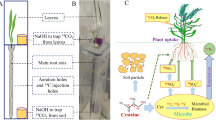
Abstract
Purpose
Nitrogen (N) and sulphur (S) are essential for plant growth and development. Cysteine (Cys) and methionine (Met) are N- and S-containing amino acids in soils. However, it is unclear whether plants possess a strong ability to utilise N- and S-containing amino acids from the plant physiology perspective, and whether they can access amino acids when facing rapid microbial decomposition in the soil.
Methods
Wheat and oilseed rape were cultivated using a sterilised hydroponic solution in the laboratory and field conditions with 13C-, 14C-, 15N-, and 35S-labelled Cys and Met.
Results
With sterilised hydroponic cultivation, wheat and oilseed rape possessed a greater ability for Cys and Met uptake than for SO42− uptake, but these compounds did not support plant growth at high concentrations. The uptake rate of Cys and Met in oilseed rape was almost 20-fold higher than that in wheat, while the transportation ratio was even higher, indicating that oilseed rape not only possesses a great ability for S-containing amino acid uptake but also metabolises and transports them to the shoot quickly. A short-term labelling uptake test (6 h) in the field showed that 0.6–2.2% of total added Cys and Met were utilised by wheat and oilseed rape in the intact form owing to fierce competition from soil microorganisms.
Conclusions
Wheat and oilseed rape possess a great ability for Cys and Met uptake but can access limited amounts owing to rapid microbial decomposition in soil.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Even though nitrogen (N) and sulphur (S) are both important plant macronutrients, S has received limited research attention. Sulphur accounts for 0.3–0.5% of plant biomass and plays a vital role in various metabolic processes, especially the formation of vitamins, chlorophyll, proteins, and enzymes (Aarabi et al. 2016; Romero et al. 2014). In recent decades, plant S deficiencies have occurred worldwide because of decreased SO2 emissions under strict emission policies, increased S removal from soils in highly intensive cropping systems, the high S demands of high-yielding crop varieties, and the use of triple superphosphate fertilisers containing little S (Aarabi et al. 2016; Churka Blum et al. 2013; Maruyama-Nakashita 2017). Sulphur deficiency can reduce crop yield and quality, especially in high-S demand plant species, such as oilseed rape, and thus requires urgent attention (Joshi et al. 2021).
In natural and agricultural soils, 90–95% of S is in the organic form, which is vital for cycling, leaching, and transport. Plant bioavailability is regulated by the mineralisation of organic to inorganic S in soils that receive limited amounts of atmospheric deposition (Kaiser and Guggenberger 2005; Vermeiren et al. 2018). Most previous studies have focused on organic S mineralisation and plant SO42− uptake, as only SO42− is considered to be bioavailable for terrestrial plants (Dong et al. 2017; Maruyama-Nakashita 2017). Organic N sources, such as amino acids (Hill and Jones 2019; Ma et al. 2017a, b; Näsholm et al. 1998), peptides (Farrell et al. 2013), and proteins (Paungfoo-Lonhienne et al. 2008), can be utilised by plant roots to bypass microbial decomposition. Plant root transporters mediating amino acid uptake have been identified, including amino acid permease 1, lysine histidine transporter 1, and amino acid permease 5 (Näsholm et al. 2009). The herbaceous model plant Arabidopsis thaliana and woody heathland plant Hakea actites (both do not form mycorrhizae) can use proteins as an N source by endocytosis and exuding proteolytic enzymes that digest proteins from roots (Paungfoo-Lonhienne et al. 2008). Although some studies have shown that ester-bonded S and carbon-bonded S, which are decomposed to SO42− by soil microbes (De Bona and Monteiro 2010; Kertesz and Mirleau 2004), are plant-available, it is unclear whether low-molecular-weight S-containing organic matter can be utilised directly by plant roots. In addition, it is still unclear whether the uptake of S-containing amino acids by plant roots is primarily driven by passive uptake (through diffusion, without energy consumption) or active uptake (transported across concentration gradients, with energy consumption).
The uptake of N/S by plants is regulated by several factors such as the content of the substrate, other N/S forms, and environmental conditions. Methionine (Met) and cysteine (Cys) are highly bioavailable N- and S-containing amino acids that play an important role in the synthesis of several metabolites, such as proteins, biotin, and Fe-S clusters, in all chemoautotrophic and photoautotrophic organisms (Dong et al. 2017). Previous research has shown that large amounts of Cys, but not Met, can be metabolised by potatoes (Lycopersicon esculentum Mill.) (Maggioni and Renosto 1977). The uptake of SO42− by potatoes is inhibited by Cys and Met uptake (Maggioni and Renosto 1977). Plants may tend to take up low-molecular-weight organic S as SO42− is in short supply in most natural and agricultural soils. However, whether the concentration of Cys and Met and other N/S forms regulate plant uptake of Cys and Met is not known.
Plants possess a great ability to utilise amino acids in sterilised hydroponic solutions, thereby removing the effects of microbial decomposition (Ma et al. 2017c, 2018). However, in the soil environment, low-molecular-weight organic N can be decomposed by soil microorganisms in minutes to hours, and only a limited amount of intact organic N can be captured by plant roots (Czaban et al. 2016; Hill and Jones 2019; Ma et al. 2020d). Previous studies based on RhizoTube and pot cultivation have shown that plant roots can access limited amounts of S-containing amino acids in the soil (Ma et al. 2020a, 2021a, c). However, it is unclear whether plants grown in the field can utilise organic N and S. We hypothesised that plant roots can access limited amounts of S-containing amino acids in the field owing to rapid microbial decomposition.
