Abstract
Kobresia grasslands on the Tibetan Plateau comprise the world’s largest pastoral alpine ecosystem. Overgrazing-driven degradation strongly proceeded on this vulnerable grassland, but the mechanisms behind are still unclear. Plants must balance the costs of releasing C to soil against the benefits of accelerated microbial nutrient mineralization, which increases their availability for root uptake. To achieve the effect of grazing on this C-N exchange mechanism, a 15NH4+ field labeling experiment was implemented at grazed and ungrazed sites, with additional treatments of clipping and shading to reduce belowground C input by manipulating photosynthesis. Grazing reduced gross N mineralization rates by 18.7%, similar to shading and clipping. This indicates that shoot removal by grazing decreased belowground C input, thereby suppressing microbial N mining and overall soil N availability. Nevertheless, NH4+ uptake rate by plants at the grazed site was 1.4 times higher than at the ungrazed site, because plants increased N acquisition to meet the high N demands of shoot regrowth (compensatory growth: grazed > ungrazed). To enable efficient N uptake and regrowth, Kobresia plants have developed specific traits (i.e., efficient above-belowground interactions). These traits reflect important mechanisms of resilience and ecosystem stability under long-term moderate grazing in an N-limited environment. However, excessive (over)grazing might imbalance such C-N exchange and amplify plant N limitation, hampering productivity and pasture recovery over the long term. In this context, a reduction in grazing pressure provides a sustainable way to maintain soil fertility, C sequestration, efficient nutrient recycling, and overall ecosystem stability.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The Tibetan Plateau hosts the world’s largest and highest grasslands and one of the most sensitive and fragile ecosystems, vulnerable to global environmental change and anthropogenic activities (e.g., Cui et al. 2006; Babel et al. 2014; Chen et al. 2014). The Tibetan grasslands play an important role in ecosystem functioning, e.g., soil carbon (C) storage (ca. 7.4 Pg C), water and soil conservation, and climate regulation (Yang et al. 2008; Lin et al. 2017). Tibetan grasslands support a diversity of plants, livestock, and local pastoral communities (Kang et al. 2007). The alpine Cyperaceae mats of Kobresia pygmaea are the major constituent of the Tibetan grasslands and cover an area of 450,000 km2, ranging from 3000 to nearly 6000 m in altitude (Miehe et al. 2008, 2017). The grazing lawns grow 2–4 cm high and develop a firm closed root system in the upper 30 cm of soil (termed Kobresia turf, Kaiser et al. 2008). Consequently, Kobresia root mats are characterized by high quantities of roots and rhizogenic organic matter that contribute to the large stores of C, nitrogen (N), and other nutrients (Kaiser et al. 2008). Soil C and N dynamics in response to land use change represent a potentially important feedback to climate (Harris 2010; Chen et al. 2013).
The Kobresia grasslands have developed as the consequence of over ca. 8 thousand years of moderate grazing (Miehe et al. 2009, 2014, 2017), and nowadays in Northern Tibet, they still provide forage resources for livestock equivalent to more than 1.7 million sheep (Feng et al. 2017). K. pygmaea has been evolutionarily selected due to its specific traits reflecting strategies of both resistance (through avoidance) and tolerance (through re-growth capacity): tiny leaves that prevent complete grazing removal and a very large and durable root system that ensures efficient water and nutrient uptake and storage (Xu et al. 2014; Schleuss et al. 2015) and prevents trampling damage (Kaiser et al. 2008); a mixed reproductive strategy also improves their competitiveness compared to other plants (Seeber et al. 2015).
The short-term compensatory growth of K. pygmaea may be undermined by serious restrictions on plant re-growth and reproduction due to long-term overgrazing. Indeed, semi-sedentary management has replaced the traditional migratory management and increased the number of livestock since the late 1970s; this has contributed to the degradation of about 30% of the grasslands (Zhou et al. 2005). The subsequent deterioration of the Kobresia turf induced further losses of C and N (Wang et al. 2005b; Su et al. 2015). To counter degradation, the central and regional government has launched a series of ecological restoration projects since 2004. Excluding grazing by fencing has been proposed as one restoration practice to balance the stocking rate with grassland productivity. However, the effects on ecosystem recovery of Kobresia grassland are not sufficiently understood (Yu et al. 2016).
Besides grazing stress, plant growth in such harsh alpine environments is co-restricted by numerous abiotic factors, such as high solar radiation, low temperature and precipitation, a short vegetation period, and nutrient deficiency (Callaway et al. 2002; Miehe et al. 2017). Among the nutrients, N is most limited and its availability has a major impact on net primary production in Tibetan grasslands. This has been confirmed by previous N fertilization experiments (Song et al. 2007; Seeber et al. 2015; Wang et al. 2015; Zong et al. 2016). The prevailing low N availability in Tibetan grassland is mainly because (1) most N is in organic forms and not directly plant available, (2) N mineralization from organic forms is suppressed by low temperature (Wang et al. 2016), (3) N availability has strong temporal and spatial variability during the short growing seasons (Song et al. 2007; Xu et al. 2011a, b), and (4) large amounts of N have been removed from the ecosystem, since intensive grazing hampers the natural livestock-mediated N redistribution: most of the yak dung is used as a fuel source by Tibetan nomads, and a part of urine is easily lost through volatilization and nitrate leaching (Luo et al. 2010).
K. pygmaea faces two major challenges under grazing: First, N should be captured efficiently by roots; second, mineralization of organic N forms should be enhanced (Kuzyakov and Xu 2013; Dijkstra et al. 2016). In fact, K. pygmaea invests a very high proportion of assimilated C in root production (with root to shoot ratios of up to 20) to compete for N with microorganisms and coexisting plants (Xu et al. 2011a, b; Kuzyakov and Xu 2013) and develops chemical (N forms) and/or temporal niche differentiation (Xu et al. 2011a, b; Jiang et al. 2017). In addition, it has become increasingly clear that plants can stimulate microbial growth, activity, and enzyme production by providing labile and energy-rich C compounds. The microbes accelerate soil organic matter (SOM) decomposition and increase N availability through rhizosphere priming effects (Kuzyakov 2002; Jones et al. 2009). Root exudates are known to be the key C inputs in this cold, semi-arid ecosystem (Farrar and Jones 2000; Cleveland et al. 2004). However, grazing alters the amount of photosynthetic assimilates and their allocation belowground, which, in turn, affects SOM mineralization and nutrient uptake by plants, with feedback to net primary production and C sequestration (Paterson and Sim 2000; Sun and Wang 2016a). In this N-limited ecosystem, we assume that K. pygmaea interacts with microorganisms for an improved N supply, which is highly important for sustaining regrowth after grazing. Such interactions also help to explain the existence and long-term stability of this unique ecosystem despite natural and human disturbance over the past millennia.
