Abstract
Mitogen-activated protein kinase (MAPK) signal transduction cascades play a crucial role in the response to extracellular stimuli in eukaryotes. A number of MAPK family genes have been isolated in plants, but the maize MAPK genes have been little studied. Here, we studied the role of maize MAP kinase 1 (ZmMAPK1) using gene expression, protein subcellular localization, transformation in Arabidopsis, expression patterns of the stress-responsive genes and physiological parameter analysis. Our physiological parameter analysis suggested that over-expression ZmMAPK1 can increase proline content and decrease malondialdehyde content under drought, and prevent chlorophyll loss and the production of scavenger reactive oxygen species under heat stress. The resistance characteristics of the over-expression of ZmMAPK1 were associated with a significant increase in survival rate. These results suggest that ZmMAPK1 plays a positive role in response to drought and heat stress in Arabidopsis, and provide new insights into the mechanisms of action of MAPK in response to abiotic stress in plants.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Maize (Zea mays L.) is an economically important grain crop, whose growth and yield are severely inhibited under various stress conditions (Zhang et al. 2014). One of the universal signal pathways involved in the response to external stimuli is the mitogen-activated protein kinase (MAPK) cascade (MAPK Group 2002). MAPK signaling networks are found in all eukaryotic organisms and regulate fundamental aspects of biology, including not only the initiation of developmental pathways, but also responses to abiotic and biotic stresses, such as drought, heat stress, reactive oxygen species (ROS), ultraviolet (UV) light, hormones, and pathogenic attack (Thomas 1992; Lampard et al. 2009; Pitzschke and Hirt 2009).
MAPKs are activated by a highly conserved mechanism of sequential phosphorylation events in which a MAPK kinase kinase (MAPKKK) activates a MAPK kinase (MAPKK) that then activates a MAPK by phosphorylation of tyrosine and threonine residues in the TxY (TEY or TDY) motif (Herskowitz 1995; Fu et al. 2002; Lei et al. 2014). The MAPKs can be divided into four groups (A–D): the TEY subtype can be classified into three groups A, B and C, whereas the TDY subtype forms a more distant group D (MAPK Group 2002). Activated MAPK can phosphorylate a variety of substrates including transcription factors, cytoskeleton-associated proteins and other protein kinases (Nakagami et al. 2005; Pitzschke and Hirt 2006; Zhang et al. 2010). Since the first plant MAPK gene, MsERK1, was discovered in alfalfa (Duerr et al. 1993), further MAPK genes have been identified and explored using both genetic and biochemical approaches. Recently, several putative MAPKs genes have been isolated from different plant species, such as Arabidopsis, tobacco, rice and maize (Kosetsu et al. 2010; Pan et al. 2012; Liu et al. 2013b).
At present, 19 MAPKs have been identified in maize, but only a few MAPKs genes have been characterized (Wei et al. 2014). ZmMPK3 accumulated markedly and rapidly when maize seedlings were subjected to cold, drought, UV light, salinity, heavy metal or mechanical wounding (Wang et al. 2010). Overexpression of ZmMPK4 in transgenic tobacco resulted in increased tolerance to low temperature stress (Zhou et al. 2012b). ZmMPK5 is required for NADPH oxidase-mediated self-propagation of apoplastic hydrogen peroxide (H2O2) in brassinosteroid-induced anti-oxidant defense systems in leaves of maize plants, and is also activated by abscisic acid (ABA) and cold (Berberich et al. 1999; Lin et al. 2009; Zhang et al. 2010). ZmMPK6 is able to interact with 14-3-3 proteins (Lalle et al. 2005). ZmMPK7 is responsible for the removal of ROS under ABA and H2O2 stress (Zong et al. 2009). ZmMAPK17 is transcriptionally regulated by multiple stresses, and its overexpression in tobacco produced enhanced resistance to low temperatures and viral infection (Pan et al. 2012).
In the present study, we isolated and characterized a maize MAPK gene, designated ZmMAPK1. Overexpression of ZmMAPK1 in Arabidopsis thaliana increased resistance to drought and heat. Physiological parameter analysis indicated that transgenic plants overexpression ZmMAPK1 showed a significant change compared with control lines.
Materials and methods
Plant materials and growth conditions
Seedlings of maize (Zea mays L.) inbred line B73, from Henan Agricultural University, China, were grown in Hoagland’s solution (pH = 6.0) in a greenhouse at 22/28 °C (night/day), with photosynthetically active radiation (PAR) of 200 µmol m−2 s−1, and a photoperiod of 14/10 h (day/night). Leaves of seedlings at the V4 development stage (when the fourth leaf has a visible “collar” at the base of the leaf) were collected and used for investigations. High-temperature and drought-stress treatment seedlings were grown in nutrient soil in the same greenhouse.
Arabidopsis thaliana (ecotype Columbia, Col-0) was used for experiments involving transformation and various abiotic stress conditions (Luo et al. 2013). Arabidopsis seedlings from surface sterilized seed, were grown in a chamber at 22/20 °C (night/day), with PAR of 100 µmol m−2 s−1, 60 % relative humidity, and a photoperiod of 16/8 h (day/night) in half Murashige and Skoog (MS) medium with salt and vitamins containing 3 % sucrose and 0.8 % (w/v) agar for 10 d, then transplanted into nutrient soil. The seeds used in the experiment were harvested at the same stage, and stored in the same way, to ensure that they all had the same vitality.
Isolation of total RNA and DNA
Total RNA was isolated from untreated maize leaves at the V4 development stage by using an RNeasy Plant mini kit (Qiagen, Valencia, CA, USA) according to the instructions supplied by the manufacturer. Approximately 2 µg of total RNA was used as a template for first-strand cDNA synthesize using the SuperScript III First-Strand cDNA Synthesis Kit (Invitrogen, Carlsbad, CA, USA). Genomic DNA was isolated by thecetyl-trimethyl ammonium bromide method.
Isolation of the maize MAPK1 gene
A blast search with rice MAPK1 sequences as a query revealed good homology with the products of the predicted maize gene. To isolate the full-length cDNA of this maize gene, RT-PCR was performed using a cDNA amplification kit (Takara, Tokyo, Japan) based on in silico predictions. The specific primers of this cDNA (ZmMAPK1F and ZmMAPK1R) were designed according to the relevant sequences and were shown in supporting information Table S1. PCR product was purified and subcloned into the pMD19-T vector (Takara, Tokyo, Japan) for sequencing (Wu et al. 2011a).
Phylogenetic analysis
The amino acid sequences of the plant MAPK proteins were retrieved from GenBank. A phylogenetic tree was constructed based on the sequence alignment. Protein sequences were obtained from maize, rice, Nicotiana tabacum and Arabidopsis. These proteins were used to construct a phylogenetic tree using the Neighbor-Joining method and a bootstrap test based on MEGA 5.2 software.