To explore the uptake of soil S-containing amino acids, wheat (Triticum aestivum L.) and oilseed rape (Brassica napus L.) were selected because of their different S demands and sensitivities to S deficiency. Cereals remove 10–15 kg S ha−1, whereas oilseed rape removes 20–30 kg S ha−1 (Scherer 2001). Plants cultivated in sterilised hydroponic solution were used to explore their uptake ability from the perspective of plant physiology, and plants cultivated in the field were used to explore their ability to compete with soil microorganisms based on 14C, 35S, 13C, and 15N quad labelling. 14C and 35S radioactive labelling can separate S uptake as an intact molecule or SO42− after decomposition (Ma et al. 2021c). 13C and 15N dual-labelled Cys and Met allow intact amino acid uptake to be distinguished from the uptake of inorganic N, which is derived from mineralised amino acids (Ganeteg et al. 2017). We hypothesised that plants possess a great ability to uptake and metabolise N- and S-containing amino acids but can access only a limited amount due to the rapid decomposition by soil microorganisms.
Methods and materials
Assessing plant growth under different concentrations of Cys, Met, and SO 4 2–
To test whether low and high concentrations of Cys, Met, and SO42− can support plant growth, wheat and oilseed rape seedlings were hydroponically cultivated in a sterilised environment (Ma et al. 2017a). Briefly, wheat and oilseed rape seeds were soaked in water for 10 h, sterilised with 60% ethanol for 1 min, followed by 10% H2O2 for 5 min and 0.1 M HgCl2 for 5 min, and washed with sterilised water several times. The sterilised seeds were placed in a culture dish with a 25 °C day and 20 °C night temperature, 60% day and 40% night humidity, and a 12-h light cycle (360 μmol m−2 s−1) for 3 d. Subsequently, each seedling was transferred to a 50-mL centrifuge tube containing 0.3% cooled agar and placed in a culture room under the same conditions. A 0.3-mm (diameter) hole was drilled through the tube cap; plant roots entered the agar after passing through the hole, while the leaves remained above the hole. Cooled agar (soft gelatinous shape) can support plant growth in a standing form, which facilitates the sealing of holes using silicone rubber (Ma et al. 2017a). One day after the roots entered the agar, the holes were sealed with silicone rubber. The seedlings, together with the tube caps, were transferred to a new centrifuge tube covered with silver paper and filled with a nutrient solution (Online Resource Table 1). The N and S sources used in the experiments were sterilised by filtering through a 0.22-μm membrane filter (PES Membrane, Millipore, Carrigtwohill, Ireland) and added to the nutrient solutions before use. Centrifuge tubes, culture dishes, and nutrient solutions without N and S used in the experiment were autoclaved at 121 °C for 30 min. Six treatments were conducted with seedlings of similar biomass: 50 μM Cys (low concentration, comparable to the free amino acid concentration in soil solution), 500 μM Cys (high concentration, with adequate S supply for plant growth), 50 μM Met, 500 μM Met, 50 μM Na2SO4, and 500 μM Na2SO4. Each treatment had 20 plants (4 replicates × 5 sampling events). The nutrient solutions were changed every 3 d on a clean bench (N was added as 2 mM NH4NO3 and S was added as Cys/Met/Na2SO4; other elements are shown in Online Resource Table 1). Four replicates from each treatment were collected (with the shoots and roots collected separately) to determine the dry biomass (oven-dried at 60 °C) after cultivation for 3, 6, 9, 12, and 15 d.
Uptake ability of plants for Cys, Met, and SO 4 2–
To test the ability of plants for Cys, Met, and SO42− uptake, wheat and oilseed rape were cultured in a sterilised hydroponic solution. Wheat and oilseed rape were cultivated for 10 d in an S-containing nutrient solution (S was added as 500 μM NaSO4 and N was added as 2 mM NH4NO3; other elements are shown in Online Resource Table 1) as stated above. Then, wheat or oilseed rape seedlings were cultivated with 50 mL of 35S-Cys, 35S-Met, or 35S-NaSO4 under 0.01, 0.05, 0.1, 0.2, 0.4, 0.8, 1.6, 3.2, 6.4, or 12.8 mM 35S-labelled compounds for 8 h (35S: 1.32 kBq mL−1; Sigma-Aldrich Ltd., Poole, UK) with four replicates (other elements are shown in Online Resource Table 1). Each treatment was applied to 40 plants (4 replicates × 10 substrate concentrations). The 35S-Cys/Met/SO42− solution was produced by adding 0.3 μL of 35S-Cys/Met/SO42− to unlabelled Cys/Met/SO42− solution (at 0.01, 0.05, 0.1, 0.2, 0.4, 0.8, 1.6, 3.2, 6.4, or 12.8 mM). The concentration of 35S-labelled Cys/Met/SO42− added to the unlabelled solution was less than 1 nM and did not alter the Cys/Met/SO42− concentration of the solution (Brailsford et al. 2020). The shoots and roots were harvested separately and then freeze-dried before being ground to a fine powder using a ball mill (Retsch MM301, Germany). Then, 200 μg of powder was extracted with 1.5 mL of Soluene 350 (PerkinElmer) for 24 h and centrifuged for 5 min at 5000 × g, before 35S activity in the extracts was measured using a Wallace 1404 Liquid Scintillation Counter (Wallace EG&G, Milton Keynes, UK) after mixing with 4 mL of Scintisafe 3 Scintillation Cocktail (Fisher Scientific, Loughborough, UK) (Jones et al. 2018; Ma et al 2020b). The uptake rate was calculated using the total uptake amount and root quantity (μM gDW−1 h−1).
