Abstract
Background
Soil amendment with biochar can increase biological nitrogen fixation (BNF) and improve growing conditions for legumes, while such effects may alter when biochar properties change with time (biochar aging).
Methods
We examined BNF and competitiveness of legumes in a mixed pasture (dominated by clover, Trifolium repens) after 26 months of biochar amendment (field aged biochar) and in a pot study with grass (Lolium perenne) and clover mixture using fresh and chemically aged biochars.
Results
In both studies, the fraction of N derived from symbiotic fixation (%Ndfa) was not affected by aged biochar treatments, while it reduced from 73% in the control to 68% with fresh biochar addition in the pot study. However, this reduction in %Ndfa was compensated with greater biomass production resulting in the largest amount of N fixed with fresh biochar application. The amount of N fixed was reduced by field aged biochar, although it was not affected by chemically aged biochars. Moreover, in the field study grasses became more competitive than legumes with biochar, while such an advantage by grasses was not observed in the pot study.
Conclusions
Aging of biochar can reduce BNF and competitiveness of legumes in mixed pastures with significant implications for pasture management.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Biological nitrogen fixation (BNF) is a vital ecosystem service contributing an estimated global annual N input of 17.2 × 107 t with a contribution of 1.2× 107 to 2.5× 107 t N from pastures and fodder legume production alone (Ishizuka 1992; Herridge et al. 2008). Therefore, BNF is an important source of reactive N in low input legume pastures, maintaining soil N and improving fodder quality (Unkovich 2012). In Australia, animal production is the main agricultural sector with more than 90% of agricultural lands under grazing (ABS 2016). However, legume derived N-input rates in Australian pastures are relatively low (<50 kg N ha−1 yr.−1) compared to rates of up to 682 N ha−1 yr.−1 reported elsewhere (Ledgard and Steele 1992; Unkovich 2012). The main limiting factor is the low abundance of legumes while other factors include acidic soil pH, reduced inoculation rate and water stress (Unkovich 2012). In mixed pastures, the competition between species for resources including nutrients, water, and light, as well as the capacity to form symbiotic relationships with N-fixing bacteria determine species composition, persistence, abundance and productivity (Ledgard and Steele 1992; Lucero et al. 1999; Lambers et al. 2004; Van de Voorde et al. 2014). Legume growth is advantageous in soils with low N by promoting symbiotic N fixation (Ledgard and Steele 1992; Lambers et al. 2004) while grasses may benefit in high N soils by taking up N more efficiently with their fibrous root systems (Lambers et al. 2004; Ledgard and Steele 1992; Høgh-Jensen and Schjoerring 1997). Management practices such as reseeding of legume species and liming are performed to improve the balance of species composition and resource allocation between species for the optimal productivity of pasture systems (Unkovich 2012).
Soil amendment with biochar, pyrolized organic matter rich in carbon (C), has also shown to increase BNF, i.e., both the fraction of N derived from fixation (%Ndfa) and amount of N fixed in several legumes under diverse environmental conditions (Mia et al. 2014; Quilliam et al. 2013; Rondon et al. 2007; Van de Voorde et al. 2014). Fresh biochar amendment has been shown to stimulate nodulation (Ogawa and Okimori 2010; Tagoe et al. 2008, 2010; Mete et al. 2015) and improve legumes performance in mixed pastures due to an increase in total N fixation and improved nutritional conditions (Van de Voorde et al. 2014). Therefore, soil amendment with biochar could promote pasture productivity and quality with a greater share of legumes while storing more C in the soil in the long term.
Biochar, produced through pyrolysis, is a broad term denoting a range of materials from partly charred materials to highly condensed graphite like pyrogenic C with diverse properties and inorganic constituents including nutrients (Singh et al. 2010, 2014; Enders and Lehmann 2017; Enders et al. 2017). As a consequence, its effects on crop productivity is largely inconsistent and determined by the interactions among biochar properties, its inorganic constituents and soil components (Joseph et al. 2010; Jeffery et al. 2011). In soil, biochar properties change with time (hereafter referred to as biochar aging) (Mia et al. 2017a), and include- (a) depletion of the labile C fraction, volatile organic C and nutrients, (b) blocking part of the biochar’s surface area by soil components such as organic matter, minerals etc., and (c) change in biochar’s structure and functionality, i.e., an increase of cation exchange capacity (CEC) due to formation of carboxylic and phenolic groups (Cao et al. 2017; Cheng et al. 2008; Cheng and Lehmann 2009; Mia et al. 2017b, c). As a result, aging can cause a temporal change in soil pH, nutrient retention and availability (Wang et al. 2015; Cao et al. 2017; Mia et al. 2017b). Therefore, the initial enhancement of BNF due to an increase in pH, biochar’s nutrient supply or nutrient availability (Güereña et al. 2015; Nishio and Okano 1991; Rondon et al. 2007; Tagoe et al. 2010) may change or dissipate during aging. Further, BNF may be negatively affected by the greater retention of NH4+-N at cation exchange sites of aged biochar (Wang et al. 2015; Mia et al. 2017b). For similar reasons, competitiveness of legumes may reduce when biochar is aged.
Natural aging of biochar in the soil is slow making it difficult to examine long-term aging effects of biochar (Kuzyakov et al. 2014; Wang et al. 2016). Thus, chemical oxidation of biochar has been used as a proxy for natural aging to understand long-term aging effect (Lawrinenko et al. 2016; Mia et al. 2017a, b). Chemical oxidization with H2O2 could cause somewhat similar changes in biochar properties to that of natural aging (Lawrinenko et al. 2016; Mia et al. 2017b). Therefore, aging of biochar with H2O2 can be used a proxy for field aged biochar to investigate long-term aging effects of biochar on BNF.
