Abstract
Aims
Transgenic betaine aldehyde dehydrogenase (BADH) maize that overaccumulates glycine betaine (GB) is developed to enhance tolerance to salt stress, while the ecological risk of cropping BADH transgenic maize BZ-136 on soil properties and rhizosphere microorganisms is ambiguous.
Methods
A pot experiment was conducted and soils were sampled at seedling, elongation, flowering, and mature stage. Soil chemical properties and enzyme activities were determined with conventional method and bacterial community diversity of BZ-136 rhizosphere was analyzed by high-throughput sequencing technique as compared with those of parental maize Zheng58 and conventional maize U8112.
Results
Cropping BZ-136 has a transient effect on EC, organic C or total N at some growth stage in neutral and saline-alkaline soil, a significant effect on urease activities from elongation to mature stage in saline-alkaline soil, and a slight influence on bacterial diversity at different stages in neutral soil and a significant impact at seedling stage in saline-alkaline soil.
Conclusion
Cropping BADH transgenic maize has transient or minor effects on soil chemistry, enzyme activity, and bacterial community diversity, while parallel factors, such as plant growth stage and soil type might also influence the rhizosphere microorganisms.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The impact of transgenic plants on soil ecosystem is a major concern over the risk associated with their commercial release (Wolfenbarger and Phifer 2000; Lilley et al. 2006). Transgenic metabolites may directly enter into soil via root exudates or plant residues. Microbes living in the rhizosphere are of particular importance to soil nutrient and plant growth. The accumulation of transgenic products in soil may promote or suppress the growth of microbes, alter the microbial community structure, and biogeochemical process through nutrient turnover and carbon cycling (Beare et al. 1995; Trevors et al. 2010). For example, insecticidal Cry proteins derived from cultivation of Bt (Bacillus thuringiensis) transgenic crops have an adversely non-targeting effect on soil organisms and microbe-mediated processes in soil (Sun et al. 2004). Glyphosate-tolerant transgenic soybean exerts transitory effects on the taxonomic diversity of rhizosphere bacterial communities at the vegetative and seed-filling stages under field conditions (Lu et al. 2018). The BADH (betaine aldehyde dehydrogenase) gene is a representative of abiotic stress tolerance genes that convey salt tolerance to transgenic plants as well as to microbes, and the expression of BADH gene may result in extra GB that can be taken as nutrient or energy sources by some kinds of bacteria (Kappes and Bremer 1998; Boncompagni et al. 1999), which may increase the potential of unpredictable non-target effects. Recently, some abiotic stress tolerant transgenic crops such as maize, wheat, and soybean are under field trials (Sahoo and Tuteja 2013; Liang et al. 2015; Lu et al. 2018). Therefore, a scientific debate is timely to focus on the risk of cropping transgenic plants with genes conferring abiotic stress.
Soil salinization is a serious global problem that threatens soil productivity and crop quality (Prochazkova et al. 2013). In China, about 3.6 × 107 ha accounting for 4.88% of total area is saline soil. As most crops cannot thrive in severely saline soils, researchers try to improve the salt tolerance of crop germplasm via transgenic technology (Ahsan et al. 2007). Glycine betaine (GB) is a quaternary ammonium compound that can increase the intracellular osmotic pressure in response to different stresses and stabilize the biological macromolecules in higher plants (Rhodes and Hanson 1993; Holmstrom et al. 2000). The BADH is the key enzyme during betaine biosynthesis in plants regulated by BADH gene. Overexpression of the BADH gene in transgenic plants can enhance plant tolerance to a wide range of abiotic stresses including salt and drought (Su et al. 2006; Zhang et al. 2011a; Fan et al. 2012).
The objective of this study was to evaluate the ecological risk of transgenic BADH maize BZ-136 on soil chemical property (EC, pH, organic C, total N, and available N), soil enzyme (urease, catalase, and saccharase activity), and microbial community composition and diversity at four growth stages under neutral and saline-alkaline conditions via comparison with parental maize Zheng58 and conventional maize U8112. Our study provides useful information associated with the impact of BADH transgenic maize on soil microbiome.
Materials and methods
Plant and soil materials
Three maize lines including the receptor parental maize (Zheng58), the transgenic BADH maize (BZ-136), and the inbred conventional maize (U8112) were used in this study. BZ-136 is developed by inserting betaine aldehyde dehydrogenase (BADH) gene of Atriplex micrantha into genome of inbred maize line of zheng58 under the control of ubiquitin promotor (Di et al. 2015). The plants of transgenic maize BZ-136 contain more GB (GB content in maize root of BZ-136, Zheng58, and U8112 root was stabilized at 7.24 ± 0.14 mg g−1 FW, 0.98 ± 0.15 mg g−1 FW, and 0.79 ± 0.12 mg g−1 FW on average, respectively) and grow better than the wild-type plants under NaCl stress. All maize lines were provided by agricultural college, Northeast Agricultural University, China, and planted in neutral- and saline-alkaline soil. Neutral soil (chernozem) was taken from horticulture experimental station of Northeast Agricultural University, China, and saline-alkaline soil was taken from Lindian, Heilongjiang province, China. The chemical properties of two soils were listed in Table 1. Seeds were germinated in sterile vermiculite in the greenhouse (22 °C, humidity 40–50%, with 16-h light and 8-h darkness). All plantlets were well watered to three-leaf stage, and then supplied with 100 mL 0.5 × Hoagland nutrient solution in salt-treated group and control group every 3 days (0.3 mol NaCl was added to the nutrient solution in the salt-treated group on the first day). On the seventh day, plants of each maize line treated with NaCl were transplanted in the saline-alkaline soils and non-treated plants were transplanted in the neutral soils.