Uptake patterns of Cys, Met, and SO 4 2−
Wheat and oilseed rape were hydroponically cultivated for 10 d in N- and S-containing nutrient solutions (500 μM Na2SO4 and 2 mM NH4NO3; other elements are shown in Online Resource Table 1). Then, the seedlings were cultivated with 35S-Cys, 35S-Cys + 50 μM Na2SO4, 35S-Cys + 2 mM NH4NO3, 35S-Met, 35S-Met + 50 μM Na2SO4, 35S-Met + 2 mM NH4NO3, or 35S-Na2SO4 for 8 h (the 35S-Cys/Met/Na2SO4 concentration was 50 μM, 35S: 0.98–1.32 kBq mL−1; a low concentration was selected to match the amino acid levels in the soil solutions when decomposed by plant roots or microorganisms; N and S were added as stated; other elements are shown in Online Resource Table 1). Each treatment had four replicates, and 5 seedlings were combined into one replicate (140 plants, 7 treatments × 4 replicates × 5 seedlings). Additionally, the effects of SO42− and N on the active and passive absorption of Cys, Met, and SO42− were examined using carbonyl cyanide 3-chlorophenylhydrazone (CCCP), which can inactivate the root (to cut off active uptake; thus, uptake by CCCP-treated plants is passive) (Ma et al. 2017a). The seedlings (140 plants) that were cultivated for 10 d were pre-treated with 50 μM CCCP for 1 h and then cultured with the above tracer solutions (35S-Cys/Met/Na2SO4) for 8 h. 35S in the CCCP-treated plants was the result of passive uptake and 35S in the CCCP-untreated plants minus that in the CCCP-treated plants reflected active uptake (Ma et al. 2017a). The shoots and roots were harvested separately and freeze-dried before being ground to a fine powder, and 35S activity in the extracts was measured using the Wallace 1404 Liquid Scintillation Counter.
Uptake of Cys, Met, SO 4 2− , and NH 4 + in field environments
To test whether wheat and oilseed rape can absorb organic S in the field when facing competition from soil microorganisms, an in-situ field uptake test was conducted using 13C, 15N, 14C, and 35S quad labelling. The field uptake test was conducted at the Henfaes Agricultural Research Station of Bangor University, Abergwyngregyn, UK (53°14′N, 4°01′W). The soil was classified as agricultural brown earth soil; the basic soil properties are shown in Online Resource Table 2 and were previously reported (Hill et al. 2013). Wheat and oilseed rape were cultured in fields (with 20 cm between plants) for 35 d (oven-dried root dry weight: oilseed rape 7.54 ± 0.28 g, wheat 5.48 ± 0.22 g, n = 50, 10 replicates for each treatment), and 20 mL of one of the five mixtures was added to the soil at a depth of 12 cm, five times around the plant (4 mL each time), using a 12 cm injection syringe that released slowly when lifted. The injected solution comprised one of the following five labelled mixtures: 35S,13C,15N-Cys-Met-SO42–-NH4+; 14C-Cys-Met-SO42–-NH4+; Cys-35S,13C,15N-Met-SO42–-NH4+; Cys-14C-Met-SO42–-NH4+; and Cys-Met-35SO42–-15NH4+. The concentration of Cys, Met, SO42–, and NH4+ was 50 μM (35S: 8.9–10.56 kBq mL–1; 14C: 5.1–5.6 kBq mL–1; L-13C3, 15N-Cys, L-13C5, 15N-Met: 99.8%; Sigma-Aldrich Ltd.), and there were 10 replicates for each mixture. Ten seedlings injected with 20 mL of unlabelled Cys-Met-SO42–-NH4+ (each at 50 μM) for each plant species were prepared as blank samples to detect the natural 14C and 35S radioactivity and 13C and 15N stable isotope ratios. The mixture represents the organic and inorganic S and N forms in the soil solution; NH4+ and SO42– were used to represent inorganic N and S, respectively. Cys and Met are the N- and S-containing amino acids, representing highly bioavailable organic N and S. The concentrations of Cys and Met were both 50 μM, which is comparable to the concentration of amino acids in the soil solution (Jones et al. 2002).
The 13C,15N-Cys/Met solution was produced by dissolving L-13C,15N-Cys/Met (99.8%) in purified water (50 μM); then, 2 μL of 35S-Cys/Met was added to the 13C,15N-labelled solution to produce the 13C,15N,35S-labelled solution. The 14C-Cys/Met solution was prepared by adding 2 μL of 14C-Cys/Met to 50 μM Cys/Met (unlabelled) solution (1000 mL). Similarly, 2 μL of 35S-SO42– was added to 50 μM 15NH4+ (99.8%) to produce a Met-Cys-35S-SO42–-15NH4+ solution (the unlabelled compound was dissolved in the solution at a concentration of 50 μM). After microbial decomposition, the C in the amino acids was released as CO2, whereas N and S were released into the soil solution as NH4+ (with some to be oxidised to NO3–) and SO42–, respectively. When plant roots capture intact amino acids, they not only take up N and S but also C. However, when plant roots take up N in its inorganic form after organic amino acid decomposition, no labelled C can be captured by the plant roots (Ma et al. 2021c). The uptake of intact Cys and Met was examined using two methods: 13C and 15N dual-labelling, which was used to examine N uptake from intact Cys and Met and inorganic N derived from mineralised Cys and Met (Ganeteg et al. 2017); and 14C and 35S radioactive labelling, which was used to determine S uptake from intact Cys and Met and inorganic S derived from decomposed Cys and Met. This labelling mechanism can also enable the calculation of the relative contributions of organic and inorganic N and S to plant growth (Ma et al. 2021c). The 14C and 35S activities were difficult to distinguish using the Wallace 1404 liquid scintillation counter; hence, the samples were separated into two mixtures. Moreover, as the detection methods for 13C/15N and 14C/35S are different, we used the results from the same detection method to indicate the uptake of Cys and Met.
