Abstract
Soil nitrogen cycling is intricately related to soil physicochemical properties, enzymatic activity, and microbial vitality. Biochar, containing various elements such as carbon, nitrogen, and phosphorus, possesses a porous structure with strong adsorption capabilities. This characteristic renders it useful for ameliorating acidic soils, influencing soil nitrogen cycling, and mitigating greenhouse gas emissions. To quantitatively analyze the diverse impacts of different biochar on soil nitrogen cycling and to highlight its implications for sustainable agriculture, this study collected 155 relevant articles and conducted a comprehensive meta-analysis. The results indicate that biochar can elevate the pH by 4.60% for acidic soils and significantly increase soil organic carbon content by 64.60%. Different feedstocks, such as Wooden Biochar (WB), Crop Husk (CH) Biochar, Crop Straw Biochar (CS), and Organic Waste Biochar (OW), exhibit distinct effects, with WB and OW showing the most significant increases in SOC. Pyrolysis temperature is also a critical factor, and biochar produced at medium and high temperatures enhances pH more effectively than low-temperature biochar. Additionally, biochar enhances the abundance of the nitrogen functional gene amoA-AOB by 25.58%, promoting ammonia oxidation, reducing ammonia (NH3) emissions by 16.39%. Experimental setups also influence outcomes that biochar application in woods and incubation studies significantly reduced nitrous oxide (N2O) emissions compared to pot and field experiments. The findings suggest that adding biochar to soil accelerates nitrogen cycling, thereby reducing greenhouse gas emissions. The results advocate biochar’s use in sustainable soil management practices.
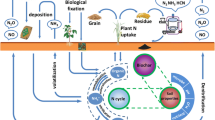
Graphical Abstract

Highlights
Biochar elevates soil pH by 4.57%, mitigating NH3 and N2O losses under certain conditions.
Biochar accelerates nitrogen cycling with 25.58% increase in amoA-AOB gene abundance.
Significant boost (64.60%) in soil organic carbon, promoting fertility.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Biochar, a carbon(C)-rich solid material derived from the pyrolysis of various biomass in a low-oxygen environment (Zhang et al. 2022), holds promise as an organic soil amendment due to its advantageous physicochemical properties (Yao et al. 2014). Its diverse raw materials, including wood, crop residues, and organic waste (Ji et al. 2022), contribute to its variability in properties and applications (Weber and Quicker 2018). However, the impact of biochar application on soil processes remains uncertain, with potential drawbacks such as increased gaseous emissions (Mukherjee and Lal 2014) and long-term effects on soil processes (Kuppusamy et al. 2016).
In biochar production, variations in raw materials and pyrolysis temperatures result in distinct physicochemical properties, influencing critical biochar characteristics, such as porosity and carbon content (Zhang et al. 2012). For instance, different feedstocks yield varying ash content and morphology, with crop residues producing biochar with lower oxygen-to-carbon (O/C) and hydrogen-to-carbon (H/C) ratios due to cellulose and lignin decomposition during pyrolysis (Ji et al. 2022). Conversely, wood-derived biochar, rich in lignin, exhibits lower ash content and enhanced pore formation.
Pyrolysis temperature further shapes biochar properties, with low temperatures yielding mainly aromatic, aliphatic, and carbonyl carbon, whereas high temperatures favoring aromatic carbon structures (Tomczyk et al. 2020). This temperature dependence extends to functional groups, with low temperature biochar characterized mainly by hydroxyl group (-OH), carbonyl group (C = O), and ether functional group (C-O-C), while high temperature biochar exhibits more carbon–carbon double bond (C = C) and methylene group (-CH2) (Li et al. 2020). Generally, elevated temperatures also augment carbon and ash content, impacting properties such as gas production, and microporous structure formation (Zeng et al. 2022), as well as surface area (Wang et al. 2021).
The nitrogen cycle encompasses crucial processes in soil ecosystems, including nitrogen mineralization (Curtin et al. 2019), nitrification, denitrification, and nitrogen fixation (Martínez-Espinosa et al. 2011). The introduction of biochar significantly influences soil nitrogen cycling, affecting processes like nitrification, denitrification, and NH3 emissions. For instance, applying biochar to paddy fields resulted in reduced soil nitrogen loss, accompanied by an increased in NH3 emission (Feng et al. 2018). It was observed that biochar promoted the reduction of N2O to nitrogen (N2) in calcareous soils while concurrently inhibiting N2O production (Dong et al. 2020).
This influence extends to microbial communities, with biochar addition influencing the abundance of functional genes associated with nitrogen-cycling microorganisms. For instance, functional gene expressions, including nifH, amoA-AOA, amoA-AOB, narG, napA, nirK, nirS, and nosZ, typically signify nitrogen fixation, nitrification and denitrification processes. Conversely, nirB, nirD, nrfA, and nrfH functional gene abundance indicates nitrate assimilation reduction processes. Sanjutha et al. observed an enrichment of microbial functional genes (nifD, amoA, amoB, norB, nrfA, nirK, and nosZ) in sandy loam soil following biochar addition (Shanmugam et al. 2021). Lan et al. demonstrated enhanced diversity in microbial nitrogen functional genes (amoB, narG, nirS, and nosZ) by introducing wood-derived biochar from high-temperature pyrolysis into acid soil (Lan et al. 2018). Continuous straw biochar addition over three years in temperate and subtropical regions increased the abundance of amoA and amoB genes, with a more pronounced effect at higher application rates, reaching 9.55 and 22.0 times gene copies in soil treated with 2.25 Mg·ha− 1 and 22.5 Mg·ha− 1 of biochar, respectively, compared to the control treatment (He et al. 2016). Additionally, pig manure biochar increased the diversity of bacterial genes (amoA, amoB, nirK, nirS, and nosZ) in soil, exhibiting a more significant effect at higher concentrations (Zhong et al. 2022). However, the precise mechanisms underlying these changes and their dependence on biochar properties remain areas of investigation.
The ammonia oxidation pathway, crucial for nitrification, is closely tied to N2O emission, a potent greenhouse gas (Chen et al. 2019). For example, intermediate nitrification products, NO2-N, and NO3−-N, can generate the greenhouse gas N2O through denitrification by functional bacteria with genes like nirK, nirS, and napA. Literature indicated the close association of bacterial denitrification genes nirK, nirS, and nosZ with N2O flux change (Qin et al. 2020).In 2019, atmospheric N2O, a major contributor to non-CO2 warming, reached 332 ppb (IPCC 2023). Although biochar application generally reduce N2O emissions (Edwards et al. 2018), its efficacy varies with soil conditions (He et al. 2018).
To comprehensively assess biochar’s impact on soil nitrogen cycling, meta-analysis offers a robust approach, synthesizing diverse studies and addressing existing limitations in the literature (Gascon et al. 2017). In contrast to isolated regional studies, meta-analysis eliminates subjective interference, employing quantitative methods for stronger statistical foundations. It enables synthesizing diverse studies, even with contradictory findings, to derive more general and universal research conclusions. Nguyen et al. compiled 56 articles on the impact of biochar on soil NH4+-N and NO3−-N, considering biochar feedstock, pyrolysis temperature, and application duration (Nguyen et al. 2017). Liu et al. (2018) analyzed biochar effects on soil organic nitrogen, NH4+-N, NO3−-N, plant nitrogen absorption, NH3 volatilization, and N2O emissions. Xiao et al. (2019) collected 36 articles on the influence of biochar on the microbial functional genes (nifH, amoA, nirK, nirS, and nosZ) in soil nitrogen transformation processes. Despite previous research, gaps remain regarding long-term effects and the influence of biochar properties on soil nitrogen dynamics (Zhang et al. 2021). Therefore, this meta-analysis aims to provide more specific and widely applicable insights into biochar’s influence on soil nitrogen cycling, spanning diverse environmental contexts and biochar properties.
This study conducts a meta-analysis spanning January 2008 to December 2023 to explore the novel implications of biochar’s impact on soil nitrogen cycling, focusing on its unique properties and mechanisms that enhance soil health and mitigate greenhouse gas emissions, thus contributing to sustainable agricultural practices. Key research questions addressed include (1) Biochar’s effects on soil pH and soil organic carbon (SOC), recognizing pH regulation and SOC enhancement as crucial mechanisms influencing soil nitrogen-related processes; (2) The influence of biochar addition on different nitrogen forms in soil and the abundance of nitrogen cycling functional genes; (3) The variation in the content of various nitrogen forms and the abundance of nitrogen-cycling functional genes in soils based on biochar derived from different raw materials; and (4) The impact of biochar pyrolysis temperature on the content of different nitrogen forms and the abundance of nitrogen cycling functional genes in soil.