Nonetheless, major changes in pasture management (i.e., intensive grazing) and climate in recent decades have strongly impacted the plant-soil system, with unknown consequences for N pools and cycles (e.g., exchange mechanisms). This could be an important mechanism underlying degradation in Kobresia grasslands. Identification of the role of grazing on the exchange mechanisms for the Tibetan grassland is challenging because the grazing activities have inconsistent definitions and various standards. Exclusion experiments provide a feasible way for studying grazing effects based on the assumption of inverse process direction and ecosystem recovery. To further clarify the exchange mechanisms, we investigated gross mineralization, N uptake by plants and N partitioning in the plant-soil system on grazed and ungrazed sites. Clipping (simulating defoliation by grazing) and shading, that reduce the leaf area and the light-dependent reactions, respectively (Detling et al. 1979, Schmitt et al. 2013), were used to manipulate photosynthesis and hence the C allocation into belowground (i.e., root and soil).We hypothesized that (1) grazing-driven removal of shoots lowers photosynthesis and belowground C translocation, reduces microbial activities in the rhizosphere, and thus decreases soil gross N mineralization; (2) N uptake rates of plants and N retention in root biomass will be higher in the grazed Kobresia grassland, in order to ensure N delivery for an efficient regrowth of new shoots. To test these hypotheses, we conducted an in situ 15N labeling study at grazed and ungrazed sites. Considering the long-term grazing impacts on Kobresia grasslands, we expected that K. pygmaea developed specific strategies for N uptake and partitioning to facilitate plant re-growth after grazing. The 15N dilution approach was used to quantify in situ gross N mineralization at grazed and ungrazed sites.
Materials and methods
Study area
The study site is located in the core area of alpine K. pygmaea grasslands adjacent to the Naqu Ecological and Environmental Observation and Research Station (Xizang, China; 31° 16′ 45″ N, 92° 59′ 37″ E; 4410 m above sea level). The Research Station was established in 2007 as the “Kobresia Experimental Monitoring Area” (KEMA), close to the village Kema, and now managed by the Institute of Tibetan Plateau Research, Chinese Academy of Science. This area is influenced by a moderately continental monsoonal climate with mean summer precipitation of 276 mm (June–September). Average annual temperature was − 1.2 °C in the previous 5 years (Miehe et al. 2011). Our experiment was conducted from July to August 2012 during the growing season, which lasted from mid-May to mid-September. A mean temperature of 10.4 °C was recorded (Babel et al. 2014).
The sites are dominated by K. pygmaea (Cyperaceae), which covers more than 80% of the surface area. K. pygmaea is accompanied (remaining 20%) by other graminoid species, such as Carex ivanoviae, Carex spp., Poa spp., Festuca spp., Stipa purpurea, and Trisetum spp., as well as a small number of rosette and cushion plants (Arenaria bryophylla, Arenaria kansuensis, Androsace tapete, Leontopodium nanum, Lamiophlomis rotata). The K. pygmaea lawn has an average height of 2 cm at the grazed site, overlying a dense root mat of approximately 14 cm. The soil is classified as a stagnic Cambisol (humic, eutric) (IUSS Working Group WRB 2006). The upper soil horizons (Afe horizons, according to Kaiser et al. 2008) consist of humified organic matter and a large quantity of living and dead roots. Soils are free of carbonates in the upper 15 cm and have mean pH values of 6.4. A detailed description of soil characteristics is reported by Schleuss et al. (2015).
The study site is traditionally used as a continuously grazed pasture. However, livestock stocking exceeds the carrying capacity, and thus, the pasture in this region is intensively grazed (Ingrisch et al. 2015; Wei and Chen 2001). To assess the effect of grazing exclusion on pasture restoration, a grassland area of 100 × 250 m was fenced in 2010 (excluding livestock such as yaks and goats, but allowing minor grazing by small mammals). The experiment was established within the fence (referred to as the ungrazed site) and outside but near to the fenced area (referred to as the grazed site). Two percent more vegetation coverage was observed after grazing exclusion, but there was no significant difference between plant communities.
15N labeling experimental setup, sampling, and analysis
The in situ 15N labeling experiment was started in August 12. On each site (grazed and ungrazed), 16 square plots (15 × 15 cm) were randomly selected; 12 of them were randomly chosen for three treatments: control, clipping, and shading with four replicates. The remaining four plots were reference plots, which were injected only with water to determine plant and soil properties as well as 15N natural abundance.
The in situ gross N mineralization rate at 0–10 cm depth was estimated by a 15N dilution approach (Davidson et al. 1991; Hart et al. 1994; Murphy et al. 2003). An amount of 4.562 mg 15N was added as (15NH4)2SO4 (10 atom% 15N) to each plot. We used the same labeling grid with 16 injection points to ensure a uniform distribution of tracer in each plot. (15NH4)2SO4 was dissolved in water and 1 ml of this tracer solution was injected at 5 cm soil depth for each injection point with a dispensing pipette. The “initial” soil cores (two soil cores per plot, 5 cm diameter × 10 cm depth) from each 15N-labeled plot were sampled 3 h after labeling (the t0 point). After that, four labeled plots as replicates were subjected to clipping or shading under both grazing regimes. For clipping, the plant shoots were clipped at 1 cm above the soil surface and thereafter allowed to grow continuously. For shading, the light intensity was reduced by more than 90% by a fine black mesh cover throughout the experimental period of 28 days. The four remaining labeled plots with untreated plants were referred to as control (without clipping and shading, but with tracer injection). Previous studies showed that a period of 6 days allowed an accurate quantification of gross mineralization by 15N pool dilution approach (Mikan et al. 2000; Murphy et al. 2003). Since the N mineralization rate in alpine grasslands is slow, we took two further soil cores from each plot after 6.5 days of in situ incubation (the t1 point). The soil samples were transferred immediately to the lab and well mixed after removing the roots. Fresh soil subsamples (8 g from each replicate) were extracted for 1 h with 30 ml 0.5 M K2SO4. The concentrations of soil NO3− and exchangeable NH4+ were determined with an autoanalyzer (AA3, Bran-Luebbe, Germany). NH4+ in the extracts was determined by a diffusion process (Murphy et al. 2003), and the 15N enrichment was determined with an isotope ratio mass spectrometer (Mat 253, Finnigan MAT).
Nitrogen uptake by plants and 15N partitioning in the plant-soil system were traced by in situ 15NH4+ labeling because NH4+ is the dominating mineral N form in the soil. Following the 15N labeling and the treatments (clipping and shading), samples of shoots, roots, and soil were taken from each plot 3 h and 7, 14, and 28 days after labeling. The shoot biomass was cut within an area of 25 cm2 on each small plot close to the soil surface. After sampling cutting, root and soil samples were taken by soil cores of 0–10 cm soil depth, because over 70% of the total root biomass is concentrated within this horizon (Schleuss et al. 2015). Fresh soil samples were sieved through a 2-mm mesh. The separated roots were washed with tap water and soaked into 0.5 mM CaCl2 solution for 30 min, then rinsed with distilled water to remove 15N absorbed on the root surface. Plant materials and soil samples were dried at 65 °C for 72 h and ground to a fine powder using a ball mill (MM2, Fa. Retsch, Haan, Germany). They were weighed into tin capsules to analyze total C and N contents and stable isotope signatures using an elemental analyzer (EA 1112, CE Instruments, Milan, Italy), ConFlo III device (Finnigan MAT, Bremen, Germany), and continuous-flow gas isotope ratio mass spectrometry (MAT253, Finnigan MAT, Bremen, Germany). Bulk density was determined by oven-drying undisturbed soil at 105 °C for 48 h (Van Reeuwijk 2002). Soil pH was measured by a dry soil-water ratio of 1:2. Total C and N stocks of plant and soil as well as the contents of NO3− and exchangeable NH4+ (unlabeled) were determined on the reference plots.
Calculations and statistics
Soil gross N mineralization rate was calculated from the measured NH4+ pool and atom % 15N of NH4+ as follows (Murphy et al. 2003):
where m is the gross mineralization rate (mg N kg−1 day−1), M 0 is the initial exchangeable NH4+ pool (mg N kg−1), M 1 is the post-incubation exchangeable NH4+ pool (mg N kg−1), AP 0 is the initial atom-%-15N of NH4+, AP 1 is the post-incubation atom-%-15N of NH4+, AP C is the atom-%-15N of NH4+ in unlabeled control, and t 0 and t 1 are the time points (in day) of initial and post-incubation soil core sampling, respectively. Net mineralization and net nitrification were determined from the difference in the N pool of exchangeable NH4+ and of NO3−, respectively, after 6.5 days of incubation (Hart et al. 1994).
