Abstract
Serious soil erosion has resulted in widespread land degradation in the Loess Plateau of China. In the past two decades, great efforts have been made to restore degraded soil such as reconverting croplands into forestlands or grasslands. A comparison of soil qualities of different revegetation types has important implications in soil reclamation. Our study investigated the effect of different revegetation types on the physicochemical and microbial soil properties in the Loess Plateau, with the aim of determining which revegetation type has the best capacity for soil recovery. The vegetation types included two shrublands (Caragana korshinskii and Hippophae rhamnoides), two grasslands (Astragalus adsurgens and Panicum virgatum), and two species from croplands that were abandoned for natural recovery (Artemisia capillaries and Heteropappus altaicus). Among the plants studied, H. altaicus and A. capillaries had the highest values of soil organic C, total N, total P, available N, available P, moisture content, microbial biomass C (MBC), substrate-induced respiration, saccharase, urease, catalase, and peroxidase. Soil sampled from the A. adsurgens plot had the highest bulk density and microbial biomass N, and soil from the H. rhamnoides plot had the highest metabolic quotient (basal respiration/MBC). The soil quality index, which was obtained based on the available N, metabolic quotient, MBC, urease, polyphenol oxidase, and bulk density, shows that the abandoned cropland for natural recovery had the highest soil quality, followed by grassland, and then shrubland. Vegetation types affect the physicochemical and microbial properties of soils in arid climatic conditions. Abandoned cropland for natural recovery has the best capacity for improving soil quality in the Loess Plateau among all studied revegetation types. Our study suggests that in the Loess Plateau, natural recovery is the best choice for soil revegetation of sloping croplands.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Land degradation is intensifying in many parts of the world because of pool soil management practices and policies. According to a 2008 estimation of the Food and Agriculture Organization (FAO) of the United Nations, there are approximately 1.5 billion people (quarter of the world’s population) who depend directly on land that is being degraded. Land degradation not only induces the deterioration of the ecological function and productivity of land through soil erosion, desertification, and salinization, but also threatens socio-economic and cultural development at regional and global scales (Lal 2001). The Loess Plateau of China has a typical semiarid climate area and has experienced serious soil degradation for the past fifty years. In most parts of the Loess Plateau, many erosion-susceptible slope areas and native grasslands have been converted into farmlands because of the pressures of an increasing population. Severe soil erosion has resulted in the loss of most of the topsoil in many locations, thus exposing parent materials or soils with low nutrient content (Wei et al. 2006; Zhou et al. 2006). The Chinese Government acknowledged the severity of this problem and launched in 1999 a series of nationwide conservation projects focusing on ecological restoration mainly by reconverting croplands into forestlands and grasslands. These reconversions have improved the soil physical properties (Li and Shao 2006; An et al. 2008; Zhu et al. 2010), nutrient status (Jia et al. 2005; Cao et al. 2008), and microbial properties (Jiang et al. 2009; Fu et al. 2009a, b). Different vegetation types influence soil quality in different magnitudes, so a comparison of the soil qualities of different vegetation types is important for successful soil reclamation in the Loess Plateau.
Physical and chemical properties have been extensively used to evaluate soil quality. These include soil organic matter, available N, and water holding capacity (Parr and Papendick 1997). These properties, however, usually change slowly and thoroughly reflecting soil changes through these properties is impossible. On the other hand, microbial properties rapidly respond to soil changes caused by both natural and anthropogenic factors, and some enzymes are closely related to soil energy flow and nutrient cycles. For instance, soil microbial biomass is considered to be a transformation agent of organic soil materials and a labile pool for plant nutrients (Lin et al. 2004). Urease and polyphenol oxidase play essential roles in catalyzing reactions necessary for the transformation of N nutrients and decomposition of humus (Benitez et al. 2000; Cullen and Kersten 1996). The metabolic quotient, basal respiration (BR)/microbial biomass C (MBC), has been used to evaluate microbial community in reclamation soils. Soil quality, though, cannot be assessed with one property alone, but with a variety of properties (Nannipieri et al. 1990). Soil quality indicators have been developed because of the complex nature of soils and the exceptionally large number of soil properties that must be determined. The selection of indicators that appropriately reflect the overall change of soil quality is important. Bastida et al. (2006) developed a microbial degradation index based on five properties: dehydrogenase, water-soluble carbohydrates, urease, water-soluble C, and respiration. A rhizosphere soil microbial index (RSMI) employing principal component analysis to assess rhizosphere health has been developed by Sinha et al. (2009). They showed that tree species with higher RSMI values should be used in the revegetation of a degraded coal mining area. Trasar-Cepeda et al. (1998) found that in native soils with climax vegetation, total N can be expressed as a combination of several microbiological and biochemical properties including microbial biomass C, mineralized N, phosphomonoesterase, glucosidase, and urease. However, there is little available information on soil quality evaluation by an integrated index in the Loess Plateau.
The present study investigates the ability of six common vegetation restoration species in three land types, namely, shrubland, grassland, and natural fallow, to regenerate soil quality. Our objectives are: (1) to study the effects of these six species on the physicochemical and microbial properties of soil in the Loess Plateau, and (2) to establish a soil quality index (SQI) that will determine which vegetation type is most capable of soil quality restoration. We not only provide specific information on the effects of changing land use on physicochemical and microbial soil properties, but also the implications of reasonable utilization of slope cropland in the region.
