Abstract
Biochar application to soil has been proposed as a potential management strategy to enhance soil carbon (C) sequestration, reduce greenhouse gas emission, improve soil quality, and increase crop productivity. The effects of biochar on soil microbial and enzyme activities are integrally linked to the potential of biochar in achieving these benefits. We conducted a global meta-analysis to assess the effects of biochar on soil microbial biomass C and nitrogen (N) and the activities of 12 enzymes, and identify key factors affecting those soil microbial properties using 964 data points from 72 papers. We found that biochar effects on enzyme activities vary widely with soil type, biochar property and the type of enzyme studied. Biochar significantly increased microbial biomass C (MBC) and urease, alkaline phosphatase and dehydrogenase activities by 21.7%, 23.1%, 25.4% and 19.8%, respectively, with no significant negative effects on any of the enzymes analyzed in this study. Biochar application was more effective in increasing MBC and enzyme activities in soils with low pH (< 6.5), TC (< 20 g kg−1), TN (< 2 g kg−1), and a fine texture (including clay, clay loam and silt clay). Biochars produced at pyrolysis temperature of 350–550 °C with a high pH (> 10) and low C/N ratio (< 50) increased MBC and urease and dehydrogenase activities. Biochar increased MBC and N-acquisition enzyme activities in the field but not in lab incubation experiments. Urease was increased in short-term studies (within 100 days of biochar application) while alkaline phosphatase was increased in long-term studies that span more than 1 year. The increase in MBC and activities of some soil enzymes in response to biochar application with no negative effects on any hydrolytic and oxidative enzymes illustrate its potential to enhance soil quality particularly in the degraded soils with low nutrient availability and fertility due to limited soil microbial and enzymatic activities. This study also shows that biochars can be designed to achieve specific properties for enhancing microbial and enzymatic activities for specific soils.
Graphic abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The soil application of biochar, a product of pyrolysis of biomass in partial or complete absence of oxygen, has been proposed as a potential management strategy to improve soil quality, support the resilience of agroecosystems, mitigate global climate change by increasing soil organic carbon (C) and fertility (Woolf et al. 2010; Biederman and Harpole 2013; Lu et al. 2014), and reducing greenhouse gas emissions (Spokas and Reicosky 2009; Crombie et al. 2015; Liu et al. 2016). These goals can be achieved by changing soil processes such as soil organic matter (SOM) decomposition and nutrient mineralization, in association with changed microbial and enzymatic activities through biochar application (Sohi et al. 2009; Lehman et al. 2011). Biochar application affects microbial and enzymatic activities by changing the availability of resources by adding labile C (Kuzyakov et al. 2009; Zimmerman et al. 2011) to the soil as well as by changing the soil environment (Verheijen et al. 2010). Soil enzymes catalyze the rate-limiting steps of SOM decomposition and nutrient cycling, and their activities are very sensitive to changes in the soil environment (Sinsabaugh 1994; Burns et al. 2013) that can be brought on by biochar application. Therefore, many studies have assessed the effect of biochar application on microbial and enzymatic activities.
A wide range of soil enzymes (extra- and intracellular) has been studied in biochar application experiments, including hydrolases and oxidases that decompose macromolecules of varying composition and complexity into soluble substrates for microbial assimilation. These enzymes target different groups of substrates present in soils for SOM decomposition (Sinsabaugh 2010) and their activities are substantially influenced by biochar application (Paz-Ferreiro et al. 2013; Zhang et al. 2014; Song et al. 2018). In biochar application studies, the most widely assayed soil hydrolytic enzymes for C cycling (C-acquisition) are β-1,4-glucosidase, β-d-cellobiohydrolase and β-1,4-xylosidase; β-1,4-N-acetyl-glucosaminidase, leucine amino peptidase and urease for N cycling (N-acquisition); and acid phosphatase and alkaline phosphatase for P cycling (P-acquisition) (Chen et al. 2013; Song et al. 2016; Pukalchik et al. 2018). The phenol oxidase and peroxidase are the most studied oxidizing soil enzymes while dehydrogenase is the most studied intracellular enzyme in biochar application studies (Ouyang et al. 2014; Chen et al. 2017). Most biochar studies that involved the above-mentioned enzyme activities are focused on soils that are severely deteriorated by extensive agricultural practices or contaminated by heavy metals. The low microbial and enzymatic activities often impede nutrient cycling and productivity of these soils (de Mora et al. 2005; Thavamani et al. 2012; Araujo et al. 2013). The use of biochar as a ‘soil conditioner’ can improve the quality of those soils by increasing microbial growth and enzyme activities that are associated with C and nutrient cycling (Verheijen et al. 2010).
Biochar application can have contrasting effects on soil enzyme activities. For instance, biochar application was found to significantly increase (Pukalchik et al. 2018), decrease (Chen et al. 2013; Zhang et al. 2014; Zheng et al. 2016; Benavente et al. 2018) or not change (Yoo and Kang 2012; Song et al. 2016) β-1,4-glucosidase activities in upland agricultural soils. Similarly, biochar application has been shown to increase (Song et al. 2018), decrease (Bamminger et al. 2014; Chen et al. 2017) or have no effect (Chen et al. 2019) on the activities of β-1,4-N-acetyl-glucosaminidase, which is involved in N-acquiring activities of microorganisms (Parham and Deng 2000). The responses of acid and alkaline phosphatases that are associated with the cleavage of P-containing organic compounds to biochar application also varied widely in both the direction and magnitude (Ouyang et al. 2014; Purakayastha et al. 2015). The wide variation in the response of enzyme activities to biochar application is associated with soil type and biochar property (Sohi et al. 2010; Gul et al. 2015).
Biochar application to the soil can change the physical (e.g., soil aeration, aggregation and water holding capacity) and chemical properties (e.g., soil pH, CEC and C/N ratio) of soil (Verheijien et al. 2010; Gul et al. 2015; Wang et al. 2017). Changes in these soil properties eventually alter microbial community composition and enzyme activities in the soil (Zhang et al. 2018a). However, the change in soil properties and their subsequent effects on microbial and enzymatic activities following biochar application is a function of soil texture, land use type and initial soil property (Sohi et al. 2010; Xie et al. 2015). Biochar application increased water holding capacity and enzymatic activities (catalase, dehydrogenase and invertase) in coarse-textured but not in fine-textured soils (Khadem and Raiesi 2017). Wu et al. (2018) observed an increase in activities of C cycling related enzymes in alkaline soil with no significant change in N cycling-related enzyme activities in alkaline and acidic soils following biochar addition.