2 Materials and Methods
2.1 Data Source and Selection Criteria
A literature search was conducted on the Web of Science and Google Scholar databases using keywords “biochar” AND “soil” OR “nitrogen,” OR “ammonia” OR “N2O"OR “nitrogen cycling functional genes” from January 2008 to December 2023. The literature selection criteria include: (1) The literature must investigate at least one nitrogen form of biochar in soil (NH4+-N, NO3−-N, TN (Total nitrogen)), nitrogen cycling functional genes (e.g., nifH, amoA-AOA, amoA-AOB, narG, napA, nirK, nirS, nosZ, nirB, nirD, nrfA, and nrfH), or urease; (2) The experiment must include a clear control group and experimental treatment group; (3) Replication must be included (n ≥ 3); (4) Data in the literature must be presented in the form of figures or tables in the article or supplementary materials. Exclusion criteria include evaluative, review, or simulated data literature. Based on these conditions, a total of 155 valid articles were obtained.
Data extraction in this study was conducted as follows: Extract the average values and standard deviations (SD) of treatments and controls from tables in the respective articles. The GetData Graph Digitizer V2.25 software (https://www.53xt.com/soft/11953.html) was used to extract data in cases presented graphically. If an article included multiple independent experimental treatments (e.g., two different biochar materials and various pyrolysis temperatures), each experiment was treated as an independent study and included in the dataset. The collected data included soil NH4+-N, NO3−-N, TN, urease activity (UA), amoA, amoB, and other nitrogen functional gene data.
2.2 Meta-Analysis
The magnitude of the impact of biochar on soil nitrogen was calculated using the natural logarithm of the response ratio (RR), which is the natural log of the ratio of a given variable between the treatment group (xt) and the control group (xc), defined as Eq. (1) (Xi et al. 2023).
The variance (v) of the effect ratio is calculated as Eq. (2):
Where st, sc, nt, and nc represent the treatment and control groups’ standard deviations and sample sizes, respectively.
In order to obtain the overall response effect of the treatment group relative to the control group, the mean effect size (RR++) is calculated as Eq. (3):
Where w = 1/v.
The standard error of RR++ is calculated using Eq. (4):
The 95% confidence interval (95% CI) for RR++ is expressed as Eq. (5):
95%CI=\(\:{\text{R}\text{R}}_{++}\)±1.96×\(\:{\text{S}}_{\left({\text{R}\text{R}}_{++}\right)}\) (5)
If the 95% CI includes 0, it indicates no significant difference between the experimental and control groups.
In order to provide a visual understanding of the transition from weighted response ratio to percentage change, the percentage change is calculated by logarithmizing the weighted response ratio, defined by Eq. (6):
Percentage Change=[exp(RR++)-1]×100% (6)
2.3 Data Analysis
From the literature collected, it was observed that the carbon content of Wooden Biochar (WB) ranged from 68.1 to 80.2%. Crop Husk (CH) Biochar, Crop Straw (CS) Biochar, and Organic Waste (OW) Biochar exhibited carbon contents of 45.4–64.8%, 52.9–57.2%, and 42.5–61.7%, respectively (Lan et al. 2018). However, OW had the highest nitrogen content at 2.0–5.2%, while WB had the lowest at 0.1–1.7%, and CH and CS showed nitrogen contents of 0.7–1.6% and 1.1–1.6%, respectively (Lan et al. 2018). It was noted that the carbon content of biochar increased with the rise in pyrolysis temperature, whereas the nitrogen content exhibited an opposite trend (Zhou et al. 2017).
Therefore, the main indicators investigated in this study were determined as follows: (1) Biochar Feedstock: Since biochar physicochemical properties exhibit variations based on different raw materials, biochar was categorized into WB, CS, Crop CH, and OW (Ji et al. 2022). WB was derived from tree components such as branches, trunks, bamboo pieces, and sawdust. CS was produced from straw residues of crops such as rice, wheat, sugarcane, corn, and sorghum. CH utilized raw materials like coconut shells, peanut shells, and cottonseed husks. OW incorporated animal manure, compost, sludge, and animal remains. (2) Pyrolysis Temperature: Different pyrolysis temperatures also impact biochar properties. Accordingly, biochar was classified into low temperature (250–400 °C, LOW), moderate temperature (420–550 °C, MOD), and mega temperature (600–950 °C, MEG) (Ghorbani et al. 2023). (3) Experimental Setup: Based on the experimental conditions reported in the collected articles, analyses were categorized into four groups: Woods, Field, Pot, and Incubation for comparative analysis.
The “metafor” package in R (version 4.2.2) was used to conduct a random-effects model for calculating the weighted response ratio (RR++) and its 95% CI. A 95% CI < 0 for the effect value was considered to indicate a significant inhibitory effect of biochar on soil nitrogen or nitrogen functional genes (p < 0.05). Conversely, a 95% CI > 0 suggested a significant promoting effect of biochar on soil nitrogen or nitrogen functional genes (p < 0.05). The impact was insignificant if the 95% CI included 0 (p ≥ 0.05). The percentage change in soil nitrogen or nitrogen functional genes due to biochar was calculated by logarithmizing the mean effect size (RR++). It is important to note that percentage change and mean effect size serve different purposes: percentage change quantifies the degree of biochar impact on various study subjects, while mean effect size assesses whether biochar significantly affects the study subjects (Zhang et al. 2019). Regression analyses on the RR values of various indicators were performed to explore potential linear relationships among different indicators under the influence of biochar.
3 Results
3.1 Effect of Biochar on Soil pH
Our meta-analysis reveals that biochar can elevate soil pH by 4.60%. Most biochar is alkaline due to their production processes and raw materials, influencing soil acidity. The impact of WB and CH on soil pH was not significant (Fig. 1A). CS and OW significantly elevated soil pH by 5.34% and 10.97%, with mean effect sizes of 0.0520 (95% CI: 0.0310–0.0730) and 0.1040 (95% CI: 0.0694–0.1387), respectively. The pairwise comparison between CH and OW showed a highly significant difference (p < 0.01) in their impact on soil pH. Compared with CS or WB, OW exhibited a significant difference in soil pH (p < 0.05).
Effect of Biochar on Soil pH and Soil Organic Carbon (SOC). In the boxplots, the symbol ○ represents the mean of the Response Ratio (RR) for different biochar in soil, with the error bars indicating the 95% confidence interval. Mean effect sizes of different biochar on soil-related indicators are represented by ♦ in the figure, with error bars indicating the 95% confidence interval. On the left of each figure, ‘n’ denotes the sample size; on the right, the percentage figure represents the percentage change. Statistical significance between pairwise comparisons, determined by independent sample t-tests, is denoted as ***p < 0.001, **p < 0.01, *p < 0.05
Different pyrolysis temperatures of biochar had varying effects on soil pH. Biochar pyrolyzed at LOW temperature did not significantly affect soil pH. However, biochar pyrolyzed at MOD and MEG temperature significantly increased soil pH by 5.68% and 6.86%, with mean effect sizes of 0.0552 (95% CI: 0.0368–0.0737) and 0.0664 (95% CI: 0.0284–0.1043), respectively, with no significant differences between the two temperatures.
The percentage change in pH due to biochar application in Woods and Field was insignificant. In Pot and Incubation, biochar significantly increased pH by 8.91% and 5.65%, with mean effect sizes of 0.0854 (95% CI: 0.0607–0.1100) and 0.0550 (95% CI: 0.0333–0.0767), respectively. The pairwise comparison between Field and Pot showed a highly significant difference (p < 0.001) in biochar’s impact on soil pH. Additionally, the pairwise comparison between Field and Incubation indicated a significant difference (p < 0.05) in the impact of biochar on soil pH.
3.2 Effect of Biochar on Soil Organic Carbon (SOC)
Biochar, rich in carbon elements, significantly enhances SOC content, reaching up to 64.60%, with a mean effect size of 0.4983 (95% CI: 0.3696–0.6270). WB, CS, and OW significantly increased SOC content by 117.89%, 56.97%, and 108.04%, with mean effect sizes of 0.7788 (95% CI: 0.4098–1.1478), 0.4508 (95% CI: 0.2951–0.6066), and 0.7325 (95% CI: 0.2502–1.2149), respectively. The effect of CH on soil SOC was insignificant. The impact of WB and CH on soil SOC content showed a significant difference in pairwise comparisons (p < 0.05) (Fig. 1B).
