Abstract
Aims
Purple nonsulfur bacteria (PNSB), Rhodopseudomonas palustris strains (TLS06, VNW02, VNW64 and VNS89), were investigated to increase rice growth and grain yield in acid sulfate soils (ASS) with low available phosphorus (Pavail).
Methods
P-solubilizers were tested in vitro. A 4 × 3 factorial design consisted of PNSB at 5.4 × 104 cells g−1 dry soil weight (mixture of 4 strains, VNW64 singly, or no-PNSB) and P fertilizer levels (0, 30, 45 and 60 kg P2O5 ha−1) that were used with the rice variety OM5451in pots with two types of ASS (Hon Dat and Phung Hiep).
Results
The four PNSB mixture had the ability to dissolve insoluble P from variscite and strengite. The combination of mixed culture with 45 P was the most effective, increasing grain yield by 34%. Enhancement of rice growth and yield in both soils corresponded to the maximal levels of Pavail, total P and NH4+, and the lowest levels of Alexch and Fe2+. Soil health with this treatment was significantly improved, with a strong positive correlation between PNSB population and phosphatase activity in both soil types.
Conclusions
The combination of PNSB mixture with P fertilizer reduced the amount of chemical fertilizer needed for maximal grain yield, provided safe rice, and maintained soil health.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
It has long been known that extractable aluminum and ferrous ions in acid sulfate soils (ASS) drastically decrease rice growth and reduce rice root length (Huang et al. 2016) consequently reducing rice yield (Onaga et al. 2016; Soomro et al. 2015). Normally, insufficient phosphorus (P) for rice has been observed in ASS due to the immobilized P that has precipitated with free aluminum and iron ions to form aluminum phosphate (AlPO4) and iron phosphate (FePO4) (Margenot et al. 2017). These forms of P are poorly available to plants (Rengel and Marschner 2005) and the soil microbial community structure is altered (Ragot et al. 2016; Soman et al. 2017). The ways to resolve this problem include chemical, physical and biological approaches. Regarding the chemical approaches, P fertilizers have been heavily applied in intensive farming areas, which reduces soil quality (Liu et al. 2017) and causes contamination by heavy metals, particularly cadmium with long term use (Kelliher et al. 2017). As a result, this approach is not suitable for a sustainable agricultural system. As a bio-based approach, the rhizosphere soil microorganisms are capable of solubilizing complex-structured phosphates and improve the availability of P to plants. The solubilizing of P by bacteria has been reported to employ various mechanisms (Alori et al. 2017). For instance, microorganisms secrete organic or inorganic acids, such as hydrochloric, nitric and sulfuric acid, lowering the pH and also chelating the metal ions Ca2+, Al3+, and Fe2+ (Alori et al. 2017; Arif et al. 2017). By this means the soil bacteria can dissolve inorganic P compounds in soils and provide P that is available to plants.
Recently, plant growth promoting bacteria (PGPB) have been selected for their resistance to Al stress, with the focus on upland soils (de la Luz Mora et al. 2017). However, no prior work has addressed the use of PGPB on ASS in wetland soils. We have recently succeeded in obtaining acid-resistant Rhodopseudomonas palustris strains, isolated from flooded rice fields on ASS, with a high potential to reduce Al3+ and Fe2+ by biosorption according to in vitro tests, and they increased the pH in strongly acidified conditions with toxic metals at critical levels to rice (Khuong et al. 2017). Among the PGPB, the purple nonsulfur bacteria (PNSB) are versatile organisms with a variety of growth modes, such as photoautotrophy, photoorganotrophy, and heterotrophy; and they are also N2 fixers (Madigan et al. 1984). They are considered potent candidates for solving the ASS problem. Interestingly, the PNSB release plant growth promoting substances such as 5-aminolevulinic acid (ALA) (Kantha et al. 2015; Nunkaew et al. 2014) and phytohormones including NH4+ (Sakpirom et al. 2017). It should be noted that potent strains of R. palustris were successfully applied as biofertilizers improving rice growth and yield in a wide range of conditions, including organic and saline soils (Kantachote et al. 2016; Kantha et al. 2015). However, they have not been examined in acidic conditions, although a previous study found that the solubilization of insoluble P by PNSB may be responsible for growth promotion of tomato seedlings; but the solubilization of P has not been elucidated (Koh and Song 2007). The PNSB have also been tested to solubilize Ca3(PO4)2 (Lakshmi 2012). However, to date the ability of PNSB to solubilize variscite (AlPO4•2H2O) and strengite (FePO4•2H2O) has not been tested; these are present in high quantities in ASS. In addition, the use of P-solubilizing bacteria could increase the activity of soil phosphatase further increasing Pavail for the plants (Pradhan et al. 2017).
As previously mentioned, our acid-resistant PNSB act as bioremediators and also biofertilizers, and could enable cultivating rice in flooded conditions by environmentally friendly sustainable agriculture, instead of by using P fertilizers. The challenge posed was therefore to investigate our PNSB strains for their potential to enable rice cultivation in ASS, with focus on solubilizing P compounds. Investigating the potential to solubilize P was done in pot assays with ASS, evaluating the alleviation of Al and Fe toxicities along with P uptake. The ultimate goals are to promote rice growth and yield, reduce toxic metals in rice, and improve soil health.
Materials and methods
In vitro evaluation of ability to solubilize Al-P and Fe-P compounds by the potent acid-resistant PNSB
The potent PNSB used in this experiment were the anoxygenic phototrophic R. palustris strains TLS06, VNW02, VNW64 and VNS89, isolated from ASS paddy fields by using basic isolation medium (BIM) as described by Brown (2013). The PNSB strains were grown in modified BIM that excluded K2HPO4, at pH 4.50, under optimal growth conditions (Khuong et al. 2017) with supplementation of either 0.3 g AlPO4•2H2O L−1 or 1.0 g FePO4•2H2O L−1. Both phosphate sources were tested individually, at equal concentrations, to assess their solubilization. The optimal incubating conditions for PNSB growth were 35 °C, 150 rpm for aerobic dark culturing; and light intensity in the range 3000–4000 lx for microaerobic culturing with light. The inoculum size of each culture was 10% in 18 ml of the medium, either in a serum bottle (100 ml) for aerobic dark conditions, or in a culture tube (15 × 150 mm) for microaerobic light conditions. To evaluate the solubilizing capacity, the released P concentration was measured daily for 6 days, and thereafter on days 9, 12 and 15; and uninoculated modified BIM medium was used as a control. The soluble P concentration was determined by ascorbic acid method (Murphy and Riley 1962). In short, culture broths were centrifuged at 12,000 rpm for 10 min. The supernatants were measured at 880 nm wavelength for colored molybdenum blue, formed by the reaction of ammonium molybdate and antimony potassium tartrate with orthophosphate to form phosphomolybdic acid. In addition, the pH of the culture supernatants was measured to assess mechanisms of P-solubilization. This experiment was carried out in triplicate.