The biochar-induced changes in soil properties and their subsequent effects on microbial and enzymatic activities also depend on the feedstock type used, the pyrolysis condition and biochar application rate (Singh et al. 2010; Gul et al. 2015). Biochar properties such as pH, C/N ratio, surface area and labile C content that have a direct influence on enzyme activities (BřEndová et al. 2012) are functions of feedstock type and pyrolysis condition. High pyrolysis temperature produces biochars with higher pH, surface area and aromatic C, and application of such biochars to the soil increases enzymatic activities associated with C cycling in a fluvo-aquic soil (Wang et al. 2015). Biochars produced from manure- and wood-based feedstocks are different in their nutrient content and pH (Lee et al. 2013; Novak et al. 2013). Generally, the application of biochars produced from manures and crop residues have higher pH, labile C and nutrient contents than that produced from wood feedstocks (Novak et al. 2013) that can increase activities of enzymes regulating C and N cycling in the soil (Bailey et al. 2011).
The number of studies that assess soil enzymatic and microbial activities in response to biochar application is rapidly increasing but the large number of such studies with contrasting results have made it difficult to reach a conclusion on the potential roles of biochar application in achieving the desired ecological benefits. With the surge in biochar amendment studies in recent years that involve assessment of soil microbial and enzymatic activities, quantitative reviews using meta-analysis procedure are helpful to critically analyze biochar’s effects on microbial and enzymatic activities on a global scale. Zhang et al. (2019) showed an increase in N- and P-cycling enzymes by biochar application in their meta-analysis based on data from 43 papers that covered publications prior to 2016. However, Zhang et al. (2019) did not include the assessment of the relationship between change in microbial biomass and enzymatic activities and did not analyze dehydrogenase (intracellular enzyme) activity in biochar-amended soils. Analyzing dehydrogenase activities in the soil is critical to understand the effect of biochar on the metabolic activities of microorganisms in the soil (Serra-Wittling et al. 1995). This global meta-analysis is based on more data (from 72 papers) on microbial and enzymatic activities than the Zhang et al. (2019) meta-analysis by including relevant papers published after 2016. This study has the following objectives: (i) to quantitatively assess the effect size of biochar application on microbial biomass, activities of intra-and extracellular enzymes that are involved in C, N and P acquisitions, (ii) to assess the relationship between changes in microbial biomass and changes in C and N acquisition enzyme activities in biochar-amended soils, and (iii) to identify key factors of soil and biochar that influence the response of intra- and extracellular soil enzymatic activities to biochar application.
2 Materials and methods
2.1 Literature search
A literature search was conducted to collect data for this meta-analysis using Web of Science and Google Scholar using the following keywords: biochar or char or pyrolyzed char or black carbon and soil and enzyme or enzymatic activities. Papers were selected based on the following criteria: (1) studies having at least three replicates in the experiment, (2) studies with treatment (biochar applied) effects paired with a control (no biochar applied) in the same experimental condition, and (3) studies reporting at least one of the following enzyme activities (given below). Studies that used (i) biochars modified by steam or citric and tartaric acid activation, denaturing stress and photochemical weathering, and (ii) biochars used in combination with other additives such as compost and lime with their control treatment not reported, were excluded. In addition, papers that reported incomplete units of enzyme activities (such as enzyme activities with no time in the unit) were also excluded. The authors of a few of the papers were contacted to get additional information such as the unit and absolute values of enzyme activities (when only relative values were reported), standard deviation (SD) or standard error (SE) in the data (if not reported in the paper).
2.2 Data collection and compilation
A total of 72 papers (published until February 18, 2019) each with an independent study were selected to collect the data used in this meta-analysis (ESM Appendix 1). Data sets for enzyme activities including mean values with the number of replicates (n) and SD or SE for the control and biochar application treatments were extracted from the tables and figures of the papers. The mean and SD (or SE) were extracted from figures using GetData Graph Digitizer 2.26 (http://getdata-graph-digitizer.com/download.php). The SD was calculated as SD = SE × √n2. If the experiment included different organic amendments, only the data for the biochar application alone and its control were extracted from that experiment. If there were data from multiple sampling times in a study, we used the data of the last sampling. In addition, in field experiments that involved multiple depths of soil to examine biochar’s effect on enzyme activities, we used only data for the uppermost soil layer to avoid potential bias caused by different soil layers being sampled (Jian et al. 2016) since biochar is generally applied to the upper 10–20 cm soil. The soil pH, total carbon (TC), total nitrogen (TN) and soil texture, and feedstock type, pyrolysis temperature, biochar pH, C/N ratio and biochar application rate (in percentage) data were also extracted from the papers. The latitude and longitude of the study location were also collected to help plot global distribution of study sites in this meta-analysis.
A total of 12 enzymes (11 extracellular and 1 intracellular) that represent the most common hydrolytic and oxidative enzymes in the soil were considered to examine the effect of biochar application on soil enzyme activities (Table 1). The extracellular enzymes included in this study are α-1,4-glucosidase, β-1,4-glucosidase, β-d-cellobiohydrolase, β-1,4-xylosidase, β-1,4-N-acetyl-glucosaminidase, leucine amino peptidase, urease, acid phosphatase, alkaline phosphatase, phenol oxidase, peroxidase and the intracellular enzyme studied is dehydrogenase. The hydrolytic extracellular enzymes were further integrated into C-acquisition (C-acq), N-acquisition (N-acq) and P-acquisition (P-acq) enzymes based on the targeted substrate or nutrients they act on. The activities of C-acq represent the average of α- and β-glucosidase, cellobiohydrolase and xylosidase, the activities of N-acq represent the average of acetyl-glucosaminidase, leucine amino peptidase and urease, and that of P-acq represent the average of acid and alkaline phosphatase activities. Soil microbial biomass C and N data were also extracted from the papers as dependent variables.