Biochar pyrolyzed at MEG, MOD, and LOW temperatures significantly increased SOC content by 106.05%, 48.85%, and 65.44%, with mean effect sizes of 0.7229 (95% CI: 0.4411–1.0048), 0.3977 (95% CI: 0.2396–0.5559), and 0.5035 (95% CI: 0.1971–0.8098), respectively. However, there were no significant differences in the impact of biochar pyrolyzed at different temperatures on soil SOC content.
The application of biochar in Woods did not significantly affect SOC content. In Field, Pot, and Incubation, biochar significantly increased SOC content by 50.83%, 116.91%, and 45.31%, with mean effect sizes of 0.4101 (95% CI: 0.2244–0.5959), 0.7743 (95% CI: 0.5144–1.0342), and 0.3737 (95% CI: 0.1725–0.5748), respectively. The impact of biochar on soil SOC content in Woods and Pot treatments showed a highly significant difference (p < 0.01). Additionally, significant differences were observed in pairwise comparisons between Field and Woods, Field and Pot, and Pot and Incubation treatments (p < 0.05).
3.3 Effects of Biochar on Soil Total Nitrogen (TN), NO3 −-N, and NH4 +-N
Adding biochar to the soil, except for CH, which exhibited no significant impact on TN, WB, CS, and OW significantly increased soil TN content by 18.14%, 14.88%, 26.21%, and 17.56%, respectively. The effect of CH and CS on soil TN content showed highly significant differences in pairwise comparisons (p < 0.01), while WB and CH exhibited a significant difference in their influence on soil TN content (p < 0.05) (Fig. 2A). Biochar pyrolyzed at MEG, MOD, and LOW temperatures significantly increased TN by 15.80%, 14.14%, and 23.53%, with mean effect sizes of 0.1467 (95%CI: 0.0401–0.2532), 0.1322 (95%CI: 0.0789–0.1856), and 0.2113 (95%CI: 0.0458–0.3769), respectively. However, there were no significant differences in the effect of biochar pyrolyzed at different temperatures on soil TN in-between temperatures. Applying biochar in Woods and Field did not significantly affect TN, with 18.61% and 4.20% percentage changes. In Pot and Incubation treatments, biochar significantly increased TN content by 24.80% and 32.84%, with mean effect sizes of 0.2216 (95%CI: 0.1289–0.3142) and 0.2840 (95%CI: 0.1417–0.4263), respectively. Highly significant differences were observed in pairwise comparisons between Field and Pot or Field and Incubation treatments regarding the effect of biochar on soil TN content (p < 0.01).
Effect of biochar on different nitrogen forms of soil (A. total nitrogen (TN), B: NH4+-N, C: NO3−-N). In the boxplots, the symbol ○ represents the mean of the Response Ratio (RR) for different biochar in soil, with the error bars indicating the 95% confidence interval. Mean effect sizes of different biochar on soil-related indicators are represented by ♦ in the figure, with error bars indicating the 95% confidence interval. On the left of each figure, ‘n’ denotes the sample size; on the right, the percentage figure represents the percentage change. Statistical significance between pairwise comparisons, determined by independent sample t-tests, is denoted as ***p < 0.001, **p < 0.01, *p < 0.05
The overall effect of biochar derived from different raw materials on NH4+-N was not significant. WB, CH, OW, and CS exhibited insignificant percentage changes in NH4+-N. The influence of WB and CS, derived from different sources, on NH4+-N showed a significant difference (p < 0.05), while there were no significant differences between pairs of biochar from different raw materials (Fig. 2B). Biochar pyrolyzed at MEG temperature significantly decreased soil NH4+-N content by 23.85%, with a mean effect size of -0.2724 (95%CI: -0.4245 - -0.1204). The changes introduced by MOD and LOW temperature pyrolyzed biochar on soil NH4+-N content were not significant. Significant differences were observed in pairwise comparisons between MEG and MOD or MEG and LOW, indicating that biochar pyrolyzed at different temperatures significantly impacted soil NH4+-N content (p < 0.05). Applying biochar in Woods, Field, Pot, and Incubation did not show significant differences in pairwise comparisons regarding NH4+-N. The influence of biochar on NH4+-N under the four different soil treatment conditions was not significantly different.
The influence of biochar derived from different raw materials on NO3−-N was not pronounced. There were no significant differences in the effect of biochar from different raw materials on NO3−-N between pairwise comparisons (Fig. 2C). Biochar pyrolyzed at MEG, MOD, and LOW temperatures did not significantly affect soil NO3−-N content. No significant differences were observed in the pairwise comparisons of the effect of biochar pyrolyzed at different temperatures on soil NO3−-N content. The application of biochar in Woods, Field, Pot, and Incubation under different soil treatment conditions did not show a significant impact on NO3−-N. The influence of biochar on NO3−-N under different soil treatment conditions was not significantly different in pairwise comparisons.
3.4 Effects of Biochar on Soil Nitrogen Cycling Functional Genes
The effect of biochar on soil nitrogen functional genes is depicted in Fig. 3A. Adding biochar to the soil did not significantly affect the abundance of the soil nitrification functional gene amoA-AOA. In contrast, amoA-AOB significantly increased by 25.58%, with an average effect size of 0.2278 (95%CI: 0.0719–0.3837). Among the denitrification genes, no significant changes were detected for the abundance of narG, norB, nosZ-I, nor napA, nirK, and nosZ, with the exception for the addition of biochar significantly increased the gene abundance of nirS by 14.78%, with an average effect size of 0.1378 (95%CI: 0.0204–0.2553). The abundance of nitrogen fixation genes nifH and nifG in the soil were not significantly affected, nor did nifD. In addition, adding biochar significantly enhanced UA’s abundance by 11.00%, with an average effect size of 0.1044 (95%CI: 0.0330–0.1758). However, the gene abundance of ureC, responsible for urease production, decreased by 15.75%. Adding biochar to the soil resulted in insignificant impact in the abundance of the nrfA gene, which is responsible for nitrate reduction to ammonium.
Effect of biochar on soil nitrogen cycling functional genes (A) and soil N2O emission (B). In the boxplots, the symbol ○ represents the mean of the Response Ratio (RR) for different biochar in soil, with the error bars indicating the 95% confidence interval. Mean effect sizes of different biochar on soil-related indicators are represented by ♦ in the figure, with error bars indicating the 95% confidence interval. On the left of each figure, ‘n’ denotes the sample size; on the right, the percentage figure represents the percentage change. Statistical significance between pairwise comparisons, determined by independent sample t-tests, is denoted as ***p < 0.001, **p < 0.01, *p < 0.05
3.5 Effects of Biochar on Gas Emissions
Both nitrification and denitrification are major microbial metabolic pathways leading to N2O production in agricultural soils. The influence of biochar derived from different raw materials and pyrolysis temperatures and the application environment on soil greenhouse gas N2O emission is depicted in Fig. 3B. In addition, biochar significantly reduced NH3 emissions by 16.39% and contributed to a 15.70% increase in N2 emissions. (Fig. 4).
Effect of biochar on soil gas emission. Mean effect sizes of biochar on different soil gas emissions are represented by ♦ in the figure, with error bars indicating the 95% confidence interval. On the left of each figure, ‘n’ denotes the sample size; on the right, the percentage figure represents the percentage change
Analysis indicates that different feedstocks did not affect soil N2O emissions significantly. MEG pyrolyzed biochar significantly reduced soil N2O emission by 23.15%, with an average effect size of -0.2633 (95%CI: -0.5045 - -0.0221). However, MOD and LOW pyrolyzed biochar did not significantly change soil N2O emission. There were no significant differences in the effect of biochar pyrolyzed at different temperatures on soil N2O emission. In Woods and Incubation, the application of biochar significantly reduced N2O emissions by 24.37% and 21.17%, with average effect sizes of -0.2793 (95%CI: -0.3914 - -0.1672) and − 0.2378 (95%CI: -0.4256 - -0.0501), respectively. However, the effect of biochar application in Field and Pot on N2O emissions was insignificant. Significant differences were observed in the effect of biochar application between Field and Incubation and between Field and Woods (p < 0.05).
3.6 Regression Analysis
Adding biochar to the soil alters the soil pH and SOC content. Regression analyses were performed to understand the effect of biochar addition on nitrogen cycling in the soil. The results indicate that the correlation between RR(pH) and RR(TN)/RR(NO3−-N) is not significant. However, RR(pH) exhibits a significant negative correlation with RR(NH4+-N) (R2 = 0.1034, p = 0.0157) (Fig. 5A).