Pot experiment for observing toxic metals and P uptake in the soil-plant system
The experimental design and pot preparation
Two soils were used in this experiment, sampled from Phung Hiep district, Hau Giang province, Depressed of Hau River area; and from Hon Dat district, Kien Giang province, Long Xuyen Quadrangle area. These areas were selected for their eco-agricutural systems with actual ASS cultivated paddy fields, based on the classification of agro-ecological areas by Xuan and Matsui (1998). Soil was collected from a variety of soil profiles; and 0–15 cm depth samples were used in the soil pot experiments. The initial soil physicochemical parameters are shown in Table 1. The physicochemical properties determined prior to use in pot experiments were pHH2O, pHKCl, electrical conductivity (EC), total iron (Fetot), copper, zinc, manganese, total dissolved iron (Fedis), ferrous iron (Fe2+), ferric oxide (Fe2O3), acidity, exchangeable aluminum (Alexch), total nitrogen (Ntot), ammonium ion (NH4+), total phosphorus (Ptot), available phosphorus (Pavail), fractions of soil P (Al-P, Fe-P, Ca-P compounds), organic matter (OM), and cation exchange capacity (CEC). Three sub-samples from each type of soil were analyzed for each parameter.
The methods of analysis, as described by Sparks et al. (1996), were as follows. Briefly, soil samples were separately extracted using 1 M KCl (pHKCl) or deionized water (pHH2O) at 1:5 ratio of soil: solvent for measuring pH; however, only the supernatant extracted with deionized water was used to measure EC. Total Fe, Cu, Zn and Mn were determined by using inductively coupled plasma – optical emission spectroscopy (ICP-OES). Alexch in the soil samples was extracted by 1 M KCl; and the spectrophotometric method was employed for Al3+ quantitation from supernatant, in fluorometric analysis of aluminum content with 8-hydroxyquinoline and butyl acetate. Feactive (Fe2O3) in the soil samples was extracted with diethylenetriaminepentaacetic acid (DTPA). The digest solution was used for amorphous iron oxide analysis: acid ammonium oxalate formed in darkness was detected by modified Tamm’s reagent. The ferrozine method for the determination of Fe2+ concentration used 1, 10-phenanthroline reagent and visible spectrophotometry at 562 nm. The available P was determined by the Bray II method, and fractions of inorganic P were extracted by 0.5 M NH4F, 0.1 M NaOH and 0.25 M H2SO4 for Al-P, Fe-P, and Ca-P fractions, respectively. Ntot was quantified by the regular Kjeldahl method, after destruction of organic N state prior to determination of inorganic N. NH4+-N concentration was determined by a colorimetric procedure. Dichromate oxidation in sulfuric acid with thermal conductivity measurement was utilized to convert organic C to inorganic, prior to titration with ferrous sulfate heptahydrate for total C determination. The compulsive exchange method has been recommended for the determination of CEC in acid soils. This extract solution was also used for the analysis of K, Na, Ca, and Mg, by atomic absorption spectrophotometry. Soil texture was analyzed by Robinson’s pipette method determining the granulometric fraction (Robinson 1922).
The PNSB bioremediators/biofertilizers used in this experiment included a mixed culture of four strains (TLS06, VNW02, VNW64 and VNS89); and the strain VNW64 was also used as a single culture as it is the most effective strain for reducing Al3+ and Fe2+ toxicities (Khuong et al. 2017). Each strain was separately cultured in a BIM broth, at pH 4.5 under microaerobic light conditions for 48 h. Then, the culture broth was centrifuged at 6000 rpm for 15 min to obtain a cell pellet that was washed twice with 0.1% peptone water (Panwichian et al. 2010). Sterile distilled water was used to make a cell suspension for which OD660 was adjusted to 0.8 using a spectrophotometer, to obtain cell density 108 cells ml−1 for preparing inoculums. To obtain equal cell counts in the mixture of 4 strains, 25 ml of each culture was mixed together to 100 ml of inoculum; similarly, 100 ml of strain VNW64 as a single culture was used as inoculum. Each PNSB inoculum was applied at 1 ml time−1 pot−1 for 3 times, to provide 5.0 × 104 PNSB cells g−1 dry soil weight (DSW). The low cell density was designed for evaluating the role of PNSB when combined with P fertilizer, and also for inexpensive inoculations that are attractive to the farmers.
Rice seeds were sterilized using 70% ethanol and 1% sodium hypochlorite solution for 3 and 10 min, respectively, and were then washed with sterile distilled water. The seeds were incubated for 24 h under dark conditions for germination. Subsequently, to inoculate the seeds with PNSB, they were soaked in flasks containing a bacterial cell suspension at 108 CFU ml−1, prepared as previously described, and placed in a reciprocal shaker at 60 rpm for 1 h, and then held at room temperature for 1 h for stability. After soaking, the seeds were taken out and dried under laminar air flow before sowing; the rice seeds were coated with approximately 6.3 × 106 PNSB cells per seed. The control seeds without inoculants were similarly prepared, but with sterile distilled water replacing the inoculating suspensions.
The 4 × 3 factorial experiment was carried out in a completely randomized block design, including as main factor the inoculants (mixed, or single at 5.4 × 104 cells g−1 DSW for each and no-PNSB) and as minor factor the P fertilizer rate (0, 30, 45 and 60 kg P2O5 ha−1) to a total of 12 sets: mixed with 0, 30, 45, 60; single with 0, 30, 45, 60; and no PNSB with 0, 30, 45, 60. All cases used ASS collected from the two sources, with four replicates in a greenhouse at An Giang University, Vietnam. Each pot (25 cm diameter × 30 cm height) had approximately 6 kg soil, with rice straw residue removed before mixing and drying in ambient atmosphere. Then, the soil in pot was moistened with roughly 2 L pot−1of tap water to saturate the soil for 2 days before seeding. The rice (Oryza sativa L.) variety OM5451 was cultivated in the pots. The recommended dose of fertilizers for this rice variety was 100 N - 60 P2O5-30 K2O (kg ha−1). However, the P fertilizer was modified to follow the designed levels. The actually used chemicals were urea for N (46% N), superphosphate fertilizer for P (16% P2O5), and potassium chloride for K (60% K2O).
Rice cultivation and monitoring of plant growth, element uptake and soil properties
Rice seeds were cultivated with a density of 4 seeds pot−1; this provided 4.2 × 103 PNSB cells g−1 DSW (4 × 6.3 × 106 cells seed−1, dry soil 6 kg−1 pot). Prior to growing rice, inorganic P fertilizer was added for half of the designed level as a basal application, and the second half was provided on day 20 of cultivation. Each PNSB inoculant (mixture or single) was applied at 1 ml to provide roughly 1.6 × 104 cells g−1 DSW for each stage on days 1, 20 and 65 of cultivation. Therefore, each soil pot had inoculated PNSB at roughly 5.4 × 104 cells g−1 DSW (4.73 log cells). The rice was flooded to maintain at least 3 cm depth during the vegetative and reproductive stages, and tap water was used for irrigation every week. All the rice plants were harvested on day 95 for determining plant growth parameters, rice yield and elements in straw and grain.
Rice growth was measured on the basis of plant height and harvest index by following the manual of the International Rice Research Institute (IRRI 2014). For agronomic traits that include rice yield components and yield, the observed quantities were the number of panicles per pot, the number of grains per panicles, grain filled percentage; panicle length; thousand-grain oven-dry weight, grain yield (14% moisture content), and dry biomass components of rice straw and grain. The harvest index was calculated as follows: HI = grain yield/ total rice biomass (straw weight + grain weight).
Straw and grain samples from each pot were collected and dried at 65–70 °C for 72 h. Then, they were ground to pass through a 0.5 mm sieve for the total analysis of the following elements: P, Al and Fe in rice straw and grain. To determine Fe, Al and P, all the samples were digested following Walinga et al. (1989). Fe and Al concentrations were determined using ICP-OES, while the P concentration was determined by a colorimetric procedure. The uptakes of P, Al and Fe were calculated based on their concentrations and biomass of grain and straw.