The selected soil and biochar data (as independent variables) were categorized into groups to facilitate meta-analysis and to help identify major factors affecting soil microbial and enzymatic activities. Following the classification of soil used in previous meta-analyses of biochar’s effects on soil microbial and enzymatic activities (Zhang et al. 2018a, 2019), soil pH was categorized into acidic (< 6.5), neutral (6.5–7.5 inclusive) and alkaline (> 7.5), TC and TN were categorized into three groups (< 10, 10–20 inclusive and > 20 g kg−1 for TC and < 1, 1–2 inclusive and > 2 g kg−1 for TN). Soil textural classes were divided into three groups: fine (clay, clay loam, silty clay loam and silty clay), medium (silt, loam, silt loam and sandy silt loam) and coarse (sandy loam, sandy clay loam, loamy sand, sand) following the USDA soil classification system. If soil textural classes were not reported but only percentages of the soil particles were given in the paper, the textural classes were determined by the percentage of clay, silt and sand. The experiments were divided into three types: lab incubation, greenhouse and field experiments. To assess the effect of time since biochar application, studies were categorized based on experiment duration into short- (experiments that span up to 100 days of biochar application), medium- (101–365 days inclusive) and long-term studies (> 365 days). Biochar feedstock types were categorized into wood, crop residue (including rice, wheat and soybean straw, maize silage, rice husk, oilseed rape and weeds), urban wastes (municipal solid waste and sewage sludge) and manure (poultry, cattle and swine). Pyrolysis temperature was categorized into low (> 350 °C), medium (350–550 °C inclusive) and high (> 550 °C); biochar pH was categorized into < 8, 8–10 inclusive and > 10; biochar C/N ratio into < 50, 50–100 inclusive and > 100. Biochar application rate was converted to a percentage (w/w) if needed using bulk density of the soil and depth of soil to which biochar was applied. If soil bulk density was not reported, it was estimated by the standard bulk density calculator based on soil texture (https://www.nrcs.usda.gov/wps/portal/nrcs/detail/soils). The biochar application rate was then categorized into four: < 1%, 1–3% inclusive, 3–5% inclusive and > 5%.
2.3 Data analysis
To assess the effect size of biochar application on soil enzymatic activities and microbial biomass, we used the natural log-transformed response ratio (ln RR: the ratio of treatment over control) as commonly used in other meta-analyses (Jian et al. 2016; Liu et al. 2016) because it improves statistical behaviors (Hedges et al. 1999). The ln RR was calculated as:
where \(\overline{\rm X}_{\text{t}} \;{\text{and}}\;\overline{\rm X}_{\text{c}}\) are the observed values of a selected variable (enzyme activities or microbial biomass C and N) under treatment (biochar application) and control, respectively. Estimation of effect size in meta-analysis largely depends on the weighting of the individual observation that can subsequently affect the inferences that can be made from a meta-analysis (Ma and Chen 2016). Various weighting functions have been used in previous meta-analyses (Jian et al. 2016; Ma and Chen 2016; Zhang et al. 2018a). The use of variance estimates in weighting functions are often unreliable because of large variances due to diverse site conditions and small sample sizes (common in many published studies we have considered in this meta-analysis). Following these previous studies (Ma and Chen 2016; Zhang et al. 2018b), we used the number of replications for the weighting function of observations as they found that this weighting function assigned less extreme weight and gave less weight to studies with multiple non-independent observations than any other weighting function. The weighting factor was calculated as:
where Wr is the weight associated with ln RR of observations of each variable, Nt and Nc are the number of replications in the treatment and control, respectively. The meta-analysis was conducted using maximum likelihood estimation with the lme4 package in R. We bootstrapped the estimates of weighted response ratio (ln RR′) to generate 95% confidence intervals (Liu et al. 2016) using the ‘confint ()’ function in the ‘boot’ package in R (Adams et al. 1997; Canty and Ripley 2012). The following equation was used to transform the log-transformed weighted response ratio back to the percentage change for ease of interpretation which is commonly used in other meta-analyses (Luo et al. 2006; Jian et al. 2016).
We consider the effect of biochar application on enzyme activities and microbial biomass to be significantly different from control if the 95% confidence interval of ln RR’ does not overlap with zero (Luo et al. 2006).
3 Results
3.1 Overall effects of biochar application on soil enzyme activities and microbial biomass
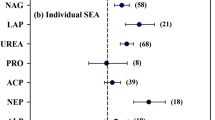
Biochar application significantly increased the activities of urease, alkaline phosphatase and dehydrogenase by 23.1%, 25.4% and 19.8%, respectively, as compared to the control, but did not affect the activities of other enzymes (Fig. 1; Table 2). Biochar application increased the activities of N-acq enzymes by 23.3% but did not affect the activities of C-acq and P-acq enzymes. Biochar application also significantly increased MBC by 21.7% but had no effect on MBN (Fig. 1; Table 2). Regression analyses showed that RR of MBC had significant linear relationships with RR of C-acq and N-acq enzymes in acidic soils but not in neutral and alkaline soils (P <0.05). In acidic soils, RR of MBC had a negative relationship (P =0.02) with RR of C-acq and a positive relationship (P <0.01) with N-acq enzymes (Fig. 2).
3.2 Effect of biochar on activities of urease, alkaline phosphatase, dehydrogenase and N-acq enzymes and MBC in different soils
The effects of biochar on soil enzyme activities were dependent on soil characteristics (Table 3). Urease activities were increased by biochar application by 33.3% and 31.2% in soils having TC less than 10 and between 10 and 20 g kg−1, respectively. Biochar also increased urease activities in soils having TN < 2 g kg−1 but not in soils having TN > 2 g kg−1 or TC > 20 g kg−1. The increase in urease activities by biochar application was significant in fine but not in coarse-textured soils. Similarly, biochar also increased dehydrogenase activities by 40% in neutral soils while there were no effects in acidic and alkaline soils (Table 3). The activities of alkaline phosphatase were dependent on soil pH, TC, TN and texture: an increase of 52% in acidic soils, but not in neutral and alkaline soils; increases of 25.7% and 36.6% in soils with TN < 10 and 10–20 g kg−1, respectively, and an increase of 67.4% in field experiments but not in lab incubation and greenhouse experiments (Table 3).
The magnitude of biochar’s effect on N-acq enzyme and MBC was also dependent on soil characteristics (Fig. 3). Biochar significantly increased the activities of N-acq enzyme by 26.6% in acidic and 27.3% in alkaline soils; by 34.8% in soils with TC < 10 g kg−1; 32.7% in soils with TN < 1 g kg−1; and 23.5% and 21% in field and greenhouse experiments, respectively (Fig. 3). The MBC was significantly increased by biochar in most cases in soils with different pH, TC, TN and texture: 16.6% and 38.7% in acidic and alkaline soils, respectively; 23.6% and 23.2% in soils with TC < 10 and 10–20 g kg−1, respectively; and 22.1% and 18.6% in coarse and fine-textured soils, respectively (Fig. 3).