Materials and methods
Study sites
The present study was conducted in the Dunshan watershed at the Ansai Research Station of Soil and Water Conservation, Shaanxi Province, in the northern Loess Plateau (109°19′23″E, 36°51′30″N) in China. The area is characterized by a semiarid climate, with a mean annual temperature of 8.8°C and an average annual precipitation of 510 mm, mostly from July to September. The annual evaporation ranges from 1,500 to 1,800 mm and the average frost-free period is approximately 203 day. The soil is mainly Huangmian soil (Calcaric Cambisols, FAO), developed on wind-deposited loessial parent material and characterized by a yellow colour, absence of bedding, silty texture, looseness, macroporousness, and wetness-induced collapsibility. The soil texture is 64% sand, 24% silt, and 12% clay. The study area is representative of soil subjected to both wind and water erosion.
Widespread vegetation restoration has been implemented in the region during the past decade to remedy the soil degradation problem (Fu et al. 2009a, b). The vegetation restoration types are predominately perennial, including shrubs such as Caragana korshinskii and Hippophae rhamnoides, grasses such as Astragalus adsurgens and Panicum virgatum, and natural vegetation from abandoned croplands.
Experiment design and soil sampling
The experimental area covered a total area of 8800 m2. In 2000, six vegetation types were established on the sloping cropland: two shrubs, C. korshinskii and H. rhamnoides; two grasses, A. adsurgens and P. virgatum; and two species from abandoned croplands for natural recovery. The six locations were all similar in terms of the slope aspect, gradient, altitude, and previous farming practices before initiating the experiment, hence the assumption that the differences in soil properties could be attributed to the type of vegetation (Fu et al. 2009a, b; Marzaioli et al. 2010). Three replicate plots (20 × 20 m) were established for each vegetation type. C. korshinskii and H. rhamnoides were seeded with a row spacing of 70 and 80 cm, respectively. A. adsurgens and P. virgatum were both seeded with 45 cm row spacing. In 2008, the predominant species in one of the abandoned croplands for natural recovery was Artemisia capillaries, and in another, Heteropappus altaicus. All the plants developed under semiarid conditions, without irrigation, fertilization, and disturbance after planting (Zhang et al. 2011). The description of each treatment is shown in Table 1.
In 2008, five subplots were randomly established in each plot for coverage measurement: 2.5 m × 2.5 m for C. korshinskii and H. rhamnoides; and 1 × 1 m for A. adsurgens, P. virgatum, A. capillaries, and H. Altaicus. Soil samples were collected from the top 20 cm of the soil profile with a 5 cm diameter stainless steel corer after litter horizons were removed. Soil was collected along an “S” pattern from six sampling points at each plot. Roots, stones, and debris were removed, and then each sample was divided into two parts. One part was air-dried for analysis of physicochemical soil properties, and the other one was immediately sieved through a 2 mm mesh and then stored at 4°C until soil microbial properties could be analyzed.
Laboratory analysis
Soil organic carbon (SOC) was determined using the Walkley-Black method (Nelson and Sommers 1982), and total nitrogen (TN) by the Kjeldahl method (Bremner and Mulvaney 1982). Total phosphorus (TP) was determined colorimetrically after wet digestion with H2SO4 + HClO4, and available phosphorus (AP) was determined by the Olsen method (Olsen and Sommers 1982). Available nitrogen (AN), available potassium (AK), and bulk density were determined according to ISSCAS (1981). AN was determined with a micro-diffusion technique after alkaline hydrolysis. AK was measured in 1 M NH4OAc extracts by flame photometry. Soil bulk density was determined using a soil core (stainless steel cylinders with a diameter and a height of 5 cm each) at each sampling point. Soil moisture content was determined gravimetrically by drying the samples at 105°C overnight, and the water content was expressed as a percentage of the dry weight. An automatic acid–base titrator (Metrohm 702) was used to determine soil pH in 1:2.5 soil: water suspensions.
MBC and microbial biomass N (MBN) were measured by the fumigation extraction method (Vance et al. 1987). Oven-dry equivalent field-moist soil (25 g) was fumigated for 24 h at 25.8°C with ethanol-free CHCl3. Following fumigant removal, the soil was treated with 100 ml of 0.5 M K2SO4 by horizontal shaking for 1 h at 200 rpm, and was then filtered. The other non-fumigated 25 g soil sample was extracted simultaneously at the time fumigation commenced. SOC in the extracts was measured using a Liqui TOCII analyzer (Elementar, Germany). TN in the extracts was measured using the Kjeldahl method. MBC was calculated using a kEC factor of 0.38, and MBN using a kEN factor of 0.54 (Vance et al. 1987). All analyses were performed on soil in triplicate at field capacity moisture status, and the extracted volumes took into account the water contained in the moist soil.
Soil BR was estimated via CO2 evolution at 25.8°C in samples incubated for 14 day, adjusted to 50% of field water holding capacity (Jenkinson and Powlson 1976). The CO2 respired was trapped in NaOH, and the residual NaOH was titrated with HCl. Soil substrate-induced respiration (SIR) was determined using the same method as BR but with actual addition of glucose to the soil (Zhang et al. 2006). The metabolic quotient BR/MBC was calculated as BR per unit of MBC (Anderson and Domsch 1993). The microbial quotient was calculated by MBC/SOC.
