Abstract
Aims
Half of field crop nitrogen (N) is often derived soil organic matter (SOM) mineralization, yet we do not fully understand the extent to which plant genotypic differences influence SOM mineralization dynamics across different soil N contexts. We explored the effects of rapeseed (Brassica napus) genotypic diversity on N uptake from organic and inorganic N sources.
Methods
In a greenhouse study, we applied dual 15N labeled ammonium-nitrate fertilizer to examine N uptake patterns of rapeseed in different N environments. Ten varieties were grown in a full factorial experiment with four treatments, including combinations of high and low N fertilizer and SOM.
Results
We found limited varietal differences in total biomass or N uptake across soil environments. Across all varieties, SOM was an important, additive N source even as N fertilizer availability increased. High SOM/High Fertilizer treatment plants obtained 64% of N from SOM, while plants grown with High SOM/Low Fertilizer obtained 89% of total N from SOM. Under low SOM availability, the high fertilizer addition increased overall N uptake from SOM by 42% relative to the low N fertilizer treatment.
Conclusions
Integrating plant reliance on SOM-N sources into crop breeding and NUE estimates has potential to improve crop productivity and improve overall system N use efficiency.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Over the last half century, the application of mineral nitrogen (N) fertilizer has supported increased crop production while also more than doubling the availability of reactive N in the global environment (Zhang et al. 2015). Only about 50% of N fertilizer is taken up by crops in the year of application despite decades of research aimed at improving the delivery of fertilizer to plants (Yan et al. 2019) and large N losses from agricultural systems continue to impact water quality, contribute to greenhouse gas emissions, and negatively impact surrounding ecosystems (Sobota et al. 2015). Reducing reliance on synthetic N inputs and improving plant nitrogen use efficiency (NUE) are essential to improve agricultural sustainability.
Globally, N mineralized from soil organic matter (SOM) is estimated to provide about 50% of N taken up by global cereal crops even in intensive management systems (Chen et al. 2014; Gardner and Drinkwater 2009; Yan et al. 2019). While SOM is recognized as a source of plant available N in soil testing and fertilizer recommendations, there has been limited research focused on managing N mineralization processes through targeted crop management or crop breeding (although see recent examples such as Kelly et al. 2022; Mwafulirwa et al. 2021). As a result, we have a limited understanding of belowground activity that affects crop utilization of N derived from the mineralization of SOM. Most modern crops have been primarily bred for high grain yield and field agronomic performance in systems where N is not a limiting factor. While roots are known to mediate N uptake through physiological and morphological mechanisms (direct effect) and microbial activity (indirect effect), belowground traits are challenging to introduce into traditional breeding programs and have largely been ignored (Bouchet et al. 2016; Dawson et al. 2008; Jilling et al. 2018; Stahl et al. 2016).
Crop NUE is typically defined as the total plant biomass or seed yield divided by applied N. However, NUE is a complex trait that integrates the efficiency of plant N uptake from the soil environment as well as N utilization patterns within the plant (Bouchet et al. 2016; Kant et al. 2011; Perchlik and Tegeder 2017; Rathke et al. 2006). Due to the challenges of directly measuring soil N mineralization from SOM, N management and breeding efforts have primarily focused on either N fertilizer uptake efficiency (REN, the proportion of plant N uptake to N fertilizer applied) or the efficiency of internal N utilization (the proportion of N uptake translocated to produce seed at maturity) (Cassman et al. 2002; Kessel et al. 2012).
Root biomass and structure affect soil carbon (C) inputs and root exudates, which can have profound influences on the crop’s ability to obtain nitrogen from diverse sources (Bouchet et al. 2016). Root morphology is also important for nutrient uptake and has been shown to have a significant effect on aboveground growth and vigor (Garnett et al. 2009). A number of studies support that root traits, such as root-shoot ratios, root length density, and root N transport and metabolism could contribute to higher NUE in crops (Bingham et al. 2012; Bowles et al. 2015; Garnett et al. 2009; Ju et al. 2015). Belowground traits are recognized as important for N uptake and may be particularly important for improving utilization of N from SOM (Kelly et al. 2022).
The mineralization of soil N from SOM into plant available forms is highly complex and requires multiple steps. The depolymerization of SOM from more complex N-containing molecules, such as lignin and proteins, to more simple monomers, such as amino acids, has been proposed as the rate limiting step in most soil environments (Daly et al. 2021; Schimel and Bennett 2004). From these monomers, N is typically converted rapidly to the most common plant available mineral forms of ammonium and then, under aerobic environments via nitrification, to nitrate. Each of these processes is mediated by microbially-derived extracellular enzymes. Indirectly, roots can enhance or inhibit these processes via their effects on soil microbial communities and activity (Coskun et al. 2017a; Jilling et al. 2018). Root exudates can enhance microbial mineralization of C, N, and other essential nutrients, such as through increasing the availability of mineral-associated N-containing compounds for plant uptake (Faucon et al. 2017; Fontaine et al. 2003; Jilling et al. 2018). Plants can also influence the rate of SOM decomposition by stimulating or inhibiting microbial activity in general (Huo et al. 2017), or by inhibiting specific processes such as nitrification (Coskun et al. 2017b).
Rapeseed (Brassica napus), also known as canola and oilseed rape, is the second most important oilseed crop in the world. Rapeseed is highly valued for its widely used, high quality vegetable oil as well as high protein meal used in livestock feed (Stahl et al. 2016). Generally, rapeseed is considered a high N demanding crop with a low NUE compared to other field crops (Bouchet et al. 2016; Kessel et al. 2012; Rathke et al. 2006). The relatively low NUE is attributed primarily to a narrow acquisition efficiency of the crop (Rathke et al. 2006; Stahl et al. 2016). The focus on improving N management has led to more interest in breeding rapeseed varieties with increased NUE capacity. This research has demonstrated genetic variation in both REN and internal N utilization efficiency (Kessel et al. 2012; Stahl et al. 2016). Kessel et al. (2012) suggested that rapeseed REN showed more genetic variation at lower fertilizer N levels whereas N translocation dynamics within the plant contributed more to variation in NUE at high fertilizer N levels.