Change in soil nitrogen acquisition (N-acq) enzyme activities and microbial biomass carbon (MBC) in biochar amended soils under different edaphic and experimental conditions. The bars represent 95% confidence intervals and the number besides each bar represents sample size with the number of studies noted in parentheses
3.3 Effect of biochar properties on activities of urease, alkaline phosphatase, dehydrogenase and N-acq enzymes and MBC
The effects of biochar on increasing activities of urease, alkaline phosphatase, dehydrogenase and N-acq enzymes and MBC were also dependent on pyrolysis temperature and feedstock type and the properties of biochar (such as pH and C/N ratio) associated with pyrolysis conditions and feedstock type used while the activities of other enzymes were not significantly affected by those factors (Table 4). Urease activities were increased by 25.9% by biochar produced at high pyrolysis temperature (> 550 °C), 23.9% by manure-based biochar and 33.5% by biochar with high pH (> 10) with no significant change in urease activities by any biochar application rates. But alkaline phosphatase activities were significantly increased by lower rates of biochar application (< 3%) (Table 4) and by the biochars produced from crop residues and wood. Dehydrogenase activities were increased by biochars produced at low pyrolysis temperature (< 350 °C) and biochars with low C/N ratio (< 50) but not affected by any biochar pH ranges, feedstock types and biochar application rates (Table 4). In greenhouse experiments, biochar application significantly increased dehydrogenase activities by 31.8%. Biochar significantly increased urease activities in short term studies while increased alkaline phosphatase activities only in long-term studies.
Significant positive changes in N-acq enzymes were observed with the application of manure-based biochars, biochars produced at medium pyrolysis temperatures (350–500 °C), biochars with high pH (> 10), and biochars applied at low rates (< 1%) (Fig. 4). Activities of N-acq enzymes were found to be significantly increased by biochars with lower C/N ratios (< 100), and by field and greenhouse experiments but not in lab incubation experiments. Similarly, MBC was significantly increased by biochars produced from crop residues and urban wastes, by biochars with high pH (< 8) and low C/N ratios (> 100), and by all biochar application rates except the rate of 3–5%. The MBC was found to be significantly increased in the field but not in lab incubation and greenhouse experiments (Fig. 4).
Change in soil nitrogen acquisition (N-acq) enzyme activities and microbial biomass carbon (MBC) in soils amended with biochars with different properties. The bars represent 95% confidence intervals and the number besides each bar representing sample size with the number of studies noted in parentheses
4 Discussion
We showed that microbial biomass C and activities of urease, alkaline phosphatase, dehydrogenase and the enzymes involved in N-acquiring activities were significantly increased by biochar application to the soil although the magnitude of those increases varied widely with soil properties, the characteristics of biochar associated with feedstock, pyrolysis temperature, biochar application rate and experiment type. This meta-analysis also showed that none of the individual enzymes we studied and C-, N- and P-acq enzymes activity were significantly reduced by biochar application although there are studies showing decreases in some of these enzyme activities in several experiments. The neutral and significantly positive effects of biochar application on soil microbial biomass and enzyme activities shown by this study along with the negative effects of biochar on CH4 and N2O emission (e.g., Jeffery et al. 2016; Borchard et al. 2018) suggest the crucial roles biochars can play in enhancing soil quality while mitigating global climate change.
4.1 Biochar application increases soil microbial biomass C and some extra- and intracellular enzyme activities
In this meta-analysis, biochar application was found to significantly increase microbial biomass C and activities of some extracellular enzymes including N cycling (urease), P cycling (alkaline phosphatase) and intracellular enzyme (dehydrogenase). Similar to the results of Zhang et al. (2019), N-acq enzymes activities were found to be significantly increased in this meta-analysis, however, P-acq enzymes activities were not significantly changed, the result is different from Zhang et al. (2019) where P-acq activities were shown to be significantly increased by 11% by biochar application. Probably the inclusion of more data points in our study (166) caused the disappearance of the effects of biochar on P-acq activities observed in Zhang et al. (2019) based on 76 data points.
The observed increase in these enzyme activities could be due to the increase in the availability of resources such as labile organic C (Kuzyakov et al. 2009) or the increase in reaction kinetics by improving soil matrix pH through addition of biochar (Van Zwieten et al. 2010; Gul et al. 2015). The increase in microbial and enzyme activities in the soil has also been referred to as the priming effect caused by biochar application to soil (Wardle et al. 2008; Zimmerman et al. 2011). Although the amount of labile C present in the biochar is generally much lower than the recalcitrant C present, the stimulation of short-term microbial growth and enzyme activities by addition of biochar to the soil have been reported in previous studies (Zimmerman et al. 2011; Farrell et al. 2013). The surfaces and pores of biochar provide habitat for microorganisms as well as increase the movement of air, water and nutrients within the soil matrix that can help promote microbial abundance and activities (Gul et al. 2015). The protection of soil microorganisms (bacteria and fungi) from grazers or competitors on biochar pores has also been pointed out for the increase in microbial biomass and the activities of enzymes secreted by these microorganisms (Theis and Rillig 2009). In addition, the increase in soil temperature by trapping heat due to biochar’s black color may speed up microbial growth and enzyme activities. However, further studies are warranted to assess the effect of biochar on increasing soil temperature that subsequently affected microbial and enzyme activities in the soil.
The increase in alkaline phosphatase activity in biochar-amended soils suggests that (i) microbial demand for P increased, (ii) P availability in soil for microbial growth became limiting, or (iii) a combination of both occurred (Nannipieri et al. 2002; Schimel and Weintraub 2003) in biochar-amended soils. Dehydrogenase activity that is considered to be a good indicator of metabolic activity was enhanced by the addition of labile organic C through biochar application (Serra-Wittling et al. 1995). The oxidative enzymes that mediate oxidation of phenolic compounds using oxygen were almost unchanged (although phenol oxidase tended to decrease slightly) by biochar application, suggesting that biochar does not play crucial roles in key ecosystem functions of lignin degradation, humification of aromatic ring-containing xenobiotic chemicals and dissolved organic C export (Sinsabaugh 2010). Phenol oxidase is primarily produced by fungi (Burke and Cairney 2002), the decreasing tendency of this enzyme activity can potentially be linked to the decrease in fungal biomass due to the increase in soil pH by biochar addition (Rousk et al. 2009).