Quantitative real-time PCR analyses
To analyze levels of ZmMAPK1 gene expression in maize leaves, quantitative real-time PCR (qRT-PCR) was performed with RNA samples harvested at the indicated times after exposure to various stresses. The transcript levels of stress-related genes in T3 A. thaliana (transgenic lines and wild type) before and after stress treatment were also analyzed (Wang et al. 2014), such as the AB1, ABA2, ABA3, ABI1, ABI2, NCED3, ACS5 and ICS1. Each PCR reaction contained 7.5 µL SYBR Premix Ex Taq (TaKaRa), 0.5 µL of each primer (10 µM), 5.6 µL water, 0.4 µL of passive reference Dye III and 0.5 µL of each reverse-transcribed cDNA product. PCR was performed in 96-well optical reaction plates using iQ5 (Bio-Rad, Richmond, CA, USA) after pre-incubation for 3 min at 95 °C, followed by 40 cycles of denaturation at 95 °C for 20 s, annealing at 56 °C for 15 s, and extension at 72 °C for 30 s, following the manufacturer’s instructions. The maize 18S ribosome RNA (rRNA) and Arabidopsis ACTIN2 genes were used as internal controls to normalize the relative gene expression levels in maize and Arabidopsis, respectively. Technical triplicates were performed for each biological replicate, and the average values were used for quantification (the PCR primers are listed in supporting information Table S2). RT-PCR was performed using SYBR and run on an Applied Biosystems 7900HT Real-Time PCR System (Applied Biosystems, Foster City, CA, USA).
Subcellular localization of the ZmMAPK1 protein
To investigate the subcellular localization of ZmMAPK1, the entire coding sequence without the stop codon was cloned into the NcoI and SpeI sites of the pCAMBIA1302 vector to yield the final plasmid 35S:ZmMAPK1:GFP. The open reading frame (ORF) of ZmMAPK1 was amplified by PCR with NcoI and SpeI linker primers (ZmMAPK1F + NcoI and ZmMAPK1R + SpeI, the underlined nucleotide sequence indicates the NcoI and SpeI restriction enzyme cutting site in supporting information Table S1). The recombinant fusion ZmMAPK1-green fluorescent protein (GFP) fused plasmid was introduced into onion epidermal cells by particle bombardment. After transformation, tissues were incubated on MS agar medium in the dark at 23 °C for 16 h. Transformed onion cells were observed by confocal microscopy (Olympus, Tokyo, Japan). This experiment was performed in triplicate with identical results.
Vector construction and Arabidopsis transformation
The coding sequence of the ZmMAPK1 gene was amplified using primers (ZmMAPK1F’ + NcoI and ZmMAPK1R’ + SpeI), and cloned into a binary vector pCAMBIA1304 under control of the cauliflower mosaic virus (CaMV) 35S promoter. The resulting vector was mobilized into Agrobacterium tumefaciens strain GV3101 Transformation of Arabidopsis plants was carried out by the floral dip method (Bechtold and Pelletier 1998). Transgenic plants were first screened on half MURASHIGE and SKOOG medium supplemented with 50 mg/L hygromycin. Seeds from each T1 plant were individually collected. Selected T2 plants were propagated, and homozygous overexpression lines were confirmed by RT-PCR analysis. T3 progeny homozygotes were obtained for further analysis.
Plant abiotic stress treatments
To prepare the V4 development stage maize seedlings for qRT-PCR to analyze ZmMAPK1 gene expression levels under different abiotic stresses, they were incubated with Hoagland’s solution containing 350 mM NaCl, 10 mM H2O2, 20 % PEG6000 (w/v). A 37 °C high temperature and drought stress treatment was applied to the soil. Each experiment was performed three times with each replicate containing 30 seedlings.
Arabidopsis thaliana homozygous T3 seedlings of the transgenic overexpression lines and WT seedlings were used for stress treatments. For the water-deficit treatment, watering was withheld from three-week-old seedlings for 14 d and then the plants were re-watered. For the salt-tolerance assay, the three-week-old seedlings were treated with 250 mM NaCl for 7 d. For the heat-stress treatment assay, the three-week-old seedlings were exposed to 37 °C for 3 d, and then returned to the normal growth environment (Liu et al. 2013a; Lv et al. 2011). All the treatments were performed in triplicate. The phenotypic and statistical aspects of the survival rates of the plants were observed one week after each treatment.
Physiological characterization of T3 ZmMAPK1 transgenic plants
Quantification of proline content
Three-week-old Arabidopsis WT and transgenic lines under drought, heat-stress or salt-stress conditions were used for measurement of the different physiological indices under different treatments. All experiments were performed in triplicate.
Arabidopsis leaf samples (0.1 g) for the measurement of proline were treated with 3 % (w/v) sulphosalicylic acid followed by boiling for 1 h. The amounts of proline were measured with ninhydrin, which was detected at 520 nm, and the standard liquid proline was used as a reference (Bates et al. 1973). Approximately 0.3 g of plant material was homogenized in 6 mL of 3 % aqueous sulfosalicylic acid and the homogenate filtered through. A total of 2 mL of filtrate was reacted with 2 mL of ninhydrin and 2 mL of glacial acetic acid in a test tube for 1 h at 100 °C, and the reaction terminated in an ice bath. The reaction mixture was extracted with 4 ml toluene, mixed vigorously with a test tube stirrer for 15–20 s. The chromophore containing toluene was aspirated from the aqueous phase, warmed to room temperature and the absorbance read at 520 nm using toluene for a blank. The proline concentration was determined from a standard curve and calculated on a fresh weight basis as follows: [(μg Pro ml−1 × ml toluene)/115.5 μg μmol−1]/[(g sample)/5] = μmol Pro g−1 fresh weight material.
Measurement of malondialdehyde content
Malondialdehyde (MDA) content was assayed according to Lv (2007). About 0.1 g of maize leaf was used and absorbance values at 450, 532, and 600 nm were determined with a spectrometer (Perkin-Elmer Lambda 25, Boston, MA, USA). The concentration of MDA was calculated using the following formula: C (μmol/L) = 6.45 (OD532 − OD600) − 0.56 OD450.
Determination of hydrogen peroxide
The Arabidopsis seedlings were grown at 37 °C for 3 d. Then, after recovering under normal conditions for 1 d, their phenotype was observed and leaf samples were harvested for determination. The hydrogen peroxide (H2O2) content after heat stress was measured as described by Zhou et al. (2012a).