Physicochemical properties of soil after rice harvest were also examined to assess the final soil quality. These were pHKCl, Fetot, Fedis, Fe2+, Fe2O3, acidity, and Alexch. On the other hand, soil fertility was assessed from the following parameters: pHH2O, EC, Ntot, NH4+, Ptot, Pavail, Al-P, Fe-P, and Ca-P. The methods used were the same as in the determination of initial soil properties.
Cell density of PNSB in the soil on day 95 was estimated by the most probable number (MPN) technique in acidic BIM broth with 3 tubes (n = 12 = 3 × 4) under microaerobic light conditions, as described by Harada et al. (2001) and modified by Kantachote et al. (2016). Soil phosphatase enzyme activity was determined using the method described by Tabatabai and Bremner (1969). In brief, 1 g soil was incubated with 1 ml of 0.115 M p-NPP (p-nitrophenylphosphate), 0.25 ml of toluene, and 4 ml of 0.1 M Tris buffer (pH 6.5) for 1 h in dark. Then, 1 ml of 0.5 M CaCl2 and 4 ml of 0.5 M sodium hydroxide were added to stop the reaction before the mixture was filtered. Concentration of p-nitrophenyl (p-NP) in the supernatant was measured by a spectrophotometer at 400 nm. Phosphatase activity was calculated from the mg of p-NP produced during 1 h per g of dry soil.
Statistical analysis
The data shown are means of four replications, unless otherwise stated. The data were subjected to two-way analysis of variance (ANOVA) using SPSS software version 13.0. Means were separated by ANOVA and the significance of differences was assessed by Duncan’s post-hoc test at P < 0.05. Statistical analysis was also applied to identify the most effective conditions, with only P added at the appropriate level, for the single culture, the mixed culture and the control (no addition of both PNSB and P fertilizer). Correlation between the PNSB population and the soil phosphatase activity was assessed from Pearson’s correlation coefficient, using SPSS.
Results
In vitro evaluation of ability to solubilize Al-P and Fe-P compounds by the potent acid-resistant PNSB
No soluble P in the uninoculated medium indicates that almost all the tested PNSB are P solubilizers; Fig. 1 shows no differences in concentrations of insoluble P to soluble P for most cell-free supernatants on day 1, with the exception being the mixture of both insoluble P types under both incubating conditions. From this point onwards, there was a dramatic increase in soluble P from all insoluble sources under aerobic dark conditions, although sometimes a decrease was observed. In contrast, for most treatments in microaerobic light conditions a sharp drop of P solubilization was found from day 1 to day 5, then tending to increase until the end of incubation. Increasing soluble P and pH had similar patterns for all PNSB culture broths under both incubating conditions, and the initial pH 4.5 changed to around 5.0–6.5 with the increase in soluble P (Fig. 1).
Phosphate solubilization and pH changes in modified acidic BIM broth by acid - resistant Rhodopseudomonas palustris strains with access to various P sources; (a, b): 0.3 g L−1 AlPO4•2H2O, (c, d): 1.0 g L−1 FePO4•2H2O and (e, f): mixed P sources (at the same level for each P source) under conditions of aerobic dark and microaerobic light for 15 cultivation days. The bars represent the standard deviation of the mean from three determinations
All the tested PNSB showed fluctuation in Al-P solubilization capacity under both incubating conditions (Fig. 1a-b). With Al-P as the P source in culture broths, strain TLS06 provided the most soluble P (25.9 mg L−1) by day 15 under aerobic dark conditions; while strain VNW64 had the highest ability under microaerobic light conditions, with 17.1 mg P L−1. However, the strain VNS89 showed higher capacity than other strains on days 4–5, with 18.3–20.0 mg P L−1 under aerobic dark conditions, and on days 9–12 with 15.2–15.7 mg P L−1 under microaerobic light conditions. The highest quantity of solubilized P from Fe-P source was achieved by strains TLS06 and VNW64 (Fig. 1c-d). The soluble phosphate released by these strains was 32.5 and 32.8 mg P L−1 under aerobic dark conditions, and under microaerobic light conditions 22.1 and 22.5 mg P L−1, respectively. When both Al-P and Fe-P were simultaneously added in modified BIM broth, strain TLS06 showed the highest amount of P solubilization among the strains, releasing 35.5 mg P L−1 by day 12 under aerobic dark conditions (Fig. 1e). Moreover, on day 15 under microaerobic light conditions strain TLS06 also released the most soluble phosphate at 19.2 mg P L−1, followed by VNW64 at 19.0 mg P L−1 (Fig. 1f).
Effects of added P and inoculated PNSB on rice growth and yield in ASS
Both types of soil had low pH below 4.0 (pHKCl), with a high acidity of up to 57.4 cmolc H+ kg−1 in Hon Dat soil (Table 1a). However, the acidity of both ASS soil types from 0 to 15 cm depth was roughly 12 cmolc H+ kg−1. The initial insoluble P concentration in soil profiles from Hon Dat and Phung Hiep was 12.9–48.4 and 12.4–16.9 mg P L−1 from AlPO4, and 5.9–86.1 and 4.8–11.5 mg P L−1 from FePO4, respectively (Table 1b). In particular, these concentrations in the cultivated horizon (0–15 cm) were high due to the precipitation of Al-P and Fe-P after use of P fertilizer. Moreover, the results also show that indigenous plant nutrients in both soil types were not sufficient to meet the nutrient requirements for rice growth, particularly as N source (0.3 and 0.5%). Therefore, in the pot experiments to assess P requirement also N and K were supplemented in recommended doses (Cho et al. 2017) to ensure that nutrients are not limiting grain yield. The soil collected from Hon Dat, Long Xuyen Quadrangle was silty clay, while the soil from Phung Hiep, Depressed of Hau River was clay (Table 1c).
The rice growth based on plant height, yield components, and grain yield was significantly increased in the treated cases relative to the control (Table 2). Regarding the P level, it was found that plant height, number of panicles, and percentage of filled grains increased significantly, with a remarkable improvement in rice grain yield for the two types of ASS (Table 2a-b). In soil from Hon Dat, treated by addition of 30–60 kg P2O5 ha−1 the 21.6–22.8 g pot−1 grain yield (Table 2a) was significantly higher than in the control (no added P fertilizer) at 18.8 g pot−1. A similar trend was also found for the soil from Phung Hiep, as the grain yield range was 16.0–17.2 g pot−1 with added P, compared with 14.4 g pot−1 for the control (Table 2b).
The mixed culture (TLS06, VNW02, VNW64 and VNS89) showed significant increases in plant height, number of panicles, panicle length, total spikelets per panicle, and grain yield with both soil types (Table 2a-b). However, the single culture (VNW64) used as bioremediator and biofertilizer improved the grain yield only with the silty clay soil from Hon Dat. Specifically, the treatments with mixed and single PNSB reached 21.4–23.4 g pot−1 grain yields that were higher than the 19.2 g pot−1 control with Hon Dat soil (Table 2a). The grain yields with mixed, single and no-PNSB were 17.5, 15.7, and 15.0 g pot−1, respectively, with Phung Hiep soil (Table 2b).