Biochar addition shows contrasting effects on C- and N-acquiring enzyme activities in response to its effect on microbial biomass increase particularly in acidic soils although the effects were not significant in neutral and alkaline soils (Fig. 2). The decrease in RR_C-acq enzyme with an increase in RR_MBC indicates that the increase in MBC by biochar addition tends to decrease C-acq enzyme activities in the soil. Biochar addition increases labile C content in the soil that leads to an increase in microbial biomass. With an increase in easily available C source, microorganism allocate less energy to produce C-acq enzymes to reduce costs and maximize resource returns (Allison and Vitousek 2005). Since N contained in the biochar added to the soil is generally not easily available for microbial consumption, microorganisms have to produce more N-acq enzymes to meet the increasing microbial demand of N when external N added to the soil (such as through biochar addition) is not readily available (Moorhead and Sinsabaugh 2006). The increase in N-acq enzyme activities by biochar addition indicates that microorganisms in these soils are N-limited (Talbot and Treseder 2012) possibly caused by high C/N ratios of biochars that can lead to N immobilization in soil (Bengtsson et al. 2003).
4.2 Biochar-induced changes in soil MBC and enzyme activities vary with soil conditions
Similar to results in a previous meta-analysis (Zhang et al. 2019) and other published studies, this meta-analysis also shows that biochar-induced changes in soil MBC and enzyme activities vary widely with soil conditions. Biochar’s effect was more pronounced in soils with acidic pH than the soils with neutral and alkaline pH as demonstrated by the significant increase in MBC and N-acq in the acidic soils. Most of the biochars have alkaline pH, the addition of biochar thus may increase the pH of the soil by its liming effects (Clough et al. 2013; Gul et al. 2015; Nguyen et al. 2017), making the soil condition more favorable for microbial and enzymatic activities. The increase in N-acq enzyme might be linked to the decreased N availability to microorganisms by biochar addition because of the high metabolism of microorganisms due to the increased pH as limited N availability can stimulate enzyme production (Allison and Vitousek 2005). Since enzyme production is N and energy-intensive process, microorganisms produce enzymes at the expense of growth and metabolism of microorganisms at lower nutrient availability (Allison and Vitousek 2005). Contrary to this, the theory of stimulation of enzyme production by the addition of complex sources to mobilize nutrients from these sources (Sinsabaugh and Moorhead 1994) can also explain the reason for increased N-acq enzyme in biochar-amended soil. The increase in N-acq enzyme activities in acidic soil by biochar application has an important implication in maintaining soil health particularly in agricultural soils that are often severely degraded and acidified because of excessive use of inorganic fertilizer.
The significant increase in alkaline phosphatase (by 53%) but not in acid phosphatase activities by biochar application in acidic soil shows highly sensitive nature of alkaline phosphatase with pH change in biochar-amended soil. Acosta-Martinez and Tabatabai (2000) showed that alkaline phosphatase activities were increased by 97 times with the increase of a unit pH change resulting from liming in agricultural soil. On the other hand, dehydrogenase activity was significantly increased only in the soil having pH range between 6.5 and 7.5 with no significant effects on acidic and alkaline soils. Since dehydrogenase activity can be used as an indicator of metabolic activity in the soil (Moeskops et al. 2010), biochar was found to be ineffective to change the metabolic activity in acidic and alkaline soils. Although soil pH has been found to be the best predictor of dehydrogenase activity in different soils (Quilchano and Maranon 2002), the result of this meta-analysis suggests that dehydrogenase activities in biochar-amended soils are likely be affected more by factors other than the liming factor of biochar.
Another important soil factor that significantly affects MBC and enzymatic activities after biochar application is the native SOM. Biochar increased MBC and N-acq, urease and alkaline phosphatase activities in soils having relatively lower SOM. Although we were not able to assess the change in soil organic C and N by biochar application in this meta-analysis as only a few studies (we considered in this study) have reported it, we assume that the addition of biochar might have increased the soil organic C significantly (as shown in a meta-analysis study by Liu et al. 2016) that could increase microbial and enzyme activities in the soils where these activities were limited by low availability of substrate as in the case of soil with low SOM (Ameloot et al. 2015). Soil MBC and N-acq, urease, alkaline phosphatase and dehydrogenase activities were found to increase in greenhouse and field experiments but not in lab incubation. In lab incubation, effects of biochar are assessed in a controlled environment, but field experiments involve many environmental factors that are not under control such as soil moisture and temperature that can have significant effects on enzyme activities (Steinweg et al. 2012), the effect of increasing soil temperature and moisture by biochar addition might be the cause for the increase in these activities in field experiments.
4.3 Biochar-induced changes in soil MBC and enzyme activities vary with biochar properties
The overall response of biochar application on soil MBC and activities of most of the enzymes (we considered in this study) were positive, but the response differed in magnitude among C, N and P cycling enzymes as well as the biochar types. Biochar itself is a heterogeneous material (Czimczik et al. 2002; Downie et al. 2009; Keiluweit et al. 2010); the variations in biochar’s properties are induced by feedstock type and pyrolysis conditions (Kloss et al. 2012). The major variations occur in biochar pH, C/N ratio, surface area and porosity that can substantially change the microbial and enzymatic activities in biochar-amended soil. The multiple regression analysis (data not shown) showed that biochar’s pH and C/N ratio and pyrolysis temperature and application rate could explain only a part (3–42%) of the total variation in weighted response ratios of microbial and enzyme activity change in biochar-amended soils. This result suggests that other attributes such as surface area, porosity and labile C present in the biochar should also be considered to assess the effect of biochar application on microbial and enzyme activities in the soil. Among the biochar properties we studied in this meta-analysis, biochar’s pH has pronounced effect in changing MBC, N-acq and urease activities. Biochar with high pH (> 10) made a significant increase but other biochars did not. Increasing pyrolysis temperature often produces biochar with higher pH that might be useful in increasing N-releasing enzyme activity in the soil (Gul et al. 2015). The biochars produced at a temperature range of 350–550 °C showed a significant increase in these enzymes but not the biochar produced at lower pyrolysis temperature. The activities of dehydrogenase, however, was increased by biochar produced at low temperature (< 350 °C), biochars produced at low temperature can have significant amounts of volatile organic matter in the biochar that can stimulate dehydrogenase production to increase metabolic activity of the soil microorganisms for volatile organic matter decomposition (Moeskops et al. 2010).