Understanding the belowground dynamics of N recovery from SOM, as well as applied N fertilizer, could be an important new direction for improving the sustainability of this globally important crop. Recent research has focused on root exudate effects on nitrification (Coskun et al. 2017b), but limited research has focused on plant mediation of upstream N mineralization processes such as depolymerization. We evaluated the effects of rapeseed genotypic diversity on N uptake from organic and inorganic N sources. We used 15N enriched ammonium-nitrate (NH4NO3) fertilizer and soil enzyme activity to determine differences in N acquisition and microbially-mediated N cycling from organic and inorganic N pools as influenced by rapeseed varieties. We hypothesized that different rapeseed varieties would differ in N source uptake patterns and soil enzyme activity.
Materials and methods
Experimental design
A greenhouse pot study was conducted at the Colorado State University Plant Growth Facility in Fort Collins, CO (40.5717° N, 105.0812° W) from June to September 2016. Ten rapeseed varieties were selected from the 51 founder lines of the Parkin et al. (2017) project, that were selected for development of a germplasm resource to dissect complex traits in B. napus. This project collected diverse rapeseed lines from around the world to make a nested association mapping (NAM) population that could be used to introduce new diversity into breeding germplasm (Parkin et al. 2017). The ten varieties for this study were selected using 12,612 single nucleotide polymorphisms (SNP) markers to capture the widest possible general genetic diversity based on genetic distance, and geographic locations of the markers while controlling for common flowering time (Table 1, Fig. 1).
Dendrogram of genetic relationships and distances of Brassica napus founder lines of Nested Association Mapping (NAM) project (Parkin et al. 2017). Genetic distances are presented on the Y axis and variety on the X axis. Blue text indicates varieties selected for this study
The rapeseed plants were grown in soil mixtures that represented 2 levels of SOM and 2 levels of N fertilizer for a total of 4 treatments (High SOM/High Fert, High SOM/Low Fert, Low SOM/High Fert, and Low SOM/Low Fert). To generate the SOM treatments, a high organic matter field soil was added to a non-soil mixture to create almost a 7-fold difference in soil organic matter levels across the high and low SOM treatments (Table 2). The field soil was a fine loamy Aridic Argiustoll with 67 g kg−1 organic matter (37 g kg−1 C, 3.8 g kg−1 N) collected from a farm near Fort Collins, Colorado, with a history of organic vegetable production. The field topsoil (0–10 cm) was collected and sieved to 8 mm. The non-soil mixture of 25% sand, 37.5% calcined clay, and 37.5% vermiculite by volume was homogenized. The field soil was mixed with this non-soil mixture at two different proportions by volume to create high SOM and low SOM treatments (Table 2) and homogenized using a clean cement mixer. Initial soil treatment properties were analyzed for texture, organic matter (loss on ignition), and extractable nutrients by Ward Lab (Lincoln, Nebraska) and results are presented in Table 2. The SOM levels were selected with the general assumption than 1–2% of total N in SOM would be mineralized during the short time period of the study, resulting in mineralization of approximately 50–100 mg N in high SOM and 10–20 mg N in low SOM treatments.
The plants were planted in randomized complete blocks with 5 blocks of each of the 4 treatments with each of the 10 varieties for a total of 200 pots. Each block was planted 1 week apart for 5 weeks. The planting was staggered to allow for staggered harvest and time-sensitive soil analyses to be completed for each block. Greenhouse conditions included 16 h of light per day and approximately 15.6 °C nighttime and 26.7 °C daytime temperatures.
Four seeds were planted into a 3.8-l pot (16.5 cm top diameter, 12.7 cm bottom diameter, 16.5 cm height) and thinned to one plant per pot one week after planting. Fertilizer treatments were initiated at 2 weeks after planting when the first true leaves were beginning to emerge. Once per week, 50 ml of N-free Hoagland’s nutrient solution was applied to each pot to ensure that nutrients other than N were not limiting across all treatments. A 15N enriched N fertilizer solution using 98% 15N enriched dual labeled NH4NO3, diluted down to 8% 15N enrichment, was applied weekly as a liquid solution to the soil surface to obtain the specified total N additions for the high and low fertilizer rates (Balint and Rengel 2008; Damon et al. 2007). Fertilizer rates of 50 mg N pot−1 for Low Fert treatments and 150 mg N pot−1 for High Fert treatments were chosen to provide sufficient N through vegetative growth based on estimates from Balint and Rengel (2008). Based on the treatment, supplemental 15N enriched fertilizer was applied weekly to achieve desired N rates. The second week after planting, 100 ml of N-fertilizer solution was applied, and 50 ml was applied in all subsequent weeks for a total of 5 fertilizer applications. For the Low Fert treatment, 10 mg N were added each week for a total of 50 mg N. For the High Fert treatment, 50 mg N were added with the first application and 25 mg N was added in each subsequent week for a total of 150 mg N. Any liquid that ran through the pot was caught in the trays below the pot and added back into the pot to eliminate N loss by leaching.
The irrigation system was started three weeks after planting and water was applied for two minutes each day using drip emitters. No additional irrigation water was given on days when liquid N fertilizer was applied. Due to differences in soil texture across treatments, a soil moisture probe (Hydrosense II, Campbell Scientific, Logan, UT) was used in the pots twice a week to measure pot moisture and the irrigation amounts were adjusted to equalize volumetric water content to approximately 20% across all treatments and blocks.