Understanding the biochar effects on BNF and competitiveness of legumes at different levels of aging (short and long term) is important in systems where biochar amendments are used. However, as far as we know, there has been no studies to investigate the effects of fresh and aged biochars on BNF and competitiveness of legumes. To examine long and short-term biochar aging effects on BNF and legume performance in mixed pasture systems, we formulated the following hypotheses:
-
H1: Compared to the control treatment (no biochar), fresh biochar amendment will increase BNF, while aged biochar will reduce BNF, particularly at higher levels of aging (greater chemical oxidation);
-
H2: Compared to the control treatment, application of fresh biochar will be more advantageous to legumes than grass, while both chemically and field aged biochars will reduce the relative advantage of legume growth, particularly at higher levels of aging.
To test these hypotheses, we examined the biochar effects on BNF and competitiveness of legumes in a grassland after 26 months of biochar amendment, and in a grass-clover (Lolium perenne and Trifolium repens) mixture in a pot study using fresh and chemically aged biochar (oxidation with 5 and 15% H2O2).
Materials and methods
Biochar field study
A biochar field experiment was established at the Lansdowne Farm, Cobbitty (34.023314 °S, 150.663314 °E), The University of Sydney in January, 2013. Details of the experiment can be found elsewhere (Keith et al. 2016; Mia et al. 2017c). In brief, an eucalyptus- wood derived biochar (550 °C) was applied to two different soil types, i.e., Dermosol and Tenosol (Australian Soil Classification, Isbell 2002), equivalent to Arenosol and Cambisol, respectively according to the world refernce base soil classification. The biochar was applied at two different rates (10 and 20 t ha−1) with a control (no biochar addition). Fresh biochar had a pH of 8.98 and a CEC of 50 cmolc kg−1. The C:N ratio was ~51 (Table 1). The Dermosol was a sandy clay loam (sand: 51.5%, silt: 19.7% and clay: 28.8%) and nearly neutral in reaction (pH = 6.81) (Mia et al. 2017c). A grassland was established with a mixture of grasses (Phalaris aquatica L., Fescuta arundinacea Schreb., Bromus wildenowii Kunth) and legumes (Medicago sativa L., Medicago polymorpha L., Trifolium subterraneum ssp. brachycalycinum Katzn. and Morley, Trifolium subterraneum ssp. subterraneum L., Trifolium vesiculosum L., Trifolium repens L., Trifolium fragiferum L., Trifolium spumosum L.) with a seeding ratio of 60% grasses and 40% legumes (by seed weight). Half of the treatments received 50% while the rest received 100% of the recommended rate of fertilizers (Keith et al. 2016). This grassland was managed by mowing and removing aboveground biomass. After 26 months of biochar application, we used two treatments from this experiment, i.e., (a) biochar applied at 20 t ha−1 and (b) without biochar in the Dermosol, all receiving 50% fertilization. Fertilizers were applied in two instalments; the first instalment after 4 months of sowing with 50 kg ha−1 of Granulock containing 14.3% N, 12% P (phosphorus), 10.5% S (sulfur) and 27.5 kg ha−1 of urea (46% N), and a second instalment after 1 year of sowing with 75 kg ha−1 SuPerfect containing 8.8% P, 11% S and 19.1% Ca (calcium). All treatments were replicated four times. We refer this biochar to as field-aged biochar (FABC) assuming that biochar was aged substantially during its 26 months of field exposure. The climatic data during the growth period are presented in Fig. S1. On March 5, 2015 the aboveground biomass was harvested from a total area of 0.5 m2 using a quadrate (0.5 m × 0.5 m) from two random locations within each plot of 9.88 m−2. This biomass harvest occurred 110 days after the last mowing event. The aboveground biomass was separated into grass and legume biomass. Soil samples from 0 to 15 cm were also collected at the same time.
Pot study
Biochar used in the pot study was also produced at 550 °C from eucalyptus wood under more controlled conditions (oxygen limitation and temperature control) than the field study biochar, and thus the two biochars had different properties (Table 1). Biochar was oxidized or aged with 5% and 15% H2O2 (1:30, m/v) for 6 h at 80 °C in a water bath and excess H2O2 was evaporated at 120 °C. The aged biochars were then purified with thorough washing (until biochar had an electrical conductivity (EC) of ~5 μS cm−1) after adjustment of pH to that of fresh biochar (6.20). Ash of the fresh biochar was also removed with washing up to an EC of <5 μS cm−1. The aging and characterization of biochars are discussed elsewhere (Mia et al. 2017b). The CEC of fresh biochar (FBC), 5% oxidized biochar (5%OBC) and 15% oxidized biochar (15%OBC) were 24, 76 and 99 cmolc kg−1, respectively (Table 1). The C:N ratios were high, between 598 and 768.