After uptake for 6 h, the plants were removed from the soil and the roots and shoots were collected separately. After gentle shaking, the wheat and oilseed rape roots were separated from the soil, washed with 0.01 M CaCl2 for 2 min, and thoroughly washed with distilled water to remove the tracers on their surface. After freeze-drying, the plant tissues were ground into a powder using a ball mill. The 14C-labelled plant tissues were combusted in an OX400 Biological Oxidiser (Harvey Instruments Co., Hillsdale, NJ, USA), liberated 14CO2 was captured using an Oxosol Scintillant (National Diagnostics, Atlanta, GA, USA), and 14C activity was measured by liquid scintillation counting. The 35S in the plant tissues was detected as stated above. The C and N contents and 13C and 15N incorporation into wheat and oilseed rape were determined using an elemental analysis-isotope ratio mass spectrometer (IsoPrime100, Isoprime Ltd., Cheadle Hulme, UK).
Calculations and statistical analysis
The ratio of 13C and 15N uptake by the wheat and oilseed rape, derived from the labelled Cys and Met, was calculated by the amount of 13C and 15N in treated seedlings minus the amount of 13C and 15N in ‘blank’ seedlings; the calculation of the 13C uptake ratio (the calculation of the 15N uptake ratio was similar to that of the 13C uptake ratio) is shown in Eq. (1) (Ma et al. 2021c).
where 13Cuptake ratio is the ratio of 13C uptake from the labelled Met or Cys (%), CTotal-C is the amount of C in the plants (μg), Ac is the abundance of 13C in the ‘blank’ seedlings (%), As is the abundance of 13C in the 13C-Met/Cys-treated plants (%), and 13CTotal is the total amount of 13C added to the soil (μg).
The 15N uptake ratio (%) of the plants after mineralisation (15N uptake ratio-min) was calculated as the 15N uptake ratio minus the 13C uptake ratio (organic Cys or Met uptake), as shown in Eq. (2):
The ratio of 14C uptake (14Cuptake ratio) by oilseed rape and wheat from labelled Cys and Met was calculated as shown in Eq. (3):
where Ac is the 14C activity in the ‘blank’ seedlings, As is the 14C activity in the 14C-Cys/Met treated plants (kBq), and 14CTotal is the total amount of 14C activity added to the soil (kBq; the calculation of 35S was similar to that of 14C).
The 35S uptake ratio (%) of the plants after mineralisation (35S uptake ratio-min) was calculated as the 35S uptake ratio minus the 14C uptake ratio (organic Cys or Met uptake), as shown in Eq. (4):
The transportation ratio of 35S from Cys and Met from the root to the shoot was calculated as 35S in the shoot / (shoot + root).
The contribution of S from intact or mineralised Cys, Met, and SO42− to total labelled S uptake was calculated using Eq. (5):
where Suptake-Cys is the S uptake amount from Cys (intact and inorganic S after mineralisation; μg plant−1), Suptake-Met is the S uptake amount from Met (μg plant−1), and Suptake-SO4 2- is the S uptake amount from SO42− (μg plant−1). Suptake is the uptake of intact or mineralised Cys, Met, and SO42− (μg plant−1; the contribution of N was similar to that of S).
One-way analysis of variance (ANOVA) was conducted using Tukey’s post-hoc test (p < 0.05) to evaluate the differences among the treatments, and the normality and homogeneity were checked using the Shapiro–Wilk test and F-test. Plant uptake rates for Cys and Met were adapted to the Michaelis–Menten equation to calculate the parameters of the affinity constant (Km) and maximal velocity (Vmax), and their differences were analysed using one-way ANOVA (the Akaike Information Criterion was used to decide whether a linear model or Michaelis–Menten model is the best). The exponential decay equation was fitted to the experimental data in SigmaPlot 10.0 (SPSS Inc., Chicago, IL, USA), and figures were created using Origin 8.1 (OriginLab, Northampton, MA, USA).
Results
Plant growth under different concentrations of Cys, Met, and SO 4 2–
Cys and Met at high concentrations (500 μM) reduced wheat and oilseed rape growth after cultivation for 9 d compared with SO42– (p < 0.05). Plants grown under lower concentrations (50 μM) of Cys and Met had higher biomass than those grown under higher concentrations (500 μM) after cultivation for 15 d (p < 0.05). Additionally, SO42– concentrations had limited effects on wheat and oilseed rape biomass (p > 0.05) (Fig. 1).
Uptake ability of plants for Cys, Met, and SO 4 2–
Under hydroponic conditions, the uptake of Cys and Met by oilseed rape and wheat increased with increasing concentrations, which fitted Michaelis–Menten kinetics, whereas SO42– uptake linearly increased with its concentration in wheat (Fig. 2). The uptake of Cys and Met was faster than that of SO42– in wheat, whereas in oilseed rape, the uptake of SO42– was faster than that of Cys and slower than that of Met. Met uptake was faster than Cys uptake in both wheat and oilseed rape, as shown by the Vmax (Table 1).