Because our research question was about N acquisition from the soil environment and not internal N translocation patterns, we sampled plants at peak biomass. Each block was destructively harvested at 39–41 days after planting when about 75% of the plants in a block were at the elongation stage before flowering. Each individual pot in the block was photographed, weighed, and notes were recorded for the number of leaves per plant and the growth stage (pre-bud, bud, or early flowering). The plant was clipped at the base of the stem. The clipped plant shoot was put in a paper bag and dried at 55 °C for at least 5 days and weighed for dry shoot biomass.
The pot of soil was turned upside down in a clean tub. The loose soil was gently brushed off leaving the root ball and the rhizosphere soil surrounding the roots. The rhizosphere soil and root ball and the bulk soil were placed in separate zip lock bags and placed in a cooler with ice until they were placed in cold storage for further processing. A subsample of the bulk soil from each pot was weighed and dried at 105 °C for a minimum of 48 h to determine soil gravimetric water content.
Enzyme activity
The activity of four soil enzymes involved in SOM decomposition and soil nutrient cycling were measured using fluorescence-based enzyme activity assays. We included enzymes involved in C, N, and P mineralization processes (Table 3). In particular, we focused on enzymes involved in catalyzing the conversion of more complex N-containing compounds to amino sugars or amino acids (NAG, LAP, Table 3). We included corresponding C and P cycling enzymes to evaluate whether enzyme activity effects were specific to N-cycling processes or broader shifts in soil microbial activity. Rhizosphere soil samples from each pot were analyzed using a microplate fluorescence-based MUB (4-methylumbelliferone) and MUC (7-amino-4-methylcoumarin) substrate protocol (Bell et al. 2013). Briefly, the day after the plants were harvested, 1.1–1.3 g of soil was weighed from the rhizosphere soil sample. The soil was blended to homogenize sample with a 50 mM sodium acetate buffer solution, that had been adjusted to the average soil pH of 7.5 to make a soil slurry. Soil slurry was pipetted into black, 96-well microplates with compound-specific fluorescing substrates. Samples were analyzed using a Tecan Infinite M200 plate reader (Tecan Austria GmbH, Salzburg, Austria).
Inorganic nitrogen
A 10 g sample of the rhizosphere soil from each pot was extracted with 100 mL of a 2 M potassium chloride (KCl) solution to analyze levels of extractable ammonium (NH4+) and nitrate (NO3−) in the soil at the time of harvest using the microplate colorimetric method (Sims et al. 1995). The vanadium (III) chloride (VCl3) protocol was used to determine soil NO3−, where 30 μL of the KCl extracted sample was pipetted into microplates with 0.4 M VCl3 solution (Doane and Horwáth 2003). The salicylate-hypochlorite method was used to determine soil NH4+, where 70 μL of KCl extracted sample was used in each of the microplate wells. Both assay reactions were read on a microplate reader (BioTek Instruments, Winooski, VT). Inorganic N values are not dependent on dry plant biomass so, all 5 blocks of data were used for analysis.
Nitrogen source analysis
Isotopic values of the dried plant shoots were analyzed to determine the relative contributions of the fertilizer and SOM to plant N content. Dried plant samples were ground to 2 mm in a Wiley Mill and then roller ground until the sample was homogenized. All samples were analyzed for total C, total N and 15N at EcoCore Analytical Services Lab, at Colorado State University, Fort Collins, CO, using an Elemental-Analyzer – Isotope Ratio Mass Spectrometry (Costech, Valencia, CA).
The contributions of the N from the labeled inorganic 15N and the organic N acquired from the SOM were calculated by applying the isotopic mixing model (Hauck and Bremner 1976). The fraction of fertilizer-derived N (ffertilizer) was calculated using Eq. 1:
Where 15N sample, 15N soil, and 15N fertilizer represent the atom % 15N of the total sample, natural abundance of the soil mixture, and fertilizer (8 atom % 15N) respectively. The value for 15N sample was the sample value output from EA-IRMS analysis. The 15N Soil was the background atom % 15N of the soil mixture, 0.3681 atom % 15N for low SOM and 0.3699 atom % 15N for High SOM treatments. The contribution of soil derived N was calculated using Eq. 2:
Where fSOM is the fraction of plant aboveground N from SOM.
The REN was calculated using Eq. 3:
Root biomass
The root biomass was obtained by washing the growth media away from the bulk and rhizosphere samples of blocks 1, 2 and 3. The washed roots were dried for a minimum of 5 days at 55 °C and weighed for dry biomass. The samples were a mixture of roots, vermiculite, and particulate organic matter. The root samples were homogenized in a ball grinder. Each root sample was analyzed for organic content using the ash correction protocol to obtain an estimated root biomass for each sample (Nelson and Sommers 1996). Briefly, the ground sample was weighed in tin weigh boats and placed into a 105 °C oven for 24 h. The sample was weighed again and then placed into a cold muffle furnace and heated to 450 °C for 4 h. Once samples were cooled to at least 200 °C, they were weighed again. The difference in the sample weights were used to correct root weights for inorganic compounds and get an ash corrected estimated root biomass for each sample (Harmon et al. 1999). The root-shoot ratio was calculated as the ash corrected root biomass estimate divided by the dry shoot weight.