Another key factor to significantly affect N-acq, urease and dehydrogenase is C/N ratio of biochar. Biochar with low C/N ratio had significant positive effects but not the biochars with high C/N ratio. Although C/N ratio of soil is negatively correlated with enzyme activities (Geisseler and Horwath 2009), addition of biochars (which have generally much higher C/N ratio than that of soil) did not cause significant negative impacts on enzyme activities. In biochar-amended soil, the effect of biochar addition may not be enough to have a substantial increase in soil’s C/N ratio for the significant negative impact on enzyme activities. Under feedstock type categories, manure-based biochars were found to increase urease, crop residue-based and wood-based biochars to increase alkaline phosphatase activities. One possible mechanism for the increase in these enzymes by adding biochars is the stimulation of corresponding enzyme production due to addition of organic N and P rich biochars to the soil as micro-organisms can produce more enzymes to mobilize mineral N and P from these added organic matters (Allison and Vitousek 2005).
5 Conclusions
Biochar application increased soil microbial biomass and activities of some of the enzymes we studied although the magnitude of increase in microbial biomass and those enzymatic activities differed widely with soil type and biochar property. Biochar application is not equally useful in increasing microbial biomass and enzymatic activities in the soil over a wide range of soil pH, SOC and soil texture, as this study shows that biochar can increase microbial biomass and enzymatic activities in soils with lower pH, TC and TN, and in fine-textured soils but not in neutral, alkaline or coarse-textured soils. Before biochar application, determining some of the key soil characteristics such as pH, SOC and texture is thus important to achieve the anticipated result of improving soil quality through increasing microbial biomass and stimulating enzymatic activities in biochar-amended soils. Similarly, due to availability of a wide range of feedstock types and pyrolysis conditions, biochars with diverse characteristics have been produced; optimizing biochar characteristics by selecting a particular feedstock and pyrolysis temperature can yield substantial benefit in improving soil quality, as this study shows that biochars with a higher pH, lower C/N ratio or produced at pyrolysis temperatures of 350–550 °C had greater effects on microbial biomass and enzymatic activities. The increase in N-acq and alkaline phosphatase activities by biochar application have important implications for agricultural soils that are extensively cultivated and have low crop productivity as such soils may have limited N and P availabilities.
Change history
20 April 2020
We regret that several typos/mistakes have slipped in our article.
References
Acosta-Martinez V, Tabatabai MA (2000) Enzyme activities in a limed agricultural soil. Biol Fertil Soils 31(1):85–91
Adams DC, Gurevitch J, Rosenberg MS (1997) Resampling tests for meta-analysis of ecological data. Ecology 78(4):1277–1283
Allison SD, Vitousek PM (2005) Responses of extracellular enzymes to simple and complex nutrient inputs. Soil Biol Biochem 37(5):937–944
Ameloot N, Sleutel S, Das KC, Kanagaratnam J, De Neve S (2015) Biochar amendment to soils with contrasting organic matter level: effects on N mineralization and biological soil properties. GCB Bioenergy 7(1):135–144
Araújo ASF, Cesarz S, Leite LFC, Borges CD, Tsai SM, Eisenhauer N (2013) Soil microbial properties and temporal stability in degraded and restored lands of Northeast Brazil. Soil Biol Biochem 66:175–181
Bailey VL, Fansler SJ, Smith JL, Bolton H Jr (2011) Reconciling apparent variability in effects of biochar amendment on soil enzyme activities by assay optimization. Soil Biol Biochem 43(2):296–301
Bamminger C, Marschner B, Jüschke E (2014) An incubation study on the stability and biological effects of pyrogenic and hydrothermal biochar in two soils. Eur Soil Sci 65(1):72–82
Benavente I, Gascó G, Plaza C, Paz-Ferreiro J, Méndez A (2018) Choice of pyrolysis parameters for urban wastes affects soil enzymes and plant germination in a Mediterranean soil. Sci Total Environ 634:1308–1314
Bengtsson G, Bengtson P, Månsson KF (2003) Gross nitrogen mineralization, immobilization, and nitrification rates as a function of soil C/N ratio and microbial activity. Soil Biol Biochem 35(1):143–154
Biederman LA, Harpole WS (2013) Biochar and its effects on plant productivity and nutrient cycling: a meta-analysis. GCB Bioenergy 5(2):202–214
Borchard N, Schirrmann M, Cayuela ML, Kammann C, Wrage-Mönnig N, Estavillo JM, Fuertes-Mendizábal T, Sigua G, Spokas K, Ippolito JA, Novak J (2018) Biochar, soil and land-use interactions that reduce nitrate leaching and N2O emissions: a meta-analysis. Sci Total Environ 651:2354–2364
BřEndová K, Tlustoš P, Száková J, Habart J (2012) Biochar properties from different materials of plant origin. Eur Chem Bull 1(12):535–539
Burke RM, Cairney JWG (2002) Laccases and other polyphenol oxidases in ecto-and ericoid mycorrhizal fungi. Mycorrhiza 12(3):105–116
Burns RG, DeForest JL, Marxsen J, Sinsabaugh RL, Stromberger ME, Wallenstein MD, Weintraub MN, Zoppini A (2013) Soil enzymes in a changing environment: current knowledge and future directions. Soil Biol Biochem 58:216–234
Canty A, Ripley B (2012) Package ‘boot’. http://cran.r-project.org/web/packages/boot/index.html
Chen J, Liu X, Zheng J, Zhang B, Lu H, Chi Z, Pan G, Li L, Zheng J, Zhang X, Wang J (2013) Biochar soil amendment increased bacterial but decreased fungal gene abundance with shifts in community structure in a slightly acid rice paddy from Southwest China. Appl Soil Ecol 71:33–44
Chen J, Li S, Liang C, Xu Q, Li Y, Qin H, Fuhrmann JJ (2017) Response of microbial community structure and function to short-term biochar amendment in an intensively managed bamboo (Phyllostachys praecox) plantation soil: effect of particle size and addition rate. Sci Total Environ 574:24–33