Water-loss measurements
For the rate of water loss assay, detached leaves (from three-week-old plants) of transgenic Arabidopsis lines and WT plants were placed in a growth chamber. Their fresh weight (FW) was recorded immediately and then every 30 min for 8 h until it failed to decrease. The proportion of water lost was calculated as (initial fresh weight − final fresh weight)/initial fresh weight × 100 % (Yoo et al. 2010).
Chlorophyll measurement
Total chlorophyll content was measured with a chlorophyll meter (SPAD 502 Plus; Konica Minolta Sensing) as described previously (Abe et al. 2012; Tian et al. 2013). Measurements of chlorophyll content under stress conditions were performed after applying a treatment of 37 °C for 3 days. Transgenic Arabidopsis and WT type leaves had to be fully expanded at the time of measurement. Thirty plants of each line were used for the chlorophyll content assays.
Statistical analyses
To determine the statistical significance of stress treatments in various genotypes, we performed one-way ANOVAs using Dunnett’s t test with SPSS 17.0 statistics software (SPSS Corp., Chicago, IL, USA). The difference was considered significant if P < 0.05.
Results
Cloning and sequence analysis of ZmMAPK1
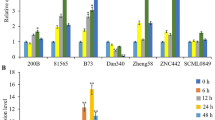
A 1871 bp full-length cDNA sequence was obtained by in silico cloning and sequencing in the Z. mays B73 inbred line and has been submitted to GenBank (accession No. KM386659). The CDS contains an 1125 bp open reading frame (ORF), which encodes a protein of 375 amino acids with a predicted molecular weight of about 42.44 kDa and a PI of 6.51. The amino acid sequence of ZmMAPK1 exhibits a molecular structure typical of plant MAPKs. The ZmMAPK1 protein contains 11 subdomains of protein kinases with serine/threonine specificity that is conserved in eukaryotic organisms (Figure S1). The TEY motif is also conserved in the ZmMAPK1 protein sequence, including the threonine and tyrosine residues whose phosphorylation is necessary for MAPK activation. To elucidate the phylogenetic relationships among MAPK proteins in various species, phylogenetic analysis was performed using MEGA 5.2 (Fig. 1). ZmMAPK1 is homologous to Oryza sativa OsMPK6 and A.thaliana AtMPK4, both of which are classified as group A MAPKs.
Phylogenetic analysis of ZmMAPK1with other MAPKS from Arabidopsis, rice, maize, and Nicotiana tabacum using the amino-acid sequence alignment function in the MEGA 5.2 program. Bootstrap analysis was performed using 1000 replicates to evaluate the reliability of the various phylogenetic groups A, B, C and D. GenBank Accession numbers: ZmMAPK1 KM386659, ZmMPK2 NM_001111373.1, ZmMPK3 EU130900.1, ZmMPK4 AB016801.1, ZmMPK5 AB016802.1, ZmMAPK17 NM_001154688.1, AtMPK1 NP_172492.1, AtMPK2 NP_564746.1, AtMPK4 NP_192046.1, AtMPK5 NP_567378.4, AtMPK6 NP_181907.1, AtMPK7 NP_179409.1, AtMPK9 NP_974331.1, AtMPK10 NP_191538.1, AtMPK11 NP_001117210.1, AtMPK12 NP_182131.2, AtMPK13 NP_001030990.1, AtMPK14 NP_195363.1, AtMPK19 NP_188090.2, OsMPK2 AAS79349.1, OsMPK3 AAG40581.1, OsMPK4 CAB61889.1, OSMPK5 AF479883, OsMPK6 EF174189.1, OsMPK18 AAT39148,OsBWMK1 AAD52659.1, OsBWMK2 AF177392.1, OsBIMK2 NP_001045815.1, OsMSRMK3 CAD54741.1, NtNTF3 CAA49592.1, NtWIPK BAA09600.1
Subcellular localization of ZmMAPK1
Previous studies have shown that different MAPKs have different subcellular localizations, including the plasma membrane, nucleus, cytoplasm, or protoplasts (Zong et al. 2009; Kosetsu et al. 2010; Shen et al. 2010; Zeng et al. 2011; Pan et al. 2012). In silico analysis, we used the softberry database (http://linux1.softberry.com/berry.phtml) to predict that ZmMAPK1 was localized in the nucleus (Figure S2). To confirm the subcellular localization, the ORF of the ZmMAPK1 was fused in frame to the GFP marker gene under the control of the CaMV 35S promoter. The 35S: GFP control exhibited GFP signals evenly in the plasma membrane, cytoplasm and nucleus, whereas the 35S: ZmMAPK1: GFP fusion protein emitted green fluorescence specifically localized in the nucleus (Fig. 2). This result was consistent with the prediction.
Expression patterns of ZmMAPK1 in maize leaves exposed to abiotic stresses and signaling molecules
To investigate the expression profile of ZmMAPK1, we performed quantitative real time PCR (qRT-PCR) to analyze changes in maize leaves at the V4 development stage under various stresses. Exogenous application of 10 mM H2O2 significantly increased the transcription of ZmMAPK1 in the following 24 h, and reached a peak at 24 h (Fig. 3a). After treatment with 250 mM NaCl, the transcript level of ZmMAPK1 was slightly reduced at 3 h, but then increased to a maximum at 24 h; there was about a two-fold change in expression level (Fig. 3b). This pattern was similar to that of ZmMAPK5 in maize under salt stress (Zhang et al. 2014). Treatment with 20 % PEG6000 increased ZmMAPK1 expression in maize seedling leaves, and high temperature (37 °C) led to an increase in expression after 12 h of treatment (Fig. 3c, d). There was no significant change in ZmMAPK1 transcript levels with water treatment (CK) during the 24 h cycle (Fig. 3e). Drought stress, applied by withholding watering of the soil for 5 d until the maize seedling leaves had significant ‘rolling’, increased ZmMAPK1 transcription in the following 5 d (Fig. 3f). Both the 20 % PEG6000 and drought stress results indicate ZmMAPK1 may play a positive role in the plant’s response to drought stress.
Expression patterns of ZmMAPK1 in maize seedling leaves under different abiotic stresses were detected with qRT-PCR. V4 stage maize seedlings were exposed to different treatments: a 10 mM H2O2, b 350 mM NaCl, c 37 °C high temperature, d 20 % PEG6000 (w/v), e control (no treatments), f drought stress in soil
Identification of transgenic Arabidopsis
To investigate the effect of ZmMAPK1 in response to multiple stresses in Arabidopsis, transgenic plants containing ZmMAPK1 under the control of the CaMV35S promoter in the pCAMBIA1304 vector were generated, and the pCAMBIA1304 vector was also used for transformation in Arabidopsis as a control. Independent transgenic lines were obtained by homomycin-resistance selection and confirmed by semi-quantitative RT-PCR. The RT-PCR analysis revealed that the ZmMAPK1 gene was expressed normally in the transgenic lines, but no expression was detected in the pCAMBIA1304 vector control lines and WT plants (Fig. 4). The homozygous T3 generation of three independent overexpression lines, namely OE-1, OE-3, OE-5, and the control lines CL-1, CL-2, CL-3 were used for further analysis.