The pot experiment consisted of four treatments, i.e., application of 20 t ha−1 (11.4 g kg−1 soil) of FBC, 5%OBC, 15%OBC and a control (C, no biochar addition) and each treatment was replicated 16 times. The biochar was mixed with the Dermosol, collected from the field experimental site at a depth of 0–20 cm. Each pot made of polyvinyl chloride pipe (6.50 cm × 20 cm, diameter and length) was filled with 597 g of soil-biochar mixture (bulk density of 1.2 g cm−3). Pots were placed in the controlled environment facility at the Centre for Carbon, Water and Food, The University of Sydney. A mixed pasture was established with ryegrass -Lolium perenne and white clover -Trifolium repens, which was also the dominant legume species in the field study. Seeds were inoculated with Bradyrhizobium sp. After germination, five grass and five clover plants were kept in each pot. Soil water was maintained at 60% water holding capacity by regular watering the pots to compensate for the weight loss due to evapotranspiration. The pots received an initial supply of nutrients to ensure that plants were not limited by nutrients (Table S1). Plants were grown in 25 °C/15 °C (day/night) temperature under 1000 W metal halide lamp for 12 h at 60% relative humidity. After 42 days of growth, the plants were harvested at the soil surface. Soil samples were also collected from the top 10 cm (while the remainder of the pots were kept for another experiment not discussed here).
Soil and plant analysis
Soils collected both from the field and pot study were extracted with 1 M KCl (1:5, w/v) and inorganic N (NH4+, NO3−) was measured on a flow injection analyser (QuickChem FIA+, Lachat Instruments, Loveland, CO, USA) (Mia et al. 2017c). Soil available P was measured using the ammonium paramolybdate/stannous chloride colorimetric method after extraction with 0.03 M NH4F - 0.025 M HCl (1:10, w/v) (Bray and Kurtz, 1945; Mia et al. 2017b). Soil pH in water (1:5, w/v) was also determined. The harvested plant samples were dried at 60 °C for 72 h and weighed. The dried samples were ground with a ball mill (Retsch MM 400, Haan, Germany) for 6 min and packed into tin foil to determine total C, total N and δ15N using an isotope ratio mass spectrophotometer (Thermo Fisher Scientific, Bremen, Germany). Additionally, plant samples were analysed in four replicates (after combining 16 to four replicates for the pot study) for macronutrients- P, potassium (K), Ca, magnesium (Mg), sodium (Na) and micronutrients- Iron (Fe), copper (Cu), cobalt (Co), boron (B), manganese (Mn), molybdenum (Mo) and zinc (Zn). We also determined aluminium (Al) concentration assuming that it would be an important element affecting plant performance since the soil is rich in Fe and Al bearing minerals. An amount of 0.5 g of each sample was digested with 10 ml of 70% HNO3 using a microwave digester (MARS 6, Thermo Fisher Scientific, North Carolina, USA). Nutrient concentrations were then measured using a MP-AES (Agilent 4200, California, USA).
BNF calculation
The %Ndfa in the Dermosol of the field and pot study was calculated using the following equation:
where, δ15Nref is the δ15N value of reference plants (grass); δ15Nleg is the δ15N value of the legume plants; B is the δ15N signature of N-fixing plants completely relying on BNF. For the B value, we collected values used in the literature for white clover (either determined in N-minus medium or the lowest value within the legumes examined) and fitted a normal distribution to the data (n = 57) (West et al. 2005). We then estimated B as the average value with the highest probability density (−1.527‰, Fig. S2, see SI for details). There may have been transfer of fixed N from the legumes to the grasses in the mixed pasture of the field experiment. We acknowledge that this may have affected the δ15N of the reference plants and therefore our calculation of %Ndfa, but we assume that this effect was small considering that aboveground biomass was removed every 2 months through mowing and both soil and grass δ15N values did not differ between the two biochar treatments (also see SI for details).
The amount of N fixed (Nfix, g N m−2) was calculated using the following equation:
where Biom leg is the legume biomass production (g m−2) and N leg is the N concentration in legume shoot biomass (g/100 g).
Statistical analysis
We used one-way analysis of variance (ANOVA) to test for biochar addition effects on plant and soil parameters in the field and pot experiments using the statistical software JMP pro 11 (SAS, USA). Model assumptions of normality and homogeneity of variance were checked and data were log transformed if the assumptions were not met. The means in the pot study were separated using Tukey’s HSD, α = 5%. We used principal component analysis (PCA) to examine inter-relationships between soil properties and nutrient concentrations in aboveground biomass, BNF (for legumes only) and biomass production of legumes and grasses. We then used ANOVA on the first and second principal components to examine whether biochar addition and aging had significant effects on these combined plant and soil parameters.
Results
Biochar and soil properties
After field aging, the biochar treatment did not significantly affect the soil pH, inorganic N and available P (Table 2). However, the overall P status of the soil (including control) was high (>190 mg available P kg−1 soil). In contrast to the field study, the biochar treatment significantly affected NH4+-N and NO3−-N concentrations in the pot study (p < 0.001). The highest NH4+-N concentration was in the 15%OBC treatment while NO3−-N concentration was highest in the FBC treatment. In the pot study, we did not observe a significant effect on available P with the different biochar amendments.
Aboveground biomass production
The aboveground biomass of legumes and grasses in the field study was significantly affected by the FABC treatment causing a clearly opposite effect (p = 0.007 and p = 0.010, respectively, Fig. 1a). Legume biomass reduced by 36% while grass biomass increased by 47% with FABC, although the total biomass production was not affected (p = 0.15). Therefore, the legume to grass biomass ratio reduced from 0.94 to 0.39 with the FABC treatment (p = 0.02). In the pot study, the aboveground legume biomass was significantly affected (p = 0.001) while grass biomass production did not change (p = 0.98) with biochar amendments. The FBC treatment yielded the maximum legume biomass production (301 g m−2), which was 19% greater than in the control treatment. However, the aboveground legume biomass production in the treatments with chemically aged biochar was statistically similar to the control (Fig. 1b). In the pot study, biochar treatment had a significant effect on total biomass production (p = 0.003) and the highest biomass production (532 g m−2) was in the FBC treatment. The relative abundance of legume compared to grass was significantly reduced when biochar was aged (p = 0.023), i.e., the legume to grass ratio reduced from 1.46 in the FBC treatment to 1.05 in the 15%OBC treatment (Fig. 1b).