15N uptake by plants (or 15N allocation to soil) was calculated by an isotope mixing model (Robinson 2001), as the product of the N content of the pool, the mass of the component per square meter, and its APE (atom-%-excess 15N). APE is the 15N difference between 15N-labeled and unlabeled plants or soil. The proportion of 15N recovered in plants (or soil) was calculated by dividing plant 15N uptake (or 15N incorporation of soil) by total amount of 15N injected into the soil. Based on previous studies (Xu et al. 2011a, b), an assumption was made that the N forms in Tibetan grasslands do not change during the 3-h period of labeling. The NH4+ uptake rate by plants was calculated by dividing the actual NH4+ uptake amount by time and root biomass. The actual NH4+ uptake from soil by plants was calculated as follows:
where Ulabeled is the amount of 15NH4+ uptake by plants, munlabeled is the content of total exchangeable NH4+ in the unlabeled soil, and mlabeled is the total mass of injected 15N (McKane et al. 2002; Xu et al. 2011a, b).
The relative growth rate (RGR) of shoots (or roots) for each treatment was determined by their initial and final biomass during 28 days of experiment period, calculated as follows (Hunt et al. 2002):
Normality (Shapiro-Wilk test, p > 0.05) and homogeneity of variance (Levene test, p > 0.05) were examined before the statistical analysis. Data were log-transformed prior to analysis if they were non-normally distributed. Repeated measures analysis of variance (ANOVA) with the sampling date as the within-subject factor and grazing and treatments (control, clipping, and shading) as the between-subject factors was calculated for shoot and root biomass; 15N recovery in shoot, root, and total biomass; and NO3− and exchangeable NH4+ contents. One-way ANOVA was used to assess the effect of grazing and then the effects of control, clipping, and shading nested within each of the grazing treatment on the parameters of plants (C and N stocks, RGR of shoot/root, total biomass, NH4+ uptake rate, total plant 15N recovery) and those of soil (C and N stocks, the mineral N contents, gross and net N mineralization, net N nitrification, bulk density, and pH). ANOVAs were followed by post hot test for multiple comparison using least significant differences. All statistical analyses were performed with SPSS 16 (SPSS Inc., Chicago, IL, USA) and considered significance at a p < 0.05 level.
Results
Above- and belowground C and N stocks
In general, shoot biomass made only a minor contribution to the overall stocks of C (1.5%) and N (0.9%) on average, whereas the majority of the total C and N was stored belowground, mainly in root mats (root + soil). The root biomass contributed 13.5 and 4.0% of the total C and N storage, and the soil accounting for 85.0 and 95.2%, respectively.
Three years after grazing exclusion, C and N stocks in shoots increased by 46.2% (p < 0.01) and 29.7%, respectively, compared to those in the grazed site. In contrast, C and N stocks of the root biomass decreased by 14.6 and 10.4% (p < 0.05; Fig. 1). Grazing exclusion increased soil C and N stocks by 14.7 and 18.2%, respectively (p < 0.05). As a result, the overall total C and N stored in the upper 10-cm root mats (root + soil) were 10.2 and 16.9% larger in the ungrazed site than those in the grazed site (p < 0.05; Fig. 1).
The photo (left) shows the characteristics of K. pygmaea and of the upper 10 cm of soils at grazed and ungrazed sites. Subfigures (right) illustrate C and N stocks in plant and soil as well as the content of mineral N (exchangeable NH4+, NO3−) in the soil. Values are means ± standard errors (n = 8). Asterisk indicates significant difference level (* p < 0.05, ** p < 0.01)
Mineral N and soil N transformation processes
NH4+ was the dominant mineral N form in this Kobresia pasture, and its content in the unlabeled soil was about four times higher than that of NO3− (Fig. 1). Grazing exclusion increased the content of NO3− and exchangeable NH4+ in unlabeled soil by 32 and 72%, respectively (p < 0.05; Fig. 1). The combined mineral N pool (exchangeable NH4+, NO3−) was on average 1.4 times higher in ungrazed site than that in grazed site (p < 0.05). After tracer addition, the NO3− and exchangeable NH4+ contents were similar during 28 days after labeling under both grazing regimes, despite their differences before labeling (Fig. S1; Table S2). The NO3− content fluctuated with time under both grazing regimes (ranged from 2.1 to 4.7 μg N g−1 soil), but that of exchangeable NH4+ strongly decreased (ranged from 80.4 to 12.5 μg N g−1 soil).
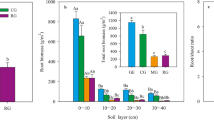
The gross N mineralization rate was averagely lower for the grazed site compared to that for the ungrazed site among all treatments (p < 0.05; Fig. 2). In the grazed site, the highest N mineralization was for control (10.4 mg N kg−1 soil day−1), followed by clipping (8.7 mg N kg−1 soil day−1) and by shading (5.6 mg N kg−1 soil day−1). A similar pattern was evident for the ungrazed site: 12.8, 10.7, and 9.2 mg N kg−1 soil day−1 for control, clipping, and shading, respectively (Fig. 2). Linear regression between gross N mineralization rates and the mean increase in N stock in plant biomass showed a weak relationship at the grazed site (r2 = 0.3, p = 0.07), but the relationship was much stronger at the ungrazed site (r2 = 0.6, p = 0.003; Fig. 2).
Left: gross N mineralization rate (mg N kg−1 soil day−1) in soil of the grazed and ungrazed sites. Capital letters indicate significant differences (post hoc LSD test, p < 0.05) between grazed and ungrazed sites. Lowercase letters indicate significant differences (post hoc LSD test, p < 0.05) between treatments (control, clipping, and shading) nested within each grazing regime. Right: relationship between gross N mineralization rate (mg N m−2 day−1) and the mean increment of N stock in plant biomass (mg N m−2 day−1) for the grazed and ungrazed sites. Each point represents the individual replicates of each treatment (control, clipping, and shading)
The negative values of net N mineralization under both grazing regimes (Table S1) indicated that NH4+ consumption (including immobilized NH4+, NH4+ oxidization, gaseous losses, and plant uptake) exceeded gross mineralization. The highest average net mineralization rate was observed for control, which was followed by clipping and then shading, under both grazing regimes. Net N nitrification rates at the grazed site were on average 1.7 times higher than at the ungrazed site (Table S1), indicating higher potential N losses by emission, denitrification, and leaching.
Shoot and root biomass production
Grazing exclusion increased shoot biomass (p < 0.001, Fig. 3; Table S4). For example, the shoot biomass was about 26% higher in the control of the grazed sites (160 g m−2) compared with that in the ungrazed control (202 g m−2; Fig. 3). Yet, grazing exclusion had only a small effect on the relative growth rate (RGR) of shoot biomass (for control and shading, Fig. 4), whereas clipping (simulated grazing) increased the RGR of shoot biomass for both grazing regimes (p < 0.05). The clipping effect on the RGR was significantly higher at the intensively grazed site compared to that at the grazing exclosure (p < 0.05; Fig. 4).