Effects of added P and inoculated PNSB on rice uptakes of P and toxic metals
Table 3 shows significant effects of both added P and added PNSB on almost all of the detected parameters in rice (straw and grain) with both soil types (Hon Dat and Phung Hiep). Regarding Al toxicity, both mixed and single inoculants reduced the absorbed concentration of Al in both parts of the rice plant, with the two soil types. For Hon Dat soil pots, Al concentration (mg kg−1 DW) in straw was 391.5 and 416.6 for mixed and single treatments, and 528.5 for the control cases (uninoculated); while in the grain, it was only 37.4 and 39.1 for the inoculated cases and 51.9 for the control cases (Table 3a). A similar trend was observed in Phung Hiep soil pots as Al concentrations in straw were at 421.1 and 437.9 mg kg−1 DW for the inoculated cases, and 587.6 mg kg−1 DW for control; whereas Al found in the grain was at 28.3 and 29.1 with inoculations and at 54.2 mg kg−1 DW for the controls (Table 3b). With Hon Dat soil, the total uptake of Al in rice (straw and grain) was significantly reduced at 15.0 and 14.4 mg pot−1 for mixture and single strain treatments, in comparison with 15.9 mg pot−1 without PNSB. This was similar with Phung Hiep soil, as the total uptake of Al in rice was 9.5 and 10.1 mg pot−1 for inoculated cases compared with 11.1 mg pot−1 for controls. Hence, both the mixed and single PNSB inoculants significantly reduced the total Al uptake in rice, by up to 9.8% for Hon Dat and 14.4% for Phung Hiep ASS. Both PNSB inoculants (mixed and single) significantly reduced Fe in both straw and grain with both soil types (Table 3a-b). For Hon Dat soil, the toxic Fe in rice straw was 399.0 mg kg−1 DW with the mixed culture, compared with 478.1 mg kg−1 DW for the controls; while in Phung Hiep soil Fe was at 285.7 mg kg−1 DW with the mixed culture compared with 383.4 mg kg−1 DW in the controls. The single culture showed similar trends to, although with poorer efficiency than the mixed culture. The total uptake of Fe in rice with mixed PNSB treatment was 6.8 mg pot−1 compared with the control cases at 7.1 mg pot−1 with Phung Hiep soil (Table 3b), so the total uptake of Fe by rice was significantly reduced by approximately 4.0%.
The data in Table 3 on P nutrient uptake by rice reveals that P content in the rice grain significantly increased with all treatments by harvest day with both soil types. Table 3b shows that the application of P from 30 to 60 kg P2O5 ha−1 with Phung Hiep soil significantly increased total P uptake by rice (38.1–41.4 mg pot−1) from the 28.9 mg pot−1 in the control cases, although there was no significant difference between these P fertilizer levels. However, with Hon Dat soil the application of 30–45 kg P2O5 ha−1 gave significantly lower P uptake than 60 P2O5 ha−1, at 77.7 and 80.8 mg pot−1 compared with 90.1 mg pot−1 (Table 3a). On the other hand, with Hon Dat soil the PNSB inoculants significantly increased the total uptake of P from 66.1 mg pot−1 in the controls to 77.8 and 89.8 mg pot−1 with the single and the mixed culture, respectively (Table 3a). A similar result was observed with Phung Hiep soil, as the control cases had only 31.1 mg pot−1 compared with 37.4 and 43.5 mg pot−1 when inoculated with the single and the mixed culture, respectively (Table 3b). To sum up, PNSB contributed to uptake of P nutrient by rice, the increment being 35.8% with Hon Dat soil and 40.2% with Phung Hiep soil.
Effects of added P and inoculated PNSB on soil quality and soil fertility
Soil quality on the basis of acidity, Alexch and Fe (Fetot, Fe2+, Fedis and Fe2O3) for both the ASS types after rice harvest on day 95 is presented in Table 4. Only the PNSB inoculants, particularly with the mixed culture, significantly increased pHKCl with both soil types; however, the pH still remained roughly at 4.0. The increases in pH indicate reduced acidity when inoculated with PNSB. This caused significantly decreased Alexch in both soil types with the inoculated PNSB cases, especially with the mixed culture. The level of Alexch in Hon Dat and Phung Hiep soils was 1.3 and 2.8 cmolc kg−1 DSW with the mixed culture; while it was up to 1.6 and 3.4 cmolc kg−1 DSW in the control cases without added PNSB (Table 4a-b). Regarding ferrous concentration, the mixed inoculants gave 50.3 mg Fe2+ kg−1 DSW which is significantly below the control (55.9 mg Fe2+ kg−1 DSW) for Hon Dat soil. However, with Phung Hiep soil both the mixed and single PNSB inoculants significantly reduced Fe2+ in the soil to 160.5 and 222.6 mg Fe2+ kg−1 DSW, from the 247.5 mg Fe2+ kg−1 DSW for the controls.
The effects of different treatments on soil physicochemical properties that indicate soil fertility are illustrated in Table 5a-b. Soil pHH2O significantly increased with PNSB application from 4.3 in the controls to 4.4 and 4.5 for inoculated cases with Hon Dat soil; and with Phung Hiep soil it was 4.0 for the controls and changed to 4.1 for inoculated cases. In contrast, no significant difference in pH was found between the alternative P levels. The results showed no significant changes in EC. Although the N2 fixing capacity has not been examined in this study, the inoculated PNSB significantly increased Ntot and NH4+ in both soil types. For example, PNSB inoculants showed a positive effect on NH4+ concentration (mg kg−1 DSW) that increased from 20.6 in the control to 23.3 and 24.0 with Hon Dat soil, and from 46.0 in the control to 50.8 and 52.2 with Phung Hiep soil. Ptot significantly increased with added P for both soil types; however, no significant effect was observed from inoculations with PNSB. In contrast, the PNSB inoculated cases showed a significant increase in Pavail with both soil types. This corresponded to the levels of P sources (Al-P, Fe-P and Ca-P) that were significantly reduced with inoculated PNSB in both soil types. The amount of Pavail was significantly higher in inoculated PNSB cases, particularly with the mixed culture (160.5 and 162.1 mg kg−1 DSW) for both Phung Hiep and Hon Dat soils, in comparison with controls (137.8–139.5 mg kg−1 DSW). The P levels of variscite, strengite and tricalcium phosphate in Hon Dat soil were 226.4, 282.9 and 14.8 mg kg−1 DSW with the mixed culture, compared with 261.2, 302.1 and 16.5 mg kg−1 DSW in the controls (Table 5a). The trend was similar in Phung Hiep soil containing 227.5, 262.3 and 21.9 mg kg−1 DSW in the mixed culture cases, and 243.6, 281.9, 27.0 mg kg−1 DSW in the controls without inoculation (Table 5b).
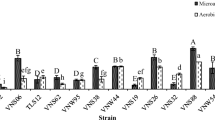
The PNSB population in the inoculated cases was significantly higher than without inoculation in both soil types, but no significant effect was observed from the level of added P (Fig. 2a-b). The PNSB population in Hon Dat soil showed significant differences between the treatments, in the rank order mixed culture > single > control at 6.0, 5.4 and 2.0 log MPN g−1 DSW, respectively (Fig. 2a). A similar result was found for Phung Hiep soil that had PNSB in single and mixed culture treatments at 4.5 and 5.0 log MPN g−1 DSW, compared with the controls at 1.8 log MPN g−1 DSW (Fig. 2b). A remarkable increase of soil phosphatase activity was found in all cases inoculated with PNSB compared with the controls, for both soil types, and no significant differences were found between the P levels (Fig. 2c-d). In Hon Dat soil, inoculated PNSB cases achieved 1.7 mg g−1 h−1 with both mixed and single cultures, above that without PNSB inoculation (0.3 mg g−1 h−1) (Fig. 2c). The Phung Hiep soil attained 1.3 and 1.5 mg g−1 h−1 with single and mixed cultures, and 0.2 mg g−1 h−1 for the controls (Fig. 2d). A strong positive correlation between PNSB population and phosphatase activity was found in the two soil types with r = 0.964 for Hon Dat soil (Fig. 2e) and r = 0.981 for Phung Hiep soil (Fig. 2f).