Experimental design
The pot experiment was conducted at experimental station of the Northeast Agricultural University, China in 2016 (longitude 126° 73′, latitude 45° 75′). Each pot was placed at 75 cm spacing into a 3.0 m × 3.4 m plot (a total of four plots) in a greenhouse, watered every 3 days, fertilized five times (on 10, 30 June; 20 July; and 10, 30 August), applying a similar fertilizer regime (pure nitrogen 150 kg hm−2, P2O5 content 70 kg hm−2, K2O content 80 kg hm−2) to ensure a basic fertility for plant growth in pot experiment and similarly external interference on the soil chemical properties and bacterial activities.
Soil samples collection
The type of soil management complied with conventional agronomic management practices for maize. A total of 72 samples (3 maize lines × 3 reps × 4 stages × 2 soil types) were collected in this study. The rhizosphere soils of three maize lines were sampled at seedling (20 June), elongation (25 July), flowering (28 August), and mature (30 September) stage, respectively. At each sampling time, soil samples were collected using sterile techniques. After weeds were manually removed, plants and bulk soil were gently pulled out and sent to the laboratory. Loosely, adhering soil was removed from the roots surface and the root that containing rhizosphere soil was obtained. The rhizosphere soil was shaken off from the roots and collected in a plastic bag. To ensure representativeness of samples, rhizosphere soil for each replicate was a composite of soil from three plants. Soil samples were sieved through 2-mm mesh to remove roots and stones and then divided into two parts. Samples in the first part were dried at room temperature and stored at 4 °C until analysis. Soil samples in the second part were stored at − 80 °C for DNA extraction.
Soil chemical property analysis
Soil pH was measured using a pH meter (Sartorius/PB-10, Germany) at a ratio of 1:5 (dry soil:deionized water, w:v), and electrical conductivity was determined using electrical conductivity meter (Leici/DDS-11A, China) at a ratio of 1:5 (dry soil:deionized water, w:v) (Gupta 2004). The supernatant was collected by centrifugation at 10,000×g for 5 min. Soil organic C was determined according to Walkley and Black method based on the reaction with K2Cr2O7 and sulfuric acid (Walkley and Black 1934). Soil total N content was determined by Kjeldahl method using Kjeldahl™ 8400 Auto Sample Systems (FOSS Tecator AB, Sweden) (Bremner and Mulvaney 1982). The available nitrogen (AN) content was determined with the alkali hydrolysis and diffusion method (Cornfield 1960).
Soil enzyme activity determination
The catalase activity was evaluated using titration method (Jin et al. 2009). Briefly, 2 g of soil with 40 mL of distilled water and 5 mL of 0.3% H2O2 were shaken in a concussion incubator at 150 rpm for 20 min and the filtrate was titrated with 0.1 mol L−1 KMnO4 under the condition of 5 mL of 3 N H2SO4 and the catalase activity was expressed as milligram·0.1 mol L−1 KMnO4 g−1 soil. The urease activity was measured by the method of the loss of added urea (Zhang et al. 2014). Briefly, 5 g of soil was incubated with 10 mL of 10% urea solution and 5 mL of sodium citrate buffer (pH 6.7) for 24 h at 37.8 °C, then the mixture was diluted to 50 mL with distilled water, filtered and reacted with sodium phenol, and 0.9% sodium hypochlorite solution. The released ammonium was quantified by Ultraviolet Spectrometer Subsystem (UVS) (PGENARAL T6) at 578 nm and the urease activity was expressed as mg·NH4+-N g−1·soil·24 h−1. Saccharase activity was determined by the method of colorimetric 3,5-dinitrylsalicylate (Zhang et al. 2011b). Five grams of soil, 15 mL of 8% glucose solution, 5 mL of 0.2 M phosphate buffer (pH 5.5), and 5 drops of toluene were mixed in a volumetric flask and incubated for 24 h at 37.8 °C. The mixture was filtered and reacted with 3 mL of 3,5-dinitrylsalicylate for 5 min, then the products were quantified by a spectrophotometer at 508 nm and expressed as milligram·glucose·g−1·24 h−1.