Uptake pattern and transportation of Cys, Met, and SO 4 2–
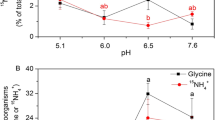
At lower concentrations (50 μM), the Met uptake amount was almost double that of the Cys and SO42– uptake amounts in wheat and oilseed rape; the uptake of Met was mostly active, whereas that of almost half of Cys was passive (Fig. 3). A higher ratio of SO42– than Cys or Met taken up by wheat was transported to the shoot, whereas oilseed rape transported Cys and SO42– at similar rates. The transportation ratio of 35S from Cys and Met in oilseed rape was higher than that in wheat, although their uptake rate was almost 20-fold higher in oilseed rape than in wheat (p < 0.05). Nitrogen addition increased Met uptake by increasing active uptake in both wheat and oilseed rape, and inorganic S addition decreased the active uptake of Met in wheat (Fig. 3).
Uptake patterns of cysteine (Cys), methionine (Met), and SO42− by wheat (a) and oilseed rape (b) and their transportation rates from roots to leaves of wheat (c) and oilseed rape (d) after 8 h. Bars indicate mean values ± SE; n = 4. Within each treatment, different letters indicate significant differences (p < 0.05)
Uptake and contribution of Cys and Met in field environments
In the field, 0.6% and 2.2% of 13C- and 5.7% and 7.9% of 15 N-Cys added to soil were taken up by wheat and oilseed rape after 6 h, respectively. Wheat and oilseed rape utilized 0.4% and 2.1% of total 13C- and 2.9% and 3.9% of the 15 N-Met added to soil after 6 h, respectively. Additionally, 2.2% and 6.1% of total NH4+ was taken up by wheat and oilseed rape, respectively. The 14C activity indicated that 0.6–1.6% of total added Cys and 0.9–1.0% of total added Met were taken up in the intact form by wheat and oilseed rape; whereas the 35S activity indicated that 0.8–6.1% of S from the total added Cys and 0.1–0.7% of S from the total added Met were taken up by wheat and oilseed rape as inorganic S derived from Cys/Met decomposition. Moreover, wheat and oilseed rape absorbed similar amounts of S from Cys and SO42–, but less from Met (p < 0.05). The linear relationship of 13C and 15 N and that between 14C and 35S in plants indicated that the ratio of 15 N and 35S uptake was in the form of Cys and Met, but not 15 N and 35S uptake from inorganic N and S derived from Cys and Met decomposition; 13–36% and 22–34% of 15 N uptake by wheat from Cys and Met was in the intact form, and they were 15–34% and 27–62% for oilseed rape (Fig. 4).
The contribution of intact Cys and Met to total N uptake was 3.9–12.1%, whereas that of inorganic N derived from Cys decomposition was 32.2–47.1%. The contribution of NH4+ was higher for oilseed rape (33.9%) than for wheat (20.5%). The contribution of intact Met and its mineralization products was almost equal for oilseed rape N uptake, and the contribution of Met was greater than that of the mineralization products for wheat and oilseed rape S uptake. SO42− contributed to 46.1–48.6% of total S uptake in both wheat and oilseed rape. The contribution of inorganic S derived from Cys decomposition was higher for oilseed rape (34.7%) than for wheat (16.8%; Fig. 5).
Contribution of N (a) and S (b) from organic amino acids and inorganic N/S, derived from the added Cys/Met/NH4+/SO42− in soil on wheat and oilseed rape growth, calculated using 13C, 15N labelling and 14C, 35S labelling. Bars indicate mean values ± SE; n = 10. Cys: cysteine; Met: methionine; IN: inorganic nitrogen; IS: inorganic sulphur. 2.22%: wheat uptake of NH4+ with respect to total addition; 6.08%: oilseed rape uptake of NH4+ with respect to total addition; 2.33%: wheat uptake of SO42− with respect to total addition; 8.08%: oilseed rape uptake of SO42− with respect to total addition
Discussion
Uptake ability of wheat and oilseed rape for organic S
Wheat and oilseed rape possess great abilities for Cys and Met uptake from a plant physiology perspective, both by active and passive uptake pathways. In hydroponic solutions, without competition from microorganisms, plants have a great ability for low-molecular-weight organic S, especially Met uptake. However, the short-term S uptake ability of wheat and oilseed rape does not necessarily reflect the long-term contribution of S to plant growth because plant growth is regulated not only through S uptake but also through its transport and subsequent metabolism (Ma et al. 2017a, c). For example, pakchoi takes up glycine at a faster rate than NO3−, but glycine at high concentrations (> 1 mM) inhibits pakchoi growth (Ma et al. 2017b). This is because glycine must be metabolised into other amino acids and metabolites before being transported to the shoot, and the limited metabolic abilities of plant roots result in high levels of amino acid accumulation in the roots, thereby inhibiting root growth (Ma et al. 2017b, 2018). In contrast, plant root uptake of SO42−, which occurs through H+-dependent co-transport processes, is vertically transferred to the shoots (Takahashi 2019). Although the uptake ability of the root for Cys and Met was much higher than that for SO42−, these amino acids reduced the long-term growth of wheat and oilseed rape compared with SO42−, which might be owing to their limited metabolism in plant roots.
The uptake rate of Cys and Met in oilseed rape was higher than that in wheat, and the transportation ratio was much higher than that in wheat, indicating that oilseed rape not only possesses a great ability for N- and S-containing amino acid uptake but also metabolises and transports them to the shoot quickly. Additionally, the assimilation of N is connected to the assimilation of S (Schneider et al. 2019). The addition of S rather than N decreased the short-term uptake of Cys and Met in both wheat and oilseed rape, suggesting that they are taken up by plants mainly as an S source and not as an N source (Fig. 3).