Data analysis
The data were analyzed in R, using a mixed model approach (R Core Team 2019). Due to a data loss of block 2 aboveground dry plant weights, only four of the five blocks were used in analyses that relied on plant biomass. Due to the loss of root biomass data, only blocks 1 and 3 were included in the root-shoot ratio estimates. Block was included as a random variable, while the fixed predictor variables were rapeseed variety and treatment. The response variables included the four different enzyme activities, plant N content, SOM and Fertilizer N uptake, percent N from SOM and fertilizer, total soil inorganic N (NH4+-N + NO3−-N), soil NH4+, soil NO3−, dry root and shoot biomass, and root-shoot ratio. We used the lme() function in the nlme package to allow for unequal variances (Pinheiro et al. 2018) with a few exceptions. The REN response variable was analyzed with the lemr() function. For NH4+ and NO3−, the data were transformed by taking the square root and soil inorganic N was log-transformed and then analyzed using lemr() function of the Lme4 package (Bates et al. 2015). Due to near zero nitrate levels in some samples, some samples had negative values after subtracting sample blanks. Therefore, a constant was added to make all values positive before applying the square root transformation. These values were then analyzed with the lme() function from the nlme package (Pinheiro et al. 2018). A type three analysis of variance Anova() with the Kenward-Roger approximation for degrees of freedom was used from the car package (Fox et al. 2019). The emmeans function, from the emmeans package, was used to make pairwise comparisons of significant predictors (Lenth et al. 2018). P values less than 0.05 were considered significant.
Results
Plant growth and development
The SOM and Fert N treatments resulted in a range of N available for plant uptake and biomass production. There was almost a 3-fold difference in biomass ranging from High SOM/High Fert treatment with an average shoot biomass of 4.46 ± 0.24 g plant−1 and plants grown in the Low SOM/Low Fert treatment that had the lowest biomass of 1.62 ± 0.19 g plant−1 (Table 4). Plant biomass production was similar for plants in the High SOM/Low Fert and Low SOM/High Fert treatments (Table 4). Variety had a modest effect on plant biomass (p = 0.04), but post-hoc tests found no differences between any two specific varieties and there were no interactions between variety and treatment (Table 5).
Plant development was impacted by soil treatment, block and variety. There were small differences in phenological stage (pre-bud, bud, early flowering) by block and variety, but no soil treatment effects (data not shown). There were, however, differences in leaf number by soil treatment, block, and variety. The Low SOM/Low Fert treatment had lower mean leaf number than all of the other soil treatments at the time of harvest (Supplemental Table 1). Root biomass also differed by soil treatment (p < 0.0001, Table 4). There was no difference in root biomass between the High SOM/High Fert and the High SOM/Low Fert treatments, but there were differences among all other treatments (Table 4). The root biomass of the High SOM treatments was 2 to 4-fold higher than the Low SOM treatments (Table 4). The root to shoot ratio also differed by treatment (p = 0.0009, Table 4). The highest average root to shoot ratio was the High SOM/Low Fert treatment, followed by the High SOM/High Fert treatment. Variety did not have a significant effect on root biomass (p = 0.42).
Plant N content and source
Total plant N content differed across all four treatments. Plants in the High SOM/High Fert treatment had the highest total N (195 ± 6 mg N), and the highest amount of N from SOM (125 ± 5 mg N) (Fig. 2). Plants in the High SOM/Low Fert treatment had 35% less total plant N content as plants in the High SOM/High Fert treatment but accessed a similar amount of N from SOM across both High SOM treatments (Fig. 2). Conversely, plants in the Low SOM/High Fert had 30% more N uptake from SOM than plants in the Low SOM/Low Fert treatment (Fig. 2). Variety did not have a significant effect on total plant N uptake, the amount of N taken up from Fert, or N from SOM (p = 0.57, p = 0.65, p = 0.33, respectively).
Least squares means of total plant nitrogen (N) from fertilizer (Fert) and soil organic matter (SOM) across 10 rapeseed varieties by treatment from mixed model ANOVA with block as a random factor and soil treatment as a fixed factor. Error bars represent standard error (n = 10). Letters denote a significant difference between treatments for each N source (SOM or fertilizer) (p < 0.05)
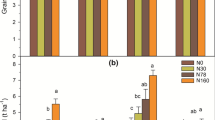
The proportion of N acquisition from fertilizer and SOM sources differed among all four treatments (Fig. 3). SOM was an important source of N for plants in all four treatments, ranging from 33% to 89% of total plant N. The extreme treatments High SOM/High Fert and Low SOM/Low Fert both obtained 64% of their total N uptake from SOM (Fig. 3), indicating a similar balance in N availability between fertilizer and SOM sources at the high and low end of the N gradient created across the 4 treatments. In contrast, High SOM/Low Fert obtained 89% of the total N from SOM, and only 11% from fertilizer, which was more than double the Low SOM/High Fert treatment that obtained 33% of N from SOM (Fig. 3).
Percentage of plant nitrogen (N) obtained from fertilizer (Fert) and soil organic matter (SOM) across 10 rapeseed varieties from mixed model ANOVA with block as a random factor and soil treatment as a fixed factor. Error bars represent standard error of % N from SOM (n = 10). Letters denote a significant difference between treatments for each N source (p < 0.05)
The REN was also affected by treatment (p = 0.012, Table 4). However, the REN values had a relatively narrow range from 44% to 49% across treatments (Table 4). The Low SOM/High Fert treatment had the highest fertilizer REN. Pairwise comparisons between treatments show that only the Low SOM/High Fert and the Low SOM/Low Fert were significantly different from each other. Variety did not have a significant effect on REN (Table 4).
Variety had a significant effect on the percentage of total plant N that came from fertilizer and SOM (p = 0.0022, Table 5). Although variety was significant in the percentage of total plant N, the interaction between variety and total plant N was not significant (p = 0.34). Similar to the plant biomass results and likely due to the high number of varieties evaluated, no single variety was significantly different from another in post-hoc analyses that adjusted for the multiple comparisons (Table 5). Variety did not influence any other aspects of N content or source (p > 0.05).