DNA extraction and high-throughput sequencing analysis
Total genomic DNA was extracted using the FastDNA spin kit for soil (MP Biomedicals, LLC, Solon, USA), further purified using DNA Purification Kit (Promega, Madison, WI, USA) according to the manufacturer’s instructions. Next generation sequencing library preparation and Illumina MiSeq sequencing were performed at Majorbio Bio-Pharm Technology (Shanghai, China). The V3-V4 region of the 16S rRNA gene was amplified using bacterial primers 338F (5′-ACT CCT ACG GGA GGC AGC AG-3′) and 806R (5′-GGA CTA CHV GGG TWT CTA AT-3′) (Petrosino et al. 2009). PCR products were pooled and purified using the Gel Extraction Kit (Axygen). QuantiFluor-ST Fluorometer was used to quantify the purified amplicons (Promega, Madison, WI, USA), and then a composite sequencing library was constructed by combining equimolar ratios of amplicons from all samples. The shorter sequences were removed, then all the remaining sequences were considered in the subsequent analyses. DNA libraries were multiplexed and loaded on an Illumina MiSeq instrument (Majorbio Bio-Pharm Technology, Shanghai, China). Raw fastq files were de-multiplexed and quality-filtered using QIIME (version 1.8.0, http://bio.cug.edu.cn/qiime/). UPARSE embedded in QIIME was used to cluster valid sequences into operational taxonomic units (OTUs) with 97% sequence identity. For alpha and beta diversity analyses, OTU tables were rarefied in accordance with minimum sample sequences (30,735 reads). Alpha diversity was calculated by Ribosomal Database Project (RDP) pipeline (http://pyro.cme.msu.edu/) at 97% sequence identity. Beta diversity analysis was calculated using weighted and unweighted UniFrac distances among groups, and non-metric multidimensional scaling (NMDS) was performed in R software to compare bacterial community structure across all samples. Unweighted pair group method with arithmetic mean (UPGMA) clustering tree was constructed to compare the hierarchical relationships among groups. The Mann–Whitney U test was performed to compare diversity indices between two groups. Heat maps were performed using Mothur and R software (http://www.mothur.org/wiki/MainPage).
Multivariate statistical analysis
Statistical analysis was implemented using one-way analysis of variance (ANOVA). Biochemical characteristics data were analyzed with SPSS 18.0. Statistical significance was calculated by Student’s t test and a probability value p < 0.05 was considered to be significant. PERMANOVA differences were calculated using the vegan package in R software. Data were expressed as mean or percentage ± standard deviation (SD).
Results
Soil chemical properties
To compare the difference in soil chemical properties, EC, pH, organic C, total N, and available N in neutral and saline alkaline were measured at different plant growth stages (Table 2). Transgenic BZ-136 showed a significant difference in EC from conventional U8112 at elongation and flowering stage in neutral soil, and a significant difference from conventional U8112 and parental Zheng58 at mature stage in saline-alkaline soil. The pH values of rhizosphere soil of transgenic BZ-136 were not significantly different from those of conventional U8112 and parental Zheng58 at all stages in neutral soil, but significantly different from conventional U8112 at seedling stage. Organic C and total N in rhizosphere soil of transgenic BZ-136 at seedling, elongation, and flowering stage exhibited a significant difference from those of conventional U8112 or parental Zheng58 in neutral soil and only a difference at elongation stage in saline alkaline. The contents of available N were almost the same at all the stages in both neutral and saline-alkaline soil. The results indicated that no definite regularity existed in chemical properties of saline-alkaline and neutral soil between transgenic BZ-136 and two non-transgenic maize lines at all growth stages.
Soil enzyme activities
The effects of transgenic BZ-136 on urease, catalase, and saccharase activity compared with parental Zheng58 and conventional U8112 in neutral and saline-alkaline soil were displayed in Fig. 1. Urease activity in rhizosphere soil of transgenic BZ-136 showed no difference from that of parental Zheng58 and conventional U8112 in neutral soil (Fig. 1a), but a significant difference in saline-alkaline soil at elongation, flowering, and mature stage (Fig. 1b). As for catalase activity in rhizosphere soil of transgenic BZ-136, no significant difference was found at all growth stages in neutral soil (Fig. 1c), whereas a significant difference (p < 0.05) in saline-alkaline soil was found at seedling stage (Fig. 1d). Saccharase activity of BZ-136 was not significantly different from that of other two maize lines in both neutral and saline-alkaline soil (Fig. 1e, f). Apparently, the differences in soil enzymes between transgenic BZ-136 and other two non-transgenic maize lines existed at certain stage in saline-alkaline soil; cropping transgenic maize had a potential risk in soil enzyme under saline-alkaline stress during the growth process.