RT-PCR analysis of ZmMAPK1 overexpression in Arabidopsis. The transcript levels of ZmMAPK1 in different overexpression lines and pCAMBIA1304 vector control lines analyzed by RT-PCR. Total RNA extracted from leaf samples collected from T2 Arabidopsis plants grown under normal conditions. The Arabidopsis ACTIN2 gene was selected as an internal control. 1 overexpression line 1; 2 overexpression line 2; 3 overexpression line 3; 4 overexpression line 4; 5 overexpression line 5; 6 pCAMBIA1304 vector control line 1; 7 pCAMBIA1304 vector control line 2; 8 pCAMBIA1304 vector control line 3; 9 wild type
Overexpression of ZmMAPK1 improves tolerance to drought and heat stress in Arabidopsis
Since there is much evidence for cross-talk between signaling pathways regulating the response to abiotic stress (Jin et al. 2011; Frey et al. 2012; Wang et al. 2013), transgenic Arabidopsis plants overexpression the ZmMAPK1 gene were generated. Three-week-old A. thaliana homozygous T3 seedlings of the transgenic lines and WT plants were used for drought, heat and salt stress experiments. After 14 d drought stress, the transgenic ZmMAPK1 lines had a higher drought tolerance than the pCAMBIA1304 vector control lines and WT plants (Fig. 5a, b), as shown in Fig. 5b, the pCAMBIA1304 vector control lines and WT plants became severely wilted and impaired after dehydration stress. However, the transgenic ZmMAPK1 lines showed more open, green leaves (Fig. 5c). Following the dehydration treatment, the plants were re-watered for a week to determine their survival rate. The ZmMAPK1 transgenic lines had a significantly higher survival rate than the control lines and WT plants after the water deficit (Fig. 5d). The data indicate that overexpression of ZmMAPK1 enhances Arabidopsis drought stress tolerance. The drought-tolerance phenotype of the overexpression ZmMAPK1 plants was further evaluated by measuring water loss from detached leaves. We found that the detached leaves of the ZmMAPK1 plants lost water more slowly than those of the control lines (Fig. 6).
Phenotypes of ZmMPAK1 transgenic plants under drought stress. Drought tolerance assay of the ZmMAPK1 overexpression (OE) lines, OE-1, OE-3, OE-5 plants; pCAMBIA1304 vector control lines (CL), CL-1, CL-2, CL-3; and wild type (WT) plants. a The three-week-old plants were grown in nutrient soil before drought, deprived of water for 2 weeks (b), and then recovered with water (c). Similar results were observed in three independent experiments. d Survival rate of the plants in (c) under drought stress. The data presented are the mean ± SD (n = 81); Asterisks indicate significant differences compared with control line values (*P < 0.05; **P < 0.01). Similar results were observed in three independent experiments
Rate of water loss from detached leaves of transgenic and control plants. a Rate of water loss from detached leaves of the OE-1, CL-1 and WT plants. b Rate of water loss from detached leaves of the OE-3, CL-2 and WT plants. c Rate of water loss from detached leaves of the OE-5, CL-3 and WT plants. The data presented are the mean ± SD of three replicates (n = 27); Asterisks indicate significant differences compared with control line values (*P < 0.05; **P < 0.01)
For the heat stress treatment assay, we observed that the phenotype of the overexpression lines showed a notable tolerance after treatment with 37 °C for 3 d. All the leaves of the control line were rolled while those of the transgenic lines were not (Fig. 7a, b). The survival rate of the ZmMAPK1 lines was significantly higher than that of the control lines after they were transferred to normal growth conditions for one week (Fig. 7c). These results suggest that overexpression of ZmMAPK1 also has a positive effect on heat stress tolerance.
Phenotypes of ZmMPAK1 transgenic plants under heat stress. Heat tolerance assay of the ZmMAPK1 overexpression (OE) lines, OE-1, OE-3, and OE-5; pCAMBIA1304 vector control lines (CL), CL-1, CL-2, CL-3; and wild type (WT) plants. a The three-week-old transgenic and control plants were grown in nutrient soil before heat stress was applied. b The seedlings were kept at 37 °C for 3 d, and then transferred to normal growth conditions for one week. Similar results were observed in three independent experiments. c Survival rate of the plants in (b) after heat stress. The data presented are the mean ± SD (n = 27); Asterisks indicate significant differences compared with control line values (*P < 0.05; **P < 0.01). Similar results were observed in three independent experiments
As for the salt-tolerance assay, after the transgenic and WT Arabidopsis seedlings were treated with 250 mM NaCl for 7 d, almost all of the transgenic and WT Arabidopsis seedlings had a similar appearance (data not shown), and the survival rates were not significantly different.
Overexpression ZmMAPK1 altered expression patterns of stress responsive genes
The induction of numerous stress-inducible marker genes is a hallmark of stress adaptation in plants (Zhu et al. 1997; Bao et al. 2014). To indentify the possible genes involved in the ZmMAPK1-mediated drought stress responsive pathway, we performed qRT-PCR to examine the expression profiles of the ABA biosynthesis or ABA signaling regulators responsive genes under drought stress treatment, such as ABA1, ABA3, ABI1 and ABI2. Sample were analyzed following the 0, 1, 3, 6 d and recovered (RE) with water for one week. The qRT-PCR analyses demonstrated that the ABA1, ABA3, ABI1 were significantly induced by drought stress treatment, these results imply that they were acting as the positive factor in regulating the expression of these stress-responsive genes (Fig. 8a, b, c), while the ABI2 gene was slightly changed compare with the control lines (Fig. 8d).