Furthermore, RR(SOC) shows a significant positive correlation with RR(TN) (R2 = 0.0883, p = 0.0449) and a highly significant negative correlation with RR(NH4+-N) (R2 = 0.2415, p = 0.0016). Additionally, RR(SOC) demonstrates a significant positive correlation with RR(NO3−-N) (R2 = 0.0873, p = 0.0415) (Fig. 5B).
The correlations between RR(pH) and RR(amoA-AOA), RR(amoA-AOB), and RR(N2O) are not significant (Fig. 5C). In addition, there is no significant correlation between RR(SOC) and RR(amoA-AOA), RR(amoA-AOB). However, RR(SOC) shows a highly significant negative correlation with RR(N2O) (R2 = 0.4300, p = 0.0058) (Fig. 5D). No significant correlation between RR(amoA-AOA) or RR(amoA-AOB) and RR(NH4+-N), RR(UA), and RR(NO3−N) (Fig. 6).
4 Discussion
4.1 Soil pH Affected by Biochar
Most biochar typically falls within the pH range of 7 to 11, containing alkaline substances (Shi et al. 2017), rendering them valuable amendments for improving soil acidity and elevating pH (Dai et al. 2017). OW and CS biochar significantly increased soil pH (p < 0.05), while WB and CH biochar did not exhibit a pronounced effect on soil pH elevation, potentially due to the ash content and ions like calcium (Ca) and magnesium (Mg) in biochar (Ippolito et al. 2020). Ash is an inorganic residue during the preparation of biochar, and its content is usually associated with the pH of the biochar (Yang et al. 2019).
As the pyrolysis temperature of biochar increased, soil pH gradually rose, except for LOW-temperature pyrolysis biochar, causing a pH decrease, consistent with Yang et al.‘s (Yang et al. 2019). Higher temperatures remove acidic functional groups, making biochar surfaces more alkaline and contributing to soil pH elevation (Song et al. 2018). Conversely, biochar produced at lower temperatures may have numerous charged functional groups, enhancing soil buffering capacity (Ahmad et al. 2012). Caution is recommended when applying biochar to alkaline soils. This is related to the functional groups of biochar under different temperature conditions. In high temperature pyrolyzed biochar, alkaline substances mainly exist in the form of carbonates, and the total amount of carbonates increases with the pyrolysis temperature. In contrast, in low-temperature pyrolyzed biochar, alkaline substances are primarily oxygen-containing functional groups such as carboxyl and hydroxyl groups. These groups carry negative charges and can bind hydrogen ions in soil solution (Ma et al. 2017). This study linked pyrolysis temperatures with biochar efficiency, providing a novel insight into optimizing biochar production processes for improved soil management outcomes.
Biochar significantly influenced soil pH in relatively controlled environments like Pot and Incubation, whereas its impact in Woods and Field was less pronounced, likely due to natural conditions affecting Field and Woods, such as rainfall event. The differences in aeration and permeability between the soils in Field and Woods may lead to the leaching and migration of biochar, affecting its efficacy (Guo et al. 2024). These findings underscore the contribution of different biochar in ameliorating soil acidity, which are critical for sustainable agriculture.
4.2 Soil Organic Carbon (SOC) Content Affected by Biochar
The soil properties, encompassing physical, chemical, and biological aspects, intricately link to SOC content (Šimanský et al. 2016), which is crucial for enhancing soil structure (Leelamanie and Mapa 2015), maintaining soil quality, and supporting ecosystem functionality (Benbi et al. 2015). SOC closely correlates with soil aeration, respiratory intensity, available nitrogen content, and microbial communities.
The type and composition of biochar feedstock directly influence produced biochar characteristics, including yield, ash content, and morphology (Zhang et al. 2012). Biochar from three sources (WB, CS, and OW) significantly increased soil SOC content. Consistent with Islam et al., it was found that biochar from WB, CS, and OW sources elevated soil aggregate content, improving soil structure and increasing SOC content upon soil application (Islam et al. 2021).
Pyrolysis temperature significantly influences biochar characteristics. Low-temperature Biochar comprises aromatic, aliphatic, and carbonyl carbon, while high-temperature biochar predominantly comprises aromatic carbon (Tomczyk et al. 2020). This study’s biochar from three different pyrolysis temperatures significantly increased soil SOC content. Biochar, with its high carbon content, promotes soil humus, carbon compounds, and organic macromolecules formation, influencing soil microbial communities and augmenting SOC’s recalcitrant fraction (Cen et al. 2021). Higher carbon content in applied biochar leads to a more pronounced increase in soil SOC.
Biochar application significantly increased soil SOC content in three soil environments - Field, Pot, and Incubation. However, in the Woods condition, soil SOC content decreased, potentially due to the high organic matter content in Wood soil, intensifying induced effects and potentially reducing efficiency in SOC sequestration (Kirkby et al. 2014). These findings highlight the originality of our study in identifying specific biochar types that optimize soil carbon content.
4.3 Soil Nitrogen Affected by Biochar
Biochar derived from different feedstocks exhibits variations in the carbon-to-nitrogen ratio (C/N), which increases with rising pyrolysis temperatures. The C/N ratio of soil directly or indirectly influences organic carbon mineralization, consequently affecting the soil’s NH4+-N, NO3−-N, or TN. Limited information has been found for nitrite nitrogen, which was not considered in the current study. Meta-analysis reveals a more pronounced influence of biochar on TN, possibly attributed to its reduced accessibility to plants and microbes after incorporation into the soil. Adding biochar in Incubation and Pot situations significantly increases soil TN, likely associated with soil aeration and permeability. The porous structure of biochar can adsorb nitrogen, mitigating the volatilization and leaching of ammonium and reducing nitrogen losses. Studies indicate that biochar enhances soil water retention (Sun et al. 2018) and increases plant N retention (Wan et al. 2023).
Biochar’s porous structure also contributes to an elevated trend in TN mineralization in soil (Ahmad et al. 2012). It may be due to the increased microbial abundance facilitated by biochar, promoting the degradation of soil organic nitrogen (Anderson et al. 2011). Additionally, it enhances the fixation of soil TN, possibly due to introducing biochar with a high C/N (Deenik et al. 2010). However, biochar might increase net nitrogen mineralization in the long run, reducing the soil’s organic nitrogen pool (Deenik et al. 2010).
Soil NH3 volatilization is closely related to soil pH (Hawthorne et al. 2017), with current meta-analysis revealing an inhibition in NH3 volatilization with rising pH levels. The intricate interplay between biochar, soil nitrogen dynamics, and pH underscores the need for a comprehensive understanding of these relationships for effective soil management and sustainable agriculture.
The ammonia volatilization process first converts NH4+-N in the soil into liquid form, which then transforms into liquid NH3 and subsequently into gaseous NH3, eventually volatilizing into the atmosphere. This process is influenced by various factors when biochar is added. Current research on the impact of biochar on soil ammonia emissions yields inconsistent results. Some studies indicate that applying biochar increases soil ammonia emissions by raising soil pH and enhancing soil permeability. Additionally, in acidic soils, biochar addition has been shown to inhibit soil NH4+-N nitrification, leading to high concentrations of NH4+-N in the soil, which promotes ammonia emissions (Wang et al. 2015). Conversely, other studies suggest that the oxygen-containing functional groups on biochar surfaces not only adsorb NH3 but also increase the protonation of H+, converting adsorbed NH3 into NH4+-N, thereby reducing ammonia volatilization (Sha et al. 2019). The effect of biochar on soil ammonia volatilization may depend on the delicate balance between biochar’s NH3 adsorption capacity, its impact on soil pH, and its influence on soil NH4+-N nitrification. In our study, biochar significantly reduced ammonia volatilization, likely due to the adsorption of NH3 by its oxygen-containing functional groups, despite an increase in soil pH. Furthermore, biochar promoted soil NH4+-N nitrification, further reducing ammonia volatilization.
4.4 Nitrogen Functional Genes Affected by Biochar
Soil microorganisms predominantly regulate soil nitrogen transformation and cycling through the expression of functional genes and the activity of extracellular enzymes (Liu et al. 2018). The meta-analysis in this study revealed that after biochar application, the nitrogen-fixing functional gene nifD increased by 3.54%, while nifH and nifG decreased by 4.13% and 2.51%, respectively. This result contrasts with the findings of Ducey et al., who observed a threefold increase in the relative abundance of nifH after applying a biochar (Ducey et al. 2013).