Inorganic nitrogen
The SOM and fertilizer treatments influenced extractable soil inorganic N. The highest to lowest average soil inorganic N by treatment was as follows: High SOM/High Fert, High SOM/Low Fert, Low SOM/High Fert, Low SOM/Low Fert (Table 4). The two extreme treatments differed from one another, but inorganic N availability was similar for the middle treatments (High SOM/Low Fert and Low SOM/High Fert). While total inorganic N was significantly affected by treatment, there was no treatment effect on the individual amounts of soil extractable NH4+ or NO3− (Table 4). Total extractable soil inorganic N, NO3−, or NH4+ did not differ by rapeseed variety (Table 4).
Enzyme activity
Enzyme activity was consistently higher in High SOM soils relative to Low SOM soils (Fig. 4). Variety was not a significant predictor for any of the measured enzymes (p > 0.05). Due to the strong effect of SOM level on background soil enzyme activity, we analyzed correlations between soil enzyme activity and plant N uptake from SOM within each SOM level and found weak positive associations between soil enzyme activity and plant N uptake from SOM only within the low SOM treatment (R2 = 0.04, 0.08, 0.09, 0.07; p = 0.06, 0.01, 0.01, 0.01 for NAG, LAP, BG, and TAP enzymes respectively). There were no significant correlations between soil enzyme activity and plant N from SOM within the high SOM treatments (p > 0.05).
Least squares means of soil enzyme activity (nmol per gram of dried soil per hour) across 10 rapeseed varieties from mixed model ANOVA with block as a random factor and soil treatment as a fixed factor. Error bars represent standard errors (n = 10). Enzyme types are represented as Leucine amino peptidase (LAP), B-1, 4-n-acetyl-glycosaminidase (NAG), B-1, 4-glucosidase (BG), and tobacco acid pyrophosphatase (TAP). Letters indicate the significant differences between treatments for each enzyme (p < 0.05)
Discussion
Our results highlight the potential importance of organic N sources for rapeseed growth but did not identify specific genotypic traits to enhance N uptake from SOM. Across all treatments, rapeseed plants effectively accessed N from SOM for crop growth and the effect was additive and not a substitute for fertilizer. The enhanced productivity when plants were supplied with both SOM and fertilizer N sources suggests than an integrated nutrient management (INM) system is likely to be an effective approach for improving N uptake and reducing N losses in rapeseed cropping systems. This approach actively manages organic and inorganic N pools and strategically uses all available nutrient sources (Drinkwater and Snapp 2007).
Rapeseed studies have primarily focused on enhancing REN and improving inorganic N fertilizer management in conventional management systems (Chamorro et al. 2002; Gan et al. 2008; Ma and Herath 2016; Stahl et al. 2016) and have rarely directly measured N derived from SOM. Regardless of total N available, only 44%–49% of the applied N fertilizer was taken up into plant shoots (Table 4). This low rate of REN is within the range of REN from field-based studies of other grain crops worldwide (Yan et al. 2019). As N fertilizer losses from agricultural systems continue to have major environmental impacts there has been an increased interest in INM. The goal of INM is to integrate the use of synthetic and biological plant nutrient sources to optimize crop production and resource conservation (Gruhn et al. 2000). Most INM studies have focused on developing countries with degraded soils and on rice, maize and wheat crops (Zhang et al. 2011). In INM systems with higher SOM, crops show improved performance, while reducing the need for inorganic N additions (Zhou et al. 2019). While oilseed rape is a major worldwide crop, fewer INM studies to this point have focused on rapeseed. Studies looking at INM in rapeseed systems have focused on organic fertilizer additions and agronomic practices, and have not focused on the importance of SOM as an N source.
There was an interactive effect between N acquisition from SOM and fertilizer that suggested that any plant-mediated effects on SOM decomposition likely shift as plants become more N limited. The rhizosphere priming effect is dependent on the relative availability of mineral N and labile C (Kuzyakov 2002). Previous studies have found a net positive priming effect with N fertilizer additions, but generally only when there is also an addition of labile C such as from root exudates (Chen et al. 2014. In our study, plants growing in the Low SOM/Low Fert treatment had significantly less N derived from SOM than in the Low SOM/High Fert treatment (Fig. 2). The higher degree of N limitation in the Low SOM/Low Fert treatment reduced plant development and root growth, likely limiting root foraging and rhizosphere effects on SOM mineralization (Kuzyakov 2002). In contrast, the Low SOM/High Fert treatment had an average plant biomass that was twice that of the Low SOM/Low Fert treatment and a root biomass that was about 70% greater (Table 4). Larger root systems can contribute more labile C, indirectly stimulating N mineralization via microbial processes, or directly enhancing N availability by destabilizing mineral-associated organic compounds (Jilling et al. 2018; Kuzyakov 2002). This larger root system also likely contributed to the modest increase in REN from 44% to 49% of plants in the Low SOM/Low Fert and Low SOM/High Fert treatments, respectively (Table 4).
However, plants increased the relative allocation of resources to support belowground biomass under greater N limitation. We found greater root-shoot ratio under the Low SOM/Low Fert relative to the Low SOM/High Fert (Table 4). Within the high SOM treatments, the High SOM/High Fert treatment also obtained more N from SOM than the High SOM/Low Fert treatment and had slightly lower root-shoot ratio, but the differences were not significant (Table 4). This suggests that plants in the High SOM treatments were not as N limited and the added N fertilizer had less of an effect on belowground C dynamics.