Enzyme activities were assayed as described by Guan et al. (1991). The moisture content was determined after drying at 105.8°C for 48 h. For all enzyme assays, controls were included without substrate and without soil samples. Saccharase activity was measured using 5 g of fresh soil (sieved to <1 mm), 15 ml of 8% glucose solution, 5 ml of 0.2 M phosphate buffer at pH 5.5, and 5 drops of toluene. After incubation for 24 h at 37.8°C, the soil solution was filtered, and a 1 ml aliquot was transferred to a volumetric flask with 3 ml of 3,5-dinitrylsalicylate, and then heated for 5 min. After the solution reached room temperature, the product was quantified colorimetrically in a spectrophotometer (Hitachi, UV2300) at 508 nm. Results were expressed as mg glucose released ∙ g−1 soil ∙ h−1.
Urease activity was determined using 5 g of fresh soil (sieved to < 1 mm), 5 ml of citrate solution at pH 6.7, and 5 ml of 10% urea solution. The samples were incubated at 37.8°C for 3 h, and then diluted to 50 ml with distilled water. The suspension was filtered, and then a 1 ml aliquot was treated with 4 ml of sodium phenol solution (100 ml of 6.6 M phenol solution and 100 ml of 6.8 M NaOH) and 3 ml of 0.9% sodium hypochlorite solution. The released ammonium was quantified colorimetrically at 578 nm. Results were expressed as mg NH +4 –N ∙ g−1 soil ∙ h−1.
Alkaline phosphatase activity was determined using 2 ml of toluene, 10 g of fresh soil (sieved to <1 mm), 10 ml of disodium phenyl phosphate solution, and 10 ml of 0.05 M borate buffer at pH 9.6. The suspensions were incubated for 3 h at 37.8°C. The samples were then filtered and the filtrate was colored with 0.5 ml of 2% 4-aminoantipyrine and 8% potassium ferrocyanide. The phenol released was determined colorimetrically at 510 nm. Results were expressed as mg phenol ∙ g−1 soil ∙ h−1.
Catalase activity was determined using 2 g of fresh soil with 40 ml of distilled water and 5 ml of 0.3% H2O2, shaken for 20 min at 150 rpm, and then immediately filtered through Whatman 2 V. The filtrate was titrated with 0.1 M KMnO4 in the presence of sulfuric acid. The results were expressed as mol KMnO4 ∙ g−1 soil ∙ h−1.
Polyphenol oxidase activity was determined using 5 g of fresh soil incubated for 2 min in a water bath at 30.8°C, with 10 ml of distilled water, 6 ml of 0.1% ascorbic acid, and 10 ml of 0.02 M catechol. A volume of 3 ml of 10% phosphoric acid was then added to the suspension, and the filtrate was titrated with 0.01 M iodine. Results were expressed as ml 0.01 M I2 ∙ g−1 soil ∙ h−1. For the measurement of peroxidase, the same method was adopted.
Cellulase activity was determined using 5 g of fresh soil incubated with 1.0% carboxymethyl-cellulose in 5 ml of 0.2 M phosphate buffer (pH 5.5) at 37°C for 24 h. The soil solution filtrate was transferred to a volumetric flask with 3 ml of 3,5-dinitrylsalicylate and was heated for 5 min. After the solution reached room temperature, the product was analyzed colorimetrically at 540 nm. The cellulase activity was expressed as mg glucose ∙ g−1 soil ∙ h−1.
Soil quality index (SQI)
As previously mentioned, soil quality comprises a variety of metabolic processes that cannot be assessed with one property alone, but with a variety of properties (Nannipieri et al. 1990; Bastida et al. 2006). However, because some parameters correlated significantly with each other and usually reflect the same information, thus it is necessary to eliminate the parameters. Based on the method described by Bastida et al. (2006) and Masto et al. (2008), an integrated SQI was established in the present study. The method mainly involves three steps: (1) selecting the appropriate parameters, (2) transforming and weighing the parameters, and (3) combining the scores into an index (Bastida et al. 2006; Sinha et al. 2009). The parameters that differed significantly were chosen for the SQI calculation. The choice of appropriate parameters and their weighing were determined by principal component analysis (PCA). In each principal component (PC), only the parameters loading higher values were chosen for indexing, and high factor loadings were defined as having absolute values within 10% of the highest factor loading (Andrews et al. 2002). If there were more than one parameter with high loading in a single PC, only the parameters that did not correlate with each other (as determined by Pearson correlation analysis) were considered to be important and were therefore selected. If the parameters were well correlated, the one with higher loading was finally chosen for the determination of the SQI (Sinha et al. 2009). To transform the parameters’ values into scores (Y), a sigmoidal type equation was used (Bastida et al. 2006; Masto et al. 2008), with an asymptote tending to 1 and another tending to 0.
In Eq. 1, Y is the score of the proposed parameter after conversion, a is the maximum score (in the present case, a = 1), x is the value of the parameter, x 0 is the mean value of the parameter, and b is the value of the equation’s slope. In the PCA, two kinds of parameters, “+” (meaning “more is better”) and “−” (meaning “less is better”), were used to obtain a sigmoidal curve tending to 1 for all the proposed parameters, −2.5 and 2.5 were used as the b values for “+” and “−”, respectively.
In Eq. 2, W is the weighing factor of the parameter selected through the PCA. Since the parameters are not equal in their importance in soil quality, each PC explains a certain amount of variance (%) in the total dataset, which provides the weight for the parameters chosen in a PC (Masto et al. 2008; Sinha et al. 2009). The equation was finally normalized to get a maximum SQI of one.
Statistical analysis
Data is expressed as mean ± standard deviation (SD). Differences between mean values were examined by a one-way analysis of variance. Comparison among means was made using the Duncan multiple range test, calculated at p < 0.05. All statistical analyses were performed using the software program SPSS 15.0.