Ammonia oxidation is the initial and rate-limiting step in nitrogen cycling. Ammonia-oxidizing archaea and bacteria carrying the amoA gene are a diverse and widely distributed group of soil microorganisms that significantly contribute to the ammonia oxidation process in various environments (Wan et al. 2024). The meta-analysis in this study found an increase of 0.57% and 25.58% in the diversity of ammonia-oxidizing bacteria (amoA-AOA and amoA-AOB) genes after adding biochar to the soil. It aligns with the results of Xiao et al.‘s meta-analysis (Xiao et al. 2019), which reported an increased abundance of amoA-AOA and amoA-AOB in soil with added biochar, possibly due to the improved soil aeration (Zhang et al. 2010) and increased soil pH (Lin et al. 2017), which are factors associated with the abundance of ammonia oxidation genes (amoA-AOA, amoA-AOB) (French et al. 2021).
Denitrification is the stepwise reduction of NO3−-N to NO2−-N, NO, N2O, and ultimately N2, occurring primarily under anaerobic conditions (Sapkota et al. 2024). The pathway from NO3−-N to NO2−-N in denitrification involves a series of genes encoding reductases (such as narG and napA), serving as proxies for assessing the potential of nitrate reduction processes in the environment (Chen et al. 2024). The meta-analysis in this study found that biochar addition reduced the abundance of narG by 2.43% while increasing the abundance of nirK and nirS by 14.78% and 11.53%, respectively. It may be attributed to biochar’s improved soil aeration and subsequent reduction of soil bulk density, stimulating the growth and diversity of denitrifying bacteria (Gul et al. 2015). However, the mediating effect of biochar on nirK is influenced by various external or internal factors. For instance, adding biochar from MOD pyrolysis increased the abundance of the nirK gene, accompanied by a change in soil pH from 5.5 to 6.5 (Zhang et al. 2015). This increase in nirK gene abundance may be stimulated by the pH improvement induced by the added biochar.
Nitrification and denitrification involve redox reactions (Fei et al. 2024), and the production or consumption of N2O is closely related to soil redox potential (Eh) (DeAngelis et al. 2010). Biochar contains electroactive functional groups (e.g., polyethylene, quinone, hydroquinone) and redox-active minerals (such as Fe or Mn), which can act as electron acceptors or donors during redox processes (Graber et al. 2014), facilitating electron transfer to microorganisms (Kappler et al. 2014). The changes in soil redox reactions mediated by biochar depend on pyrolysis temperature, biochar feedstock, and soil type. In a rice soil system undergoing flooding and drying cycles, biochar addition decreased soil Eh and the availability of Fe, influencing the expression of nitrogen-cycling genes (Wang et al. 2018). The intricate interplay between biochar, nitrogen cycling genes, and soil redox dynamics highlights the importance of understanding these relationships for effective soil management and sustainable agricultural practices.
4.5 Gas Emissions Affected by Biochar
Applying biochar to calcareous soils is a strategy to reduce N2O emissions and mitigate global warming (Dong et al. 2020). In the current study, we observed reduced soil N2O emissions after applying biochar r produced at MEG pyrolysis temperatures. Furthermore, adding biochar also decreased N2O emissions in the Woods and Incubation soil environments, although the same size of Woods application is small (n = 2). These findings advocate for biochar’s application in specific environments to maximize its environmental benefits. However, the mechanisms behind these responses remain unclear. Petter et al. proposed that biochar improves soil aeration, reducing anaerobic environment where denitrifying bacteria thrive (Petter et al. 2016).
Additionally, the increase in soil pH induced by biochar enhances the activity of N2O reductase (Cayuela et al. 2014). Moreover, the fixation of inorganic N by biochar, along with its restrictions on soil nitrifying and denitrifying bacteria, contributes to the reduction in N2O emissions (Bruun et al. 2012), and the porous structure of biochar can directly adsorb N2O (Cornelissen et al. 2013). Some studies suggest that biochar can absorb NH4+-N through negatively charged functional groups (carboxyl and phenolic hydroxyl), reducing soil NH3 volatilization (Taghizadeh-Toosi et al. 2012). It promotes the shift of N2O towards N2, alleviating soil N2O emissions (Cayuela et al. 2013).
Our meta-analysis revealed a significant promotion of UA, nirS, and amoA-AOB in soil by adding biochar. While amoA-AOB participates in the rate-limiting step of the ammonia oxidation process (Chu et al. 2022), an increase in amoA-AOB accelerates ammonia oxidation. Meanwhile, the ratio of nosZ to amoA-AOB and nirS reflects the thoroughness of nitrogen cycling, leading to an increase in N2, a decrease in N2O, and a reduction in NH4+-N. Biochar increases the efficiency of nitrogen cycling, resulting in a more complete and effective process. However, some studies suggest that adding biochar may increase soil alkalinity, leading to contrasting effects on soil NH3 emissions (Sun et al. 2014) and accelerating soil N2O emissions by promoting nitrification (Sánchez-García et al. 2014). Based on the results of our meta-analysis, it is understood that biochar regulates soil denitrifying enzymes, promoting denitrification to completion, ultimately increasing N2 emissions and reducing N2O emissions under certain conditions, which is aligning with the findings of Cayuela (Cayuela et al. 2013). It should be cautioned that few studies were available to trace the effect of biochar on N2 emissions (n = 5). In addition, this discrepancy might be related to the age of the biochar application. Literature indicated that fresh biochar application increased NH3 volatilization while aged biochar decreased NH3 volatilization (Dong et al. 2019). The intricate interactions between biochar, denitrification enzymes, and nitrogen cycling contribute to our understanding of the complex role of biochar in greenhouse gas dynamics within soils.
4.6 Relationship Between Nitrogen Cycling Genes and Soil Nitrogen
In our meta-analysis, adding biochar to the soil decreased NH4+-N and NO3−-N, with a decreasing trend in their percentage changes. In addition, the abundance of ammonia-oxidizing bacteria genes (amoA-AOB) increased significantly with biochar addition, promoting further transformation of NH4+-N and NO3−-N. Additionally, biochar addition increased the abundance of nirS functional genes by 14.78%, closely linked to N2O emissions. This result suggests that the reduction in N2O emissions associated with biochar addition may be related to the increased abundance of nosZ in the soil (Van Zwieten et al. 2014). The elevated abundance of nosZ, involved in denitrification, results in more efficient conversion of N2O to N2, ultimately leading to a 15.70% increase in N2 emissions.
Furthermore, we observed a relationship between the abundance of nirK, nirS and N2O emissions. The lack of these denitrifying genes (nirS and nirK) is associated with reduced genetic capacity to decrease N2O (Philippot et al. 2011), as they are considered major contributors to N2O production during denitrification. The overall result indicated a 9.62% reduction in N2O emissions due to adding biochar. This finding aligns with earlier studies, suggesting that N2O emissions could typically be reduced in soils treated with biochar (Hagemann et al. 2017).
The urea in the soil is converted to NH4+-N through urease, and biochar addition increases soil UA by 11.00%. However, the soil microbial gene ureC, which controls urease synthesis, decreased by 15.75%. Literature indicated that extracellular urease activity could be inhibited with increased soil ureC gene (Jiang et al. 2023). This result suggests that biochar may enhance the urease content released by crop roots. Biochar influences soil processes, including nitrification, denitrification, nitrate reduction, and N2 fixation.
5 Conclusions
This study elucidates the multifaceted role of biochar in modulating soil nitrogen cycling and improving soil health. Biochar significantly elevates soil pH by 4.57%, UA by 11.00%, amoA-AOB gene abundance by 25.58%, and nirS gene abundance by 14.78%. In addition, it significantly reduces NH3 emissions by 16.39%. While biochar is beneficial for ameliorating acidic soils by increasing pH, it tends to elevate alkalinity in alkaline soils. Biochar substantially increases SOC content by 64.60%. However, the overall impact of biochar on NH4+-N and NO3−-N contents is not significant.
Different biochar sources and their pyrolysis temperatures also introduce variations in the effects on soil nitrogen, pH, and SOC content. Biochar application to soil, sourced from different feedstocks and subjected to varying pyrolysis temperatures, provides carbon sources to the soil.
Moreover, biochar enhances the abundance of amoA-AOB genes, promoting nitrification and accelerating soil nitrogen cycling. The reduction in N2O emissions under certain conditions and elevated nirS gene abundance indicate that biochar addition accelerates nitrogen cycling, leading to a significant decrease in greenhouse gas emissions.
Although the specific mechanisms behind these effects are not fully understood, the current study includes a correlation analysis with soil pH and SOC to explore the impact of biochar amendment on these soil parameters. Future research should focus on elucidating these mechanisms, particularly in relation to additional physical and chemical properties or indicators, as well as various enzymes related to nitrogen conversion, such as nitrate and nitrite reductase. Practitioners are advised to carefully select biochar types based on local conditions and production methods, tailoring applications to maximize positive outcomes for soil nitrogen dynamics while minimizing potential drawbacks.