We found that High SOM treatments had higher enzyme activity, in all four measured enzymes, than the Low SOM treatments (Fig. 4). Soil organic matter provides both habitat and a food source that can support larger and more active microbial communities. To access C and other nutrients in SOM, soil microorganisms produce exoenzymes to catalyze SOM decomposition and nutrient mineralization. Thus, it is not surprising that enzyme activity was higher in high SOM treatments as the high SOM treatments likely started with higher microbial biomass as well as potentially higher residual exoenzymes in the substrate (Kögel-Knabner 2002). In addition, soil N cycling enzymes (NAG and LAP) did not show stronger relationships with plant N content relative to the C and P cycling enzymes (BG and TAP). Thus, the soil environment had a large impact on soil microbial activity, but we were unable to link plant N acquisition to specific microbial N cycling enzyme activity.
We did not find differences between specific varieties in our study for any response variable. Rather, we found a relatively narrow range in REN across all of the varieties included. All varieties in this study were open pollinated varieties chosen based on common spring flowering time and overall genomic diversity. We had limited knowledge of the genetic variation in belowground traits of the varieties included in this study. Some studies suggest that high yielding herbicide tolerant hybrid rapeseed varieties have different N requirements and uptake patterns than open pollinated varieties. Hybrid varieties could have been included in this study to examine differences in uptake patterns (Brandt et al. 2007; Harker et al. 2012; Karamanos et al. 2007; Smith et al. 2010). While we did not find varietal differences in this study, there is evidence of genomic links between belowground traits and N uptake from other studies that suggest it is possible to incorporate NUE of root systems into a breeding program (Coque et al. 2008; Kelly et al. 2022; Mwafulirwa et al. 2021).
Although this study did not find significant effects of variety on N content and source, this study provides a new understanding of rapeseed response to different N environments and the importance of SOM as a N source and could be valuable for future studies focused on belowground trait genetics. In particular, the additive effect of these different N sources suggests that there may be distinct traits that foster uptake of these different N pools. Our study examined N content in the shoots at the plant bolting stage. After this stage, internal N translocation dynamics can influence measurements of NUE at seed maturity (Balint and Rengel 2008). However, N uptake does continue as reproduction and internal translocation processes proceed, and future studies should consider N content at multiple stages and include assessment at grain maturity. While greenhouse pot studies are valuable for more controlled mechanistic studies, further examination of N source uptake in a field setting will be needed to further guide integrated nutrient management recommendations.
Conclusion
Much of sustainable agriculture research has focused on how to decrease the need for surplus N fertilizer additions by increasing fertilizer use efficiency (REN) of crops. While many studies have focused on N fertilizer uptake and use, we focused on the belowground mechanisms in relation to N uptake from SOM. Our results indicate the importance of SOM as an N source to support crop growth, even when an abundance of mineral N is available. Our results also suggest that the interaction between plants and soil environments mediates the dynamic nature of N uptake as demonstrated by reduced fertilizer N uptake efficiency as plants became more N limited. These findings support an integrated nutrient management system that manages both synthetic and organic N sources for effective nutrient management in rapeseed cropping systems.
Availability of data and material
Data will be placed in a public database at time of publication.
Code availability
Not applicable.
References
Balint T, Rengel Z (2008) Nitrogen efficiency of canola genotypes varies between vegetative stage and grain maturity. Euphytica 164:421–432. https://doi.org/10.1007/s10681-008-9693-6
Bates D, Mächler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67(1):1–48. https://doi.org/10.18637/jss.v067.i01
Bell CW, Fricks BE, Rocca JD, Steinweg JM, McMahon SK, Wallenstein MD (2013) High-throughput fluorometric measurement of potential soil extracellular enzyme activities. J Vis Exp 81:e50961. https://doi.org/10.3791/50961
Bingham I, Karley A, White P, Thomas W, Russell J (2012) Analysis of improvements in nitrogen use efficiency associated with 75 years of spring barley breeding. Eur J Agron 42:49–58. https://doi.org/10.1016/j.eja.2011.10.003
Bouchet A-S, Laperche A, Bissuel-Belaygue C, Snowdon R, Nesi N, Stahl A (2016) Nitrogen use efficiency in rapeseed. A review. Agron Sustain Dev 36:1–20. https://doi.org/10.1007/s13593-016-0371-0
Bowles TM, Raab PA, Jackson LE (2015) Root expression of nitrogen metabolism genes reflects soil nitrogen cycling in an organic agroecosystem. Plant Soil 392:175–189. https://doi.org/10.1007/s11104-015-2412-x
Brandt S, Malhi S, Ulrich D, Lafond G, Kutcher H, Johnston A (2007) Seeding rate, fertilizer level and disease management effects on hybrid versus open pollinated canola (Brassica napus L.). Can J Plant Sci 87:255–266. https://doi.org/10.4141/P05-223