Urease, catalase, and saccharase activities in rhizosphere soil of BZ-136, Zheng58, and U8112 at different growth stages in neutral and saline-alkaline soil. Vertical bars indicate the standard deviation of the means. Same letter above each bar represents the value that is insignificantly different (p < 0.05) according to the LSD test (n = 3) at the same stage. The standard deviation is based on the average of three biological replicates
Richness and diversity of rhizosphere bacteria
Statistically significant differences (p < 0.05) in richness and diversity were observed for Chao1, ACE, and Shannon at different growth stages (Table 3). Basing on pyrosequencing analysis of V3-V4 region of 16S rRNA gene, 3,450,515 high-quality sequences from 72 soil samples with an average of 47,924 sequences per sample were recovered. Raw sequences were deposited to the NCBI Sequence Read Archive with the sample group accession no. SRP108110. A total of 195,906 operational taxonomic units (OTUs) in the range of 2115 to 3238 were identified based on the conventional criterion of 97% similarity (equal to OTU level). On average, NZS had the least OTUs (2255) and AUM had the maximum (3029). Shannon diversity index and Chao1 richness estimator indicated that rhizosphere microorganisms of transgenic BZ-136 were similar to those of other two maize lines during the most growth period, while dissimilar to those of Zheng58 at seedling stage and U8112 at mature stage in neutral soil and dissimilar to Zheng58 at elongation stage in saline-alkaline soil. In addition, the numbers of Ace and Coverage index of BZ-136 were generally similar with the control maize groups at the same stage. Between the two types of soil, the diversity and abundance of the microorganisms in the saline-alkaline soil were higher than those in the neutral soil.
Bacterial community composition
The similarity of bacterial community composition for the 72 samples was clustered by non-metric multidimensional scaling (NMDS) (Fig. 2). The NMDS ordination of the bacterial composition showed an acceptable stress level of 0.125, indicating a good representation of the bacterial taxonomic composition. Soil samples from different soil types were distributed at both ends of nmds1. Three maize lines were clustered generally closely in the same soil type, indicating that bacterial community composition in non-transgenic maize and BADH transgenic maize were similar to one another. PERMANOVA analysis was used to mirror NMDS plot as shown in Table 4. The bacterial community composition in the soils, based on the relative abundance of different OTUs, was significantly affected by soil type. The effect of BADH transgenic traits on soil bacterial community was absent.
Non-metric multidimensional scaling (NMDS) of the dissimilarity among samples. N, means neutral soil; A, means saline-alkaline soil; Z, means Zheng58 maize line; B, means BZ-136 maize line; U, means U8112 maize line; S, means seedling stage; E, means elongation stage; F, means flowering stage; M, means mature stage
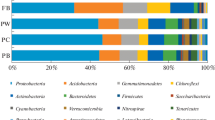
The phylogenetic classification based on 16S rRNA sequences at phylum level for all samples was showed in Fig. S1 and Table S1. There were 11 dominant phyla including Proteobacteria, Actinobacteria, Acidobacteria, Chloroflexi, Firmicutes, Bacteroidetes, Gemmatimonadetes, Nitrospirae, Verrucomicrobia, Saccharibacteria, and Planctomycetes in neutral and saline-alkaline soil samples, where Proteobacteria, Actinobacteria, and Acidobacteria were the most dominant phyla on average in both neutral and saline-alkaline soil samples. The relative abundance at class level for phylum Proteobacteria was showed in Fig. 3 and Table S2. In neutral soil, the relative abundance of Alphaproteobacteria, Betaproteobacteria, Gammaproteobacteria, and Deltaproteobacteria of BZ-136 was similar to that of parental Zheng58 and conventional U8112. There were only temporary significant differences at certain times, such as Deltaproteobacteria at seedling stage. In saline-alkaline soil, the relative abundance of Alphaproteobacteria and Gammaproteobacteria was similar to that of parental Zheng58 and conventional U8112. Significant differences in the abundance of Betaproteobacteria and Deltaproteobacteria were found at different growth stage, including Betaproteobacteria at seedling, elongation, and flowering stage and Deltaproteobacteria at elongation and flowering stage. Relative abundance of phylum Actinobacteria of BZ-136 in neutral soil was 15.98%, 31.92%, 24.09%, and 26.85% at corresponding stage, which was 3.17%, 5.73%, 1.06%, and 5.06% higher than that in saline-alkaline soil, respectively. Compared with Zheng58, the abundance of Actinobacteria of BZ-136 was significantly lower at seedling stage in neutral soil and significantly higher at flowering stage in saline-alkaline soil. Compared with U8112, the abundance of Actinobacteria of BZ-136 was 7.61% higher at flowering stages and significantly lower at mature stage in neutral soil, while significantly lower at seedling stage in saline-alkaline soil. Acidobacteria was not as rich as Proteobacteria and Actinobacteria in BZ-136 with relative abundance of 14.29%, 8.82%, 8.69%, and 14.52% at different stage in neutral soil, while significantly higher than Zheng58 at seedling stage. In saline-alkaline soil, the relative abundance of Acidobacteria in BZ-136 was 6.57%, 7.68%, 2.62%, and 5.74% higher than that in neutral soil, while significantly higher than Zheng58 and U8112 at seedling stage and significantly lower than Zheng58 at flowering stage.
Relative abundance of Proteobacteria communities of transgenic maize BZ-136, parent Zheng58, and conventional U8112 following at different growth stage. Each sample is based on the average of three replicates. N, means neutral soil; A, means saline-alkaline soil; Z, means Zheng58 maize line; B, means BZ-136 maize line; U, means U8112 maize line; S, means seedling stage; E, means elongation stage; F, means flowering stage; M, means mature stage
Community similarity at genus level and cluster analysis
The relative percentage of key genus for all samples was shown in heat maps (Fig. 4). Fifty major genera were identified for separating dominant rhizosphere microbes in both neutral and saline-alkaline soil (Fig. 4a, b). The relative value was expressed by the color intensity as illustrated in the figure. It was denoted that BZ-136 shared high similarity with Zheng58 and U8112 at each stage in neutral soil, but it showed difference from other two maize lines at seedling stage in saline-alkaline soil. Cluster analysis manifested that bacterial communities were clustered closely to one another in the same type of soil (Fig. S2), although there were some differences in different samples.