Data Availability
Data will be made available on request.
Abbreviations
- WB:
-
Wooden Biochar
- CH:
-
Crop Husk
- CS:
-
Crop Straw
- OW:
-
Organic Waste
- UA:
-
Urease Activity
References
Ahmad M, Lee SS, Dou X, Mohan D, Sung JK, Yang JE, Ok YS (2012) Effects of pyrolysis temperature on soybean stover- and peanut shell-derived biochar properties and TCE adsorption in water. Bioresour Technol 118:536–544. https://doi.org/10.1016/j.biortech.2012.05.042
Anderson CR, Condron LM, Clough TJ, Fiers M, Stewart A, Hill RA, Sherlock RR (2011) Biochar induced soil microbial community change: implications for biogeochemical cycling of carbon, nitrogen and phosphorus. Pedobiologia (Jena) 54:309–320. https://doi.org/10.1016/j.pedobi.2011.07.005
Benbi DK, Brar K, Toor AS, Sharma S (2015) Sensitivity of labile soil organic carbon pools to long-term fertilizer, straw and manure management in rice-wheat system. Pedosphere 25:534–545. https://doi.org/10.1016/S1002-0160(15)30034-5
Bruun EW, Ambus P, Egsgaard H, Hauggaard-Nielsen H (2012) Effects of slow and fast pyrolysis biochar on soil C and N turnover dynamics. Soil Biol Biochem 46:73–79. https://doi.org/10.1016/j.soilbio.2011.11.019
Cayuela ML, Sánchez-Monedero MA, Roig A, Hanley K, Enders A, Lehmann J (2013) Biochar and denitrification in soils: when, how much and why does biochar reduce N2O emissions? Sci Rep 3:1–7. https://doi.org/10.1038/srep01732
Cayuela ML, van Zwieten L, Singh BP, Jeffery S, Roig A, Sánchez-Monedero MA (2014) Biochar’s role in mitigating soil nitrous oxide emissions: a review and meta-analysis. Agric Ecosyst Environ 191:5–16. https://doi.org/10.1016/j.agee.2013.10.009
Cen R, Feng W, Yang F, Wu W, Liao H, Qu Z (2021) Effect mechanism of biochar application on soil structure and organic matter in semi-arid areas. J Environ Manage 286:112198. https://doi.org/10.1016/j.jenvman.2021.112198
Chen H, Yin C, Fan X, Ye M, Peng H, Li T, Zhao Y, Wakelin SA, Chu G, Liang Y (2019) Reduction of N2O emission by biochar and/or 3,4-dimethylpyrazole phosphate (DMPP) is closely linked to soil ammonia oxidizing bacteria and nosZI-N2O reducer populations. Sci Total Environ 694:133658. https://doi.org/10.1016/j.scitotenv.2019.133658
Chen Z, Liu Z, Song C (2024) Science of the total Environment Agricultural fertilization near marshes impacts the potential for greenhouse gas emissions from wetland ecosystems by modifying microbial communities. Sci Total Environ 930:172400. https://doi.org/10.1016/j.scitotenv.2024.172400
Chu X, Bai N, Zheng X, Wang Q, Pan X, Li S, Zhang J, Zhang H, He W, Zhong F, Lv W, Zhang H (2022) Effects of straw returning combined with earthworm addition on nitrification and ammonia oxidizers in paddy soil. Front Microbiol 13:1–11. https://doi.org/10.3389/fmicb.2022.1069554
Cornelissen G, Rutherford DW, Arp HPH, Dörsch P, Kelly CN, Rostad CE (2013) Sorption of pure N2O to biochars and other organic and inorganic materials under anhydrous conditions. Environ Sci Technol 47:7704–7712. https://doi.org/10.1021/es400676q
Curtin D, Beare MH, Qiu W, Tregurtha CS (2019) Nitrogen cycling in soil under grass-clover pasture: influence of long-term inputs of superphosphate on N mineralisation. Soil Biol Biochem 130:132–140. https://doi.org/10.1016/j.soilbio.2018.12.003
Dai Z, Zhang X, Tang C, Muhammad N, Wu J, Brookes PC, Xu J (2017) Potential role of biochars in decreasing soil acidification - A critical review. Sci Total Environ 581–582:601–611. https://doi.org/10.1016/j.scitotenv.2016.12.169
DeAngelis KM, Silver WL, Thompson AW, Firestone MK (2010) Microbial communities acclimate to recurring changes in soil redox potential status. Environ Microbiol 12:3137–3149. https://doi.org/10.1111/j.1462-2920.2010.02286.x
Deenik JL, McClellan T, Uehara G, Antal MJ, Campbell S (2010) Charcoal volatile matter content influences plant growth and soil nitrogen transformations. Soil Sci Soc Am J 74:1259–1270. https://doi.org/10.2136/sssaj2009.0115
Dong Y, Wu Z, Zhang X, Feng L, Xiong Z (2019) Dynamic responses of ammonia volatilization to different rates of fresh and field-aged biochar in a rice-wheat rotation system. F Crop Res 241:107568. https://doi.org/10.1016/J.FCR.2019.107568
Dong W, Walkiewicz A, Bieganowski A, Oenema O, Nosalewicz M, He C, Zhang Y, Hu C (2020) Biochar promotes the reduction of N2O to N2 and concurrently suppresses the production of N2O in calcareous soil. Geoderma 362:114091. https://doi.org/10.1016/j.geoderma.2019.114091
Ducey TF, Ippolito JA, Cantrell KB, Novak JM, Lentz RD (2013) Addition of activated switchgrass biochar to an aridic subsoil increases microbial nitrogen cycling gene abundances. Appl Soil Ecol 65:65–72. https://doi.org/10.1016/j.apsoil.2013.01.006
Edwards JD, Pittelkow CM, Kent AD, Yang WH (2018) Dynamic biochar effects on soil nitrous oxide emissions and underlying microbial processes during the maize growing season. Soil Biol Biochem 122:81–90. https://doi.org/10.1016/j.soilbio.2018.04.008
Fei Y, Zhang B, Zhang Q, Chen D, Cao W, Borthwick AGL (2024) Multiple pathways of vanadate reduction and denitrification mediated by denitrifying bacterium Acidovorax sp. strain BoFeN1. Water Res 257:121747. https://doi.org/10.1016/j.watres.2024.121747
Feng Y, Sun H, Xue L, Wang Y, Yang L, Shi W, Xing B (2018) Sawdust biochar application to rice paddy field: reduced nitrogen loss in floodwater accompanied with increased NH3 volatilization. Environ Sci Pollut Res 25:8388–8395. https://doi.org/10.1007/s11356-017-1059-y
French E, Kozlowski JA, Bollmann A (2021) Competition between ammonia-oxidizing archaea and bacteria from freshwater environments. Appl Environ Microbiol 87:1–11. https://doi.org/10.1128/AEM.01038-21
Gascon M, Zijlema W, Vert C, White MP, Nieuwenhuijsen MJ (2017) Outdoor blue spaces, human health and well-being: a systematic review of quantitative studies. Int J Hyg Environ Health 220:1207–1221. https://doi.org/10.1016/j.ijheh.2017.08.004
Ghorbani M, Konvalina P, Kopecký M, Kolář L (2023) A meta-analysis on the impacts of different oxidation methods on the surface area properties of biochar. L Degrad Dev 34:299–312. https://doi.org/10.1002/ldr.4464
Graber ER, Tsechansky L, Lew B, Cohen E (2014) Reducing capacity of water extracts of biochars and their solubilization of soil Mn and Fe. Eur J Soil Sci 65:162–172. https://doi.org/10.1111/ejss.12071
Gul S, Whalen JK, Thomas BW, Sachdeva V, Deng H (2015) Physico-chemical properties and microbial responses in biochar-amended soils: mechanisms and future directions. Agric Ecosyst Environ 206:46–59. https://doi.org/10.1016/j.agee.2015.03.015
Guo F, Wang C, Wang S, Zhao X, Li G, Sun Z (2024) The native SOC increase in woodland and lawn soil amended with biochar surpassed greenhouse — a seven-year field trial. Sci Total Environ 907:167924. https://doi.org/10.1016/j.scitotenv.2023.167924
Hagemann N, Harter J, Kaldamukova R, Guzman-Bustamante I, Ruser R, Graeff S, Kappler A, Behrens S (2017) Does soil aging affect the N2O mitigation potential of biochar? A combined microcosm and field study. GCB Bioenergy 9:953–964. https://doi.org/10.1111/gcbb.12390
Hawthorne I, Johnson MS, Jassal RS, Black TA, Grant NJ, Smukler SM (2017) Application of biochar and nitrogen influences fluxes of CO2, CH4 and N2O in a forest soil. J Environ Manage 192:203–214. https://doi.org/10.1016/j.jenvman.2016.12.066