Cassman KG, Dobermann A, Walters DT (2002) Agroecosystems, nitrogen-use efficiency, and nitrogen management. Ambio 31:132–140. https://doi.org/10.1579/0044-7447-31.2.132
Chamorro AM, Tamagno LN, Bezus R, Sarandón SJ (2002) Nitrogen accumulation, partition, and nitrogen-use efficiency in canola under different nitrogen availabilities. Commun Soil Sci Plan 33:493–504. https://doi.org/10.1081/CSS-120002759
Chen R, Senbayram M, Blagodatsky S, Myachina O, Dittert K, Lin X, Blagodatskaya E, Kuzyakov Y (2014) Soil C and N availability determine the priming effect: microbial N mining and stoichiometric decomposition theories. Glob Chang Biol 20:2356–2367. https://doi.org/10.1111/gcb.12475
Coque M, Martin A, Veyrieras J, Hirel B, Gallais A (2008) Genetic variation for N-remobilization and postsilking N-uptake in a set of maize recombinant inbred lines. 3. QTL detection and coincidences. Theor Appl Genet 117:729–747. https://doi.org/10.1007/s00122-008-0815-2
Coskun D, Britto DT, Shi W, Kronzucker HJ (2017a) How plant root exudates shape the nitrogen cycle. Trends Plant Sci 22:661–673. https://doi.org/10.1016/j.tplants.2017.05.004
Coskun D, Britto DT, Shi W, Kronzucker HJ (2017b) Nitrogen transformations in modern agriculture and the role of biological nitrification inhibition. Nat Plants 3:1–10. https://doi.org/10.1038/nplants.2017.74
Daly AB, Jilling A, Bowles TM, Buchkowski RW, Frey SD, Kallenbach CM, Keiluweit M, Mooshammer M, Schimel JP, Grandy AS (2021) A holistic framework integrating plant-microbe-mineral regulation of soil bioavailable nitrogen. Biogeochemistry 154:211–229. https://doi.org/10.1007/s10533-021-00793-9
Damon P, Osborne L, Rengel Z (2007) Canola genotypes differ in potassium efficiency during vegetative growth. Euphytica 156:387–397. https://doi.org/10.1007/s10681-007-9388-4
Dawson JC, Huggins DR, Jones SS (2008) Characterizing nitrogen use efficiency in natural and agricultural ecosystems to improve the performance of cereal crops in low-input and organic agricultural systems. Field Crop Res 107:89–101. https://doi.org/10.1016/j.fcr.2008.01.001
Doane TA, Horwáth WR (2003) Spectrophotometric determination of nitrate with a single reagent. Anal Lett 36:2713–2722. https://doi.org/10.1081/AL-120024647
Drinkwater LE, Snapp S (2007) Nutrients in agroecosystems: rethinking the management paradigm. Adv Agron 92:163–186. https://doi.org/10.1016/S0065-2113(04)92003-2
Faucon M-P, Houben D, Lambers H (2017) Plant functional traits: soil and ecosystem services. Trends Plant Sci 22:385–394. https://doi.org/10.1016/S0065-2113(04)92003-2
Fontaine S, Mariotti A, Abbadie L (2003) The priming effect of organic matter: a question of microbial competition? Soil Biol Biochem 35:837–843. https://doi.org/10.1016/S0038-0717(03)00123-8
Fox J, Weisberg S, An R (2019) Companion to applied regression, 3rd edn. Sage, Thousand Oaks
Gan Y, Malhi S, Brandt S, Katepa-Mupondwa F, Stevenson C (2008) Nitrogen use efficiency and nitrogen uptake of juncea canola under diverse environments. Agron J 100:285–295. https://doi.org/10.2134/agronj2007.0229
Gardner JB, Drinkwater LE (2009) The fate of nitrogen in grain cropping systems: a meta-analysis of 15N field experiments. Ecol Appl 19:2167–2184. https://doi.org/10.1890/08-1122.1
Garnett T, Conn V, Kaiser BN (2009) Root based approaches to improving nitrogen use efficiency in plants. Plant Cell Environ 32:1272–1283. https://doi.org/10.1111/j.1365-3040.2009.02011.x
Gruhn P, Goletti F, Yudelman M (2000) Integrated nutrient management, soil fertility, and sustainable agriculture: current issues and future challenges. Food, Agriculture, and the Environment- Discussion Paper 32, Intl Food Policy Res Inst
Harker KN, O'Donovan JT, Turkington TK, Blackshaw RE, Lupwayi NZ, Smith EG, Klein-Gebbinck H, Dosdall LM, Hall L, Willenborg CJ, Kutcher HR, Malhi SS, Vera CL, Gan Y, Lafond GP, May WE, Grant CA, McLaren DL (2012) High-yield no-till canola production on the Canadian prairies. Can J Plant Sci 92:221–233. https://doi.org/10.4141/cjps2011-125
Harmon ME, Nadelhoffer KJ, Blair JM (1999) Measuring decomposition, nutrient turnover, and stores in plant litter. In: Standard soil methods for long-term ecological research. Oxford University Press, New York
Hauck R, Bremner J (1976) Use of tracers for soil and fertilizer nitrogen research. Adv Agron 28:219–266. https://doi.org/10.1016/S0065-2113(08)60556-8
Huo C, Luo Y, Cheng W (2017) Rhizosphere priming effect: a meta-analysis. Soil Biol Biochem 111:78–84. https://doi.org/10.1016/j.soilbio.2017.04.003
Jilling A, Keiluweit M, Contosta AR, Frey S, Schimel J, Schnecker J, Smith RG, Tiemann L, Grandy AS (2018) Minerals in the rhizosphere: overlooked mediators of soil nitrogen availability to plants and microbes. Biogeochemistry 139:103–122. https://doi.org/10.1007/s10533-018-0459-5
Ju C, Buresh RJ, Wang Z, Zhang H, Liu L, Yang J, Zhang J (2015) Root and shoot traits for rice varieties with higher grain yield and higher nitrogen use efficiency at lower nitrogen rates application. Field Crop Res 175:47–55. https://doi.org/10.1016/j.fcr.2015.02.007
Kant S, Bi Y-M, Rothstein SJ (2011) Understanding plant response to nitrogen limitation for the improvement of crop nitrogen use efficiency. J Exp Bot 62:1499–1509. https://doi.org/10.1093/jxb/erq297