Heat maps of the soil rhizosphere bacterial composition at the genus level in neutral soil (a) and saline-alkaline soil (b). Each sample is based on the average of three replicates. N, means neutral soil; A, means saline-alkaline soil; Z, means Zheng58 maize line; B, means BZ-136 maize line; U, means U8112 maize line; S, means seedling stage; E, means elongation stage; F, means flowering stage; M, means mature stage
Discussion
Transgenic plants are known to alter root exudate composition through the introduction of a functional gene (Wu et al. 2014). As abiotic stress tolerance gene, BADH may influence the expression of other genes in adversity, which may consequently increase the potential of the persistence, invasiveness, competitiveness, and other unpredictable effects on non-target soil microflora (Mallory-Smith and Zapiola 2008; Wolt 2009). In this study, BADH transgenic maize exhibited minor or transient influences on bacterial community composition and diversity in rhizosphere soil as well as soil chemical properties and enzyme activities in saline-alkaline soil. However, to establish a novel criterion or to supplement new items to original paradigm for risk assessment of transgenic plants with abiotic stress tolerance genes, further pot experiments or field experiments need to be conducted.
One of the primary potential environmental risks associated with the transgenic plants is their effects on soil ecology. The release of the root exudates of transgenic plants may affect the soil physicochemical property, which subsequently affects microbial community and crop performance (Tao et al. 2017). While several studies found that Bt toxins from transgenic plants were decomposed quickly after they entered into the soil, the long-term persistence of Bt toxins was largely dependent on the toxin and soil type (Rauschen et al. 2008). In this study, the soil chemical properties such as organic C and total N mainly changed with growth stage of BADH transgenic maize, while pH, EC, and available N varied with soil type. And no change in soil chemical property was correlated with maize lines. In accord with our results, previous studies already showed that soil chemical property varied with the growth stage of chitinase-transgenic tobacco (Wang et al. 2013) and non-significant impacts on soil chemistry were observed in Cry1Fa2 GM maize compared to non-GM maize (Liu et al. 2010). Moreover, DNA helicase-45 transgenic rice had no detectable adverse effects on the soil physicochemical properties as compared with their wild-type counterpart (Sahoo and Tuteja 2013). Though GB is a nontoxic osmoprotectant, GB accumulation in soil may decrease the hydrophily and osmolarity but increase the lipophilicity, and promote the dewatering of soil (Yin et al. 2017). It was reported that the presence of GB strongly stimulated the growth of Bacillus subtilis under high-osmolarity conditions (Kappes and Bremer 1998). It has also been reported that Pseudomonas aeruginosa could use GB as a nutrient and energy source as well as an osmoprotectant (Boncompagni et al. 1999). Our results indicated that BADH transgenic maize has no significant effect on soil chemical properties.
Soil enzymes play an essential role in organic matter decomposition and nutrient cycling, thus they are often employed as important monitors for the quality of soil ecosystem and the health of microbial community (Velmourougane and Sahu 2013). Oxidoreductase, hydrolase, and transferase are three major categories of soil enzymes, while catalase, urease, and saccharase belonging to each category are more sensitive to saline-alkaline stress than other enzymes (Frankenberger and Bingham 1982; Maacaroun 2008). Urease, catalase, and saccharase activities are closely related to soil organic matter and the number of microorganisms (Li et al. 2006). In this study, there were no differences in soil enzyme activities among three maize lines in neutral soil at all growth stages, but significant differences between transgenic and non-transgenic maize at certain growth stages in saline-alkaline soil. For example, the urease activity of Zheng58 was 19.69% and 10.14% lower than that of BZ-136 at elongation and mature stages, while that of U8112 was 14.35% and 14.42% lower than BZ-136 at elongation and flowering stages. The occasional differences between the transgenic BZ-136 and non-transgenic maize indicated that transgenic maize had no negative impact on ecological environment in neutral soil but might have a potential risk in soil enzyme under saline-alkaline stress. The cultivation of transgenic Bt crops was reported to alter the urease activity (Jepson et al. 1994). However, soil enzyme activity is a very sensitive parameter that may be affected by fertilization, watering, crop lines, and environmental condition, which should be rigorously evaluated by a long-term continuous monitoring experiment.