Shoot and root biomass (g m−2) during 28 days after labeling. The values of bottom figures show the total plant biomass (shoots + roots) 28 days after the labeling. CK: control, SH: shading, CL: clipping. Values are means ± standard errors (n = 4). Letters indicate significant differences at p < 0.05 within treatments (control, shading, and clipping) for grazed and ungrazed sites
Left: relative growth rate (RGR) of shoots (g g−1 day−1). Values are means ± standard errors (n = 4). Capital letters indicate significant differences (post hoc LSD test, p < 0.05) between grazed and ungrazed sites. Lowercase letters indicate significant differences (post hoc LSD test, p < 0.05) between treatments (control, clipping, and shading) nested within each grazing regime. Right: relationship between the RGR of shoot (g g−1 day−1) and 15N recovery in the root biomass (%). Each point represents the individual replicates of each treatment (control, clipping, and shading) for grazed and ungrazed sites
In contrast to the shoot biomass, root biomass decreased by 12% following grazing exclusion (in the upper 10 cm depth; Fig. 3, Table S4). This resulted in a lower root to shoot investment at the ungrazed site (root to shoot ratio 7.9) than at the grazed site (root to shoot ratio 11.4). The mean biomass of the control was 1494 and 1638 g m−2 in the ungrazed and the grazed sites, respectively. Clipping did not change root biomass under both grazing regimes. Shading inhibited root growth and led to the lowest root biomass under both grazing regimes (Fig. 3). Nonetheless, no significant difference in the RGR of roots was observed between grazed and ungrazed sites (0.02 g g−1 day−1 on average, data not presented).
Plant N uptake and 15N partitioning in the soil-plant system
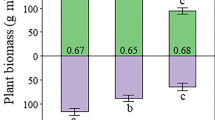
The NH4+ uptake rate by plants (within 3 h after 15N pulse labeling) was higher at the grazed sites (0.67 ± 0.03 μg N g−1 root−1 h−1) than that at the ungrazed sites (0.48 ± 0.04 μg N g−1 root−1 h−1; p < 0.05).
Overall, 86.3 and 79.7% of the 15N were recovered in the plant-soil system (the upper 10-cm root mats) at the grazed and ungrazed sites, respectively. The major portion of 15N remained in soil (0–10 cm; range from 41.8 to 62.6%) 28 days after the labeling. This was independent of grazing and treatments (control, clipping, and shading).
The total 15N recovery in plant biomass (shoots + roots) is higher at grazed site than that at ungrazed site 28 days after labeling (p = 0.01; Table S5). The total plant 15N recovery was 36.5 and 42.1% for control at the grazed and ungrazed sites, respectively (Fig. 5). The 15N recovery in plants was slightly increased by clipping at the grazed sites, but it was reduced by clipping at the ungrazed sites. Shading had the lowest 15N recovery under both grazing regimes at all times (Fig. 5) and did not differ between grazing regimes. Notably, 15N partitioning between shoot and root biomass showed deviating patterns with grazing regime, which is why both compartments are presented separately (see below).
The 15N recovery (% of input) in shoot and root biomass during 28 days after labeling. The values at the bottom figures show the 15N recovery (%) in total plant biomass at the last sampling. CK: control, SH: shading, CL: clipping. Values are means ± standard errors (n = 4). Lowercase letters indicate significant differences at p < 0.05 within treatments (control, shading, and clipping) under each grazing regime
The 15N recovery in shoots for control was about two times lower at the grazed site (11.0%) than that at the ungrazed site (19.2%). An even lower 15N recovery was observed following clipping and shading for both grazing regimes, essentially due to the lowered shoot biomass (Fig. 5). The 15N recovery in shoots correlated positively with root biomass, but negatively with soil exchangeable NH4+ content under both grazing regimes (p < 0.001; Table S3).
15N recovery in roots was higher at grazed sites than that at ungrazed sites for all treatments 28 days after the labeling (p < 0.05); the 15N recovery in roots for control was 25.5 and 22.9% at grazed and ungrazed sites, respectively (Fig. 5, Table S5). Compared to the respective control, clipping increased the 15N recovery in roots of grazed plants (28.7%), while it decreased the 15N recovery of the ungrazed plants (19.0%, Fig. 5). Under both grazing regimes, the respective lowest 15N recovery in roots was observed for shading. The 15N recovery in root biomass was negatively correlated with soil exchangeable NH4+ content under both grazing regimes (p < 0.001), but its correlation with the shoot biomass was significant only at the grazed sites (p < 0.05; Table S3), reflecting that root N uptake was important for the re-growth of photosynthetically active shoots in the grazed K. pygmaea grassland. Linear regression between the RGR of shoots and 15N recovery in roots showed a significant relationship for grazed site (r2 = 0.33, p < 0.05), but not for the ungrazed one (Fig. 4).
Discussion
Grazing effects on N stocks
Soil N stocks in the upper 10-cm root mats were 15% lower under grazing (Fig. 1). This is consistent with several studies reporting negative grazing effects on N stocks, especially within the upper soil horizon (Table S6). In the grazed Kobresia pastures, the lower N stocks are mainly attributed to (1) the increasing stocking numbers in recent decades, which increased outflows of nutrients from the soil-plant system by livestock consumption, (2) redistribution of N from the grazing sites to the captive area adjacent to the villages, (3) collection of dung for household fuel, (4) gaseous N loss, and (5) nitrate leaching (Table S1; He et al. 2011; Zhong et al. 2017). Consequently, Soil C sequestration might be limited under grazing due to the tight stoichiometric relationships between C and N (Fig. 1; Hu et al. 2016). However, there are also other studies reporting neutral or even positive grazing effects on N stocks (Table S6). This could be ascribed to grazing intensity and site differences in altitude and regional climate conditions (e.g., temperature, precipitation). For example, moderate grazing intensity has been suggested as a suitable way to preserve the capacity of Tibetan grasslands to store C and nutrients (Hafner et al. 2012; Zhang et al. 2015; Zhou et al. 2016). However, the intrinsic mechanisms controlling N stocks following grazing are less understood in this alpine environment. This requires more detailed investigation of N cycling processes such as microbial N mineralization and plant N acquisition.
Grazing effects on soil N mineralization
Mineral N that highly depends on microbial gross N mineralization (i.e., the ammonification process) and subsequent nitrification constitutes about 80% of plant-available N in Tibetan grasslands (Xu et al. 2006). We found that grazing decreased gross N mineralization (Fig. 2), causing lower contents of exchangeable NH4+ and NO3− in the soil (Fig. 1). Nitrogen mineralization is a microbially driven process and is largely determined by both quality and quantity of labile C compounds (Hart et al. 1994). Grazing decreased these labile C inputs because a permanent removal of photosynthetic shoots directly reduces rhizodeposition (Kuzyakov et al. 2002; Schmitt et al. 2013; Wei et al. 2016). Additionally, a higher allocation of C assimilates is required (a) to sustain shoot re-growth (the higher RGR, Fig. 4) and (b) to support the increasing root biomass (Fig. 3), with its associated C maintenance costs, leading to a low rhizodeposition. By means of 13C pulse labeling for the same study site, Ingrisch et al. (2015) showed that most of the 13C was recovered in the roots and only a minor portion (< 10%) remained in the soil after 2 months. Of secondary importance was that grazing caused changes to the chemical composition of roots and litter (e.g., lower cellulose, higher lignin/N ratio, and tannin content), restraining the C release via decomposition (Luo et al. 2009, 2010). We suggest that the lower input of labile C inhibited the growth and activity of microorganisms and enzyme production (Shahzad et al. 2012, 2015). Therefore, these had negative effects on gross N mineralization. Indeed, photosynthetic manipulations via clipping and shading also decreased gross N mineralization compared to the control, for both grazing regimes (Fig. 2). In summary, we confirmed our first hypothesis that intensive grazing suppresses microbial N mining and has a negative impact on gross N mineralization.
