90405-0.
Jeong M, Lee SK, Kim BG. A rice (Oryza sativa L.) MAP kinase gene, OsMAPK44, is involved in response to abiotic stresses. Plant Cell Tissue Organ Cult 2006;85:151–60. doi:10.1007/s11240-005-9064-0.
Jonak C, Kiegerl S, Ligterink W. Stress signaling in Plants: a mitogen-activated protein kinase pathway is activated by cold and drought. Proc Natl Acad Sci USA 1996;93:11274–9. doi:10.1073/pnas.93.20.11274.
Jonak C, Ökrész L, Böger L, Hirt H. Complexity, cross talk and integration of plant MAP kinase signaling. Curr Opin Plant Biol 2002;5:415–24. doi:10.1016/S1369-5266(02)00285-6.
Kim CY, Zhang S. Activation of a mitogen-activated protein kinase cascade induces WRKY family of transcription factors and defense genes in tobacco. Plant J 2004;38(1):142–51. doi:10.1111/j.1365-313X.2004.02033.x.
Knetsch MLW, Wang M, Snaar Jagalaka BE. Abscisic acid induces mitogen-actevated protein kinase activation in Barley aleurone protoplasts. Plant Cell 1996;8:1061–7.
Li YN. Study for the germplasm resources of Malus species. Beijing: China Agricultural Press; 2001. p. 121.
Ligterink W, Kroj T, Nieden UZ. Receptor mediated activation of a MAP kinase in pathogen defense of plants. Science 1997;276:2054–7. doi:10.1126/science.276.5321.2054.
Liu Q, Kasuga M, Sakuma Y. Two transcription factors, DREB1 and DREB2, with an EREBP/ AP2 DNA binding domain separate two cellular signal transcription pathways in drought and low temperature responsive gene expression, respectively, in A rabidopsis. Plant Cell 1998;10:1391–406.
Liu Q, Zhang GY, Shinozaki K. The Plant Mitogen-activated Protein (MAP) Kinase. Acta Bot Sin 2000;42:661–7.
Machida Y, Nishihama R, Kitakura S. Progress in studies of plant homologs of mitogen-activated protein (MAP) kinase and potential upstream components in kinase cascades. Crit Rev Plant Sci 1997;16:481–96. doi:10.1080/713608155.
Menke FL, Kang HG, Chen Z, Park JM, Kumar D, Klessig DF. Tobacco transcription factor WRKY1 is phosphorylated by the MAP kinase SIPK and mediates HR-like cell death in tobacco. Mol Plant Microbe Interact 2005;18(10):1027–1034.
Miao Y, Thomas ML, Anja S, Ulrike Z. Arabidopsis MEKK1 can take a short cut: it can directly interact with senescence-related WRKY53 transcription factor on the protein level and can bind to its promoter. Plant Mol Biol 2007;65(1–2):63–76. doi:10.1007/s11103-007-9198-z.
Mikolajczyk M, Awotunde OS, Muszynska G, Klessig DF, Dobrowolska G. Osmotic stress induces rapid activation of a salicylic acid-induced protein kinase and a homolog of protein kinase ASK1 in tobacco cells. Plant Cell 2000;12:165–78.
Mizoguchi T, Gotoh Y, Nishida E. Characterization of two cDNAs that encode MAP kinase homologues in Arabidopsis thaliana and analysis of the possible role of auxtin in activating such kinase activities in cultured cells. Plant J 1994;5:111–22. doi:10.1046/j.1365-313X.1994.5010111.x.
Mizoguchi T, Irie K, Hirayama T, Hayashida N, Yamaguchi-Shinozaki K, Matsumoto K, et al. A gene encoding a mitogen-activated protein kinase kinase kinase is induced simultaneously with genes for a mitogen-activated protein kinase and an S6 ribosomal protein kinase by touch, cold, and water stress in Arabidopsis thaliana. Proc Natl Acad Sci USA 1996;93:765–9. doi:10.1073/pnas.93.2.765.
Mizoguchi T, Ichimura K, Irie K, Morris P, Giraudat J, Matsumoto K, et al. Identification of a possible MAP kinase cascade in Arabidopsis thaliana based on pairwise yeast two- hybrid analysis and functional complementation tests of yeast mutants. FEBS Lett 1998;437:56–60. doi:10.1016/S0014-5793(98)01197-1.
Pagnussat GC, Lanteri ML, Lombardo MC, Lamattina L. Nitric oxide mediates the indole acetic acid induction activation of a MAPK cascade involved in adventitious root development. Plant Physiol 2004;135:279–86. doi:10.1104/pp.103.038554.
Petersen M, Brodersen P, Naested H, Andreasson E, Lindhart U, Johansen B, et al. Arabidopsis MAP kinase 4 negatively regulates systemic acquired resistance. Cell 2000;103:1111–20. doi:10.1016/S0092-8674(00)00213-0.
Seo S, Okamoto M, Seto H. Tobacco MAP kinase: a possible mediator in wound signal transduction pathways. Science 1995;270:1988–92. doi:10.1126/science.270.5244.1988.
Shoresh M, Gal-On A, Leibman D, Chet I. Characterization of a mitogen- activated protein kinase gene from cucumber required for trichoderma-conferred plant resistance. Plant Physiol 2006;142:1169–79. doi:10.1104/pp.106.082107.
Waller F, Muller A, Chung KM, Yap YK, Nakamura K, Weiler E, et al. Expression of a WIPK-activated transcription factor results increase of endogenous salicylic acid and pathogen resistance in tobacco plants. Plant Cell Physiol 2006;47(8):1169–74. doi:10.1093/pcp/pcj079.
Yang HQ, Jie YL. Studies of individual difference in seedlings of apple rootstock. J Shandong Agric Univ 1997;28:487–91.
Yap YK, Kodama Y, Waller F, Chung KM, Ueda H, Nakamura K, et al. Activation of a novel transcription factor through phosphorylation by WIPK, a wound-induced mitogen-activated protein kinase in tobacco plants. Plant Physiol 2005;139:127–37. doi:10.1104/pp.105.065656.
Zhang S, Klessig DF. Salicylic acid activates a 48-KD MAP kinase in Tobacco. Plant Cell 1997;9:809–24.
Zhang TG, Liu YB, Xue LG. Molecular cloning and characterization of a novel MAP kinase gene in Chorispora bungeana. Plant Physiol Biochem 2006;44:78–84. doi:10.1016/j.plaphy.2006.01.001.
Acknowledgments
This study was supported by projects 30571285 and 30671452 of the National Natural Science Foundation of China.
Author information
Authors and Affiliations
Corresponding author
Additional information
MhMAPK Gen-Bank accession number is EF427897.
Rights and permissions
About this article
Cite this article
Duan, K., Yang, H., Ran, K. et al. Characterization of a Novel Stress-Response Member of the MAPK Family in Malus hupehensis Rehd . Plant Mol Biol Rep 27, 69–78 (2009). https://doi.org/10.1007/s11105-008-0057-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11105-008-0057-0