Within the rhizosphere, microorganisms positively affect plant health through mineralizing nutrients, suppressing diseases, improving stress tolerance, and producing phytohormones (Mathesius 2003). Nimusiima et al. indicated that the expression of the resistance genes appears to have no consequences for non-target rhizobacteria using high-throughput sequencing technology (Nimusiima et al. 2015). Other studies also found no or transitory effects of transgenic plants on microbes (Wu et al. 2004; Griffiths et al. 2006; Shen et al. 2006; Oliveira et al. 2008; Li et al. 2011). However, the researchers also found that some transgenic plants did not influence the overall soil functions but altered microbial community composition and key functions mediated by specific microbial populations (Milling et al. 2004). In this study, high-throughput sequencing technology was used to investigate the effect of BADH transgenic maize BZ-136 on bacterial community composition and diversity in the rhizosphere at different growth stages. There was no evidence that transgenic BZ-136 adversely influenced the bacterial community composition compared with parental Zheng58 and conventional U8113. Based on the values of Chao1 and ACE, the soil bacterial community richness of transgenic maize was similar to those of non-transgenic maize lines at same stage. The Shannon–Weaver index also reflected that microbial community diversity was coincident in three maize lines. These results were consistent with previous research in β-carotene transgenic rice (Li et al. 2014). The obvious differences in NMDS between transgenic BZ-136 and non-transgenic maize mainly existed in soil type and growth stage rather than maize lines. Previous research has shown that CMV-resistant transgenic chili pepper had minor effect on NMDS of bacterial community (Chun et al. 2011).
Changes in bacterial community structure also depended on growth stage rather than maize lines. At phylum level, the top five phyla of the bacterial community of all samples were Proteobacteria, Actinobacteria, Acidobacteria, Chloroflexi, and Firmicutes. Proteobacteria are well known as critical components of soil fertility due to their roles in C, N, and S cycles (Kersters et al. 2006), while Chloroflexi is a phylum of bacteria containing a diversity of phenotypes and distributing widely. In this study, the proportion of Proteobacteria and Chloroflexi varied inconspicuously at different stages. The proportions of Firmicutes were highest at seedling stages, but they were dropped at later stages, the sudden decrease may be a result of season effect. At genus level, the proportion of a few genera changed with growth stage, obviously. The number of microorganisms in genus Rhodanobacter was increased at elongation, flowering, and mature stage in neutral soil, those in Pseudomonas and Bacillus were decreased at elongation, flowering, and mature stage in saline-alkaline soil. Obviously, the variations in bacterial community structure are correlated with plant growth stage. Icoz et al. (2008) reported that the differences in rhizosphere bacterial diversity between Bt and non-Bt maize relied on development stages. Dunfield and Germida observed variable effects on the bacterial community from one plant development stage to another (Dunfield and Germida 2003). According to the results of our study, with the extra amount of GB release from the root exudates or plant residue, planting transgenic BADH maize may have a potential impact on the soil urease enzyme activity as well as bacterial diversity and richness in long-term period. Continuous monitoring in the whole growth period is indispensable to verify whether transgenic plants have a persistent effect on the microbial community in the rhizosphere soil.
Conclusion
Cropping BADH transgenic maize BZ-136 has minor effect on soil EC, organic C, and total N at certain growth stage in neutral or saline-alkaline soil. Cropping BADH transgenic maize BZ-136 has improved the soil urease activity at certain developmental stage in saline-alkaline soil compared to other two non-transgenic maize lines. Cropping BADH transgenic maize BZ-136 has a subtle impact on the microbial community structure in neutral soil and an obvious impact at seedling stage in saline-alkaline soil. In brief, compared with non-transgenic maize, BADH transgenic maize BZ-136 has marginal effects on soil chemical property, enzyme activity, and bacterial community diversity.
References
Ahsan MH, Hussnain H, Saleem M, Malik TA, Aslam M (2007) Gene action and progeny performance for various traits in maize. Pak J Agric Sci 44:608–613
Beare MH, Coleman DC, Jr DAC, Hendrix PF, Odum EP (1995) A hierarchical approach to evaluating the significance of soil biodiversity to biogeochemical cycling. Plant Soil 170:5–22
Boncompagni E, Osteras M, Poggi MC, Rudulier DL (1999) Occurrence of choline and glycine betaine uptake and metabolism in the family Rhizobiaceae and their roles in osmoprotection. Appl Environ Microbiol 65:2072–2077