Aboveground biomass production in the (a) field study and (b) pot study. The bottom section (unfilled or different shades of grey) bars represent legume biomass while the top section bars (with diagonal lines in white or different shades of grey) represent the grass biomass, and the whole vertical bar thus represents the total biomass. The diamond symbol with error bars (units given on the right hand side in the two graphs) denotes legume to grass ratio. FABC, FBC, 5%OBC, 15%OBC and control represent, field aged biochar, fresh biochar, biochar aged (oxidized) with 5% and 15% H2O2 and no biochar addition, respectively. Error bars in the boxes represent standard error of the means (n = 4 and 16 for the field and pot study, respectively). Different letters above bars/diamonds indicate significant differences (p < 0.05)
Biological nitrogen fixation
The %Ndfa did not vary with FABC treatment in the field study (p = 0.53; Fig. 2a). However, the FABC treatment significantly reduced the total amount of N fixed, by ~45% (Fig. 2a). By contrast, the biochar aging treatment in the pot study had a significant effect on %Ndfa (p = 0.019; Fig. 2b) and the amount of N fixed (p = 0.016). Soil amendment with FBC caused slight but a significant reduction in %Ndfa (from 73% in the control treatment to 68% in the FBC) in the pot study, while this negative effect disappeared with biochar aging. The amount of N fixed was significantly lower (by 20%) in the 15%OBC compared to FBC treatment, and was also lower than in the control treatment, although not significantly (Fig. 2b). However, both %Ndfa and N fixed were similar in the 5%OBC and 15%OBC treatment of the pot study.
Biological nitrogen fixation in the (a) field study and (b) pot study. The bar plots represent the proportion of legume biomass N derived from fixation (%Ndfa) while diamond symbols with error bars represent total amount of N fixation (y-axis in the right). For explanation of treatment abbreviations, see Fig. 1. Error bars represent standard error of the means (n = 4 and 16, respectively for the field and pot study). Different letters above bars indicate significant differences (p < 0.05)
Nutrient concentrations in plants
Nutrient concentrations in the grasses of the field study were not affected by the FABC treatment except for Co (p = 0.005) and Al (p = 0.035), which was significantly lower in the FABC treatment than in the control (Table 3). However, none of the nutrient concentrations in the legumes of the field study were significantly affected by the FABC treatment. The Fe concentration in the legumes of the field study was surprisingly high (~1500 μg g−1, five-fold greater than in the pot study) although it was not affected by FABC. In the pot study, Ca and Na concentrations in the grass were significantly affected by the biochar treatments (p = 0.038 and <0.001, respectively) while all other nutrient concentrations were not affected. The lowest Ca concentration was in the 15%OBC treatment while all the other treatments were statistically similar. In contrast to Ca, the Na concentration was highest in the 15%OBC treatment. The nutrient concentrations in legumes of the pot study were not affected by the biochar treatment except for B and Na (p = 0.042 and <0.001, respectively). The Na concentrations in legumes increased in the aged biochar treatments while the B concentration decreased in the same treatment.
Principal component analysis
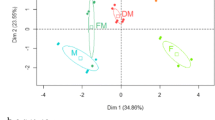
The bi-plot of first two principal components (PCs) and loading of variables in the field and pot study are presented in Fig. 3. The first and second PC explained 37.3 and 20.1% of the variation in legumes of the field study and 26.1 and 20.3% of the variation in legumes of the pot study. For grasses, the variation explained with the first two PCs were 28.9 and 27.5%, and 34.5 and 19.5% in the field and pot study, respectively. The plots treated with biochar were not separated from the control with the first two components for grasses and legumes in the field study. However, in the PCA for legumes in the pot study, the average position of the FBC treatment (2.43) was significantly higher (p < 0.001) than that of 5%OBC (−0.26) and 15%OBC (−3.25) treatments along the first PC. Separation of treatments along the first PC was largely caused by positive loadings of soil NO3−-N concentrations, legume biomass, total N fixed and B concentrations and negative loadings of soil NH4+-N and Na concentration in legumes (Fig. 3c). For the grass PCA, the average position of the 15%OBC treatment (2.05) was significantly higher than for the FBC (−1.16) and control treatment (−1.58) along the second component of the PCA. Separation along the second component for grass was largely caused by positive loadings of soil NH4+-N and Fe, Al, Mn, Mo, and Na concentrations and negative loadings of soil NO3− and P concentration in grass biomass (Fig. 3d).