He L, Liu Y, Zhao J, Bi Y, Zhao X, Wang S, Xing G (2016) Comparison of straw-biochar-mediated changes in nitrification and ammonia oxidizers in agricultural oxisols and cambosols. Biol Fertil Soils 52:137–149. https://doi.org/10.1007/s00374-015-1059-3
He T, Liu D, Yuan J, Luo J, Lindsey S, Bolan N, Ding W (2018) Effects of application of inhibitors and biochar to fertilizer on gaseous nitrogen emissions from an intensively managed wheat field. Sci Total Environ 628–629:121–130. https://doi.org/10.1016/j.scitotenv.2018.02.048
IPCC (2023) Climate Change 2023: synthesis report. Geneva, Switzerland
Ippolito JA, Cui L, Kammann C, Wrage-Mönnig N, Estavillo JM, Fuertes-Mendizabal T, Cayuela ML, Sigua G, Novak J, Spokas K, Borchard N (2020) Feedstock choice, pyrolysis temperature and type influence biochar characteristics: a comprehensive meta-data analysis review. Biochar 2:421–438. https://doi.org/10.1007/s42773-020-00067-x
Islam MU, Jiang F, Guo Z, Peng X (2021) Does biochar application improve soil aggregation? A meta-analysis. Soil Tillage Res 209:104926. https://doi.org/10.1016/j.still.2020.104926
Ji M, Wang X, Usman M, Liu F, Dan Y, Zhou L, Campanaro S, Luo G, Sang W (2022) Effects of different feedstocks-based biochar on soil remediation: a review. Environ Pollut 294:118655. https://doi.org/10.1016/j.envpol.2021.118655
Jiang D, Jiang N, Jiang H, Chen L (2023) Urease inhibitors increased soil ureC gene abundance and intracellular urease activity when extracellular urease activity was inhibited. Geoderma 430:116295. https://doi.org/10.1016/j.geoderma.2022.116295
Kappler A, Wuestner ML, Ruecker A, Harter J, Halama M, Behrens S (2014) Biochar as an Electron shuttle between Bacteria and Fe(III) minerals. Environ Sci Technol Lett 1:339–344. https://doi.org/10.1021/ez5002209
Kirkby CA, Richardson AE, Wade LJ, Passioura JB, Batten GD, Blanchard C, Kirkegaard JA (2014) Nutrient availability limits carbon sequestration in arable soils. Soil Biol Biochem 68:402–409. https://doi.org/10.1016/j.soilbio.2013.09.032
Kuppusamy S, Thavamani P, Megharaj M, Venkateswarlu K, Naidu R (2016) Agronomic and remedial benefits and risks of applying biochar to soil: current knowledge and future research directions. Environ Int 87:1–12. https://doi.org/10.1016/j.envint.2015.10.018
Lan Z, Chen C, Rezaei Rashti M, Yang H, Zhang D (2018) High pyrolysis temperature biochars reduce nitrogen availability and nitrous oxide emissions from an acid soil. GCB Bioenergy 10:930–945. https://doi.org/10.1111/gcbb.12529
Leelamanie DAL, Mapa RB (2015) Alterations in soil aggregate stability of a tropical Ultisol as mediated by changes in land use. Biol 70:1444–1449. https://doi.org/10.1515/biolog-2015-0168
Li B, Liu D, Lin D, Xie X, Wang S, Xu H, Wang J, Huang Y, Zhang S, Hu X (2020) Changes in Biochar Functional groups and its reactivity after volatile-char interactions during Biomass Pyrolysis. Energy Fuels 34:14291–14299. https://doi.org/10.1021/acs.energyfuels.0c03243
Lin Y, Ding W, Liu D, He T, Yoo G, Yuan J, Chen Z, Fan J (2017) Wheat straw-derived biochar amendment stimulated N2O emissions from rice paddy soils by regulating the amoA genes of ammonia-oxidizing bacteria. Soil Biol Biochem 113:89–98. https://doi.org/10.1016/j.soilbio.2017.06.001
Liu Q, Zhang Y, Liu B, Amonette JE, Lin Z, Liu G, Ambus P, Xie Z (2018) How does biochar influence soil N cycle? A meta-analysis. Plant Soil 426:211–225. https://doi.org/10.1007/s11104-018-3619-4
Ma Z, Yang Y, Ma Q, Zhou H, Luo X, Liu X, Wang S (2017) Evolution of the chemical composition, functional group, pore structure and crystallographic structure of bio-char from palm kernel shell pyrolysis under different temperatures. J Anal Appl Pyrol 127:350–359. https://doi.org/10.1016/j.jaap.2017.07.015
Martínez-Espinosa RM, Cole JA, Richardson DJ, Watmough NJ (2011) Enzymology and ecology of the nitrogen cycle. Biochem Soc Trans 39:175–178. https://doi.org/10.1042/BST0390175
Mukherjee A, Lal R (2014) The biochar dilemma. Soil Res 52:217–230. https://doi.org/10.1071/SR13359
Nguyen TTN, Xu CY, Tahmasbian I, Che R, Xu Z, Zhou X, Wallace HM, Bai SH (2017) Effects of biochar on soil available inorganic nitrogen: a review and meta-analysis. Geoderma 288:79–96. https://doi.org/10.1016/j.geoderma.2016.11.004
Petter FA, Borges de Lima L, Marimon Júnior BH, Alves de Morais L, Marimon BS (2016) Impact of biochar on nitrous oxide emissions from upland rice. J Environ Manage 169:27–33. https://doi.org/10.1016/j.jenvman.2015.12.020
Philippot L, Andert J, Jones CM, Bru D, Hallin S (2011) Importance of denitrifiers lacking the genes encoding the nitrous oxide reductase for N2O emissions from soil. Glob Chang Biol 17:1497–1504. https://doi.org/10.1111/j.1365-2486.2010.02334.x
Qin H, Xing X, Tang Y, Zhu B, Wei X, Chen X, Liu Y (2020) Soil moisture and activity of nitrite- and nitrous oxide-reducing microbes enhanced nitrous oxide emissions in fallow paddy soils. Biol Fertil Soils 56:53–67. https://doi.org/10.1007/s00374-019-01403-5
Sánchez-García M, Roig A, Sánchez-Monedero MA, Cayuela ML (2014) Biochar increases soil N2O emissions produced by nitrification-mediated pathways. Front Environ Sci 2:1–10. https://doi.org/10.3389/fenvs.2014.00025
Sapkota S, Ghimire R, Bista P, Hartmann D, Rahman T, Adhikari S (2024) Greenhouse gas mitigation and soil carbon stabilization potential of forest biochar varied with biochar type and characteristics. Sci Total Environ 931:172942. https://doi.org/10.1016/j.scitotenv.2024.172942
Sha Z, Li Q, Lv T, Misselbrook T, Liu X (2019) Response of ammonia volatilization to biochar addition: a meta-analysis. Sci Total Environ 655:1387–1396. https://doi.org/10.1016/j.scitotenv.2018.11.316
Shanmugam S, Jenkins SN, Mickan BS, Jaafar NM, Mathes F, Solaiman ZM, Abbott LK (2021) Co-application of a biosolids product and biochar to two coarse-textured pasture soils influenced microbial N cycling genes and potential for N leaching. Sci Rep 11:1–12. https://doi.org/10.1038/s41598-020-78843-9
Shi RY, Hong ZN, Li JY, Jiang J, Baquy MA, Al, Xu RK, Qian W (2017) Mechanisms for increasing the pH buffering capacity of an acidic Ultisol by Crop Residue-Derived Biochars. J Agric Food Chem 65:8111–8119. https://doi.org/10.1021/acs.jafc.7b02266
Šimanský V, Horák J, Igaz D, Jonczak J, Markiewicz M, Felber R, Rizhiya EY, Lukac M (2016) How dose of biochar and biochar with nitrogen can improve the parameters of soil organic matter and soil structure? Biol 71:989–995. https://doi.org/10.1515/biolog-2016-0122
Song D, Tang J, Xi X, Zhang S, Liang G, Zhou W, Wang X (2018) Responses of soil nutrients and microbial activities to additions of maize straw biochar and chemical fertilization in a calcareous soil. Eur J Soil Biol 84:1–10. https://doi.org/10.1016/j.ejsobi.2017.11.003
Sun L, Li L, Chen Z, Wang J, Xiong Z (2014) Combined effects of nitrogen deposition and biochar application on emissions of N2O, CO2 and NH3 from agricultural and forest soils. Soil Sci Plant Nutr 60:254–265. https://doi.org/10.1080/00380768.2014.885386
Sun H, Min J, Zhang H, Feng Y, Lu K, Shi W, Yu M, Li X (2018) Biochar application mode influences nitrogen leaching and NH3 volatilization losses in a rice paddy soil irrigated with N-rich wastewater. Environ Technol (United Kingdom) 39:2090–2096. https://doi.org/10.1080/09593330.2017.1349839