Bremner JM, Mulvaney CS (1982) Nitrogen total. In: Page, A.L. (Ed.), Methods of soil analysis. Part 2. American Society of Agronomy. Soil Sci. Soc. Am., Inc. Publisher, Madison, WI, USA, 595–624
Chun YJ, Kimb DY, Kimb HJ, Parkb KW, Jeongb SC, Parkc S, Leed B, Harne CH, Kimf HM, Kimb CG (2011) Do transgenic chili pepper plants producing viral coat protein affect the structure of a soil microbial community? Appl Soil Ecol 51(1):130–138
Cornfield AH (1960) Ammonia released on treating soils with n sodium hydroxide as a possible means of predicting the nitrogen-supplying power of soils. Nature 187(4733):260–261
Di H, Tian Y, Zu HY, Meng XY, Zeng X, Wang ZH (2015) Enhanced salinity tolerance in transgenic maize plants expressing a BADH gene from Atriplex micrantha. Euphytica 206:775–783
Dunfield KE, Germida JJ (2003) Seasonal changes in the rhizosphere microbial communities associated with field-grown genetically modified canola (Brassica napus). Appl Environ Microbiol 69:7310–7318
Fan WJ, Zhang M, Zhang HX, Zhang P (2012) Improved tolerance to various abiotic stresses in transgenic sweet potato (Ipomoea batatas) expressing spinach betaine aldehyde dehydrogenase. PLoS One 7(5):e37344
Frankenberger WT, Bingham FT (1982) Influence of salinity on soil enzyme activities. Soil Sci Soc Am J 46:1173–1177
Griffiths BS, Caul S, Thompson J, Birch ANE, Scrimgeour C, Cortet J, Foggo A, Hackeet CA, Krogh PH (2006) Soil microbial and faunal community responses to Bt maize and insecticide in two soils. J Environ Qual 35:734–741
Gupta PK (2004) Methods in environmental analysis: Water, Soil and Air. Agrobios
Holmstrom KO, Somersalo S, Mandal A, Palva TE, Welin B (2000) Improved tolerance to salinity and low temperature in transgenic tobacco producing glycine betaine. J Exp Bot 51:177–185
Icoz I, Saxena D, Andow DA, Zwahlen C, Stotzky G (2008) Microbial populations and enzyme activities in soil in situ under transgenic corn expressing cry proteins from Bacillus thuringiensis. J Environ Qual 37:647–662
Jepson PC, Croft BA, Pratt GE (1994) Test systems to determine the ecological risks posed by toxin release from bacillus-thuringiensis genes in crop plants. Mol Ecol 3:81–89
Jin K, Sleutel S, Buchan D, De Neve S, Cai DX, Gabriels D, Jin JY (2009) Changes of soil enzyme activities under different tillage practices in the Chinese Loess Plateau. Soil Tillage Res 104:115–120
Kappes R, Bremer E (1998) Response of Bacillus subtilis to high osmolarity: uptake of carnitine, crotonobetaine, and γ-butyrobetaine via the ABC transport system OpuC. Microbiology 144:83–90
Kersters K, Vos PD, Gillis M, Swings J, Vandamme P, Stackebrandt E (2006) Introduction to the Proteobacteria. Prokaryotes 3–37
Li CR, Xu JW, Song HY, Li CY, Zheng L, Wang WD, Wang YH (2006) Soil enzyme activities in different plantations in lowlands of the Yellow River Delta, China. J Plant Ecol 30(5):802–809 (in Chinese)
Li XG, Liu BA, Cui JJ, Liu DD, Ding S, Gilna B, Luo JY, Fang ZX, Cao W, Han ZM (2011) No evidence of persistent effects of continuously planted transgenic insect-resistant cotton on soil microorganisms. Plant Soil 339:247–257
Li P, Dong JY, Yang SF, Bai L, Wang JB, Wu GG, Wu X, Yao QH, Tang XM (2014) Impact of b-carotene transgenic rice with four synthetic genes on rhizosphere enzyme activities and bacterial communities at different growth stages. Eur J Soil Biol 65:40–46
Liang J, Meng F, Sun S, Wu C, Wu H, Zhang M, Zhang H, Zheng X, Song X, Zhang Z (2015) Community structure of arbuscular mycorrhizal fungi in rhizospheric soil of a transgenic high-methionine soybean and a near isogenic variety. PLoS One 10(12):e0145001
Lilley AK, Bailey MJ, Cartwright C, Turner SL, Hirsch PR (2006) Life in earth: the impact of GM plants on soil ecology? Trends Biotechnol 24:9–14
Liu N, Zhu P, Peng C, Kang LS, Gao HJ, Clarke NJ, Clarke JL (2010) Effect on soil chemistry of genetically modified (GM) vs. non-GM maize. GM Crops 1(3):5
Lu GH, Tang CY, Hua XM, Cheng J, Wang GH, Zhu YL, Zhang LY, Shou HX, Qi JL, Yang YH (2018) Effects of an EPSPS-transgenic soybean line ZUTS31 on root-associated bacterial communities during field growth. PLoS One 13(2):e0192008
Maacaroun A (2008) Effect of the irrigation with saline water on the behavior of 2 soil enzymes urease and saccharase, soil respiration and soil humidity. Scientific papers–University of Agronomic Sciences and Veterinary Medicine Bucharest. Series B, Horticulture 51:95–98 (in Romania)
Mallory-Smith C, Zapiola M (2008) Gene flow from glyphosate-resistant crops. Pest Manag Sci 64:428–440
Mathesius U (2003) Conservation and divergence of signalling pathways between roots and soil microbes – the rhizobium-legume symbiosis compared to the development of lateral roots, mycorrhizal interactions and nematode-induced galls. Plant Soil 255:105–119