Uptake ability of plants for organic S in field environments
Plants can take up limited amounts of organic S when facing fierce competition from soil microorganisms. In the field, only 0.4–2.2% of added Cys or Met was utilized by plants as indicated by the 14C activity and 13C abundance, as microorganisms can decompose them rapidly and a limited amount of intact Cys and Met could be captured by plant roots. As observed for Cys and Met, plant roots can take up a limited amount of intact organic N, such as glycine, alanine, and glutamine, and most of it is decomposed by soil microorganisms (Ma et al. 2018; Ganeteg et al. 2017; Hill and Jones 2019). Soil microbes are C-limited, with no significant N or S limitation in well-aerated soils, and plants can uptake a high amount of SO42−, NH4+, and NO3− but not the C-containing Cys and Met (most of them were captured by microorganisms) (Franklin et al. 2017; Hill et al. 2011; Kuzyakov and Xu 2013; Svennerstam et al. 2011).
The mineralisation of Cys and Met is rapid in soils, and these amino acids were rapidly immobilised into microbial biomass within minutes (Ma et al. 2021b, d); therefore, plants in the field can access limited amounts of intact Cys and Met. C, N, and S in microbial biomass were released in the form of CO2, NH4+, and SO42−, and higher amounts of SO42− were released from Cys, as indicated by the higher uptake of inorganic S from Cys by plants in the field. In our previous study, we have shown that after the addition of Cys and Met to the soil, the highest amount of SO42− released from Cys (37%) and Met (15%) was founded at 3 h and 24 h, respectively, while a higher amount of NH4+ was released from both Cys and Met within 15 min of addition (Ma et al. 2021b). Reduced S and N liberated during the mineralisation processes can be available for plant roots (Seegmüller and Rennenberg 2002). Therefore, the contribution of intact Met and its mineralization products is almost equal for oilseed rape N uptake due to high inorganic production; the contribution of Met was greater than that of the mineralization products for wheat and oilseed rape S uptake, as a high amount of S was not released from microorganism during the 8 h test periods. In the long run (days to months), plants outcompete microbes in terms of N and S acquisition because of the unidirectional nutrient flow from soil to roots, and Cys may be a better S source for plants than Met (Kuzyakov and Xu 2013; Ma et al. 2020c).
There were some limitations to this study. We added the labelled mixture to the soil, which can be regarded as an open system, and the area of separation was difficult to define; thus, the utilisation of the added mixtures by soil microorganisms was not explored. Uptake was mainly observed during a 6-h period in this study, and the actual contributions to plant growth were difficult to estimate. Therefore, N and S contributions from organic and inorganic N/S during the plant growth period and biotic and abiotic factors that regulate these contributions require further research.
In conclusion, wheat and oilseed rape can take up a large amount of Cys and Met from the perspective of plant physiology; however, their uptake in the intact form is limited in field soils owing to fierce competition from soil microorganisms. Plants utilise Cys, via both intact and derived inorganic S uptake, at a higher ratio than Met in the field.
References
Aarabi F, Kusajima M, Tohge T, Konishi T, Gigolashvili T, Takamune M, Sasazaki Y, Watanabe M, Nakashita H, Fernie AR, Saito K, Takahashi H, Hubberten HM, Hoefgen R, Maruyama-Nakashita A (2016) Sulfur deficiency-induced repressor proteins optimize glucosinolate biosynthesis in plants. Sci Adv 2:e1601087. https://doi.org/10.1126/sciadv.1601087
Brailsford FL, Glanville HC, Wang D, Golyshin PN, Johnes PJ, Yates CA, Jones DL (2020) Rapid depletion of dissolved organic sulphur (DOS) in freshwaters. Biogeochemistry 149:105–113. https://doi.org/10.1007/s10533-020-00669-4
Churka Blum SC, Lehmann J, Solomon D, Caires EF, Alleoni LRF (2013) Sulfur forms in organic substrates affecting S mineralization in soil. Geoderma 200–201:156–164. https://doi.org/10.1016/j.geoderma.2013.02.003
Czaban W, Rasmussen J, Nicolaisen M, Fomsgaard IS (2016) Dissipation kinetics of asparagine in soil measured by compound-specific analysis with metabolite tracking. Biol Fertil Soils 52:911–916. https://doi.org/10.1007/s00374-016-1132-6
De Bona FDD, Monteiro FA (2010) Nitrogen and sulfur fertilization and dynamics in a Brazilian entisol under pasture. Soil Sci Soc Am J 74:1248–1258. https://doi.org/10.2136/sssaj2009.0228
Dong Y, Silbermann M, Speiser A, Forieri I, Linster E, Poschet G, Allboje Samami AA, Wanatabe M, Sticht C, Teleman AA, Deragon JM, Saito K, Hell R, Wirtz M (2017) Sulfur availability regulates plant growth via glucose-TOR signaling. Nat Commun 8:1174. https://doi.org/10.1038/s41467-017-01224-w