Taghizadeh-Toosi A, Clough TJ, Sherlock RR, Condron LM (2012) A wood based low-temperature biochar captures NH3-N generated from ruminant urine-N, retaining its bioavailability. Plant Soil 353:73–84. https://doi.org/10.1007/s11104-011-1010-9
Tomczyk A, Sokołowska Z, Boguta P (2020) Biochar physicochemical properties: pyrolysis temperature and feedstock kind effects. Rev Environ Sci Biotechnol 19:191–215. https://doi.org/10.1007/s11157-020-09523-3
Van Zwieten L, Singh BP, Kimber SWL, Murphy DV, Macdonald LM, Rust J, Morris S (2014) An incubation study investigating the mechanisms that impact N2O flux from soil following biochar application. Agric Ecosyst Environ 191:53–62. https://doi.org/10.1016/j.agee.2014.02.030
Wan H, Chen Y, Cui B, Liu X, Hou J, Wei Z, Liu J, Liu F (2023) Biochar amendment increases C and N retention in the soil–plant systems: its implications in enhancing plant growth and water-use efficiency under reduced irrigation regimes of maize (Zea mays L). J Soil Sci Plant Nutr 23:1576–1588. https://doi.org/10.1007/s42729-023-01223-5
Wan X, Yang X, Zhang Y, Hu P, Duan P, Li D, Wang K (2024) Lithology-driven soil properties control of N2O production by ammonia oxidizers in subtropical forest soils. Geoderma 446:116910. https://doi.org/10.1016/j.geoderma.2024.116910
Wang Z, Zong H, Zheng H, Liu G, Chen L, Xing B (2015) Reduced nitrification and abundance of ammonia-oxidizing bacteria in acidic soil amended with biochar. Chemosphere 138:576–583. https://doi.org/10.1016/j.chemosphere.2015.06.084
Wang YQ, Bai R, Di HJ, Mo LY, Han B, Zhang LM, He JZ (2018) Differentiated mechanisms of biochar mitigating straw-induced greenhouse gas emissions in two contrasting paddy soils. Front Microbiol 9:1–19. https://doi.org/10.3389/fmicb.2018.02566
Wang W, Bai J, Lu Q, Zhang G, Wang D, Jia J, Guan Y, Yu L (2021) Pyrolysis temperature and feedstock alter the functional groups and carbon sequestration potential of Phragmites australis- and Spartina alterniflora-derived biochars. GCB Bioenergy 13:493–506. https://doi.org/10.1111/gcbb.12795
Weber K, Quicker P (2018) Properties of biochar. Fuel 217:240–261. https://doi.org/10.1016/j.fuel.2017.12.054
Xi S, Chu H, Zhou Z, Li T, Zhang S, Xu X, Pu Y, Wang G, Jia Y, Liu X (2023) Effect of potassium fertilizer on tea yield and quality: a meta-analysis. Eur J Agron 144:126767. https://doi.org/10.1016/j.eja.2023.126767
Xiao Z, Rasmann S, Yue L, Lian F, Zou H, Wang Z (2019) The effect of biochar amendment on N-cycling genes in soils: a meta-analysis. Sci Total Environ 696:133984. https://doi.org/10.1016/j.scitotenv.2019.133984
Yang X, Zhang S, Ju M, Liu L (2019) Preparation and modification of biochar materials and their application in soil remediation. Appl Sci 9. https://doi.org/10.3390/app9071365
Yao Y, Gao B, Fang J, Zhang M, Chen H, Zhou Y, Creamer AE, Sun Y, Yang L (2014) Characterization and environmental applications of clay-biochar composites. Chem Eng J 242:136–143. https://doi.org/10.1016/j.cej.2013.12.062
Zeng XY, Wang Y, Li RX, Cao HL, Li YF, Lü J (2022) Impacts of temperatures and phosphoric-acid modification to the physicochemical properties of biochar for excellent sulfadiazine adsorption. Biochar 4:1–14. https://doi.org/10.1007/s42773-022-00143-4
Zhang A, Cui L, Pan G, Li L, Hussain Q, Zhang X, Zheng J, Crowley D (2010) Effect of biochar amendment on yield and methane and nitrous oxide emissions from a rice paddy from Tai Lake plain, China. Agric Ecosyst Environ 139:469–475. https://doi.org/10.1016/j.agee.2010.09.003
Zhang M, Gao B, Yao Y, Xue Y, Inyang M (2012) Synthesis of porous MgO-biochar nanocomposites for removal of phosphate and nitrate from aqueous solutions. Chem Eng J 210:26–32. https://doi.org/10.1016/j.cej.2012.08.052
Zhang J, Liu J, Liu R (2015) Effects of pyrolysis temperature and heating time on biochar obtained from the pyrolysis of straw and lignosulfonate. Bioresour Technol 176:288–291. https://doi.org/10.1016/j.biortech.2014.11.011
Zhang P, Li B, Wu J, Hu S (2019) Invasive plants differentially affect soil biota through litter and rhizosphere pathways: a meta-analysis. Ecol Lett 22:200–210. https://doi.org/10.1111/ele.13181
Zhang L, Jing Y, Chen C, Xiang Y, Rezaei Rashti M, Li Y, Deng Q, Zhang R (2021) Effects of biochar application on soil nitrogen transformation, microbial functional genes, enzyme activity, and plant nitrogen uptake: a meta-analysis of field studies. GCB Bioenergy 13:1859–1873. https://doi.org/10.1111/gcbb.12898
Zhang Y, Fan S, Liu T, Fu W, Li B (2022) A review of biochar prepared by microwave-assisted pyrolysis of organic wastes. Sustain Energy Technol Assessments 50:101873. https://doi.org/10.1016/j.seta.2021.101873
Zhong L, Li G, Qing J, Li J, Xue J, Yan B, Chen G, Kang X, Rui Y (2022) Biochar can reduce N2O production potential from rhizosphere of fertilized agricultural soils by suppressing bacterial denitrification. Eur J Soil Biol 109:103391. https://doi.org/10.1016/j.ejsobi.2022.103391
Zhou Y, Berruti F, Greenhalf C, Tian X, Henry HAL (2017) Increased retention of soil nitrogen over winter by biochar application: implications of biochar pyrolysis temperature for plant nitrogen availability. Agric Ecosyst Environ 236:61–68. https://doi.org/10.1016/j.agee.2016.11.011
Acknowledgements
The authors would like to thank Yaobing Song for proofreading the article.
Funding
This research was financially supported by the National Natural Science Foundation of China (No.31500425) and the Natural Science Foundation of Jiangsu Province, China (No. BK20150452). K. Yang’s involvement in this work was supported by Yangzhou University Student Innovation and Entrepreneurship Program, Jiangsu Province, China (NO.X20220589).
Author information
Authors and Affiliations
Contributions
Binbin Yu: Conceptualization, Funding acquisition, Investigation, Project administration, Resources, Writing– original draft, Writing– review & editing, Supervision. Keming Yang: Data curation, Methodology, Project administration, Software, Visualization. Min Cui: Data curation, Methodology, Visualization. Zilong Chen: Data curation, Formal analysis, Visualization. Yuanyuan Dai: Validation, Supervision, Writing– review & editing. Xiaoqing Qian: Funding acquisition, Resources, Writing– review & editing, Supervision. Zhongzhi Chen: Conceptualization, Investigation, Visualization, Writing– original draft, Writing– review & editing.
Corresponding author
Ethics declarations
Declaration of Generative AI in Scientific Writing
While preparing this manuscript, the authors used ChatGPT to improve readability and language. After using ChatGPT, the authors reviewed and edited the content as needed and take full responsibility for the content of the publication.
Competing Interests
The authors have no competing interests to declare that are relevant to the content of this article.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yu, B., Yang, K., Cui, M. et al. Meta-Analysis Study on the Role of Biochar on Soil Nitrogen Cycling. J Soil Sci Plant Nutr (2024). https://doi.org/10.1007/s42729-024-01931-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42729-024-01931-6