Results
Soil physicochemical properties
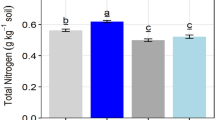
Table 2 shows the effects of the different vegetation types on SOC, TN, TP, AN, AP, AK, pH, bulk density, and moisture content. The highest SOC, TN, AN, and moisture content were found in the soil of H. altaicus, followed by A. capillaries and P. virgatum, whereas the lowest was in C. korshinskii. TP and AP were highest in the soil of A. capillaries and H. altaicus, followed by P. virgatum. AK ranged from 106.55 mg kg−1 in H. rhamnoides to 73.86 mg kg−1 in A. adsurgens. Bulk density was highest in A. adsurgens and lowest in A. capillaries. All pH values were above 8.50; the highest was in P. virgatum and A. capillaries, whereas the lowest was in H. rhamnoides and H. altaicus.
Soil microbial biomass and respiration
From Fig. 1, the highest MBC value was found in the soil of H. altaicus (72.9 mg kg−1). A. adsurgens, C. korshinskii, and A. capillaries came second with 61.2 mg kg−1, and H. rhamnoides had the lowest value of 43.9 mg kg−1. MBN was highest in the soil of A. adsurgens, followed by H. altaicus, and A. capillaries, and lowest in C. korshinskii. BR was generally highest in H. altaicus (52.5 mg CO2–C kg−1 day−1), followed by A. capillaries, H. rhamnoides, and P. virgatum (42.5 mg CO2–C kg−1 day−1), and was lowest in C. korshinskii (36.3 mg CO2–C kg−1 day−1). SIR, specifically glucose-amended respiration, behaved similar to BR. MBC/SOC and BR/MBC were within 1.6–3.6% and 24.7–43.4 mg CO2–C kg−1 day−1, respectively (Fig. 2). C. korshinskii soil had the highest MBC/SOC and H. rhamnoides soil had the highest BR/MBC.
Soil MBC and MBN (a), BR (b), and SIR (c) among revegetation types, Results are given as mean ± SD. MBC is microbial biomass C; MBN is microbial biomass N; BR is basal respiration; and SIR is substrate-induced respiration. Values with the same letter are not significantly different at the p < 0.05 level
Soil enzymes
Changes in soil enzymes (saccharase, urease, catalase, peroxidase, alkaline phosphatase, polyphenol oxidase, and cellulase) are shown in Fig. 3. The overall metabolic activity indicated by saccharase was generally grouped into three plant types. The first group (H. altaicus and A. capillaries) had the highest value (> 2.0 mg glucose g−1 h−1). The second group (H. rhamnoides, A. adsurgens, and P. virgatum) had intermediate values (1.0−2.0 mg glucose g−1 h−1). The third group (C. korshinskii) had the lowest value (0.7 mg glucose g−1 h−1). The highest urease activity was found in the soil of H. altaicus, followed by A. capillaries, and the lowest was in C. korshinskii and H. rhamnoides. Soil catalase and peroxidase were highest in H. altaicus and A. capillaries. Alkaline phosphatase was highest in H. altaicus (4.0 mg phenol g−1 h−1) and lowest in P. virgatum (2.5 mg phenol g−1 h−1). Polyphenol oxidase was highest in P. virgatum, followed by C. korshinskii and A. adsurgens. No significant difference was observed on cellulase activity for the different soils.
Soil quality index (SQI)
All studied soil parameters were chosen through the PCA for calculating the SQI, with the exception of cellulase because its content did not significantly differ among the vegetation types. As shown in Table 4, eigenvalues of the first four PCs were above 1, and the highly weighted parameters in PC-1 were SOC, TN, AN, SM, BR/MBC, and saccharase. AN had the highest loading and highly correlated with SOC, TN, SM, and saccharase (r > 0.85; Table 3); thus, SOC, TN, and saccharase were eliminated from the SQI. No significant correlation was found between AN and BR/MBC (r = 0.02); therefore, both AN and BR/MBC were selected for the SQI determination. In PC-2, the highly loaded parameters included MBC and urease, and they were retained for indexing because both of them did not correlate significantly (r = 0.45). Likewise, polyphenol oxdiase and BD were chosen for calculating the SQI due to their higher loading in PC-3 and PC-4. In summary, the most critical parameters as determined from the PCA for the SQI determination were AN, BR/MBC, MBC, urease, polyphenol oxdiase, and BD (Table 4). The final polynomial for the SQI was calculated using Eq. 2, as follows:
where Y are the scores of the critical parameters and were determined based on Eq. 1. Table 5 shows the mean value of these parameters and their curve type. As shown in Fig. 4, the SQI differed significantly among the different plants. H. altaicus (0.63) and A. capillaries (0.61) had the highest values, followed by P. virgatum (0.50) and A. adsurgens (0.47), and the lowest was found in the soil of H. rhamnoides (0.36).
Discussion
Soil physicochemical properties
Vegetative cover has fundamental effects on soil properties (Rutigliano et al. 2004), mainly due to its contribution of organic matter to the soil. In the present study, all the physicochemical soil properties differed significantly among the vegetation types. This is probably related to changes in the amounts and forms of organic materials added to the soils of the different vegetation types (Condron and Newman 1998). H. altaicus and A. capillaries showed the highest SOC, TN, AN, TP, AP, and moisture content, suggesting that naturally grown vegetation could more significantly improve the soil C, N, P components and the water content. This is in accordance with the report of Li et al. (2010), who found that the contents of SOC and TN in natural grasslands was 3.0 times higher than that in artificial grasslands, and 3.3 times higher than artificial shrublands in the Loess Plateau. Albaladejo et al. (1994) reported that the increase in SOC and nitrogen could be accompanied by improvement in the soil structure and diminution of bulk density, consistent with our results. These effects could be attributed to the extensive fine root systems in grasslands that also enhance the soil porosity (Wei et al. 2009). The various acid exudates released from roots could be responsible for the small differences in the pH values (Garcia et al. 2005).