Since N mineralization is also restricted by abiotic environmental conditions such as low precipitation and temperature, decreasing gross N mineralization rates might aggravate prevailing N limitations, with respective feedbacks for plant and microbial N uptake (Song et al. 2007, 2011; Wang et al. 2012). In agreement with this, net N mineralization was negative, indicating a strong N limitation to both plants and microorganisms (Table S1; Lang et al. 2016). The correlation between gross N mineralization in soil and the N stock in plant biomass was weak under grazing, but it was strengthened by grazing exclusion (Fig. 2) due to the higher available N supply via the increased rate of gross mineralization. We suggest that increased grazing pressure may disturb the exchange between plant C input and microbial N releases, further exacerbating N limitation in this deficient, vulnerable ecosystem and decreasing C sequestration.
Grazing effects on plant growth
Several studies report that plants respond to grazing by increasing their growth rates to compensate for the lost tissues (Hilbert et al. 1981; Oesterheld and McNaughton 1991; Kuzyakov et al. 2002). This physiological mechanism is referred to as compensatory growth (Mcnaughton 1979; Wang et al. 2005a). We found that K. pygmaea increased the relative growth rate (RGR) of shoots after simulated grazing (clipping) and induced compensatory growth under both grazing regimes. An increase in the RGR of shoots was especially evident at the grazed sites, suggesting that K. pygmaea efficiently develops new photosynthetic biomass. A fast regrowth of shoots is an important plant trait to cover the high metabolic C costs of root maintenance (Poorter et al. 1990; Chapin et al. 2002). To sustain the continual renewal of shoot biomass, however, K. pygmaea faces increasing demands for resource uptake, which is especially challenging in these N and P co-limited environments (Wise and Abrahamson 2015; Miehe et al. 2017). From this perspective, K. pygmaea develops a large amount of root biomass in order to exploit nutrients at the times and depths of their availability. Indeed, Kobresia pygmea has a far higher root than shoot biomass, i.e., root to shoot ratio of ~ 8 for the ungrazed sites (in 0–10 cm), and the ratio can be even higher (up to 20) when the entire rooting zone is considered (ca. 0–25 cm, Li et al. 2008; Schleuss et al. 2015). Such root to shoot ratios are common in other grazing-driven ecosystems (Milchunas and Lauenroth 1993; Mcnaughton et al. 1998; Yan et al. 2013) and in cold and dry climates (e.g., arctic tundra, Callaghan et al. 2005), or for ecosystems with extremely low nutrient status (McConnaughay and Coleman 1999; Lambers et al. 2011). A very higher root biomass production or root activity is a trait of K. pygmaea to obtain nutrients (Schleuss et al. 2015) and to facilitate shoot re-growth under prevailing deficient nutrient conditions. Given a lower soil N availability at the grazed sites, we found that the root biomass (0–10 cm) was about 13% higher for grazed site than that for ungrazed site. Kobresia pygmaea even sustained root growth after clipping, presumably to counteract nutrient limitation (Chapin 1980; Sun and Wang 2016). In summary, a dense root network for nutrient uptake and efficient compensatory growth are important traits contributing to the high grazing tolerance of K. pygmaea. This particularly matters in this pastoral ecosystem, where plants have had to cope with grazing impacts for more than 8000 years (Miehe et al. 2009). These traits at least partly explain the wide dominance of K. pygmaea on the Tibetan Plateau (Miehe et al. 2017).
Grazing effects on plant N uptake and 15N partitioning
Although 15NH4+ can be lost via NH3 volatilization (Cameron et al. 2013) or via leaching after nitrification, more than 80% of added 15N was recovered in the upper plant-soil system after 28 days. About 40% of added 15N was recovered in total plant biomass. This is in a similar range to other studies for the Kobresia grasslands (Song et al. 2007; Xu et al. 2004, 2011a, b; Schleuss et al. 2015; Jiang et al. 2017) and indicates efficient plant N uptake in this N-limited ecosystem. Higher NH4+ uptake rates at grazed site than at ungrazed site are ascribed to upregulation of NH4+ transport systems (i.e., the most dominant N form) in K. pygmaea roots in response to grazing (McKane et al. 2002; Jackson et al. 2008; Nacry et al. 2013). Clipping even slightly increased the plant 15N recovery (shoot + root) at grazed sites. This agrees with our second hypothesis that grazing increases plant N acquisition to sustain shoot biomass (i.e., for compensatory growth). A negative correlation between total plant 15N recovery and soil exchangeable NH4+ content demonstrated that plants utilized NH4+ for re-growth (Table S3). In fact, the mineral soil N contents (NO3− + exchangeable NH4+, Fig. 1) were lower for grazed site than those for ungrazed site, presumably due to stimulated plant N uptake and decreasing N mineralization rates following grazing (Figs. 1 and 2). Since low N availability can constrain N absorption by roots, K. pygmaea increased root biomass to occupy larger soil volumes and to extend the root surface for acquiring as much N as possible, especially in response to grazing (Fig. 3). Consequently, a positive correlation between the total plant 15N recovery and the root biomass was observed for both grazing regimes (Table S3). Besides root biomass, grazing also increased root N uptake and N retention (as reserves) in roots (root 15N recovery: grazed > ungrazed, Fig. 5) and ultimately caused a higher belowground investment than for ungrazed sites. This is highly beneficial in this N and P co-limited environment because K. pygmaea can reallocate N stored in remnant leaves or roots to support re-growth after grazing (Freschet et al. 2015). In agreement with this, a linear correlation between RGR of shoots and root 15N recovery was observed for the grazed sites, but not for ungrazed sites (Fig. 4), which is consistent with our second hypothesis. Overall, our findings support the concept of very efficient above-belowground interaction of K. pygmaea in response to grazing (Schleuss et al. 2015). Grazing stimulates a higher N uptake rate for compensatory growth of photosynthetic shoots, to cover the high plant C costs. Simultaneously, K. pygmaea develops a dense root network with efficient N uptake mechanisms to meet the high nutrient demands for continually replacing lost shoots.
Conclusions
The soil N stock, N mineralization, and N availability in Kobresia pasture were decreased under intensive grazing. The decrease in N mineralization was attributed to the reduced belowground inputs of labile C due to decreased photosynthesis. This aggravated the prevailing N limitation, challenging plant N acquisition. Nevertheless, plant N uptake was higher at grazed site compared to that at ungrazed site, mainly due to the compensatory growth of lost photosynthetic organs. K. pygmaea has evolved specific traits (efficient above-belowground interactions) to cope with nutrient limitations and simultaneous grazing pressure. However, intensive grazing disturbs the critical balance between plant C input and microbial N mineralization (Fig. 6). This might establish a negative feedback to plant productivity over the long term. Therefore, a reduction of grazing pressure (moderate grazing or short-term grazing exclusion) benefits soil fertility, C sequestration, and overall ecosystem stability.
Several previous studies have reported positive effects of moderate grazing on N cycles. From this perspective, the traditional migratory grazing system of the Tibetan nomads seems to be well adapted to maintain grassland stability, despite low levels of ecosystem productivity. The increase in stocking numbers due to sedentarization programs on the one hand and the long-term complete grazing exclusion by fencing programs on the other might both have contributed to the vast changes in pasture conditions (i.e., changing vegetation composition, intensified degradation). This study, therefore, provides mechanistic knowledge to understand the existence, resilience, functioning, and future development of this sensitive and vulnerable ecosystem against the background of land use and climate changes. Further research that compensates the mechanisms of ecosystem vulnerability (e.g., the roles of plant-microbial interactions, microbial communities, and soil fauna) is required, focusing on the tipping point regarding ecosystem resilience under variable degree of grazing intensity and pasture management to avoid serious degradation of these unique alpine ecosystems.