Bi-plot of PCA analysis showing the first two principal components (PCA1 and PCA2). For PCA analysis, data included were soil properties, plant parameters, and nutrient concentrations in (a) legumes and (b) grasses in the field study and in (c) legume and (d) grass in the pot study. The circles, triangles, diamonds and squares in panel c and d represent control, fresh biochar, 5% oxidized biochar and 15% oxidized biochar treatments in the pot study, respectively, while triangles and diamonds in panel a and b represent control and field aged biochar treatments in the field study
Discussion
Biochar aging effects on BNF
Biochar effects on BNF (%Ndfa and total N fixed) in the field and pot studies were somewhat similar. In the pot study, we observed the largest amount of N fixed in the fresh biochar (FBC) treatment despite having the lowest %Ndfa, while the total N fixed in the chemically aged biochar treatments were similar or somewhat lower than in the control without significant changes in %Ndfa. In the field study, we also observed a significant reduction in total N fixed in the FABC treatment compared to the control without significant changes in %Ndfa (Fig. 2). In terms of total N fixed, these results partly support our first hypothesis (H1): fresh biochar addition increased (although not significantly) while field (but not chemically) aged biochar decreased BNF compared to the control. However, unexpectedly, fresh biochar reduced BNF per unit plant biomass (%Ndfa) in the pot study and there were no effects of natural and chemical aging on %Ndfa. Increases in total N fixed and %Ndfa have been reported with fresh biochar amendment by several investigators (Mia et al. 2014; Rondon et al. 2007; Van de Voorde et al. 2014), while effects of biochar aging on BNF have not been reported before.
Biochar mediated changes in nutrient supply may have affected the reduced %Ndfa observed in the fresh biochar treatment in the pot study. Soil N supply is one of the primary nutrients, that determines whether legumes would rely on soil N or acquire N through fixation (Ledgard and Steele 1992; Lambers et al. 2004). The reduction in %Ndfa in the FBC treatment was possibly caused by greater soil NO3− supply (Macduff et al. 1996), as the FBC treatment showed the highest soil NO3− concentration (Table 2) and showed PC1 and PC2 loadings that were opposite to loadings of %Ndfa (Fig. 3c). It is also possible that the greater N uptake may enrich the clover 15N signature resulting in a decrease of %Ndfa. A fresh biochar mediated increase in B availability has also shown to stimulate %Ndfa (Rondon et al. 2007). Although the B concentration (56 μg g−1) in the FBC treatment was similar to the control, the negative relationship with %Ndfa when B concentrations exceed 45 μg g−1 in legume biomass (Fig. S3) suggest an inhibitory effect on %Ndfa at relatively high B concentrations (Arora 2005; Linse et al. 2015). A fresh biochar mediated increase in P, K, Mg and Mo concentrations can also increase %Ndfa (Mia et al. 2014; Rondon et al. 2007; Scheifele et al. 2017; Van Zwieten et al. 2015). However, concentrations of these nutrients did not change in legume biomass after FBC application in our study. It was further suggested that biochar addition could affect %Ndfa through an increase in soil pH (Van Zwieten et al. 2015). However, pH was not affected by the biochar treatments in our studies (Table 2). Thus, it is likely that the reduction in %Ndfa was primarily caused by greater soil NO3− supply.
Legumes can meet their N demand through BNF when access to soil N is limited (Ledgard and Steele 1992; Lambers et al. 2004). The legume N concentration and %Ndfa were much lower in the field than in the pot study (averaged across treatments ~1.6% vs. 4% N and 44 vs.72 %Ndfa), suggesting that BNF (%Ndfa) was downregulated in the field, possibly by the toxic effect of Fe and Al (Edmeades et al. 1991; Van Zwieten et al. 2015). We observed a surprisingly high Fe concentration (~1.5 mg g−1) and a relatively high concentration of Al (~300 μg g−1) in the legumes that could have inhibited nodulation (Bouma et al. 1981; Jarvis and Hatch 1985; Whelan and Alexander 1986; MacPherson 2000). Given that soil pH was neutral (6.8) throughout the soil profile (0–30 cm), only polynuclear Al species (Hunter and Ross 1991) or increased availability of Fe and Al, possibly enhanced by humic fractions including from aged biochar, may have imposed such inhibition (Gerke et al. 1994).
The total amount of N fixed is contributed by N concentration in legumes, its biomass production and %Ndfa. Biomass production was an important contributor to total N fixed in both pot and field study (Fig. 3A, C) although N concentration in the pot study and %Ndfa in the field study also played important roles. While a greater N uptake (3.85 vs. 2.71 g m−2 in the control treatment, p < 0.001) may have reduced %Ndfa in the FBC treatment in the pot study, this may also have increased legume productivity (more so than grass productivity, discussed below) and thus the total N fixed. The reduction of N fixed in the field study possibly occurred due to reduced legume biomass production. Legumes in the field had somewhat limited access to soil N supply, as suggested by the relatively low N concentration in aboveground biomass, while this low acquisition of soil N was not compensated for by N fixation per unit plant biomass (a low %Ndfa, as discussed above).
We may have underestimated %Ndfa in the field study because of a possible direct N transfer from legumes to grasses. However, we removed aboveground biomass by mowing every 2 months, suggesting a decreased likelihood of direct N transfer (Peoples et al. 2015). The soil δ15N was also not significantly different between the control and biochar addition treatment (7.50 and 7.92‰ respectively), while the δ15N in grasses was also similar (2.32 and 2.83‰, respectively). These results suggest that the biochar treatment did not change soil available δ15N. While N fixation may have diluted the soil available N pool in both treatments, this dilution may only have had limited effects on our BNF determination, since both legumes and grasses had access to similar soil N enrichment (Unkovich et al. 2008). For future studies, establishment of a grass monoculture should be considered to avoid dilution of δ15N in the reference plant due to N transfer.