Effects of PNSB inoculum (5.4 × 104 cells g−1 dry soil weight: DSW) and added phosphorus (kg P2O5 ha−1) levels on PNSB population, phosphatase activity and their linear correlation coefficient in acid sulfate soils collected (a, c, e) from Hon Dat, and (b, d, f) from Phung Hiep sites after rice harvest on day 95. Mean of four determinations and its standard deviation (S.D.) are presented; while different letters above the bars indicate significant differences at P < 0.05 (ns is for “no significant difference”, P > 0.05)
Among the 12 treatments, combining the mixed culture with P at 45 kg P2O5 ha−1 (45 P) gave the most Pavail for providing maximal rice yield with the least toxic metals in rice grain, and achieving improved soil quality and soil fertility (Table 6). A remarkable increase in rice grain yield with Hon Dat soil had the rank order of combined mixed PNSB and 45 P (34.3%) > 45 P (8.3%) ~ mixed PNSB (6.2%) ~ single PNSB (5.7%) > control. A similar result was observed with Phung Hiep soil as the rice grain yield ranking was combined mixed PNSB and 45 P (33.6%) > 45 P (10.0%) ~ mixed PNSB (6.4%) > single PNSB (4.5%) > control. The results on rice yield were similar in pattern with Pavail, total P uptake, and NH4+. On the other hand, the levels of Alexch and Fe2+ were significantly lower in treated cases, particularly for the combination of mixed PNSB and 45 P; this corresponded to reduced concentration of Al and Fe in grain. The reduction percentages were 41.9 and 41.5 for Al, and 58.1 and 20.9 for Fe, with Hon Dat and Phung Hiep soils, respectively.
Discussion
Effects of added P and inoculated PNSB on rice growth and yield in ASS
Ranking behind N, P is an essential macronutrient that affects growth and metabolism of plants. This is the first report on four acid-resistant PNSB that in this study were able to solubilize the insoluble phosphates variscite (AlPO4•2H2O) and strengite (FePO4•2H2O), which are present in high amounts in ASS (Wisawapipat et al. 2017). The P solubilization from FePO4 was higher than that from AlPO4 in both incubating conditions (Fig. 1a-d). It should be noted that most bacteria cannot process insoluble phosphates (Perez et al. 2007) or release only a limited amount of P for plants (Sulbarán et al. 2009). Interestingly, all the strains of R. palustris in this study produced more Pavail from mineral P-sources than other bacterial genera such as Burkholderia, Serratia, Ralstonia and Pantoea reported on by Perez et al. (2007), and also more than fungi (Spagnoletti et al. 2017). Furthermore, our PNSB as phosphate-solubilizers were effective for growing rice in P deficient ASS samples from Hon Dat and Phung Hiep sites.
According to Horneck et al. (2011), it was found that Pavail content in Phung Hiep soil (15.5 mg kg−1 DSW) was comparatively low, while the high level in Hon Dat soil was 49.7 mg kg−1 DSW (Table 1b). This corresponded to the higher contents of toxicants Alexch and Fe2+ as well as Mn2+ in Phung Hiep compared with Hon Dat (Table 1a and c), which contributed to the higher rice yield with the latter soil. This is in accordance with previous reports (Huang et al. 2016; Onaga et al. 2016) because in ASS a great portion of P from chemical fertilizers becomes insoluble by precipitation with the cations, such as Al3+ and Fe2+, so that less is available to rice. However, additions of P and PNSB significantly improved rice growth and grain yield with both soil types (Table 2a-b). This can be explained through improvements in root growth and P uptake (our unpublished data). The differences in grain yield resulted from properties of soil as previously explained. Moreover, soil texture might affect on applying P fertilizer and PNSB inoculants, causing the poorer efficiency of Phung Hiep clay soil than of the silty clay from Hon Dat (Table 1c). Soil texture strongly affects yield, particularly for rice (Dou et al. 2016). For example, silty clay loam soil produced a higher grain yield than sandy loam (Yadav et al. 2017). Although there are no prior reports comparing silty clay soil and clay soil, a higher yield in the silty soil can be explained by its moderate particle size that allows better root development than in clay soil with smaller particles. In this study, rice yield without P application was remarkably lower than with application of 30 kg P2O5 ha−1 for Hon Dat (Table 2a) and 45 kg P2O5 ha−1for Phung Hiep soils (Table 2b). However, the increase in Pavail by inoculated PNSB (Figs. 1 and 2) markedly increased rice yield. Hence, the PNSB inoculants, particularly the mixed culture, produced a similar effect as added P in increasing rice yield (Table 2). This suggests that our PNSB converted insoluble P forms into soluble forms, increasing the availability of phosphate to plant roots. Our results indicate that in both soil types the application of PNSB inoculants with P fertilizer reduced the required P fertilizer level by about half from the recommendation (60 kg P2O5 ha−1), as previously stated. For instance, in Hon Dat soil the combined application of 30 kg P2O5 ha−1 and the mixed culture produced grains (23.6 g pot−1) performed similarly as the sole application of 60 kg P2O5 ha−1 (20.0 g pot−1). In Hon Dat soil, the inoculation with mixed PNSB and 45 or 60 kg P2O5 ha−1significantly increased yield (24.3 and 26.3 g pot−1) over the application of only 60 kg P2O5 ha−1 (20.0 g pot−1). This is in agreement with Khan et al. (2017), who reported that the application of P-solubilizing bacteria in pot experiments reduced by up to 50% the P fertilizer need from the recommendation. The increase in grain yield by inoculated PNSB relative to uninoculated cases matched increases in the yield components: number of panicles per pot, panicle length and total spikelets per panicle with Hon Dat soil (Table 2a); and number of panicles per pot, panicle length, total spikelets per panicle and percentage of filled grains with Phung Hiep soil (Table 2b). This is similar to Bakhshandeh et al. (2015), who found that yield and P uptake by rice were more pronounced when P fertilizer was supplemented with the inoculation of P-solubilizing bacteria. This might be due to better utilization of P from the pool of soil nutrients by the action of P-solubilizing bacteria (Khalimi et al. 2012).
The inoculated PNSB either as the mixed culture or as single culture showed remarkable increases in plant height, panicle length, panicles per pot, number of grains per panicle, and rice grain yield, with both soil types, although the single culture showed less efficiency, particularly with the Phung Hiep soil (Table 2a-b). This may be due to synergistic action to solubilize P from unavailable P forms by the mixed culture. This nutrient stimulated root development significantly improving the absorption of nutrients. For example, the single culture in this experiment was VNW64; when it was mixed with other strains including TLS06, this was most effective for releasing the available phosphate (Fig. 1) for plant roots to absorb under acidic conditions. This is in accordance with Duarah et al. (2011), who found that the use of consortia of P solubilizers significantly improved rice growth. This suggests that PNSB inoculants or combination of PNSB with P fertilizer increased Pavail in both ASS types for plant uptake relative to the control cases. This explains why treatments with PNSB had higher rice grain yield and higher nutrient uptakes; and our PNSB showed higher efficiency than other previously studied bacterial genera that seem to solubilize less of the insoluble P forms in soil (Perez et al. 2007; Sulbarán et al. 2009). The use of PNSB for providing Pavail was not only cost-effective but also environmentally friendly, as the P fertilizers contain heavy metals as summarized by Gupta et al. (2014). While the P-solubilizing bacteria act by various mechanisms, the main ones are by the release of organic acids, siderophores, exopolysaccharides, and phosphatase, including phytase-phosphatase enzymes (Arif et al. 2017).