Farrell M, Hill PW, Farrar J, Deluca TH, Roberts P, Kielland K, Dahlgren R, Murphy DV, Hobbs PJ, Bardgett RD, Jones DL (2013) Oligopeptides represent a preferred source of organic N uptake: A global phenomenon? Ecosystems 16:133–145. https://doi.org/10.1007/s10021-012-9601-8
Franklin O, Cambui CA, Gruffman L, Palmroth S, Oren R, Näsholm T (2017) The carbon bonus of organic nitrogen enhances nitrogen use efficiency of plants. Plant Cell Environ 40:25–35. https://doi.org/10.1111/pce.12772
Ganeteg U, Ahmad I, Jämtgård S, Aguetoni-Cambui C, Inselsbacher E, Svennerstam H, Schmidt S, Näsholm T (2017) Amino acid transporter mutants of Arabidopsis provides evidence that a non-mycorrhizal plant acquires organic nitrogen from agricultural soil. Plant Cell Environ 40:413–423. https://doi.org/10.1111/pce.12881
Hill PW, Farrar J, Roberts P, Farrell M, Grant H, Newsham KK, Hopkins DW, Bardgett RD, Jones DL (2011) Vascular plant success in a warming Antarctic may be due to efficient nitrogen acquisition. Nat Clim Change 1:50–53. https://doi.org/10.1038/nclimate1060
Hill PW, Jones DL (2019) Plant–microbe competition: does injection of isotopes of C and N into the rhizosphere effectively characterise plant use of soil N? New Phytol 221:796–806. https://doi.org/10.1111/nph.15433
Hill PW, Marsden KA, Jones DL (2013) How significant to plant N nutrition is the direct consumption of soil microbes by roots? New Phytol 199:948–955. https://doi.org/10.1111/nph.12320
Jones DL, Magthab EA, Gleeson DB, Hill PW, Sánchez-Rodríguez AR, Roberts P, Ge T, Murphy DV (2018) Microbial competition for nitrogen and carbon is as intense in the subsoil as in the topsoil. Soil Biol Biochem 117:72–82. https://doi.org/10.1016/j.soilbio.2017.10.024
Jones DL, Owen AG, Farrar JF (2002) Simple method to enable the high resolution determination of total free amino acids in soil solutions and soil extracts. Soil Biol Biochem 34:1893–1902. https://doi.org/10.1016/S0038-0717(02)00203-1
Joshi N, Gothalwal R, Singh M, Dave K (2021) Novel sulphur-oxidizing bacteria consummate sulphur deficiency in oil seed crop. Arch Microbiol 203:1–6. https://doi.org/10.1007/s00203-020-02009-4
Kaiser K, Guggenberger G (2005) Dissolved organic sulphur in soil water under Pinus sylvestris L. and Fagus sylvatica L. stands in northeastern Bavaria, Germany variations with seasons and soil depth. Biogeochemistry 72:337–364. https://doi.org/10.1007/s10533-004-0155-5
Kertesz MA, Mirleau P (2004) The role of soil microbes in plant sulphur nutrition. J Exp Bot 55:1939–1945. https://doi.org/10.1093/jxb/erh176
Kuzyakov Y, Xu X (2013) Competition between roots and microorganisms for nitrogen: mechanisms and ecological relevance. New Phytol 198:656–669. https://doi.org/10.1111/nph.12235
Ma Q, Cao X, Ma J, Tan X, Xie Y, Xiao H, Wu L (2017a) Hexavalent chromium stress enhances the uptake of nitrate but reduces the uptake of ammonium and glycine in pak choi (Brassica chinensis L.). Ecotoxicol Environ Saf 139:384–393. https://doi.org/10.1016/j.ecoenv.2017.02.009
Ma Q, Cao X, Tan X, Si L, Wu L (2017b) Effects of cadmium stress on pakchoi (Brassica chinensis L.) growth and uptake of inorganic and organic nitrogenous compounds. Environ Exp Bot 137:49–57. https://doi.org/10.1016/j.envexpbot.2017.02.001
Ma Q, Cao X, Xie Y, Gu Y, Feng Y, Mi W, Yang X, Wu L (2017c) Effect of pH on the uptake and metabolism of glycine in pak choi (Brassica chinensis L.). Environ Exp Bot 133:139–150. https://doi.org/10.1016/j.envexpbot.2016.10.013
Ma Q, Hill PW, Chadwick DR, Wu L, Jones DL (2021a) Competition for S-containing amino acids between rhizosphere microorganisms and plant roots: the role of cysteine in plant S acquisition. Biol Fertil Soils 57:825–836. https://doi.org/10.1007/s00374-021-01572-2
Ma Q, Kuzyakov Y, Pan W, Tang S, Chadwick DR, Wen Y, Hill PW, Macdonald A, Ge T, Si L, Wu L, Jones DL (2021b) Substrate control of sulphur utilisation and microbial stoichiometry in soil: results of 13C, 15N, 14C, and 35S quad labelling. ISME J 15:3148–3158. https://doi.org/10.1038/s41396-021-00999-7
Ma Q, Luo Y, Wen Y, Hill PW, Chadwick DR, Wu L, Jones DL (2020a) Carbon and sulphur tracing from soil organic sulphur in plants and soil microorganisms. Soil Biol Biochem 150:107971. https://doi.org/10.1016/j.soilbio.2020.107971
Ma Q, Pan W, Tang S, Sun X, Xie Y, Chadwick DR, Hill PW, Si L, Wu L, Jones DL (2021c) Maize and soybean experience fierce competition from soil microorganisms for the uptake of organic and inorganic nitrogen and sulphur: A pot test using 13C, 15N, 14C, and 35S labelling. Soil Biol Biochem 157:108260. https://doi.org/10.1016/j.soilbio.2021.108260