Aging of biochar affects competitiveness of legumes
Competitiveness of legumes in the mixed pastures in our study was different in the different biochar treatments. In the pot study, the legume gained benefits with the FBC application producing the highest biomass, but this advantage disappeared with biochar aging, while grass biomass production remained unaffected. In contrast to the pot study, legumes were disadvantaged in the FABC treatment of the field study incurring a 36% biomass reduction, while grasses increased their biomass production by 47%. These results mostly confirm our second hypothesis (H2) since the relative abundance of the legume increased with the FBC treatment in the pot study while legume abundance was reduced by FABC in the field study. In contrast to our expectations, legume to grass ratios were not reduced in the chemically aged biochar treatments and no stronger effects were observed with increased levels of aging. Consistent with our results, legumes were more competitive than the grasses after fresh biochar application (produced at 400 and 600 °C from pasture biomass) in a temperate mixed pasture (Van de Voorde et al. 2014) while reports on the competitiveness of legumes after aged biochar treatments are not available.
Soil inorganic N and preference of species to different N forms, i.e., NO3− vs. NH4+ can determine the competitiveness of legumes when grown with grasses (Ledgard and Steele 1992; Lambers et al. 2004). Legume biomass production may have been favoured by the greater soil NO3− availability in the FBC treatment since clover is known to preferentially take up NO3− compared to NH4+ (Rys and Phung 1985; Macduff et al. 1996; Svenning et al. 1996). The positive association between legume biomass and soil NO3− concentration as suggested by similar loadings in the PCA supports this contention (Fig. 3c). The reduction of legume biomass in the chemically aged biochar treatments in the pot study may be due to the reduction in NO3− concentrations in these treatments (Table 2). Moreover, although soil NH4+ was more strongly associated with grass biomass in the pot study (Fig. 3d), the relatively higher soil NH4+ concentrations in the chemically aged biochar treatments may have been insufficient for the grass to become more dominant in the chemically aged biochar treatments. By contrast, the significantly higher N uptake of the grasses (3.85 g m−2 vs. 2.77 g m−2 in the control, p = 0.05, data not shown) in the FABC treatment of the field study suggests that FABC retained and supplied sufficient N to grasses boasting their biomass production (Wilman and Asiegbu 1982; Woledge 1986). Therefore, changes in the retention of different inorganic N forms with aging, i.e., a substantial NH4+-N retention in the aged biochar treatment from fertilization, soil organic matter mineralization or from BNF could promote grass production over the production of legumes.
Apart from the soil N supply, other plant nutrients may have contributed to the competitiveness of species in the mixed pasture. In the pot study, the chemically aged biochar significantly increased Na concentration in aboveground biomass (Table 3), which may have negatively affected legume biomass production (although not significantly) but not grass biomass production (Fig. 3c, d), suggesting that legumes were more sensitive to Na levels in the soil (Russell 1976; Smith et al. 1993). In the field study, grass biomass production was strongly associated with CEC and negatively with Al concentrations in plant biomass (Fig. 3b), and we speculate that the increase in CEC (and therefore an overall increase in exchangeable base cations) in the FABC treatment may have alleviated Al toxicity in grasses. Indeed, the FABC treatment significantly reduced the Al concentration in grass shoots from 123 to 86 μg g−1 (Table 3). A concentration of 104 μg g−1 Al in grasses (including the species we tested) strongly reduced their growth (Wheeler et al. 1992). Therefore, alleviation of Al toxicity in our study may have increased the competitiveness of grasses over legumes. In contrast to a study where it was suggested that greater availability of soil P and K after fresh biochar amendment increased competitiveness of legumes over grasses (Van de Voorde et al. 2014), we did not observe any changes in these or other nutrient concentrations in aboveground legume and grass biomass (Table 3).
In conclusion, our results showed that addition of fresh biochar can improve legume biomass production, the amount of N fixed and increase competitiveness of legumes over grasses in mixed pastures. However, these positive effects may be short-lived and grass species may benefit from enhanced N supply when biochar is sufficiently aged and the soil continues to receive N inputs, either from BNF or from fertilization. Thus, the change in BNF and legume performance with biochar aging will have significant implications for ecosystem services. Results from this study will guide decision making in pasture management to optimise BNF and pasture production.