Increased pH in both soil types after PNSB inoculation (Tables 4 and 5) indicates that our PNSB might use other mechanisms than the release of organic acids. Elevated pH was also observed in the culture broths of modified BIM, which in this study increased from initial 4.50 up to between 5.50 and 6.50 for the effective strains (TLS06 and VNW64); and the same happened in the BIM of our previous study (Khuong et al. 2017). This suggests that the PNSB might use siderophores, EPS, and phosphatase; and in our previous study we found that these PNSB released EPS for survival in acidic conditions with the presence of Al3+ and Fe2+ (Nguyen et al. 2018) along with siderophores (unpublished data). In addition, this study showed a positive relationship between the PNSB population and the phosphatase activity in both soil types (Fig. 2a-f). This is in agreement with Pradhan et al. (2017), who reported that P-solubilizing bacteria increased soil phosphatase activity. However, the long term use of P fertilization reduces most kinds of phosphatases (Yokoyama et al. 2017) and changes the microbial community (Tang et al. 2016). Elevated pH in both soil types was only found with inoculated PNSB; this implies that the release of substances by the PNSB, such as EPS and NH4+, should be involved. As previously described, the R. palustris strains released NH4+, although we did not investigate that with our strains in vitro. However, the evidence of a significant increase in NH4+ in the inoculated PNSB cases with both soil types (Table 5) confirms that our PNSB strains fix the N2 in soils. This is also supported by the interaction of P fertilization and the PNSB inoculation, without significant differences in promoting rice growth (Table 2), which suggests not only bacterial phosphate solubilization but also others mechanisms, i.e. N2 fixation. Hence, the exploration in this study encourages us to further investigate the efficiency of PNSB inoculants in ASS paddy fields containing various insoluble P sources, as shown in Table 1a-b. For further information relevant to field applications, in this study also the subsoil was tested as shown in Table 1a, for predicting the influences of subsoil on rice growth.
Effects of inoculated PNSB on producing safe rice by reducing metal uptake
High levels of Al3+ or Fe2+ negatively affect rice growth by reducing Pavail and by increasing toxic metal uptake in the rice. However, these problems could be solved by P-solubilizing PNSB that have mechanisms to combat toxic metals and improve soil acidity (pH increase), and reduce metal uptake (Tables 1, 3, 4, and 5) enhancing rice growth and yield. The soil pH is the most important factor affecting availability of nutrients for plant growth (Vanzolini et al. 2017) and of the numerous toxic metals (Barrow 2017). Our results are in agreement with a previous study on the use of both P fertilizer and biofertilizers at roughly 3.3 × 105 cells g−1 DSW to increase rice yield (Panhwar et al. 2016). It should be noted that in this current study a lower cell count dose of PNSB was applied, by inoculating the seeds with PNSB and then later adding cell suspension into the soil 3 times, for a total of roughly 5.4 × 104 cells g−1 DSW. This suggests that the inoculated PNSB survived in both the acidic soil types; and the populations grew by about 1.5–2.0 log by the end of cultivation (Fig. 2a-b). This means that the inoculated PNSB had important roles as previously described, and also significantly increased soil phosphatase from that of the control cases, while only little indigenous PNSB was detected in the soils (Fig. 2a-f). To the best of our knowledge, soil phosphatase produced by PNSB has not been reported earlier; it still remains a desideratum to describe the enzyme activities in a pure PNSB culture. This indicates that the inoculated PNSB might provide phosphatase or support soil indigenous microbes that dissolve P from organic P compounds, in both soil types tested.
It has been shown previously that R. palustris strains can produce plant growth promoting substances, such as phytohormones and ALA (Kantha et al. 2015; Sakpirom et al. 2017), including EPS to reduce stress from salt (Nunkaew et al. 2015) and promoting rice growth in paddy fields (Kantachote et al. 2016). It is well recognized that ALA promotes plant growth under stress conditions, i.e. cadmium and salinity, with its mediating effects on chlorophyll, ultrastructural changes and antioxidative enzymes (Ali et al. 2013; Nunkaew et al. 2014). However, to date there is no report on the role of ALA in binding or chelation of iron and aluminum ions by using its carboxylic group; unfortunately, we did not investigate ALA in this study. Hence, the role of ALA acting as a chelator that binds iron and aluminum, which can contribute to phosphate solubilization from inorganic P sources, should be investigated. In addition, the PNSB also use EPS to reduce the toxic metals Al and Fe under acidic conditions (Nguyen et al. 2018). These activities together should facilitate rice absorbing nutrients, increasing P uptake while decreasing toxic metal uptake (Table 3a-b). On the other hand, the accumulation of the toxic metals Al and Fe in rice grains dropped sharply (Table 3a-b) in the inoculated cases, and their availability in both soil types was reduced. This resulted in improved rice grain yield with low metal uptake (Tables 2 and 3). The sharp decrease by roughly 42% of Al with the combination of mixed PNSB and 45 P (kg ha−1) was much superior to the decrease by only 9% with 45 P fertilizer level without inoculation (Table 6). This indicates that the inoculated PNSB not only acted to significantly increase the grain yield, but also reduced the threat of Al toxicity in the rice grains by reducing its accumulation. This helps prevent the adverse effects of high aluminum in food on consumers’ health, in areas where rice is the staple food.
Effect of added P and inoculated PNSB on soil health
The levels of Alexch and Fe2+ usually correlate with soil acidity, as also seen in Table 4a-b. The activity of PNSB as P solubilizer was the key to improving soil fertility for the two types of ASS (Table 5a-b). Despite no significant effects on Fetot by the alternative treatments, the available forms Alexch and Fe2+ significantly decreased in both soil types with the application of P fertilizer and PNSB inoculant, particularly the latter (Table 4a-b). The PNSB reduces amounts of both Al3+ and Fe2+ in ASS via releasing EPS and also by accumulation in the PNSB biomass (Nguyen et al. 2018). In contrast, other P-solubilizing bacteria release organic acids to fix Al3+ and Fe2+, immobilizing these metals in ASS (Panhwar et al. 2016). As previously discussed, the soil phosphatase activity significantly increased with inoculated PNSB in both soil types (Fig. 2). This enzyme converts organic P into available inorganic P forms for easy absorption by the plants (Pradhan et al. 2017). In addition, several acid phosphatase genes have been investigated and identified in Gram-negative bacteria (Rossolini et al. 1998) that are capable of performing well in soil. Both plant roots and bacteria produce acid phosphatases, so it is hard to attribute their origination (Richardson et al. 2009). However, based on the evidence in this study of only little soil phosphatase activity without inoculated PNSB, the soil phosphatase activity was related to the PNSB inoculants (Fig. 2); this means that PNSB could process both inorganic and organic P sources to stimulate rice growth and grain yield.