Expression levels of stress-responsive genes by qRT-PCR in overexpression line (OE), control line (CL) and wild type (WT). a, b, c and d are under the drought stress treatment; e, f, g and h are under heat stress treatment. Gene-specific primers were used for detection of relative transcript levels of stress-responsive genes, the raw date were normalized using ACTIN2 as an internal reference. Each value is the mean ± SE (n = 27); Asterisks indicate significant differences compared with control line values (*P < 0.05; **P < 0.01). Similar results were observed in three independent experiments
To elucidate the mechanisms underlying heat stress treatment, we also characterized the expression of some genes related with heat stress. It has been reported that some plant hormones, such as ABA, SA and the ethylene biosynthetic gene, is involved in heat response signaling (Larkindale et al. 2005; Lv et al. 2011). The genes known to be involved in plant heat response, such as 9-cis-epoxycarotenoid dioxygenase 3 (NCED3), ABA2, 1-aminocyclopropane-1-carboxylate synthase 5 (ACS5) and isochorismate synthase 1 (ICS1) were chosen. The expression level of the genes were measured at five stages, namely 0, 1, 2, 3 d and recover to normal growth conditions for one week (RE). As shown in Fig. 8e, f, the transcript levels of NCED3 and ACS5 were significantly increased after heat stress for 3 days. That’s because the AtNCED3 is an important gene in ABA biosynthesis, and the AtACS5 is a member of ACS family, which has been implicated in the plant response to high temperature (Wang et al. 2005). The expression of the SA biosynthetic gene ICS1 and ABA biosynthesis gene ABA2 were suppressed during the processes of the heat stress treatment, but the control line was more suppressed in heat stress stage (Fig. 8g, h). These results indicated that the overexpression of ZmMAPK1 in Arabidopsis resulted in an alteration in the expression of stress-responsive genes.
Determination of MDA, proline, and chlorophyll content, and reactive oxygen species accumulation
The different stress tolerances of overexpression transgenic plants have been correlated with changes in proline and MDA contents (Sharma et al. 2011; Cheng et al. 2013). Previous studies showed increases the MDA content in plants subjected to drought stress (Lim and Lee 2014). To investigate the effect of drought and heat stress on lipid peroxidation, we measured the MDA content in transgenic and control plants. The MDA level in overexpression lines 1, 3 and 5 was lower than those in control lines and WT plants after drought and heat stress (Fig. 9a, b). To determine whether ZmMAPK1 overexpression affected proline accumulation, the proline content in overexpression and control lines was measured. Under drought stress, proline activity was higher in ZmMAPK1 overexpression transgenic plants, but activity in the control line and WT line was similar. As shown in Fig. 9d, under heat stress, the proline content in the ZmMAPK1 overexpression transgenic plants were also higher than that in the control lines.
Determination of the MDA, free proline, H2O2 and chlorophyll content in plants of the T3 ZmMAPK1 overexpression lines, OE-1, OE-3, and OE-5; pCAMBIA1304 vector control lines (CL), CL-1, CL-2, CL-3; and wild type (WT) plants. a Quantification of the MDA concentration in the overexpression lines, control lines and WT plants after 2 weeks drought stress. b Quantification of the MDA concentration in the overexpression lines, control lines and WT plants after 37 °C heat stress for 3 days. c Concentration of the free proline content in the overexpression lines, control lines and WT plants after 2 weeks drought stress. d Concentration of the free proline content in the overexpression lines, control lines and WT after 37 °C heat stress for 3 days. e Quantification of H2O2 content regulated by overexpression lines, control lines and WT plants under drought treatment for 2 weeks. f Quantification of H2O2 content regulated by overexpression lines, control lines and WT after 37 °C heat stress for 3 days. g Changes in chlorophyll (Chl) content in overexpression lines, control lines and WT plants after 37 °C heat stress for 3 days. Values are the mean ± SE (n > 80); Asterisks indicate significant differences compared with control line values (*P < 0.05; **P < 0.01). Similar results were observed in three independent experiments
Many studies have indicated that various abiotic stresses can result in damage to plants via oxidative stress involving the generation of ROS (Zhu 2002; Zhou et al. 2012a; Cai et al. 2014). We quantified the amount of H2O2 among the overexpression and control lines after 2 weeks of drought and growth at 37 °C for 3 d, respectively. The amount of H2O2 in all the transgenic lines and the WT plants increased after drought treatment, but the increase in the overexpression lines was smaller (Fig. 9e). There was no significant difference in H2O2 content among WT, overexpression and control lines under normal conditions (P < 0.05). The H2O2 content after heat stress was similar to that after drought stress (Fig. 9f), the results suggest that low H2O2 concentrations were beneficial for the drought and heat stress response in the overexpression transgenic lines.
To test whether endogenous chlorophyll content is involved in the heat stress response, we analyzed the chlorophyll content in Arabidopsis leaves after heat stress. After 37 °C heat stress, total chlorophyll content was examined in the transgenic line and the WT plants. Clear differences in the chlorophyll contents in overexpression lines were evident compared with the control line and the WT plants (Fig. 9g). No significant difference in chlorophyll content was found between the control line and the WT plants. The survival rates of the overexpression lines were higher than that of the control line (Fig. 7c). The results showed that overexpression of ZmMAPK1 can increase the chlorophyll content in Arabidopsis for resistance to heat stress.
Discussion
Plants have developed sophisticated signaling pathways to deal with environmental stress (Ning et al. 2010). Here, we have identified a MAPK1 homologue in maize that we named ZmMAPK1. The database search and sequence analysis suggest that ZmMAPK1 contains 11 conserved subdomains and belongs to group A of the plant MAPKs. It is well known that MAPK cascades play essential roles in the activation of plant defense signal pathways in response to various abiotic stresses (Fu et al. 2002; Colcombet and Hirt 2008; Rodriguez et al. 2010; Zhang et al.2012; Ding et al. 2013). The group A MAPKs in maize has been characterized in terms of their expression patterns under stress. Overexpression of ZmMPK4 in transgenic tobacco resulted in increased tolerance to low temperature stress by the accumulation of fewer ROS, and activated more relative gene expression (Zhou et al. 2012b). In this research, we examined the response of ZmMAPK1 to drought, high temperature, PEG, and sodium chloride. The observed changes in expression level of ZmMAPK1 in response to different stress stimuli were similar to those of group A MAPKs in maize. Therefore, we infer that ZmMAPK1 may have a similar function in resistance to various stresses. We also provided genetic and chemical evidence that ZmMAPK1 plays an important role in response to abiotic stress (Figs. 3, 9).
MAPKs can be induced by various environmental stresses (Colcombet and Hirt 2008; Andreasson and Ellis 2010; Wu et al. 2011b), so the regulation of the large superfamily gene mechanism is complex. In our study, the relative expression levels of ZmMAPK1 increased prominently in the few days before the leaf phenotype exhibited withering, and the gene expression level was about 3.8 fold higher after drought treatment (Fig. 3f). From the PEG6000 treatment of seedlings, we know that ZmMAPK1 expression increased during the following 24 h (Fig. 3d). All the results indicate that ZmMAPK1 was involved in drought stress. ABA is a major phytohormone in mediating inhibition of water loss from drought stressed leaves (Lim et al. 2014), the phytohormones may contribute to decrease water loss from the overexpreisson plants. As the ABA1 and ABA3 are the major ABA biosynthetic genes, the results showed that they were significantly expressed in the OE lines (Fig. 8a, b). The ABI1 is the ABA signaling regulators gene which was also had a great effect on the ABA biosynthesis in the plants, which plays a pivotal role in coordinating the adaptive response to abiotic stress (Hartung et al. 2005). Proline is also an important osmoprotectant that protects cells from damage under different abiotic stress conditions as previous report. Overexpression of ZmMAPK1 in Arabidopsis resulted in higher survival rates than in control lines under drought stress (Fig. 5d), and the resistance to drought was significantly different (Fig. 5a, b, c). Therefore, we conclude that ZmMAPK1 has an important effect on resistance to drought stress.