Milling A, Smalla K, Maidl FX, Schloter M, Munch JC (2004) Effects of transgenic potatoes with an altered starch composition on the diversity of soil and rhizosphere bacteria and fungi. Plant Soil 266:23–39
Nimusiima J, Köberl M, Tumuhairwe JB, Kubiriba J, Staver C, Berg G (2015) Transgenic banana plants expressing Xanthomonas wilt resistance genes revealed a stable non-target bacterial colonization structure. Sci Rep 5:18078
Oliveira AP, Pampulha ME, Bennett JP (2008) A two-year field study with transgenic Bacillus thuringiensis maize: effects on soil microorganisms. Sci Total Environ 405:351–357
Petrosino JF, Highlander S, Luna RA, Gibbs RA, Versalovic J (2009) Metagenomic pyrosequencing and microbial identification. Clin Chem 55:856–866
Prochazkova D, Sairam RK, Leckshmy S, Wilhelmova N (2013) Differential response of a maize hybrid and its parental lines to salinity. Czech J Genet Plant 49(1):9–15
Rauschen S, Nguyen HT, Schuphan I, Jehle JA, Eber S (2008) Rapid degradation of the Cry3Bb1 protein from Diabrotica resistant Bt-corn Mon88017 during ensilation and fermentation in biogas production facilities. J Sci Food Agric 88:1709–1715
Rhodes D, Hanson AD (1993) Quaternary ammonium and tertiary sulfonium compounds in higher plants. Annu Rev Plant Biol 44:357–384
Sahoo RK, Tuteja N (2013) Effect of salinity tolerant PDH45 transgenic rice on physicochemical properties, enzymatic activities and microbial communities of rhizosphere soils. Plant Signal Behav 8(8):e24950
Shen RF, Cai H, Gong WH (2006) Transgenic Bt cotton has no apparent effect on enzymatic activities or functional diversity of microbial communities in rhizosphere soil. Plant Soil 285:149–159
Su J, Hirji R, Zhang L, He CK, Selvaraj G, Wu R (2006) Evaluation of the stress-inducible production of choline oxidase in transgenic rice as a strategy for producing the stressprotectant glycine betaine. J Exp Bot 57:1129–1135
Sun CX, Chen LJ, Wu ZJ (2004) Persistence of Bt of toxin in soil and its effects of soil phosphatase activity. Acta Pedol Sin 41:761–766
Tao JM, Liu XD, Liang YL, Niu JJ, Xiao YH, Gu YB, Ma LY, Meng DL, Zhang YG, Huang WK, Peng DL, Yin HQ (2017) Maize growth responses to soil microbes and soil properties after fertilization with different green manures. Appl Microbiol Biotechnol 101:1289–1299
Trevors JT, Kuikman P, Watson B (2010) Transgenic plants and biogeochemical cycles. Mol Ecol Resour 3:57–64
Velmourougane K, Sahu A (2013) Impact of transgenic cottons expressing cry1Ac on soil biological attributes. Plant Soil Environ 3:108–114
Walkley A, Black IA (1934) An examination of Degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid tritation method. Soil Sci 37(1):29–38
Wang B, Shen H, Yang X, Guo T, Zhang B, Yan W (2013) Effects of chitinase-transgenic (McChit1) tobacco on the rhizospheric microflora and enzyme activities of the purple soil. Plant Soil Environ 59:241–246
Wolfenbarger LL, Phifer PR (2000) The ecological risks and benefits of genetically engineered plants. Science 290:2088–2093
Wolt JD (2009) Advancing environmental risk assessment for transgenic biofeedstock crops. Biotechnol Biofuels 2(1):1–13
Wu WX, Ye QF, Min H (2004) Effect of straws from Bt-transgenic rice on selected biological activities in water-flooded soil. Eur J Soil Biol 40:15–22
Wu J, Yu Z, Xu J, Du J, Ji F, Dong F, Li X (2014) Impact of transgenic wheat with wheat yellow mosaic virus resistance on microbial community diversity and enzyme activity in rhizosphere soil. PLoS One 9(6):e98394
Yin ZX, Gao Z, Feng Y, Gu SY (2017) Study on effect and mechanism of using betaine to treat expansive soil. Journal of Highway and Transportation Research and Development 34(3):1–6, 14 (in Chinese)
Zhang N, Si HJ, Wen G, Du HH, Liu BL, Wang D (2011a) Enhanced drought and salinity tolerance in transgenic potato plants with a BADH gene from spinach. Plant Biotechnol Rep 5(1):71–77
Zhang C, Xue S, Liu GB, Song ZL (2011b) A comparison of soil qualities of different revegetation types in the loess plateau, China. Plant Soil 347:163–178
Zhang YN, Xie M, Li CY, Wu G, Peng DL (2014) Impacts of the transgenic CrylAc and CpTI insect-resistant cotton SGK321 on selected soil enzyme activities in the rhizosphere. Plant Soil Environ 60:401–406
Acknowledgments
Xin Bai and Xing Zeng contributed equally to this work. This work is supported by Major Subject of Environmental Safety Assessment Technology for Genetically Modified Maize, Wheat, and Soybean, China (No.2016ZX08011-003).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible Editor: Simon Jeffery.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 608 kb)
Rights and permissions
About this article
Cite this article
Bai, X., Zeng, X., Huang, S. et al. Marginal impact of cropping BADH transgenic maize BZ-136 on chemical property, enzyme activity, and bacterial community diversity of rhizosphere soil. Plant Soil 436, 527–541 (2019). https://doi.org/10.1007/s11104-019-03941-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-019-03941-1