Karamanos R, Goh T, Flaten D (2007) Nitrogen and Sulphur fertilizer management for growing canola on Sulphur sufficient soils. Can J Plant Sci 87:201–210. https://doi.org/10.4141/P06-133
Kelly C, Haddix ML, Byrne PF, Cotrufo MF, Schipanski M, Kallenbach CM, Wallenstein MD, Fonte SJ (2022) Divergent belowground carbon allocation patterns of winter wheat shape rhizosphere microbial communities and nitrogen cycling activities. Soil Biol Biochem 165:108518. https://doi.org/10.1016/j.soilbio.2021.108518
Kessel B, Schierholt A, Becker HC (2012) Nitrogen use efficiency in a genetically diverse set of winter oilseed rape (Brassica napus L.). Crop Sci 52:2546–2554. https://doi.org/10.2135/cropsci2012.02.0134
Kögel-Knabner I (2002) The macromolecular organic composition of plant and microbial residues as inputs to soil organic matter. Soil Biol Biochem 34:139–162. https://doi.org/10.1016/S0038-0717(01)00158-4
Kuzyakov Y (2002) Factors affecting rhizosphere priming effects. J Plant Nutr Soil Sci 165:382–396. https://doi.org/10.1002/1522-2624(200208)165:4<382::AID-JPLN382>3E3.0.CO;2-%23
Lenth R, Singmann H, Love J, Buerkner P, Herve M (2018) Emmeans: Estimated marginal means, aka least-squares means. R package version 1.3. 1
Ma B, Herath A (2016) Timing and rates of nitrogen fertiliser application on seed yield, quality and nitrogen-use efficiency of canola. Crop Pasture Sci 67:167–180. https://doi.org/10.1071/CP15069
Mwafulirwa L, Baggs EM, Russell J, Hackett CA, Morley N, De la Fuente CC, Paterson E (2021) Identification of barley genetic regions influencing plant–microbe interactions and carbon cycling in soil. Plant Soil 468:165–182. https://doi.org/10.1007/s11104-021-05113-6
Nelson DW, Sommers LE (1996) Total carbon, organic carbon, and organic matter. In: PA Sparks, Helmke PA, Loeppert RH, Soltanpour PN, Tabatabai MA, Johnston CT, Sumner ME (ed) Methods of Soil Analysis: Part 3 Chemical Methods, 53. 5 edn, Madison
Parkin I, Vail S, Robinson S (2017) Development of a germplasm resource to dissect complex traits in Brassica napus. Agriculture Development Fund, Government of Saskatchewan
Perchlik M, Tegeder M (2017) Improving plant nitrogen use efficiency through alteration of amino acid transport processes. Plant Physiol 175:235–247. https://doi.org/10.1104/pp.17.00608
Pinheiro J, Bates D, Deb Roy S, Sarkar D, Team RC (2018) nlme: linear and nonlinear mixed effects models. R package version 3.1–137. Vienna, Austria
R Core Team (2019) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Rathke G-W, Behrens T, Diepenbrock W (2006) Integrated nitrogen management strategies to improve seed yield, oil content and nitrogen efficiency of winter oilseed rape (Brassica napus L.): a review. Agric Ecosyst Environ 117:80–108. https://doi.org/10.1016/j.agee.2006.04.006
Schimel JP, Bennett J (2004) Nitrogen mineralization: challenges of a changing paradigm. Ecology 85:591–602. https://doi.org/10.1890/03-8002
Sims G, Ellsworth T, Mulvaney R (1995) Microscale determination of inorganic nitrogen in water and soil extracts. Commun Soil Sci Plan 26:303–316. https://doi.org/10.1080/00103629509369298
Smith EG, Upadhyay BM, Favret ML, Karamanos RE (2010) Fertilizer response for hybrid and open-pollinated canola and economic optimal nutrient levels. Can J Plant Sci 90:305–310. https://doi.org/10.4141/CJPS09027
Sobota DJ, Compton JE, McCrackin ML, Singh S (2015) Cost of reactive nitrogen release from human activities to the environment in the United States. Environ Res Lett 10:025006. https://doi.org/10.1088/1748-9326/10/2/025006
Stahl A, Friedt W, Wittkop B, Snowdon RJ (2016) Complementary diversity for nitrogen uptake and utilisation efficiency reveals broad potential for increased sustainability of oilseed rape production. Plant Soil 400:245–262. https://doi.org/10.1007/s11104-015-2726-8
Yan M, Pan G, Lavallee JM, Conant RT (2019) Rethinking sources of nitrogen to cereal crops. Glob Chang Biol 26:191–199. https://doi.org/10.1111/gcb.14908
Zhang F, Cui Z, Fan M, Zhang W, Chen X, Jiang R (2011) Integrated soil–crop system management: reducing environmental risk while increasing crop productivity and improving nutrient use efficiency in China. J Environ Qual 40(4):1051–1057. https://doi.org/10.1016/B978-0-12-394277-7.00001-4
Zhang X, Davidson EA, Mauzerall DL, Searchinger TD, Dumas P, Shen Y (2015) Managing nitrogen for sustainable development. Nature 528:51–59. https://doi.org/10.1038/nature15743
Zhou B, Sun X, Wang D, Ding Z, Li C, Ma W, Zhao M (2019) Integrated agronomic practice increases maize grain yield and nitrogen use efficiency under various soil fertility conditions. Crop J 7:527–538. https://doi.org/10.1016/j.cj.2018.12.005
Acknowledgements
We would like to thank M. Wallenstein, R. Fletcher, and J. Vivanco for helpful comments on this manuscript. This research was supported by funding from Cargill to C. Carter and from the Colorado Agricultural Experiment Station (Project #1019781) to M. Schipanski.
Funding
This project was funded by Cargill and USDA Hatch funds.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Not applicable.
Additional information
Responsible Editor: Devrim Coskun.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 17 kb)
Rights and permissions
About this article
Cite this article
Carter, C., Schipanski, M.E. Nitrogen uptake by rapeseed varieties from organic matter and inorganic fertilizer sources. Plant Soil 474, 499–511 (2022). https://doi.org/10.1007/s11104-022-05357-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-022-05357-w