It has been previously reported that heat stress is often combined with drought stress (Macková et al. 2013) and heat stress may cause changes in the content of ROS, MDA and proline (Larkindale and Knight 2002; Lv et al. 2011). Oxidative stress is the main driver of heat stress in plants, and the more H2O2 accumulates in the cell membranes, the greater the danger to plant development, especially in the delay of plant growth (Guan et al. 2013). The results showed that ZmMAPK1 is significantly induced by exposure to 37 °C (Fig. 3c), and highly expressed after heat stress for 12 h in the leaves, which suggests that ZmMAPK1 also plays a positive role in resistance to heat stress. Overexpression of the ZmMAPK1 gene in Arabidopsis resulted in increased survival rates after heat stress, and the heat stress related genes has a significant changed (Fig. 8e, f). The survival rates of overexpression lines were about four times those of the controls. Compared with the control lines, the result shows that the transgenic plants had a higher tolerance under heat stress (Fig. 7).
In this study, we measured different physiological indices. Environmental stresses often cause physiological changes in plants, so such indices are used to evaluate abiotic stress tolerance and resistance in crops (Tian et al. 2013). ROS is the main product of heat stress, and MAPKs are the key players in ROS signaling (Pitzschke and Hirt 2009; Zhang et al. 2010). Because elevated H2O2 concentrations in plants can accelerate cell death and decrease drought tolerance and thermotolerance (Choi et al. 2007), we investigated the ROS level in the overexpression lines. The result showed that total ROS accumulation was increased after heat treatment, but the overexpression lines had lower levels than the controls (Fig. 9f). Proline is related to drought resistance and may more closely reflect the balance between osmotic stress and resistance. In this study, the rate of water loss of detached leaves from the ZmMAPK1 overexpression lines was slower than in the control line (Fig. 6), and the survival rates were higher than in controls (Fig. 5d). These results indicated that the overexpression transgenic lines had higher water retention ability. Chlorophyll content is widely used in heat tolerance assays, and is a determining factor for the accumulation of biomass. In our study, changes in chlorophyll accumulation were lower than normal, although it was higher in the transgenic line than in the control and WT plants under heat stress (Fig. 9g).
Water deficit and heat stresses induce various physiological responses in plants, especially the production of MDA. MDA is the main compound produced in plants under drought and heat stress, which can damage membranes and result in cell death (Levine et al. 1994, Liu et al. 2013a). The higher the content of MDA, the higher is the danger to plant survival under environmental stress. MDA levels in transgenic lines 1, 3 and 5 were obviously lower than those in controls and WT plants after drought stress (Fig. 9a, b). Cell membrane stability is affected by lipid peroxidation caused by ROS accumulation under stress conditions (Sudhakar et al. 2001), and heat stress-induced H2O2 is required for effective expression of heat shock genes in Arabidopsis (Volkov et al. 2006). As shown here, the ROS content was lower in the overexpression transgenic plants than in control and WT plants after drought and heat stress (Fig. 9e, f). Physiological parameters in plants are important for growth and development. The altered physiological indices may promote plant survival in conditions of environmental stress, and they help to maintain cell membrane stability by reducing ROS. This effect is partly due to the increased activities of some anti-oxidant enzymes such as superoxide dismutase (SOD), an interpretation consistent with previous studies.
In summary, a MAPK1 homologue (ZmMAPK1) has been identified and characterized in maize. Morphological and physiological evidence strongly demonstrated that the transgenic ZmMAPK1 plants acquired enhanced tolerances to drought and heat stresses. The enhanced stress tolerance was partly confirmed by higher plant survival rates, proline content, and lower MDA accumulation in the plants. Further studies are needed to understand the function of the MAPK pathway under drought and heat related stresses.
Abbreviations
- ABA:
-
Abscisic acid
- CaMV:
-
Cauliflower mosaic virus
- MDA:
-
Malondialdehyde
- ROS:
-
Reactive oxygen species
- MAPK:
-
Mitogen-activated protein kinase
- ORF:
-
Open reading frame
- SOD:
-
Superoxide dismutase
- GFP:
-
Green fluorescent protein
References
Abe A, Kosugi S, Yoshida K, Natsume S, Takagi H, Kanzaki H, Matsumura H, Yoshida K, Mitsuoka C, Tamiru M, Innan H, Cano L, Kamoun S, Terauchi R (2012) Genome sequencing reveals agronomically important loci in rice using MutMap. Nat Biotechnol 30:174–178
Andreasson E, Ellis B (2010) Convergence and specificity in the Arabidopsis MAPK nexus. Trends Plant Sci 15:106–113
Bao Y, Wang CT, Jiang CM, Pan J, Zhang GB, Liu H, Zhang HX (2014) The tumor necrosis factor receptor-associated factor (TRAF)-like family protein SEVEN IN ABSENTIA2 (SINA2) promotes drought tolerance in an ABA-dependent manner in Arabidopsis. New Phytol 202:174–187
Bates LS, Waldren RP, Teare ID (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39:205–220
Bechtold N, Pelletier G (1998) In planta Agrobacterium-mediated transformation of adult Arabidopsis thaliana plants by vacuum infiltration. Methods Mol Biol 82:259–266
Berberich T, Sano H, Kusano T (1999) Involvement of a MAP kinase, ZmMPK5, insenescence and recovery from low-temperature stress in maize. Mol Gen Genet 262:534–542