Abbreviations
- BNF:
-
Biological nitrogen fixation
- FBC:
-
Fresh biochar
- FABC:
-
Field aged biochar
- OBC:
-
Oxidized biochar
- CEC:
-
Cation exchange capacity
- %Ndfa:
-
Fraction of nitrogen derived from fixation (%)
- PCA:
-
Principal component analysis
References
Arora S (2005) Toxic effect of high boron content in soils on clover (Trifolium alexandrinum). Environ Ecol 23:225–257
Australian Bureau of Statistics (ABS) (2016) Land management and farming in Australia, 2015–16. Available via dialong. http://www.abs.gov.au/ausstats/abs@.nsf/mf/4627.0. Accessed 5 Sep 2017
Bouma D, Dowling EJ, David DJ (1981) Relations between plant aluminium content and of lucerne and subterranean clover : their usefulness in the detection of aluminium toxicities. Aust J Exp Agric Anim Husb 21:311–317
Bray RH, Kurtz LT (1945) Determination of total, organic and available forms of phosphorus. Soil Sci 59:39–45
Cao T, Chen W, Yang T et al (2017) Surface characterization of aged biochar incubated in different types of soil. Bioresources 12:6366–6377
Cheng C-H, Lehmann J (2009) Ageing of black carbon along a temperature gradient. Chemosphere 75:1021–1027
Cheng C-H, Lehmann J, Engelhard MH (2008) Natural oxidation of black carbon in soils: changes in molecular form and surface charge along a climosequence. Geochim Cosmochim Acta 72:1598–1610
Edmeades DC, Blamey FPC, Asher CJ, Edwards DG (1991) Effects of pH and aluminium on the growth of temperate pasture species. II. Growth and nodulation of legumes. Aust J Agric Res 42:893–900
Enders A, Lehmann J (2017) Proximate analyses for characterising biochar. In: Singh B, Camps-Arbestain M, Lehmann J (eds) Biochar: a guide to analytical methods, 1st edn. CSIRO publishing, Clayton South, Melbourne Victoria, pp 9–22
Enders A, Sohi S, Lehmann J, Singh B (2017) Total elemental analysis of metals and nutrients in biochar. In: Singh B, Camps-Arbestain M, Lehmann J (eds) Biochar: a guide to analytical methods, 1st edn. CSIRO Publishing, Clayton South, Melbourne Victoria, pp 95–108
Gerke J, Meyer U, Römer W (1994) Phosphate, Fe and Mn uptake of N2 fixing red clover and ryegrass from an oxisol as affected by P and model humic substances application. 1. Plant parameters and soil solution composition. Zeitschrift für Pflanzenernährung, Düngung, Bodenkd 158:261–268
Güereña DT, Lehmann J, Thies JE et al (2015) Partitioning the contributions of biochar properties to enhanced biological nitrogen fixation in common bean (Phaseolus vulgaris). Biol Fertil Soils 51:479–491
Herridge DF, Peoples MB, Boddey RM (2008) Global inputs of biological nitrogen fixation in agricultural systems. Plant Soil 311:1–18
Høgh-Jensen H, Schjoerring JK (1997) Interactions between white clover and ryegrass under contrasting nitrogen availability: N2 fixation, N fertilizer recovery, N transfer and water use efficiency. Plant Soil 197:187–199
Hunter D, Ross DS (1991) Evidence for a phytotoxic hydroxy-aluminum polymer in organic soil horizons. Science 251:1056–1058
Isbell RF (2002) The Australian soil classification, 4th edn. CSIRO Publishing, Melbourne, Victoria, Collingwood, pp 1–144
Ishizuka J (1992) Trends in biological nitrogen fixation research and application. Plant Soil 141:197–209
Jarvis SC, Hatch DJ (1985) The effects of aluminum on the growth of white clover dependent upon fixation of atmospheric nitrogen. J Exp Bot 36:1075–1086
Jeffery S, Verheijen FGA, Van der Velde M, Bastos AC (2011) A quantitative review of the effects of biochar application to soils on crop productivity using meta-analysis. Agric Ecosyst Environ 144:175–187
Joseph SD, Camps-Arbestain M, Lin Y et al (2010) An investigation into the reactions of biochar in soil. Aust J Soil Res 48:501–515
Keith A, Singh B, Dijkstra FA, Van Ogtrop F (2016) Biochar field study: greenhouse gas emissions, productivity, and nutrients in two soils. Agron J 108:1805–1815
Kuzyakov Y, Bogomolova I, Glaser B (2014) Biochar stability in soil: decomposition during eight years and transformation as assessed by compound-specific 14C analysis. Soil Biol Biochem 70:229–236
Lambers JHR, Harpole WS, Tilman D et al (2004) Mechanisms responsible for the positive diversity-productivity relationship in Minnesota grasslands. Ecol Lett 7:661–668
Lawrinenko M, Laird DA, Johnson RL, Jing D (2016) Accelerated aging of biochars: impact on anion exchange capacity. Carbon 103:217–227
Ledgard SF, Steele KW (1992) Biological nitrogen-fixation in mixed legume grass pastures. Plant Soil 141:137–153
Linse L, Dahlin S, Nadeau E et al (2015) Boron fertilisation of organically managed grass-clover swards on coarse-textured soils: effects on botanical and element composition. Agri. Food Sci 24:261–272
Lucero DW, Grieu P, Guckert A (1999) Effects of water deficit and plant interaction on morphological growth parameters and yield of white clover (Trifolium repens L.) and ryegrass (Lolium perenne L.) mixtures. Eur J Agron 11:167–177
Macduff JH, Jarvis SC, Davidson IA (1996) Inhibition of N2 fixation by white clover (Trifolium repens L .) at low concentrations of NO3 − in flowing solution culture. Plant Soil 180:287–295
MacPherson A (2000) Trace-mineral status of forages. In: Forage evaluation in ruminant nutrition. CABI, Wallingford, pp 345–371
Mete FZ, Mia S, Dijkstra FA et al (2015) Synergistice effects of biochar and NPK fertilizer on soybean yield in an alkaline soil. Pedosphere 25:713–719
Mia S, Van Groenigen JW, Van de Voorde TFJ et al (2014) Biochar application rate affects biological nitrogen fixation in red clover conditional on potassium availability. Agric Ecosyst Environ 191:83–91