Soil microbial biomass and respiration
Microbial biomass has been considered as an indicator of changes in organic matter content (Powlson and Jenkinson 1981). In the present study, MBC and MBN differed significantly among all six soils, indicating that microbial biomass seems to be strongly influenced by the nature of the plants, in agreement with the results of Garcia et al. (2005) and Sinha et al. (2009). Differences in MBC in the studied soils were higher than the differences in SOC, confirming that MBC is a more sensitive index of changes of the SOC (Powlson et al. 1987). A positive correlation between SOC and MBC is usually found (Jia et al. 2005; Arunachalam and Pandey 2003), but this correlation was not observed in the present study. Garcia et al. (2005) reported that a positive correlation between SOC and MBC is usually found in soils where C is a limiting factor and the soils have reached equilibrium. In our case, either C availability was not a limiting factor or the soils had not yet reached equilibrium.
MBC reflects the size of microbial populations and includes both metabolically active and resting-state microorganisms, whereas parameters such as BR and SIR reflect the actual and potential microbial activities in the soil. Bastida et al. (2006) reported that there is a highly positive correlation coefficient between SOC and BR, i.e., the soils with greater SOC content also showed greater BR. Indeed, in the present study, the correlation coefficient between SOC and BR was high, as well as the coefficient between SOC and SIR. This suggests that a large part of the SOC is dedicated to sustaining microbial respiration. The zones with the highest SOC values (H. altaicus) are also the zones with the highest respiration. Microbial quotient MBC/SOC, which can be interpreted as the available substrate and the portion of total soil carbon immobilized in microbial cells, is a sensitive parameter for monitoring organic carbon or microbial biomass (Garcia et al. 2002). In the present study, C. korshinskii presented the highest microbial quotient. Similarly, Zhang et al. (2010) found that microbial quotient in the soil of C. korshinskii was significantly higher compared with that in natural grassland, although the soil microbial biomass was slightly lower than in natural grassland. Jia et al. (2005) reported that microbial quotient had a positive correlation with MBC and SOC; however, in the present study, microbial quotient was negatively correlated with SOC and not at all correlated with MBC, in agreement with the results of Schipper et al. (2001). This can be explained by the fact that changes in the microbial quotient depend not only on the soil microbial biomass, but also on the SOC. This can be clearly seen from Table 2 and Fig. 1, where H. altaicus soil has the highest SOC and MBC, whereas C. korshinskii soil has the lowest SOC but has a high MBC. Consequently, microbial quotient in C. korshinskii soil was higher than in H. altaicus soil. This implies that microbial quotient is affected not only by the quantity, but also by the quality of the carbon input (Singh et al. 1991). The metabolic quotient BR/MBC has been used as an ecophysiological index to reflect the bioenergetic status of microbial community in reclamation studies (Insam and Domsch 1988). Tirol-Padre et al. (2007) found that metabolic quotient in a disturbed soil ecosystem was higher than that in a stable one. This is because the stress is severe and soil microbial activity is low in disturbed soil, and an increased energy is needed by soil microorganisms to repair the damages caused by the stress (Odum 1985). This implies that the higher metabolic quotient of H. rhamnoides soil is indicative of its poor quality.
Soil enzymes
Enzyme activity plays an important role in soil nutrient cycling and in the degradation of organic inputs, and can thus be useful for indicating the degree of change in soil fertility and microbial activity (Badiane et al. 2001; Bandick and Dick 1999). Research has shown that soil enzyme activity is closely related with the SOC because the transformations of important organic elements are facilitated by microorganisms (An et al. 2008; Jiang et al. 2009). Our results agree with this view based on the observations that saccharase, urease, catalase, and perioxidase significantly correlated with the SOC (p < 0.01 or p < 0.05; Table 3). This suggests that a large part of the SOC could contain substrates that stimulate the synthesis of these enzymes. The high amount of these enzymes in H. altaicus and A. capillaries soils could be due to the sufficient amount of C input by their root exudates. H. altaicus soil had the highest alkaline phosphatase content, probably caused by the greater adsorption of inorganic P or depletion of AP. Polyphenol oxidase, an important enzyme closely related to soil humus decomposition, did not significantly correlate with SOC. This may be related to the different components of the litter and the pathway of humus decomposition in the soils of the different species.
Many researchers have reported a significant correlation between soil enzyme activity and MBC (Sinha et al. 2009; Jiang et al. 2009; Taylor et al. 2002), but this correlation was not observed in the present study. This is probably due to soil enzymes that have significant abiotic activity (Nannipieri et al. 1990).