In this study, the levels of toxic metals remained high in the ASS, limiting the efficiency of P fertilizer. However, the applied PNSB could support the use of P fertilizer not only by using natural soil P sources but also by improving soil health. This is because the PNSB inoculants produced a big drop in both Alexch and Fe2+ for the benefit of flooded rice cultivation; and this improvement was related to the acidity both in terms of pH and soil acidity (Table 4a-b), in the two soil types. This is related to a significant increase in NH4+ by the treatments, particularly by PNSB in both soil types (Table 5a-b) as the PNSB are also biofertilizers (Kantachote et al. 2016). Physicochemical properties of rice rhizospheric soil at harvest were significantly improved by the co-treatment with P fertilizer and PNSB (Table 5). For example, Pavail (Bray II) significantly increased in both soil types (Table 5a-b). Besides, the Al-Fe-Ca phosphate compounds were still found in quite high contents, and they contribute to soil fertility in sustainable agriculture. The reduction of metal toxicities was confirmed by the high PNSB populations in inoculated cases, as well as by soil phosphatase activity in both soil types (Fig. 2). Overall, the results indicate that the mechanisms by which the PNSB inocula enhanced rice growth and grain yield in ASS were by release of EPS, siderophores, and NH4+, significantly increasing pH in both soil types and reducing toxic metals, while providing more Pavail from inorganic P sources, and possibly releasing acid phosphatase for phosphate solubilization from organic P sources.
Conclusions
The acid, Al and Fe-resistant R. palustris strains (TLS06, VNW02, VNW64 and VNS89) were effective in solubilizing the insoluble forms of variscite and strengite that are highly present in actual ASS. The use of combined P fertilizer with PNSB inoculants, particularly the mixture of PNSB strains, remarkably increased rice growth and grain yield in two different ASS types in pot experiments. This reduced metal toxicity along with increasing Pavail from both inorganic and organic P compounds. In both types of ASS, PNSB as P solubilizers could reduce P fertilizer need by 25 to 50% from the recommended dose while achieving the maximum rice grain yield. The mixed PNSB culture also improved soil health by alleviating metal toxicity of Al and Fe in grains, contributing to the production of safe-to-consume rice.
References
Ali B, Wang B, Ali S, Ghani MA, Hayat MT, Yang C, Xu L, Zhou WJ (2013) 5-Aminolevulinic acid ameliorates the growth, photosynthetic gas exchange capacity and ultrastructural changes under cadmium stress in Brassica napus L. J Plant Growth Regul 32:604–614
Alori ET, Glick BR, Babalola OO (2017) Microbial phosphorus solubilization and its potential for use in sustainable agriculture. Front Microbiol 8:971. https://doi.org/10.3389/fmicb.2017.00971
Arif MS, Shahzad SM, Yasmeen T, Riaz M, Ashraf M, Ashraf MA, Mubarik MS, Kausar R (2017) Improving plant phosphorus (P) acquisition by phosphate-solubilizing bacteria. In: Naeem M, Ansari A, Gill S (eds) Essential plant nutrients. Springer, Cham, pp 513–556
Bakhshandeh E, Rahimian H, Pirdashti H, Nematzadeh GA (2015) Evaluation of phosphate-solubilizing bacteria on the growth and grain yield of rice (Oryza sativa L.) cropped in northern Iran. J Appl Microbiol 119:1371–1382
Barrow NJ (2017) The effects of pH on phosphate uptake from the soil. Plant Soil 410(1–2):401–410
Brown JW (2013) Enrichment and isolation of purple non-sulfur bacteria. Department of Biological Sciences. College of Sciences. North Carolina State University. http://www.mbio.ncsu.edu/mb452/purple_nonsulfurs/purples.html. Accessed 15 Nov 2017
Cho MH, Kyaw N, Kyaw KW, Swe SM (2017) Assessment of soil indigenous nutrient supply as a natural resource management in rice production towards climate resilience agriculture. In Proceedings of the Tenth Agricultural Research Conference, Yezin Agricultural University, Nay Pyi Raw, Myanmar, 11–12 January 2017 (pp 159–177). Yezin Agricultural University
de la Luz Mora M, Demanet R, Acuña JJ, Viscardi S, Jorquera M, Rengel Z, Durán P (2017) Aluminum-tolerant bacteria improve the plant growth and phosphorus content in ryegrass grown in a volcanic soil amended with cattle dung manure. Appl Soil Ecol 115:19–26
Dou F, Soriano J, Tabien RE, Chen K (2016) Soil texture and cultivar effects on rice (Oryza sativa, L.) grain yield, yield components and water productivity in three water regimes. PLoS One 11(3):e0150549
Duarah I, Deka M, Saikia N, Boruah HD (2011) Phosphate solubilizers enhance NPK fertilizer use efficiency in rice and legume cultivation. 3 Biotech 1(4):227–238
Gupta DK, Chatterjee S, Datta S, Veer V, Walther C (2014) Role of phosphate fertilizers in heavy metal uptake and detoxification of toxic metals. Chemosphere 108:134–144
Harada N, Nishiyama M, Matsumoto S (2001) Inhibition of methanogens increases photo-dependent nitrogenase activities in anoxic paddy soil amended with rice straw. FEMS Microbiol Ecol 35(3):231–238
Horneck DA, Sullivan DM, Owen JS, Hart JM (2011) Soil test interpretation guide. EC 1478. Corvallis, OR: Oregon State University Extension Service
Huang Q, Tang S, Huang X, Yang S, Yi Q (2016) Characteristics of the acidity and sulphate fractions in acid sulphate soils and their relationship with rice yield. J Agric Sci 154(8):1463–1473
IRRI, International Rice Research Institute (2014) Standard procedure for determining yield components at harvest.< https://sites.google.com/a/irri.org/oryza2000/calibration-and-validation/experimental-data-collection-and-analysis/standard-procedure-for-determining-yield-components-at-harvest> (Accessed 8 Jan 2017)
Kantachote D, Nunkaew T, Kantha T, Chaiprapat S (2016) Biofertilizers from Rhodopseudomonas palustris strains to enhance rice yields and reduce methane emissions. Appl Soil Ecol 100:154–161
Kantha T, Kantachote D, Klongdee N (2015) Potential of biofertilizers from selected Rhodopseudomonas palustris strains to assist rice (Oryza sativa L. subsp. indica) growth under salt stress and to reduce greenhouse gas emissions. Ann Microbiol 65:2109–2118
Kelliher FM, Gray CW, Noble AD (2017) Superphosphate fertiliser application and cadmium accumulation in a pastoral soil. New Zeal J Agric Res 60(4):404–422
Khalimi K, Suprapta DN, Nitta Y (2012) Effect of Pantoea agglomerans on growth promotion and yield of rice. Agric Sci Res J 2(5):240–249
Khan MMA, Haque E, Paul NC, Khaleque MA, Al-Garni SM, Rahman M, Islam MT (2017) Enhancement of growth and grain yield of rice in nutrient deficient soils by rice probiotic bacteria. Rice Sci 24(5):264–273
Khuong NQ, Kantachote D, Onthong J, Sukhoom A (2017) The potential of acid-resistant purple nonsulfur bacteria isolated from acid sulfate soils for reducing toxicity of Al3+ and Fe2+ using biosorption for agricultural application. Biocatal Agric Biotechnol 12:329–340
Koh RH, Song HG (2007) Effects of application of Rhodopseudomonas sp. on seed germination and growth of tomato under axenic conditions. J Microbiol Biotechnol 17(11):1805–1810