References
Babel W, Biermann T, Coners H, Falge E, Seeber E, Ingrisch J, Schleuß PM, Gerken T, Leonbacher J, Leipold T, Willinghöfer S, Schützenmeister K, Shibistova O, Becker L, Hafner S, Spielvogel S, Li X, Xu XL, Sun Y, Zhang L, Yang Y, Ma Y, Wesche K, Graf HF, Leuschner C, Guggenberger G, Kuzyakov Y, Miehe G, Foken T (2014) Pasture degradation modifies the water and carbon cycles of the Tibetan highlands. Biogeosciences 11:6633–6656. https://doi.org/10.5194/bg-11-6633-2014
Callaghan TV, Björn LO, Chaplin III FS, Chernov Y, Christensen TR, Huntley B, Ims R, Johansson M, Riedlinger DJ, Jonasson S, Matveyeva N (2005) Arctic tundra and polar desert ecosystems. Arctic Climate Impact Assessment 1:243–352
Callaway RM, Brooker RW, Choler P, Kikvidze Z, Lortie CJ, Michalet R, Paolini L, Pugnaire FI, Newingham B, Aschehoug ET, Armas C, Kikodze D, Cook BJ (2002) Positive interactions among alpine plants increase with stress. Nature 417:844–848. https://doi.org/10.1038/nature00805.1
Cameron KC, Di HJ, Moir JL (2013) Nitrogen losses from the soil/plant system: a review. Ann Appl Biol 162:145–173. https://doi.org/10.1111/aab.12014
Chapin III FS (1980) The mineral nutrition of wild plants. Annu Rev Ecol Syst 11:233–260
Chapin FS, Matson P, Mooney HA (2002) Principles of terrestrial ecosystem ecology. Springer, New York
Chen H, Zhu Q, Peng C, Wu N, Wang Y, Fang X, Gao Y, Zhu D, Yang G, Tian J, Kang X, Piao S, Ouyang H, Xiang W, Luo Z, Jiang H, Song X, Zhang Y, Yu G, Zhao X, Gong P, Yao T, Wu JH (2013) The impacts of climate change and human activities on biogeochemical cycles on the Qinghai-Tibetan Plateau. Glob Chang Biol 19:2940–2955. https://doi.org/10.1111/gcb.12277
Chen B, Zhang X, Tao J, Wu J, Wang J, Shi P, Zhang Y, Yu C (2014) The impact of climate change and anthropogenic activities on alpine grassland over the Qinghai-Tibet Plateau. Agric For Meteorol 189:11–18. https://doi.org/10.1016/j.agrformet.2014.01.002
Cleveland CC, Neff JC, Townsend AR, Hood E (2004) Composition, dynamics, and fate of leached dissolved organic matter in terrestrial ecosystems: results from a decomposition experiment. Ecosystems 7:275–285. https://doi.org/10.1007/s10021-003-0236-7
Cui X, Graf HF, Langmann B, Cui XF, Graf HF, Langmann B, Chen W, Huang RH (2006) Climate impacts of anthropogenic land use changes on the Tibetan Plateau. Glob Planet Change 54:33–56. https://doi.org/10.1016/j.gloplacha.2005.07.006
Davidson EA, Hart SC, Shanks CA, Firestone MK (1991) Measuring gross nitrogen mineralization, immobilization, and nitrification by N15 isotopic pool dilution in intact soil cores. J Soil Sci 42:335–349. https://doi.org/10.1111/j.1365-2389.1991.tb00413.x
Detling JK, Dyer MI, Winn DT(1979) Net photosynthesis, root respiration, and regrowth of Bouteloua gracilis following simulated grazing. Oecologia 41:127–34
Dijkstra FA, Jenkins M, de Rémy de Courcelles V, Keitel C, Barbour MM, Kayler ZE, Adams MA (2016) Enhanced decomposition and nitrogen mineralisation sustain rapid growth of Eucalyptus regnans after wildfire. J Ecol 105:229–236. https://doi.org/10.1111/1365-2745.12663
Farrar JF, Jones DL (2000) The control of carbon acquisition by roots. New Phytol 147:43–53
Feng Y, Zhang X, Shi P, Fu G, Zhang Y, Zhao G, Zeng C, Zhang J (2017) Livestock dynamic responses to climate change in alpine grasslands on the northern Tibetan plateau: forage consumption and time-lag effects. J Resour Ecol 8:88–96. https://doi.org/10.5814/j.issn.1674-764x.2017.01.011
Freschet GT, Swart EM, Cornelissen JHC (2015) Integrated plant phenotypic responses to contrasting above- and below-ground resources: key roles of specific leaf area and root mass fraction. New Phytol 206:1247–1260. https://doi.org/10.1111/nph.13352
Hafner S, Unteregelsbacher S, Seeber E, Lena B, Xu X, Li X, Guggenberger G, Miehe G, Kuzyakov Y (2012) Effect of grazing on carbon stocks and assimilate partitioning in a Tibetan montane pasture revealed by 13CO2 pulse labeling. Glob Chang Biol 18:528–538. https://doi.org/10.1111/j.1365-2486.2011.02557.x
Harris RB (2010) Rangeland degradation on the Qinghai-Tibetan plateau: a review of the evidence of its magnitude and causes. J Arid Environ 74:1–12. https://doi.org/10.1016/j.jaridenv.2009.06.014
Hart SC, Nason GE, Myrold DD, Perry DA (1994) Dynamics of gross nitrogen transformations in an old-growth forest. Ecology 75:880–891
He N, Zhang Y, Yu Q, Chen Q, Pan Q, Zhang G, Han X (2011) Grazing intensity impacts soil carbon and nitrogen storage of continental steppe. Ecosphere 2:art8. https://doi.org/10.1890/ES10-00017.1
Hilbert DW, Swift DM, Detling JK, Dyer MI (1981) Relative growth rates and the grazing optimization hypothesis. Oecologia 51:14–18. https://doi.org/10.1007/BF00344645
Hu Z, Li S, Guo Q, Niu S, He N, Li L, Yu G (2016) A synthesis of the effect of grazing exclusion on carbon dynamics in grasslands in China. Glob Chang Biol 22:1385–1393. https://doi.org/10.1111/gcb.13133
Hunt R, Causton DR, Shipley B, Askew AP (2002) A modern tool for classical plant growth analysis. Ann Bot 90:485–488. https://doi.org/10.1093/aob/mcf214
Ingrisch J, Biermann T, Seeber E, Ingrisch J, Biermann T, Seeber E, Leipold T, Li M, Ma Y, Xu X, Miehe G, Guggenberger G, Foken T, Kuzyakov Y (2015) Carbon pools and fluxes in a Tibetan alpine Kobresia pygmaea pasture partitioned by coupled eddy-covariance measurements and 13CO2 pulse labeling. Sci Total Environ 505:1213–1224. https://doi.org/10.1016/j.scitotenv.2014.10.082
IUSS Working Group WRB (2006) World reference base for soil resources. World Soil Resources Report 103. FAO, Rome
Jackson LE, Burger M, Cavagnaro TR (2008) Roots nitrogen transformations, and ecosystem services. Annu Rev Plant Biol 59:341–363. https://doi.org/10.1146/annurev.arplant.59.032607.092932
Jiang L, Wang S, Zhe P, Wang C, Kardol P, Zhong L, Yu Q, Lan Z, Wang Y, Xu X, Kuzyakov Y, Luo C, Zhang Z, Jones D (2017) Effects of grazing on the acquisition of nitrogen by plants and microorganisms in an alpine grassland on the Tibetan plateau. Plant Soil 416:297–308. https://doi.org/10.1007/s11104-017-3205-1