Cai G, Wang G, Wang L, Pan J, Liu Y, Li D (2014) ZmMKK1, a novel group A mitogen-activated protein kinase gene in maize, conferred chilling stress tolerance and was involved in pathogen defense in transgenic tobacco. Plant Sci 214:57–73
Cheng MC, Liao PM, Kuo WW, Lin TP (2013) The Arabidopsis ETHYLENE RESPONSE FACTOR1regulates abiotic stress-responsive gene expression by binding to different cis-acting elements in response to different stress signals. Plant Physiol 162:1566–1582
Choi HW, Kim YJ, Lee SC, Hong JK, Hwang BK (2007) Hydrogen peroxide generation by the pepper extracellular peroxidase CaPO2 activates local and systemic cell death and defense response to bacterial pathogens. Plant Physiol 145:890–904
Colcombet J, Hirt H (2008) Arabidopsis MAPKs: a complex signaling network involved in multiple biological processes. Biochem J 413:217–226
Ding Y, Cao J, Ni L, Zhu Y, Zhang A, Tan M, Jiang M (2013) ZmCPK11 is involved in abscisic acid-induced antioxidant defence and functions upstream of ZmMPK5 in abscisic acid signalling in maize. J Exp Bot 64:871–884
Duerr B, Gawienowski M, Ropp T, Jacobs T (1993) MsERK1: amitogen-activated protein kinase from a flowering plant. Plant Cell 5:87–96
Frey A, Effroy D, Lefebvre V, Seo M, Perreau F, Berger A, Sechet J, To A, North HM, Marion-Poll A (2012) Epoxycarotenoid cleavage by NCED5 fine-tunes ABA accumulation and affects seed dormancy and drought tolerance with other NCED family members. Plant J 70:501–512
Fu SF, Chou WC, Huang DD, Huang HJ (2002) Transcriptional regulation of a rice mitogen-activated proteined kinase gene, OsMAPK4, in response to environmental stresses. Plant Cell Physiol 43:958–963
Guan Q, Lu X, Zeng H, Zhang Y, Zhu J (2013) Heat stress induction of miR398 triggers a regulatory loop that is critical for thermotolerance in Arabidopsis. Plant J 74:840–851
Hartung W, Schraut D, Jiang F (2005) Physiology of abscisic acid (ABA) in roots under stress—a review of the relationship between root ABA and radial water and ABA flows. Crop Pasture Sci 56:1253–1259
Herskowitz I (1995) MAP kinase pathways in yeast: formating and more. Cell 80:187–197
Jin YM, Jung J, Jeon H, Won SY, Feng Y, Kang JS, Lee SY, Cheong JJ, Koiwa H, Kim M (2011) AtCPL5, a novel Ser-2-specific RNA polymerase II C-terminal domain phosphatase, positively regulates ABA and drought responses in Arabidopsis. New Phytol 190:57–74
Kosetsu K, Matsunaga S, Nakagami H, Colcombet J, Sasabe M, Soyano T, Takahashi Y, Hirt H, Machida Y (2010) The MAP kinase MPK4is required for cytokinesis in Arabidopsis thaliana. Plant Cell 22:3778–3790
Lalle M, Visconti S, Marra M, Camoni L, Velasco R, Aducci P (2005) ZmMPK6, a novel maize MAP kinase that interacts with 14-3-3 proteins. Plant Mol Biol 59:713–722
Lampard GR, Lukowitz W, Ellis BE, Bergmann DC (2009) Novel and expanded roles for MAPK signaling in Arabidopsiss stomatal cell fate revealed by Cell type-specific manipulations. Plant Cell 21:3506–3517
Larkindale J, Knight MR (2002) Protection against heat stress-induced oxidative damage in Arabidopsis involves calcium, abscisic acid, ethylene, and salicylic acid. Plant Physiol 128:682–695
Larkindale J, Hall JD, Knight MR, Vierling E (2005) Heat stress phenotypes of Arabidopsis mutants implicate multiple signaling pathways in the acquisition of thermotolerance. Plant Physiol 138:882–897
Lei L, Li Y, Wang Q, Xu J, Chen YF, Yang HL, Ren DT (2014) Activation of MKK9-MPK3/MPK6 enhances phosphate acquisition in Arabidopsis thaliana. New Phytol 203:1146–1160
Levine A, Tenhaken R, Dixon R, Lamb C (1994) H2O2 from the oxidative burst orchestrates the plant hypersensitive disease resistance response. Cell 79:583–593
Lim CW, Lee SC (2014) Functional roles of the pepper MLO protein gene, CaMLO2, in abscisic acid signaling and drought sensitivity. Plant Mol Biol 85:1–10
Lim S, Baek W, Lee SC (2014) Identification and functional roles of CaDIN1 in abscisic acid signaling and drought sensitivity. Plant Mol Biol 86:513–525
Lin F, Ding H, Wang J, Zhang H, Zhang A, Zhang Y, Tan M, Dong W, Jiang M (2009) Positive feedback regulation of maize NADPH oxidase by mitogen-activated protein kinase cascade in abscisic acid signaling. J Exp Bot 60:3221–3238
Liu P, Xu ZS, Pan-Pan L, Hu D, Chen M, Li LC, Ma YZ (2013a) A wheat PI4 K gene whose product possesses threonine autophophorylation activity confers tolerance to drought and salt in Arabidopsis. J Exp Bot 64:2915–2927
Liu YK, Wang L, Zhang D, Li D (2013b) Expression analysis of segmentally duplicated ZmMPK3-1 and ZmMPK3-2 genes in maize. Plant Mol Bio Rep 31:457–463
Luo X, Bai X, Sun X, Zhu D, Liu B, Ji W, Cai H, Cao L, Wu J, Hu M, Liu X, Tang L, Zhu Y (2013) Expression of wild soybean WRKY20 in Arabidopsis enhances drought tolerance and regulates ABA signaling. J Exp Bot 64:2155–2169
Lv WT, Lin B, Zhang M, Hua XJ (2011) Proline accumulation is inhibitory to Arabidopsis seedlings during heat stress. Plant Physiol 156:1921–1933
Macková H, Hronková M, Dobrá J, Turečková V, Novák O, Lubovská Z, Motyka V, Haisel D, Hájek T, Prášil IT, Gaudinová A, Štorchová H, Ge E, Werner T, Schmülling T, Vanková R (2013) Enhanced drought and heat stress tolerance of tobacco plants with ectopically enhanced cytokine in oxidase/dehydrogenase gene expression. J Exp Bot 64:2805–2815
MAPK Group (2002) Mitogen-activated protein kinase cascades in plants: a new nomenclature. Trends Plant Sci 7:301–308
Nakagami H, Pitzschke A, Hirt H (2005) Emerging MAP kinase pathways in plant stress signaling. Trends Plant Sci 10:339–346
Ning J, Li X, Hicks LM, Xiong L (2010) A Raf-Like MAPKKK gene DSM1 mediates drought resistance through reactive oxygen species scavenging in rice. Plant Physiol 152:876–890