Lakshmi KVNS (2012) Cultured diversity of purple anoxygenic phototrophic rhizobacteria of paddy and their plant growth promoting attributes (Doctoral dissertation, Jawaharlal Nehru Technological University, Hyderabad). Page:243
Liu Z, Rong Q, Zhou W, Liang G (2017) Effects of inorganic and organic amendment on soil chemical properties, enzyme activities, microbial community and soil quality in yellow clayey soil. PLoS One 12(3):e0172767
Madigan M, Cox SS, Stegeman RA (1984) Nitrogen fixation and nitrogenase activities in members of the family Rhodospirillaceae. J Bacteriol 157(1):73–78
Margenot AJ, Sommer R, Mukalama J, Parikh SJ (2017) Biological P cycling is influenced by the form of P fertilizer in an Oxisol. Biol Fertil Soils 53(8):899–909
Murphy J, Riley HP (1962) A modified single solution method for the determination of phosphate in natural waters. Anal Chim Acta 27:31–36
Nguyen NQ, Kantachote D, Onthong J, Sukhoom A (2018) Al3+ and Fe2+toxicity reduction potential by acid-resistant strains of Rhodopseudomonas palustris isolated from acid sulfate soils under acidic conditions. Ann Microbiol 68:217–228
Nunkaew T, Kantachote D, Kanzaki H, Nitoda T, Ritchie RJ (2014) Effects of 5-aminolevulinic acid (ALA)-containing supernatants from selected Rhodopseudomonas palustris strains on rice growth under NaCl stress, with mediating effects on chlorophyll, photosynthetic electron transport and antioxidative enzymes. Electron J Biotechnol 17(1):19–26
Nunkaew T, Kantachote D, Nitoda T, Kanzaki H, Ritchie RJ (2015) Characterization of exopolymeric substances from selected Rhodopseudomonas palustris strains and their ability to adsorb sodium ions. Carbohydr Polym 115:334–341
Onaga G, Dramé KN, Ismail AM (2016) Understanding the regulation of iron nutrition: can it contribute to improving iron toxicity tolerance in rice? Funct Plant Biol 43(8):709–726
Panhwar QA, Naher UA, Shamshuddin J, Othman R, Ismail MR (2016) Applying limestone or basalt in combination with bio-fertilizer to sustain rice production on an acid sulfate soil in Malaysia. Sustainability 8(7):700. https://doi.org/10.3390/su8070700
Panwichian S, Kantachote D, Wittayaweerasak B, Mallavarapu M (2010) Factors affecting immobilization of heavy metals by purple nonsulfur bacteria isolated from contaminated shrimp ponds. World J Microbiol Biotechnol 26(12):2199–2210
Perez E, Sulbaran M, Ball MM, Yarzabal LA (2007) Isolation and characterization of mineral phosphate-solubilizing bacteria naturally colonizing a limonitic crust in the south-eastern Venezuelan region. Soil Biol Biochem 39(11):2905–2914
Pradhan M, Pradhan C, Mohanty S (2017) Effect of P- solubilizing bacteria on microbial biomass P and phosphatase activity in groundnut (ArachishypogaeaL) rhizosphere. Int J Curr Microbiol App Sci 6(4):1240–1260
Ragot SA, Huguenin-Elie O, Kertesz MA, Frossard E, Bünemann EK (2016) Total and active microbial communities and phoD as affected by phosphate depletion and pH in soil. Plant Soil 408(1–2):15–30
Rengel Z, Marschner P (2005) Nutrient availability and management in the rhizosphere: exploiting genotypic differences. New Phytol 168:305–312
Richardson AE, Barea JM, McNeill AM, Prigent-Combaret C (2009) Acquisition of phosphorus and nitrogen in the rhizosphere and plant growth promotion by microorganisms. Plant Soil 321(1–2):305–339
Robinson GW (1922) A new method for the mechanical analysis of soils and other dispersions. J Agric Sci 12(3):306–321
Rossolini GM, Schippa S, Riccio ML, Berlutti F, Macaskie LE, Thaller MC (1998) Bacterial nonspecific acid phosphohydrolases: physiology, evolution and use as tools in microbial biotechnology. Cell Mol Life Sci 54(8):833–850
Sakpirom J, Kantachote D, Nunkaew T, Khan E (2017) Characterizations of purple non-sulfur bacteria isolated from paddy fields, and identification of strains with potential for plant growth-promotion, greenhouse gas mitigation and heavy metal bioremediation. Res Microbiol 168:266–275
Soman C, Li D, Wander MM, Kent AD (2017) Long-term fertilizer and crop-rotation treatments differentially affect soil bacterial community structure. Plant Soil 413(1–2):145–159
Soomro AA, Abro MA, Leghari N, Leghari GM, Soomro AA (2015) Evaluation of aluminum toxicity tolerance in rice (Oryza sativa L.). Sci Int 27(3):2251–2255
Spagnoletti FN, Tobar NE, Di Pardo AF, Chiocchio VM, Lavado RS (2017) Dark septate endophytes present different potential to solubilize calcium, iron and aluminum phosphates. Appl Soil Ecol 111:25–32
Sparks DL, Page AL, Helmke PA, Loeppert RH, Soltanpour PN, Tabatabai MA, Johnston CT, Sumner ME (1996) Methods of soil analysis. Part 3-Chemical methods. SSSA Book Ser. 5.3. SSSA, ASA, Madison, WI. https://doi.org/10.2136/sssabookser5.3
Sulbarán M, Pérez E, Ball MM, Bahsas A, Yarzábal LA (2009) Characterization of the mineral phosphate-solubilizing activity of Pantoeaaglomerans MMB051 isolated from an iron-rich soil in southeastern Venezuela (Bolívar state). Curr Microbiol 58(4):378–383
Tabatabai MA, Bremner JM (1969) Use of p-nitrophenyl phosphate or assay of soil phosphatase activity. Soil Biol Biochem 1:301–307
Tang X, Placella SA, Daydé F, Bernard L, Robin A, Journet EP, Justes E, Hinsinger P (2016) Phosphorus availability and microbial community in the rhizosphere of intercropped cereal and legume along a P-fertilizer gradient. Plant Soil 407(1–2):119–134
Vanzolini JI, Galantini JA, Martínez JM, Suñer L (2017) Changes in soil pH and phosphorus availability during decomposition of cover crop residues. Arch Agron Soil Sci 63(13):1864–1874
Walinga I, van Vark W, Houba VJG, van der Lee JJ (1989) Plant analysis procedure, Part 7. Department of Soil Science and Plant Nutrition, Wageningen Agricultural University
Wisawapipat W, Charoensri K, Runglerttrakoolchai J (2017) Solid-phase speciation and solubility of phosphorus in an acid sulfate paddy soil during soil reduction and reoxidation as affected by oil palm ash and biochar. J Agric Food Chem 65(4):704–710
Xuan VT, Matsui S (1998) Development of farming systems in the Mekong delta of Vietnam. Ho Chi Minh City Publ. House, Ho Chi Minh City
Yadav VR, Chandra S, Singh G, Singh SP (2017) Productivity and economics of direct seeded rice (Oryza sativa L.) as affected by moisture regimes and seed priming under sandy loam and silty clay loam soils. Res Crops 18(1):1–5
Yokoyama D, Imai N, Kitayama K (2017) Effects of nitrogen and phosphorus fertilization on the activities of four different classes of fine-root and soil phosphatases in Bornean tropical rain forests. Plant Soil 416(1–2):463–476
Acknowledgements
The first author was supported by the Graduate School, Prince of Songkla University, and additional support was received from the Thailand’s Education Hub for Southern Region of ASEAN Countries (TEH-AC), grant number TEH-AC 027/2015.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None
Additional information
Responsible Editor: Tim S. George
Rights and permissions
About this article
Cite this article
Khuong, N.Q., Kantachote, D., Onthong, J. et al. Enhancement of rice growth and yield in actual acid sulfate soils by potent acid-resistant Rhodopseudomonas palustris strains for producing safe rice. Plant Soil 429, 483–501 (2018). https://doi.org/10.1007/s11104-018-3705-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-018-3705-7