Chen J, Chen D, Xu Q, Fuhrmann JJ, Li L, Pan G, Li Y, Qin H, Liang C, Sun X (2019) Organic carbon quality, composition of main microbial groups, enzyme activities, and temperature sensitivity of soil respiration of an acid paddy soil treated with biochar. Biol Fertil Soils 55(2):185–197
Clough T, Condron L, Kammann C, Müller C (2013) A review of biochar and soil nitrogen dynamics. Agronomy 3(2):275–293
Crombie K, Mašek O, Cross A, Sohi S (2015) Biochar—synergies and trade-offs between soil enhancing properties and C sequestration potential. GCB Bioenergy 7(5):1161–1175
Czimczik CI, Preston CM, Schmidt MW, Werner RA, Schulze ED (2002) Effects of charring on mass, organic carbon, and stable carbon isotope composition of wood. Org Geochem 33(11):1207–1223
de Mora AP, Ortega-Calvo JJ, Cabrera F, Madejón E (2005) Changes in enzyme activities and microbial biomass after “in situ” remediation of a heavy metal-contaminated soil. Appl Soil Ecol 28(2):125–137
Downie A, Crosky A, Munroe P (2009) Physical properties of biochar. In: Lehmann J, Joseph S (eds) Biochar for environmental management: science and technology. Earthscan, London, pp 13–32
Farrell M, Kuhn TK, Macdonald LM, Maddern TM, Murphy DV, Hall PA, Singh BP, Baumann K, Krull ES, Baldock JA (2013) Microbial utilization of biochar-derived carbon. Sci Total Environ 465:288–297
Geisseler D, Horwath WR (2009) Relationship between carbon and nitrogen availability and extracellular enzyme activities in soil. Pedobiologia 53(1):87–98
Gul S, Whalen JK, Thomas BW, Sachdeva V, Deng H (2015) Physico-chemical properties and microbial responses in biochar-amended soils: mechanisms and future directions. Agric Ecosyst Environ 206:46–59
Hedges LV, Gurevitch J, Curtis PS (1999) The meta-analysis of response ratios in experimental ecology. Ecology 80(4):1150–1156
Jeffery S, Verheijen FG, Kammann C, Abalos D (2016) Biochar effects on methane emissions from soils: a meta-analysis. Soil Biol Biochem 101:251–258
Jian S, Li J, Chen J, Wang G, Mayes MA, Dzantor KE, Hui D, Luo Y (2016) Soil extracellular enzyme activities, soil carbon and nitrogen storage under nitrogen fertilization: a meta-analysis. Soil Biol Biochem 101:32–43
Keiluweit M, Nico PS, Johnson MG, Kleber M (2010) Dynamic molecular structure of plant biomass-derived black carbon (biochar). Environ Sci Technol 44(4):1247–1253
Khadem A, Raiesi F (2017) Influence of biochar on potential enzyme activities in two calcareous soils of contrasting texture. Geoderma 308:149–158
Kloss S, Zehetner F, Dellantonio A, Hamid R, Ottner F, Liedtke V, Schwanninger M, Gerzabek MH, Soja G (2012) Characterization of slow pyrolysis biochars: effects of feedstocks and pyrolysis temperature on biochar properties. J Environ Qual 41(4):990–1000
Kuzyakov Y, Subbotina I, Chen H, Bogomolova I, Xu X (2009) Black carbon decomposition and incorporation into soil microbial biomass estimated by 14C labeling. Soil Biol Biochem 41:210–219
Lee Y, Park J, Ryu C, Gang KS, Yang W, Park YK, Jung J, Hyun S (2013) Comparison of biochar properties from biomass residues produced by slow pyrolysis at 500 C. Bioresour Technol 148:196–201
Liu S, Zhang Y, Zong Y, Hu Z, Wu S, Zhou J, Jin Y, Zou J (2016) Response of soil carbon dioxide fluxes, soil organic carbon and microbial biomass carbon to biochar amendment: a meta-analysis. GCB Bioenergy 8(2):392–406
Lu W, Ding W, Zhang J, Li Y, Luo J, Bolan N, Xie Z (2014) Biochar suppressed the decomposition of organic carbon in a cultivated sandy loam soil: a negative priming effect. Soil Biol Biochem 76:12–21
Luo Y, Hui D, Zhang D (2006) Elevated CO2 stimulates net accumulations of carbon and nitrogen in land ecosystems: a meta-analysis. Ecology 87(1):53–63
Ma Z, Chen HY (2016) Effects of species diversity on fine root productivity in diverse ecosystems: a global meta-analysis. Glob Ecol Biogeogr 25(11):1387–1396
Moeskops B, Buchan D, Sleutel S, Herawaty L, Husen E, Saraswati R, Setyorini D, De Neve S (2010) Soil microbial communities and activities under intensive organic and conventional vegetable farming in West Java, Indonesia. Appl Soil Ecol 45(2):112–120
Moorhead DL, Sinsabaugh RL (2006) A theoretical model of litter decay and microbial interaction. Ecol Monogr 76(2):151–174
Nannipieri P, Kandeler E, Ruggiero P (2002) Enzyme activities and microbiological and biochemical processes in soil. In: Burns RG, Dick RP (eds) Enzymes in the environment: activity, ecology and applications. Marcel Dekker, Inc., New York, pp 1–33
Nguyen TTN, Xu CY, Tahmasbian I, Che R, Xu Z, Zhou X, Wallace HM, Bai SH (2017) Effects of biochar on soil available inorganic nitrogen: a review and meta-analysis. Geoderma 288:79–96
Novak JM, Cantrell KB, Watts DW (2013) Compositional and thermal evaluation of lignocellulosic and poultry litter chars via high and low temperature pyrolysis. BioEnergy Res 6(1):114–130
Ouyang L, Tang Q, Yu L, Zhang R (2014) Effects of amendment of different biochars on soil enzyme activities related to carbon mineralization. Soil Res 52(7):706–716
Parham JA, Deng SP (2000) Detection, quantification and characterization of β-glucosaminidase activity in soil. Soil Biol Biochem 32(8–9):1183–1190
Paz-Ferreiro J, Gascó G, Gutiérrez B, Méndez A (2012) Soil biochemical activities and the geometric mean of enzyme activities after application of sewage sludge and sewage sludge biochar to soil. Biol Fertil Soils 48(5):511–517
Pukalchik M, Mercl F, Terekhova V, Tlustoš P (2018) Biochar, wood ash and humic substances mitigating trace elements stress in contaminated sandy loam soil: evidence from an integrative approach. Chemosphere 203:228–238
Purakayastha TJ, Kumari S, Pathak H (2015) Characterization, stability, and microbial effects of four biochars produced from crop residues. Geoderma 239:293–303
Quilchano C, Marañón T (2002) Dehydrogenase activity in Mediterranean forest soils. Biol Fertil Soils 35(2):102–107
Rousk J, Brookes PC, Bååth E (2009) Contrasting soil pH effects on fungal and bacterial growth suggest functional redundancy in carbon mineralization. Appl Environ Microbiol 75(6):1589–1596
Schimel JP, Weintraub MN (2003) The implications of exoenzyme activity on microbial carbon and nitrogen limitation in soil: a theoretical model. Soil Biol Biochem 35(4):549–563