Jones D, Nguyen C, Finlay RD (2009) Carbon flow in the rhizosphere: carbon trading at the soil–root interface. Plant Soil 321:5–33. https://doi.org/10.1007/s11104-009-9925-0
Kaiser K, Miehe G, Barthelmes A, Wang C, Kardol P, Zhong L, Yu Q, Lan Z, Wang Y, Xu X, Kuzyakov Y, Luo C, Zhang Z, Jones D (2008) Turf-bearing topsoils on the central Tibetan Plateau, China: pedology, botany, geochronology. Catena 73:300–311. https://doi.org/10.1016/j.catena.2007.12.001
Kang L, Han X, Zhang Z, Sun O (2007) Grassland ecosystems in China: review of current knowledge and research advancement. Philos Trans R Soc B 362:997–1008. https://doi.org/10.1098/rstb.2007.2029
Kuzyakov Y (2002) Review: factors affecting rhizosphere priming effects. J Plant Nutr Soil 165:382–396. https://doi.org/10.1002/1522-2624(200208)165:4<382::AID-JPLN382>3.0.CO;2-#
Kuzyakov Y, Xu X (2013) Competition between roots and microorganisms for nitrogen: mechanisms and ecological relevance. New Phytol 198:656–669. https://doi.org/10.1111/nph.12235
Kuzyakov Y, Biryukova OV, Kuznetzova TV, Molter K, Kandeler E, Stahr K (2002) Carbon partitioning in plant and soil, carbon dioxide fluxes and enzyme activities as affected by cutting ryegrass. Biol Fertil Soils 35:348–358. https://doi.org/10.1007/s00374-002-0480-6
Lambers H, Brundrett MC, Raven JA, Hopper SD (2011) Plant mineral nutrition in ancient landscapes: high plant species diversity on infertile soils is linked to functional diversity for nutritional strategies. Plant Soil 348:7–27. https://doi.org/10.1007/s11104-011-0977-6
Lang M, Li P, Han X, Han XZ, Qiao YF, Miao SJ (2016) Gross nitrogen transformations in black soil under different land uses and management systems. Biol Fertil Soils 52:233–241. https://doi.org/10.1007/s00374-015-1070-8
Li Y, Luo T, Lu Q (2008) Plant height as a simple predictor of the root to shoot ratio: evidence from alpine grasslands on the Tibetan Plateau. J Veg Sci 19:245–252. https://doi.org/10.3170/2007-8-18365
Lin X, Han P, Zhang W, Wang G (2017) Sensitivity of alpine grassland carbon balance to interannual variability in climate and atmospheric CO2 on the Tibetan Plateau during the last century. Glob Planet Chang 154:23–32. https://doi.org/10.1016/j.gloplacha.2017.05.008
Luo C, Xu G, Wang Y, Wang S, Lin X, Hu Y, Zhang Z, Chang X, Duan J, Su A, Zhao X (2009) Effects of grazing and experimental warming on DOC concentrations in the soil solution on the Qinghai-Tibet plateau. Soil Biol Biochem 41:2493–2500. https://doi.org/10.1016/j.soilbio.2009.09.006
Luo C, Xu G, Chao Z, Wang S, Lin X, Hu Y, Zhang Z, Duan J, Chang X, Su A, Li Y, Zhao X, Du M, Tang Y, Kimball B (2010) Effect of warming and grazing on litter mass loss and temperature sensitivity of litter and dung mass loss on the Tibetan plateau. Glob Chang Biol 16:1606–1617. https://doi.org/10.1111/j.1365-2486.2009.02026.x
McConnaughay KDM, Coleman JS (1999) Biomass allocation in plants: ontogeny or optimality? A test along three resource gradients. Ecology 80:2581–2593. https://doi.org/10.1890/0012-9658(1999)080[2581:BAIPOO]2.0.CO;2
McKane RB, Johnson LC, Shaver GR, Nadelhoffer KJ, Rastetter EB, Fry B, Giblin AE, Kielland K, Kwiatkowski BL, Laundre JA, Murray G (2002) Resource-based niches provide a basis for plant species diversity and dominance in arctic tundra. Nature 415:68–71. https://doi.org/10.1038/415068a
Mcnaughton SJ (1979) Grazing as an optimization process: grass-ungulate relationships in the Serengeti. Source Am Nat 113:691–703. https://doi.org/10.1086/283426
Mcnaughton SJ, Banyikwa FF, Mcnaughton MM (1998) Root biomass and productivity in a grazing ecosystem: the Serengeti. Ecology 79:587–592. https://doi.org/10.1890/0012-9658(1998)079[0587:RBAPIA]2.0.CO;2
Miehe G, Miehe S, Kaiser K, Liu J, Zhao X (2008) Status and dynamics of the Kobresia pygmaea ecosystem on the Tibetan plateau. Ambio 37:272–279. https://doi.org/10.1579/0044-7447(2008)37[272:SADOTK]2.0.CO;2
Miehe G, Miehe S, Kaiser K, Reudenbach C, Behrendes L, La D, Schlütz F (2009) How old is pastoralism in Tibet? An ecological approach to the making of a Tibetan landscape. Palaeogeogr Palaeoclimatol Palaeoecol 276:130–147. https://doi.org/10.1016/j.palaeo.2009.03.005
Miehe G, Miehe S, Bach K, Nölling J, Hanspach J, Reudenbach C, Kaiser K, Wesche K, Mosbrugger V, Yang Y, Ma Y (2011) Plant communities of central Tibetan pastures in the Alpine Steppe/Kobresia pygmaea ecotone. J Arid Environ 75:711–723. https://doi.org/10.1016/j.jaridenv.2011.03.001
Miehe G, Miehe S, Böhner J, Kaiser K, Hensen I, Madsen D, Liu J, Opgenoorth L (2014) How old is the human footprint in the world’s largest alpine ecosystem? A review of multiproxy records from the Tibetan Plateau from the ecologists’ viewpoint. Quat Sci Rev 86:190–209. https://doi.org/10.1016/j.quascirev.2013.12.004
Miehe G, Schleuss P, Seeber E, Babel W, Biermann T, Braendle M, Chen F, Coners H, Foken T, Gerken T, Graf HF, Guggenberger G, Hafner S, Holzapfel M, Ingrisch J, Kuzyakov Y, Lai Z, Lehnert L, Leuschner C, Liu J, Liu S, Ma Y, Miehe S, Mosbrugger V, Noltie HJ, Opgenoorth L, Schmidt J, Spielvogel S, Unteregelsbacher S, Wang Y, Willinghofer S, Xu X, Yang Y, Zhang S, Wesche K (2017) The Kobresia pygmaea ecosystem of the Tibetan highlands—origin, functioning and degradation of the world’s largest pastoral alpine ecosystem. bioRxiv. https://doi.org/10.1101/135558
Mikan CJ, Zak DR, Kubiske ME, Pregitzer KS (2000) Combined effects of atmospheric CO2 and N availability on the belowground carbon and nitrogen dynamics of aspen mesocosms. Oecologia 124:432–445. https://doi.org/10.1007/PL00008869
Milchunas DG, Lauenroth WK (1993) Quantitative effects of grazing on vegetation and soils over a global range of environments. Ecol Monogr 63:327–366. https://doi.org/10.2307/2937150
Murphy D, Recous S, Stockdale E, Fillery IRP, Jensen LS, Hatch DJ, Goulding KWT (2003) Gross nitrogen fluxes in soil: theory, measurement and application of 15Npool dilution techniques. Adv Agron 79:69–118. https://doi.org/10.1016/S0065-2113(02)79002-0