Soil quality index (SQI)
Many studies have analyzed soil quality using different parameters, ranging from a purely agricultural point of view to a more environmental perspective. However, the large number of studied sites and the high variability of the studied physical, chemical, and biological indicators make it difficult to clearly interpret results. The synthetic approach derived from the use of an SQI, as well as multivariate analyses (PCA), allowed us to point out a clear separation of the sites into three classes of different soil qualities. We finally chose six soil parameters for the SQI calculation, which we considered the most important indicators of soil quality: AN, BR/MBC, MBC, urease, polyphenol oxdiase, and bulk density. Almost all of these indicators were also suggested by Bastida et al. (2006), Armas et al. (2007), Erkossa et al. (2007), and Sinha et al. (2009). The species from croplands abandoned for natural recovery (H. altaicus and A. capillaries) had the highest SQI, followed by those from grasslands (A. adsurgens and P. virgatum), and then by shrublands (C. korshinskii and H. rhamnoides).
The process of reconverting slope croplands into forestlands and grasslands has been implemented to avoid the soil degradation problem of the past two decades in the Loess Plateau. Shrubs grown on slope croplands such as C. korshinskii and H. rhamnoide, as well as grasses such as A. Adsurgens and P. virgatum, are predominately revegetation measures. Many studies have demonstrated that this reconversion can significantly improve the physical, chemical, and biological soil properties.(An et al. 2008; Zhu et al. 2010) The improvement in shrubland and grassland soil qualities can be attributed to the increased plant cover and reduced decomposition rate as a consequence of tillage, which increases input of organic matter and physical protection of soil from erosion (Moscatelli et al. 2007). In the present study, the grassland species generally showed higher SQI than shrubland species, suggesting a better soil recovery capacity in grasslands than shrubland. This higher grassland soil quality is probably due to the larger herbaceous coverage (Table 1), because plant coverage plays an important role in both soil protection from erosion and contribution to soil organic matter content (Marzaioli et al. 2010). Indeed, high soil quality often occurred in the soils where the herbaceous coverage was high. Similar to our results, Caravaca et al. (2003) found that perennial grass species improved soil quality in an abandoned agroecosystem in Spain by increasing organic matter and nitrogen content, as well as by favoring the formation of stable aggregates and the development of mycorrhizal propagules. Furthermore, extensive fine root systems in grasslands can be another contributing factor to the high grassland soil quality, because abundant exudates released from roots and widely distributed in the soil improve chemical and physical soil properties (Wei et al. 2009; Zhang et al. 2010).
In the Loess Plateau, natural vegetation from abandoned croplands can both protect the soil from erosion and improve soil properties, such as organic matter content, soil structure, and infiltration rate. In the present study, abandoned cropland for natural recovery had the best soil quality on the basis of the considered parameters, indicating that it has the best capacity for soil recovery. This agrees with Li et al. (2010) who observed that soil carbon and nitrogen components was higher in natural grasslands compared with artificial shrublands and grasslands (human-planted) in the Loess Plateau. Aside from the presence of a dense herbaceous layer due to recolonization by spontaneous plants (Rutigliano et al. 2006), the excellent soil quality of abandoned croplands could be related to the nature of the plants therein. Compared with artificial vegetation, where the species are selected by humans, natural vegetation occurs through spontaneous natural succession without any anthropogenic influnence whatsoever (Zhang et al. 2011). Natural vegetation depends on the natural factors of the broader ecosystem development context of plant associations, soil, animals, and particularly soil organisms (Whisenant 1995). This results in a stronger adaptability of natural vegetation to natural environment conditions. Natural vegetation can allow more species to colonize due to the creation of high habitat diversity, which are difficult or impossible to achieve through planting (Florgard 2004).
Conclusions
Different vegetation types significantly affected in different magnitudes the physical, chemical, and microbial properties of arid climatic soils. Compared with the soils of shrubs (C. korshinskii and H. rhamnoides) and grasses (A. adsurgens and P. virgatum), the soil of natural species from abandoned cropland species (H. altaicus and A. capillaries) had the highest SOC, TN, TP, AN, AP, MBC, SIR, saccharase, urease, catalase, and peroxidase. The SQI, which was calculated by integrating all critical parameters, indicated that in the Loess Plateau, abandoned croplands for natural recovery have the best capacity for soil restoration. Thus, the present study suggests that in the Loess Plateau, natural recovery is the best choice for soil revegetation of sloping croplands. However, our suggestion was based on the natural recovery that normally takes place, if there are dangerous invasive weeds capitalizing on the bare ground, more research should be conducted.