Ma Q, Tang S, Pan W, Zhou J, Chadwick DR, Hill PW, Wu L, Jones DL (2021d) Effects of farmyard manure on soil S cycling: substrate level exploration of high- and low-molecular weight organic S decomposition. Soil Biol Biochem 160:108359. https://doi.org/10.1016/j.soilbio.2021.108359
Ma Q, Wang J, Sun Y, Yang X, Ma J, Li T, Wu L (2018) Elevated CO2 levels enhance the uptake and metabolism of organic nitrogen. Physiol Plant 162:467–478. https://doi.org/10.1111/ppl.12663
Ma Q, Wen Y, Ma J, Macdonald A, Hill PW, Chadwick DR, Wu L, Jones DL (2020b) Long-term farmyard manure application affects soil organic phosphorus cycling: A combined metagenomic and 33P/14C labelling study. Soil Biol Biochem 149:107959. https://doi.org/10.1016/j.soilbio.2020.107959
Ma Q, Wen Y, Pan W, Macdonald A, Hill PW, Chadwick DR, Wu L, Jones DL (2020c) Soil carbon, nitrogen, and sulphur status affects the metabolism of organic S but not its uptake by microorganisms. Soil Biol Biochem 149:107943. https://doi.org/10.1016/j.soilbio.2020.107943
Ma Q, Wen Y, Wang D, Sun X, Hill PW, Macdonald A, Chadwick DR, Wu L, Jones DL (2020d) Farmyard manure applications stimulate soil carbon and nitrogen cycling by boosting microbial biomass rather than changing its community composition. Soil Biol Biochem 144:107760. https://doi.org/10.1016/j.soilbio.2020.107760
Maggioni A, Renosto F (1977) Cysteine and methionine regulation of sulfate uptake in potato tuber discs (Solanum tuberosum). Physiol Plant 39:143–147. https://doi.org/10.1111/j.1399-3054.1977.tb04026.x
Maruyama-Nakashita A (2017) Metabolic changes sustain the plant life in low-sulfur environments. Curr Opin Plant Biol 39:144–151. https://doi.org/10.1016/j.pbi.2017.06.015
Näsholm T, Ekblad A, Nordin A, Giesler R, Högberg M, Högberg P (1998) Boreal forest plants take up organic nitrogen. Nature 392:914–916. https://doi.org/10.1038/31921
Näsholm T, Kielland K, Ganeteg U (2009) Uptake of organic nitrogen by plants. New Phytol 182:31–48. https://doi.org/10.1111/j.1469-8137.2008.02751.x
Paungfoo-Lonhienne C, Lonhienne TG, Rentsch D, Robinson N, Christie M, Webb RI, Gamage HK, Carroll BJ, Schenk PM, Schmidt S (2008) Plants can use protein as a nitrogen source without assistance from other organisms. Proc Natl Acad Sci U S A 105:4524–4529. https://doi.org/10.1073/pnas.0712078105
Romero LC, Aroca MÁ, Laureano-Marín AM, Moreno I, García I, Gotor C (2014) Cysteine and cysteine-related signaling pathways in Arabidopsis thaliana. Mol Plant 7:264–276. https://doi.org/10.1093/mp/sst168
Scherer HW (2001) Sulphur in crop production — invited paper. Eur J Agron 14:81–111. https://doi.org/10.1016/S1161-0301(00)00082-4
Schneider S, Schintlmeister A, Becana M, Wagner M, Woebken D, Wienkoop S (2019) Sulfate is transported at significant rates through the symbiosome membrane and is crucial for nitrogenase biosynthesis. Plant Cell Environ 42:1180–1189. https://doi.org/10.1111/pce.13481
Seegmüller S, Rennenberg H (2002) Transport of organic sulfur and nitrogen in the roots of young mycorrhizal pedunculate oak trees (Quercus robur L.). Plant Soil 242:291–297. https://doi.org/10.1023/A:1016290324076
Svennerstam H, Jämtgård S, Ahmad I, Huss-Danell K, Näsholm T, Ganeteg U (2011) Transporters in Arabidopsis roots mediating uptake of amino acids at naturally occurring concentrations. New Phytol 191:459–467. https://doi.org/10.1111/j.1469-8137.2011.03699.x
Takagi H, Ohtsu I (2017) l-cysteine metabolism and fermentation in microorganisms. Adv Biochem Eng Biotechnol 159:129–151. https://doi.org/10.1007/10_2016_29
Takahashi H (2019) Sulfate transport systems in plants: functional diversity and molecular mechanisms underlying regulatory coordination. J Exp Bot 70:4075–4087. https://doi.org/10.1093/jxb/erz132
Vermeiren C, Smolders E, McLaughlin MJ, Degryse F (2018) Model-based rationalization of sulphur mineralization in soils using 35S isotope dilution. Soil Biol Biochem 120:1–11. https://doi.org/10.1016/j.soilbio.2018.01.013
Acknowledgements
This work was supported by the National Natural Science Foundation of China (32102488, 32172674, 31872180) and the UK–China Virtual Joint Centre for Agricultural Nitrogen (CINAg, BB/N013468/1), which is jointly supported by the Newton Fund, via UK BBSRC and NERC, and the Chinese Ministry of Science and Technology.
Author information
Authors and Affiliations
Contributions
QXM and DLJ designed the research, conducted the experiments, and wrote the manuscript; ST, WKP, DRC, and LHW revised the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests.
Additional information
Responsible Editor: Ad C. Borstlap.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ma, Q., Pan, W., Tang, S. et al. Plants can access limited amounts of nitrogen- and sulphur-containing amino acids in soil owing to rapid microbial decomposition. Plant Soil 480, 57–70 (2022). https://doi.org/10.1007/s11104-022-05557-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-022-05557-4