Serra-Wittling C, Houot S, Barriuso E (1995) Soil enzymatic response to addition of municipal solid-waste compost. Biol Fertil Soils 20(4):226–236
Singh B, Singh BP, Cowie AL (2010) Characterization and evaluation of biochars for their application as a soil amendment. Soil Res 48(7):516–525
Sinsabaugh RL (1994) Enzymic analysis of microbial pattern and process. Biol Fertil Soils 17(1):69–74
Sinsabaugh RL (2010) Phenol oxidase, peroxidase and organic matter dynamics of soil. Soil Biol Biochem 42(3):391–404
Sinsabaugh RL, Moorhead DL (1994) Resource allocation to extracellular enzyme production: a model for nitrogen and phosphorus control of litter decomposition. Soil Biol Biochem 26(10):1305–1311
Sohi SP, Krull E, Lopez-Capel E, Bol R (2010) A review of biochar and its use and function in soil. Adv Agron 105:47–82
Song D, Xi X, Huang S, Liang G, Sun J, Zhou W, Wang X (2016) Short-term responses of soil respiration and C-cycle enzyme activities to additions of biochar and urea in a calcareous soil. PLoS ONE 11(9):e0161694
Song D, Tang J, Xi X, Zhang S, Liang G, Zhou W, Wang X (2018) Responses of soil nutrients and microbial activities to additions of maize straw biochar and chemical fertilization in a calcareous soil. Eur J Soil Biol 84:1–10
Spokas KA, Reicosky DC (2009) Impacts of sixteen different biochars on soil greenhouse gas production. Ann Environ Sci 3:179–193
Steinweg JM, Dukes JS, Wallenstein MD (2012) Modeling the effects of temperature and moisture on soil enzyme activity: linking laboratory assays to continuous field data. Soil Biol Biochem 55:85–92
Talbot JM, Treseder KK (2012) Interactions among lignin, cellulose, and nitrogen drive litter chemistry–decay relationships. Ecology 93(2):345–354
Thavamani P, Malik S, Beer M, Megharaj M, Naidu R (2012) Microbial activity and diversity in long-term mixed contaminated soils with respect to polyaromatic hydrocarbons and heavy metals. J Environ Manag 99:10–17
Thies JE, Rillig MC (2009) Characteristics of biochar: biological properties. In: Lehmann J, Joseph S (eds) Biochar for environmental management: science and technology. Earthscan, London, pp 85–105
van Zwieten L, Kimber S, Morris S, Chan KY, Downie A, Rust J, Joseph S, Cowie A (2010) Effects of biochar from slow pyrolysis of papermill waste on agronomic performance and soil fertility. Plant Soil 327(1–2):235–246
Verheijen F, Jeffery S, Bastos AC, Van der Velde M, Diafas I (2010) Biochar application to soils. A critical scientific review of effects on soil properties, processes, and functions. In: EUR 24099 EN, Office for the Official Publications of the European Communities, Luxembourg, p 149
Wang X, Zhou W, Liang G, Song D, Zhang X (2015) Characteristics of maize biochar with different pyrolysis temperatures and its effects on organic carbon, nitrogen and enzymatic activities after addition to fluvo-aquic soil. Sci Total Environ 538:137–144
Wang D, Fonte SJ, Parikh SJ, Six J, Scow KM (2017) Biochar additions can enhance soil structure and the physical stabilization of C in aggregates. Geoderma 303:110–117
Wardle DA, Nilsson MC, Zackrisson O (2008) Fire-derived charcoal causes loss of forest humus. Science 320(5876):629
Woolf D, Amonette JE, Street-Perrott FA, Lehmann J, Joseph S (2010) Sustainable biochar to mitigate global climate change. Nat Commun 1:56
Wu D, Senbayram M, Zang H, Ugurlar F, Aydemir S, Brüggemann N, Kuzyakov Y, Bol R, Blagodatskaya E (2018) Effect of biochar origin and soil pH on greenhouse gas emissions from sandy and clay soils. Appl Soil Ecol 129:121–127
Xie T, Sadasivam BY, Reddy KR, Wang C, Spokas K (2015) Review of the effects of biochar amendment on soil properties and carbon sequestration. J Hazard Toxic Radioact Waste 20(1):04015013
Yoo G, Kang H (2012) Effects of biochar addition on greenhouse gas emissions and microbial responses in a short-term laboratory experiment. J Environ Qual 41(4):1193–1202
Zhang QZ, Dijkstra FA, Liu XR, Wang YD, Huang J, Lu N (2014) Effects of biochar on soil microbial biomass after four years of consecutive application in the north China plain. PLoS ONE 9(7):e102062
Zhang L, Jing Y, Xiang Y, Zhang R, Lu H (2018a) Responses of soil microbial community structure changes and activities to biochar addition: a meta-analysis. Sci Total Environ 643:926–935
Zhang T, Chen HY, Ruan H (2018b) Global negative effects of nitrogen deposition on soil microbes. ISME J 12(7):1817–1825
Zhang L, Xiang Y, Jing Y, Zhang R (2019) Biochar amendment effects on the activities of soil carbon, nitrogen, and phosphorus hydrolytic enzymes: a meta-analysis. Environ Sci Pollut Res 26:22990–23001
Zheng J, Chen J, Pan G, Liu X, Zhang X, Li L, Bian R, Cheng K, Jinwei Z (2016) Biochar decreased microbial metabolic quotient and shifted community composition four years after a single incorporation in a slightly acid rice paddy from southwest China. Sci Total Environ 571:206–217
Zimmerman AR, Gao B, Ahn MY (2011) Positive and negative carbon mineralization priming effects among a variety of biochar-amended soils. Soil Biol Biochem 43(6):1169–1179
Acknowledgements
We are grateful to all the authors of papers whose primary data have been used in this study. Financial support for this work was provided by the National Science and Engineering Research Council of Canada (NSERC) and the Western Grains Research Foundation, Canada as scholarships to the first author. Partial funding was also provided by the Agricultural Greenhouse Gas Program (AGGP) of Agriculture and Agri-Food Canada. We would like to thank Dr Jin-Hyeob Kwak for his help in preparing the figures. We would also like to thank the comments and suggestions of four anonymous reviewers that were very helpful in improving the overall quality of an earlier version of this paper.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Pokharel, P., Ma, Z. & Chang, S.X. Biochar increases soil microbial biomass with changes in extra- and intracellular enzyme activities: a global meta-analysis. Biochar 2, 65–79 (2020). https://doi.org/10.1007/s42773-020-00039-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42773-020-00039-1