References
Albaladejo J, Martinez-Mena M, Castillo V (1994) Changes in soil physical properties induced by soil degradation. Transaction of the 15th World Congress of Soil Science Mexico vol. 2b ISSS Acapulco Mexico, pp 250–252
An SS, Huang YM, Zheng FL, Yang JG (2008) Aggregate characteristics during natural revegetation on the Loess Plateau. Pedosphere 18:809–816
Anderson TH, Domsch KH (1993) The metabolic quotient for CO2 (qCO2) as a specific activity parameter to assess the effects of environmental conditions, such as Ph, on the microbial biomass of the soil. Soil Biol Biochem 25:393–395
Andrews SS, Karlen DL, Mitchell JP (2002) A comparison of soil quality indexing methods for vegetable systems in Northern California. Agric Ecosyst Environ 90:25–45
Armas CM, Santana B, Mora JL, Notario JS, Arbelo CD, Rodríguez-Rodríguez A (2007) A biological quality index for volcanic Andisols and Aridisols (Canary Islands, Spain): variations related to the ecosystem development. Sci Total Environ 378:238–244
Arunachalam A, Pandey H (2003) Ecosystem restoration of Jhum fallows in northeast India: microbial C and N along altitudinal and successional gradients. Res Ecol 11:168–173
Badiane NNY, Chotte JL, Pate E, Masse D, Rouland C (2001) Use of soil enzyme activities to monitor soil quality in natural and improved fallows in semi-arid tropical regions. Appl Soil Ecol 18:229–238
Bandick AK, Dick RP (1999) Field management effects on soil enzyme activities. Soil Biol Biochem 31:1471–1479
Bastida F, Moreno JL, Hernandez T, Garcia C (2006) Microbiological degradation index of soils in a semiarid climate. Soil Biol Biochem 38:3463–3473
Benitez E, Melgar R, Sainz H, Gómez M, Nogales R (2000) Enzyme activities in the rhizosphere of pepper (Capsicum annuum, L.) grown with olive cake mulches. Soil Biol Biochem 32:1829–1835
Bremner JM, Mulvaney CS (1982) Nitrogen-total. In: Page AL, Miller RH, Keeney DR (eds) Methods of soil analysis, Part 2, chemical and microbial properties. Agronomy Society of America Agronomy Monograph 9, Madison, pp 595–624
Cao CY, Jiang DM, Teng XH, Jiang Y, Liang WJ, Cui ZB (2008) Soil chemical and microbiological properties along a chronosequence of Caragana microphylla Lam. plantations in the Horqin sandy land of Northeast China. Appl Soil Ecol 40:78–85
Caravaca F, Figueroa D, Barea JM, Azcón-Aguilar C, Palenzuela J, Roldán A (2003) The role of relict vegetation in maintaining physical, chemical, and biological properties in an abandoned Stipa-grass agroecosystem. Arid Land Res Manag 17:103–111
Condron LM, Newman RH (1998) Chemical nature of soil organic matter under grassland and forest. Eur J Soil Sci 49:597–604
Cullen D, Kersten PJ (1996) Enzymology and molecular biology of lignin degradation. In: Brambl R, Marzluf GA (eds) The Mycota III. Biochemistry and molecular biology. Springer-Verlag, Berlin, pp 295–306
Erkossa T, Itanna F, Stahr K (2007) Indexing soil quality: a new paradigm in soil science research. Aus J Soil Res 45:129–137
Florgard C (2004) Remaining original natural vegetation in towns and cities. Urban Forest & Urban Greening 3:1–2
Fu SL, Zou XM, Coleman D (2009a) Highlights and perspectives of soil biology and ecology research in China. Soil Biol Biochem 41:868–876
Fu XL, Shao MA, Wei XR, Horton R (2009b) Effects of two perennials, fallow and millet on distribution of phosphorous in soil and biomass on sloping loess land, China. Catena 77:200–206
Garcia C, Hernandez T, Roldan A, Martin A (2002) Effect of plant cover decline on chemical microbiological parameters under Mediterranean climate. Soil Biol Biochem 34:635–642
Garcia C, Roldan A, Hernandez T (2005) Ability of different plant species to promote microbiological processes in semiarid soil. Geoderma 124:193–202
Guan SY, Zhang DS, Zhang ZM (1991) Methods of soil enzyme activities analysis. Agriculture, Beijing, pp 263–271 (in Chinese)
Insam H, Domsch KH (1988) Relationship between soil organic carbon and microbial biomass on chronosequences of reclamation sites. Microb Ecol 15:177–188
Institute of Soil Science of Chinese Academy of Science (1981) Soil chemical and physical analysis. Shanghai Science and Technology, Shanghai (in Chinese)
Jenkinson DS, Powlson DS (1976) The effects of biocidal treatments on metabolism in soils—a method for measuring soil biomass. Soil Biol Biochem 8:167–177
Jia GM, Cao J, Wang CY, Wang G (2005) Microbial biomass and nutrients in soil at the different stages of secondary forest succession in Ziwuling, northwest China. Fores Ecol Manage 217:117–125
Jiang JP, Xiong YC, Jiang HM, Ye DY, Song YJ, Li FM (2009) Soil microbial activity during secondary vegetation succession in semiarid abandoned lands of Loess Plateau. Pedosphere 19:735–747
Lal R (2001) Soil degradation by erosion. Land Degrad Dev 12:519–539
Li YY, Shao MA (2006) Change of soil physical properties under long-term natural vegetation restoration in the Loess Plateau of China. J Arid Environ 64:77–96
Li JF, Chen JM, Liu W, Gu XL (2010) Distribution of soil organic carbon and total nitrogen of grassland in Yunwu mountain of Loess Plateau. Acta Agrestia Sinica 18(5):661–668 (in Chinese)
Lin XG, Yin R, Zhang HY, Huang JF, Chen RR, Cao ZH (2004) Changes of soil microbiological properties caused by land use changing from rice-wheat rotation to vegetable cultivation. Environ Geochem Health 26:119–128
Marzaioli R, D’Ascoli R, De Pascale RA, Rutigliano FA (2010) Soil quality in a Mediterranean area of Southern Italy as related to different land use types. Appl Soil Ecol 44:205–212