Pan J, Zhang M, Kong X, Xing X, Liu Y, Zhou Y, Liu Y, Sun L, Li D (2012) ZmMAPK17, a novel maize group D MAP kinase gene, is involved in multiple stress responses. Planta 235:661–676
Pitzschke A, Hirt H (2006) Mitogen-activated protein kinase and reactive oxygen species signaling in plants. Plant Physiol 141:351–356
Pitzschke A, Hirt H (2009) Disentangling the complexity of mitogen-activated protein kinases and reactive oxygen species signaling. Plant Physiol 149:606–615
Rodriguez MC, Petersen M, Mundy J (2010) Mitogen-activated protein kinase signaling in plants. Annu Rev Plant Biol 61:621–649
Sharma S, Villamor JG, Verslues PE (2011) Essential role of tissue-specific proline synthesis and catabolism in growth and redox balance at low water potential. Plant Physiol 157:292–304
Shen X, Yuan B, Liu H, Li X, Xu C, Wang S (2010) Opposite functions of a rice mitogen-activated protein kinase during the process of resistance against Xanthomonas oryzae. Plant J 64:86–99
Sudhakar C, Lakshmi A, Giridarakumar S (2001) Changes in the antioxidant enzyme efficacy in two high yielding genotypes of mulberry (Morusalba L.) under NaCl salinity. Plant Sci 161:613–619
Thomas G (1992) MAP kinase by any other name smells just as sweet. Cell 68:3–6
Tian S, Mao X, Zhang H, Chen S, Zhai C, Yang S, Jing R (2013) Cloning and characterization of TaSnRK2.3, a novel SnRK2 gene in common wheat. J Exp Bot 64:2063–2080
Volkov RA, Panchuk II, Mullineaux PM, Schöffl F (2006) Heat stress-induced H2O2 is required for effective expression of heat shock genes in Arabidopsis. Plant Mol Bio 61:733–746
Wang NN, Shih MC, Li N (2005) The GUS reporter-aided analysis of the promoter activities of Arabidopsis ACC synthase genes AtACS4, AtACS5, and AtACS7 induced by hormones and stresses. J Exp Bot 56:909–920
Wang J, Ding H, Zhang A, Ma F, Cao J, Jiang M (2010) A novel mitogen-activated protein kinase gene in maize (Zea mays), ZmMPK3, is involved in response to diverse environmental cues. J Integr Plant Biol 52:442–452
Wang LC, Wu JR, Chang WL, Yeh CH, Ke YT, Lu CA, Wu SJ (2013) Arabidopsis HIT4 encodes a novel chromocentre-localized protein involved in the heat reactivation of transcriptionally silent loci and is essential for heat tolerance in plants. J Exp Bot 64:1689–1701
Wang L, Liu Y, Cai G, Jiang S, Pan J, Li D (2014) Ectopic expression of ZmSIMK1 leads to improved drought tolerance and activation of systematic acquired resistance in transgenic tobacco. J Biotechnol 172:18–29
Wei KF, Wang YM, Zhong XJ, Pan S (2014) Protein kinase structure, expression and regulation in maize drought signaling. Mol Breeding 34:583–602
Wu LJ, Wang XT, Wu LC, Wang PA, Chen YH (2011a) Molecular cloning and expression analysis of an HINT1 homologue from maize (Zea mays L.). Plant Mol Biol Rep 29:1006–1012
Wu T, Kong XP, Zong XJ, Li DP, Li DQ (2011b) Expression analysis of five maize MAP kinase genes in response to various abiotic stresses and signal molecules. Mol Biol Rep 38:3967–3975
Yoo CY, Pence HE, Jin JB, Miura K, Gosney MJ, Hasegawa PM, Mickelbart MV (2010) The Arabidopsis GTL1 transcription factor regulates water use efficiency and drought tolerance by modulating stomatal density via transrepression of SDD1. Plant Cell 22:4128–4141
Zeng Q, Chen JG, Ellis BE (2011) AtMPK4 is required for male-specific meiotic cytokinesis in Arabidopsis. Plant J 67:895–906
Zhang A, Zhang J, Ye N, Cao J, Tan M, Zhang J, Jiang M (2010) ZmMPK5 is required for the NADPH oxidase-mediated self-propagation of apoplastic H2O2 in brassinosteroid-induced antioxidant defence in leaves of maize. J Exp Bot 61:4399–4411
Zhang M, Pan J, Kong X, Zhou Y, Liu Y, Sun L, Li D (2012) ZmMKK3, a novel maize group B mitogen-activated protein kinase kinase gene, mediates osmotic stress and ABA signal responses. J Plant Physiol 169:1501–1510
Zhang D, Jiang S, Pan J, Kong X, Zhou Y, Liu Y, Li D (2014) The overexpression of a maize mitogen-activated protein kinase gene (ZmMPK5) confers salt stress tolerance and induces defence responses in tobacco. Plant Biol (Stuttg) 16:558–570
Zhou W, Zhou T, Li MX, Zhao CL, Jia N, Wang XX, Sun YZ, Li GL, Xu M, Zhou RG, Li B (2012a) The Arabidopsis J-protein AtDjB1 facilitates thermotolerance by protecting cells against heat-induced oxidative damage. New Phytol 194:364–378
Zhou Y, Zhang D, Pan J, Kong X, Liu Y, Sun L, Wang L, Li D (2012b) Overexpression of a multiple stress-responsive gene, ZmMPK4, enhances tolerance to low temperature in transgenic tobacco. Plant Physiol Biochem 58:174–181
Zhu JK (2002) Salt and drought stress signal transduction in plants. Annu Rev Plant Biol 53:247–273
Zhu JK, Hasegawa PM, Bressan RA, Bohnert HJ (1997) Molecular aspects of osmotic stress in plants. Crit Rev Plant Sci 16:253–277
Zong XJ, Li DP, Gu LK, Li DQ, Liu LX, Hu XL (2009) Abscisic acid and hydrogen peroxide induce a novel maize group C MAP kinase gene, ZmMPK7, which is responsible for the removal of reactive oxygen species. Planta 229:485–495
Acknowledgments
We thank Prof. Zhankuan Chen (Henan Academy of Agricultural Sciences) for providing plasmid pCAMBIA1304. We also thank Prof. Hairong Zhang (Henan Agricultural University) for kind help in the Arabidopsis transformation work. This research was supported by grants from the National Nature Science Foundation of China (No. 31471503) and the National Basic Research Program of China (973 Program, No. 2011CB111500).
Author information
Authors and Affiliations
Corresponding author
Additional information
Liuji Wu and Xiaofeng Zu have contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wu, L., Zu, X., Zhang, H. et al. Overexpression of ZmMAPK1 enhances drought and heat stress in transgenic Arabidopsis thaliana . Plant Mol Biol 88, 429–443 (2015). https://doi.org/10.1007/s11103-015-0333-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11103-015-0333-y