Mia S, Dijkstra FA, Singh B (2017a) Long-term aging of biochar: a molecular understanding with agricultural and environmental implications. Adv Agron 141:1–51
Mia S, Dijkstra FA, Singh B (2017b) Aging induced changes in biochar's functionality and adsorption behavior for phosphate and ammonium. Environ Sci Technol 51(15):8359–8367
Mia S, Singh B, Dijkstra FA (2017c) Aged biochar affects gross nitrogen mineralisation and recovery: a 15N study in two contrasting soils. GCB Bioenergy 9(7):1196–1206
Nishio M, Okano S (1991) Stimulation of the growth of alfalfa and infection of mycorrhizal fungi by the application of charcoal. Bull Natl Grassl Res Inst 45:61–71
Ogawa M, Okimori Y (2010) Pioneering works in biochar research, Japan. Aust J Soil Res 48:489–500
Peoples MB, Chalk PM, Unkovich MJ, Boddey RM (2015) Can differences in 15N natural abundance be used to quantify the transfer of nitrogen from legumes to neighbouring non-legume plant species? Soil Biol Biochem 87:97–109
Quilliam RS, DeLuca TH, Jones DL (2013) Biochar application reduces nodulation but increases nitrogenase activity in clover. Plant Soil 366:83–92
Rondon MA, Lehmann J, Ramírez J, Hurtado M (2007) Biological nitrogen fixation by common beans (Phaseolus vulgaris L.) increases with bio-char additions. Biol Fertil Soils 43:699–708
Russell JS (1976) Comperative salt tolerance of some tropical and temperate legumes and tropical grasses. Aust J Exp Agri Ani Husb 16:103–109
Rys GJ, Phung T (1985) Comparison of the nitrogen nutrition of 2 white clover cultivars. New zeal. J Agric Res 28:1–5
Scheifele M, Hobi A, Buegger F et al (2017) Impact of pyrochar and hydrochar on soybean (Glycine max L.) root nodulation and biological nitrogen fixation. J Plant Nutr Soil Sci 180:199–211
Singh B, Singh BP, Cowie AL (2010) Characterisation and evaluation of biochars for their application as a soil amendment. Aust J Soil Res 48:516–525
Singh B, MacDonald LM, Kookana RS et al (2014) Opportunities and constraints for biochar technology in Australian agriculture: looking beyond carbon sequestration. Soil Res 52:739–750
Smith CJ, Chalk PM, Noble CL et al (1993) Nitrogen fixation in a white clover-grass pasture irrigated with saline groundwater. Irrigation Sci 13:189–194
Svenning MM, Junttila O, Macduff JH (1996) Differential rates of inhibition of N2 fixation by sustained low concentrations of NH4 + and NO3 − in northern ecotypes of white clover (Trifolium repens L.) J Exp Bot 47:729–738
Tagoe SO, Horiuchi T, Matsui T (2008) Effects of carbonized and dried chicken manures on the growth, yield, and N content of soybean. Plant Soil 306:211–220
Tagoe SO, Horiuchi T, Matsui T (2010) Effects of carbonized chicken manure on the growth, nodulation, yield, nitrogen and phosphorus contents of four grain legumes. J Plant Nutr 33:684–700
Unkovich M (2012) Nitrogen fixation in Australian dairy systems: review and prospect. Crop Pasture Sci 63:787–804
Unkovich MJ, Herridge DF, Peoples MB, Cadisch G, Boddey RM, Giller KE et al (2008) Measuring plant-associated nitrogen fixation in agricultural systems. ACIAR, Canberra Australia, Monograph no. 136, pp 1–258
Van de Voorde TFJ, Bezemer TM, Van Groenigen JW et al (2014) Soil biochar amendment in a nature restoration area: effects on plant productivity and community composition. Ecol Appl 24:1167–1177
Van Zwieten L, Rose T, Herridge D et al (2015) Enhanced biological N2 fixation and yield of faba bean (Vicia faba L.) in an acid soil following biochar addition: dissection of causal mechanisms. Plant Soil 395:7–20
Wang B, Lehmann J, Hanley K et al (2015) Adsorption and desorption of ammonium by maple wood biochar as a function of oxidation and pH. Chemosphere 138:120–126
Wang J, Xiong Z, Kuzyakov Y (2016) Biochar stability in soil: meta-analysis of decomposition and priming effects. GCB Bioenergy 8:512–523
West JB, HilleRisLambers J, Lee TD et al (2005) Legume species identity and soil nitrogen supply determine symbiotic nitrogen-fixation responses to elevated atmospheric [CO2]. New Phytol 167:523–530
Wheeler DM, Edmeades DC, Christie RA (1992) Effect of aluminum on plant chemical concentrations in some temperate grasses grown in solution culture at low ionic strength. J Plant Nutr 15:387–402
Whelan AM, Alexander M (1986) Effects of low pH and high Al, Mn and Fe levels on the survival of Rhizobium trifolii and the nodulation of subterranean clover. Plant Soil 371:363–371
Wilman D, Asiegbu JE (1982) The effects of clover variety, cutting interval and nitrogen application on herbage yields, proportions and heights in perennial ryegrass-white clover swards. Grass and Forage Sceince 37:1–13
Woledge J (1986) Competition between grass and clover as affected by nitrogen fertilizer. Ann Appl Biol 112:175–186
Acknowledgements
We gratefully appreciate The University of Sydney for funding the PhD project. We are thankful to Michael Turner, Hero Tahaei, Dr. Paola Corneo, Abdul Muktadir and Md. Ashraful Islam for their help in either collecting data or analysis of results.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Per Ambus.
Electronic supplementary material
ESM 1
(DOCX 88 kb)
Rights and permissions
About this article
Cite this article
Mia, S., Dijkstra, F.A. & Singh, B. Enhanced biological nitrogen fixation and competitive advantage of legumes in mixed pastures diminish with biochar aging. Plant Soil 424, 639–651 (2018). https://doi.org/10.1007/s11104-018-3562-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-018-3562-4