Masto RE, Chhonkar PK, Singh D, Patra AK (2008) Alternative soil quality indices for evaluating the effect of intensive cropping, fertilization and manuring for 31 years in the semi-arid soils of India. Environ Monit and Assess 136:419–435
Moscatelli MC, Di Tizio A, Marinari S, Grego S (2007) Microbial indicators related to soil carbon in Mediterranean land use systems. Soil Till Res 97:51–59
Nannipieri P, Greco S, Ceccanti B (1990) Ecological significance of the biological activity in soil. In: Bollag JM, Stozky G (eds) Soil biochemistry vol. 6. Marcel Dekker, New York, pp 293–354
Nelson DW, Sommers LE (1982) Total carbon, organic carbon, and organic matter. In: Page AL, Miller RH, Keeney DR (eds) Methods of soil analysis, Part 2, Chemical and microbial properties. Agronomy Society of America, Agronomy Monograph 9, Madison, pp 539–552
Odum EP (1985) Trends expected in stressed ecosystems. Bioscience 35:419–422
Olsen SR, Sommers LE (1982) Phosphorous. In: Page AL, Miller RH, Keeney DR (eds) Methods of soil analysis Part 2, chemical and microbial properties. Agronomy Society of America, Agronomy Monograph 9, Madison, pp 403–430
Parr JF, Papendick RI (1997) Soil quality: relationship and strategies for sustainable dryland farming systems. Ann Arid Zone 36:181–191
Powlson DS, Jenkinson D (1981) A comparison of the organic matter, biomass, adenosine triphosphate and mineralizable nitrogen contents of ploughed and direct-drilled soils. J Agric Sci 97:713–721
Powlson DS, Brookes PC, Christensen BT (1987) Measurement of soil microbial biomass provides an early indication of changes in total soil organic matter due to straw incorporation. Soil Biol Biochem 19:159–164
Rutigliano FA, Ascoli RD, Virzo De Santo A (2004) Soil microbial metabolism and nutrient status in a Mediterranean area as affected by plant cover. Soil Biol Biochem 36:1719–1729
Rutigliano FA, De Pascale RA, Bronzo E, D’Ascoli R, Strumia S (2006) Soil and plant community properties as affected by two different types of land management in Southern Italy. In: La Fortezza R, Sanesi G (eds) Patterns and processes in forest landscapes. Consequences of human management. Accademia Italiana di Scienze Forestali, Firenze, pp 53–58
Schipper LA, Degens BP, Sparling GP, Duncan LC (2001) Changes in microbial heterotrophic diversity along five plant successional sequences. Soil Biol Biochem 33:2093–2103
Singh RS, Srivastava SC, Raghubanshi AS, Singh JS, Singh SP (1991) Microbial C, N and P in dry tropical savanna: effects of burning and grazing. J Appl Ecol 28:869–878
Sinha S, Masto RE, Ram LC, Selvi VA, Srivastava NK, Tripathi RC, George J (2009) Rhizosphere soil microbial index of tree species in a coal mining ecosystem. Soil Biol Biochem 41:1824–1832
Taylor JP, Wilson B, Mills MS, Burns RG (2002) Comparison of microbial numbers and enzymatic activities in surface soils and subsoils using various techniques. Soil Biol Biochem 34:387–401
Tirol-Padre A, Ladha JK, Regmi AP, Bhandari AL, Inubushi K (2007) Organic amendments affect soil parameters in two long-term rice-wheat experiments. Soil Sci Soc Am J 71:422–452
Trasar-Cepeda C, Leirós C, Gil-Sotres F, Seoane S (1998) Towards a biochemical quality index for soils: an expression relating several biological and biochemical properties. Biol Fertil Soils 26:100–106
Vance ED, Brookes PC, Jenkinson D (1987) An extraction method for measuring microbial biomass carbon. Soil Biol Biochem 19:703–707
Wei J, Zhou J, Tian JL, He XB, Tang KL (2006) Decoupling soil erosion and human activities on the Chinese Loess Plateau in the 20th century. Catena 68:10–15
Wei XR, Shao MA, Fu XL, Horton R, Li Y, Zhang XC (2009) Distribution of soil organic C, N and P in three adjacent land use patterns in the northern Loess Plateau, China. Biogeochemistry 96:149–162
Whisenant SG (1995) Landscape dynamics and arid land restoration. In: Roundy BR, McArthur ED, Haley JS, Mann DK (eds) Proceedings: Wildlife shrub and arid land restoration symposium. USDA, Ogden, pp 26–34
Zhang PJ, Li LQ, Pan GX, Ren JC (2006) Soil quality changes in land degradation as indicated by soil chemical, biochemical and microbiological properties in a karst area of southwest Guizhou, China. Environ Geo l51:609–619
Zhang YY, Qu LY, Chen LD, Wei W (2010) Soil microbial properties under different vegetation types in Loess hilly region. Chinese Journal of Applied Ecology 21(1):165–173 (in Chinese)
Zhang C, liu GB, Xue S, Song ZL (2011) Rhizosphere soil microbial activity under different vegetation types on the Loess Plateau, China. Geoderma 161:115–125
Zhou ZC, Shangguan ZP, Zhao D (2006) Modeling vegetation coverage and soil erosion in the Loess Plateau Area of China. Ecol Model 198:263–268
Zhu BB, Li ZB, Li P, Liu GB, Xue S (2010) Soil erodibility, microbial biomass, and physical–chemical property changes during long-term natural vegetation restoration: a case study in the Loess Plateau, China. Ecol Res 25:531–541
Acknowledgements
We acknowledge the support of the National Natural Sciences Foundation of China (40801094), the National 973 Program (No. 2007CB407205), the CAS Action Plan for West Development Program (No.KZCX2-XB2-05) and West Light Foundation of CAS. We thank all our partners and previous researchers who have also been involved in this project, as well as the editor and reviewers of this paper.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Johan Six.
Rights and permissions
About this article
Cite this article
Zhang, C., Xue, S., Liu, GB. et al. A comparison of soil qualities of different revegetation types in the Loess Plateau, China. Plant Soil 347, 163–178 (2011). https://doi.org/10.1007/s11104-011-0836-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-011-0836-5