Nacry P, Bouguyon E, Gojon A (2013) Nitrogen acquisition by roots: physiological and developmental mechanisms ensuring plant adaptation to a fluctuating resource. Plant Soil 370:1–29. https://doi.org/10.1007/s11104-013-1645-9
Oesterheld M, McNaughton SJ (1991) Effect of stress and time for recovery on the amount of compensatory growth after grazing. Oecologia 85:305–313. https://doi.org/10.1007/BF00320604
Paterson E, Sim A (2000) Effect of nitrogen supply and defoliation on loss of organic compounds from roots of Festuca rubra. J Exp Bot 51:1449–1457. https://doi.org/10.1093/jexbot/51.349.1449
Poorter H, Remkes C, Lambers H (1990) Carbon and nitrogen economy of 24 wild species differing in relative growth rate. Plant Physiol 94:621–627
Robinson D (2001) δ15N as an integrator of the nitrogen. Trends Ecol Evol 16:153–162. https://doi.org/10.1016/s0169-5347(00)02098-x
Schleuss PM, Heitkamp F, Sun Y, Miehe G, Xu X, Kuzyakov Y (2015) Nitrogen uptake in an alpine Kobresia pasture on the Tibetan Plateau: localization by 15N labeling and implications for a vulnerable ecosystem. Ecosystems 18:946–957. https://doi.org/10.1007/s10021-015-9874-9
Schmitt A, Pausch J, Kuzyakov Y (2013) Effect of clipping and shading on C allocation and fluxes in soil under ryegrass and alfalfa estimated by 14C labelling. Appl Soil Ecol 64:228–236. https://doi.org/10.1016/j.apsoil.2012.12.015
Seeber E, Miehe G, Hensen I, Yang Y, Wesche K (2015) Mixed reproduction strategy and polyploidy facilitate dominance of Kobresia pygmaea on the Tibetan Plateau. J Plant Ecol 9:87–99. https://doi.org/10.1093/jpe/rtv035
Shahzad T, Chenu C, Repinçay C, Mougin C, Ollier JL, Fontaine S (2012) Plant clipping decelerates the mineralization of recalcitrant soil organic matter under multiple grassland species. Soil Biol Biochem 51:73–80. https://doi.org/10.1016/j.soilbio.2012.04.014
Shahzad T, Chenu C, Genet P, Barot S, Perveen N, Mougin C, Fontaine S (2015) Contribution of exudates, arbuscular mycorrhizal fungi and litter depositions to the rhizosphere priming effect induced by grassland species. Soil Biol Biochem 80:146–155. https://doi.org/10.1016/j.soilbio.2014.09.023
Song M, Xu X, Hu Q, Tian Y, Ouyang H, Zhou C (2007) Interactions of plant species mediated plant competition for inorganic nitrogen with soil microorganisms in an alpine meadow. Plant Soil 297:127–137. https://doi.org/10.1007/s11104-007-9326-1
Song MH, Jiang J, Xu XL, Shi PL (2011) Correlation between CO2 efflux and net nitrogen mineralization and its response to external C or N supply in an alpine meadow soil. Pedosphere 21:666–675. https://doi.org/10.1016/S1002-0160(11)60169-0
Su X, Wu Y, Dong S, Wen L, Li Y, Wang X (2015) Effects of grassland degradation and re-vegetation on carbon and nitrogen storage in the soils of the Headwater Area Nature Reserve on the Qinghai-Tibetan Plateau, China. J Mt Sci 12:582–591. https://doi.org/10.1007/s11629-014-3043-z
Sun J, Wang H (2016) Soil nitrogen and carbon determine the trade-off of the above- and below-ground biomass across alpine grasslands, Tibetan Plateau. Ecol Indic 60:1070–1076. https://doi.org/10.1016/j.ecolind.2015.08.038
van Reeuwijk LP (2002) Procedures for soil analysis. Technical Paper 9. 6th edn. International Soil Reference and In- formation Centre (ISRIC), Food and Agriculture Organization of the United Nations (FAO), Wageningen, pp. 120
Wang S, Niu H, Cui X, Jiang S, Li Y, Xiao X, Wang J, Wang G, Huang D, Qi Q, Yang Z (2005a) Plant communities: ecosystem stability in Inner Mongolia. Nature 435:E5-6–E5-7. https://doi.org/10.1038/nature03862
Wang W, Wang Q, Wang C, Shi H, Li Y, Wang G (2005b) The effect of land management on carbon and nitrogen status in plants and soils of alpine meadows on the Tibetan Plateau. Land Degrad Dev 16:405–415. https://doi.org/10.1002/ldr.661
Wang S, Duan J, Xu G, Wang Y, Zhang Z, Rui Y, Luo C, Xu B, Zhu X, Chang X, Cui X (2012) Effects of warming and grazing on soil N availability, species composition, and ANPP in an alpine meadow. Ecology 93:2365–2376. https://doi.org/10.1890/11-1408.1
Wang C, Wang G, Wang Y, Rafique R, Ma L, Hu L, Luo Y (2015) Urea addition and litter manipulation alter plant community and soil microbial community composition in a Kobresia humilis meadow. Eur J Soil Biol 70:7–14. https://doi.org/10.1016/j.ejsobi.2015.06.003
Wang J, Zhang J, Müller C, Cai Z (2016) Temperature sensitivity of gross N transformation rates in an alpine meadow on the Qinghai-Tibetan Plateau. J Soils Sediments 17:423–431. https://doi.org/10.1007/s11368-016-1530-2
Wei Y, Chen Q (2001) Grassland classification and evaluation of grazing capacity in Naqu Prefecture, Tibet Autonomous Region, China. N Z J Agric Res 44:253–258. https://doi.org/10.1080/00288233.2001.9513482
Wei J, Liu W, Wan H, Cheng J, Li W (2016) Differential allocation of carbon in fenced and clipped grasslands: a 13C tracer study in the semiarid Chinese Loess Plateau. Plant Soil 406:251–263. https://doi.org/10.1007/s11104-016-2879-0
Wise MJ, Abrahamson WG (2015) Resource levels continuum: environmental the compensatory Beyond of herbivory and plant tolerance. Oikos 109:417–428
Xu X, Ouyang H, Cao G, Richter A, Wanek W, Kuzyakov Y (2004) Nitrogen deposition and carbon sequestration in alpine meadows. Biogeochemistry 71:353–369. https://doi.org/10.1007/s10533-004-0371-z
Xu X, Ouyang H, Kuzyakov Y, Richter A, Wanek W (2006) Significance of organic nitrogen acquisition for dominant plant species in an alpine meadow on the Tibet plateau, China. Plant Soil 285:221–231. https://doi.org/10.1007/s11104-006-9007-5
Xu X, Ouyang H, Cao G et al (2011a) Dominant plant species shift their nitrogen uptake patterns in response to nutrient enrichment caused by a fungal fairy in an alpine meadow. Plant Soil 341:495–504. https://doi.org/10.1007/s11104-010-0662-1
Xu X, Ouyang H, Richter A, Wanek W, Cao G, Kuzyakov Y (2011b) Spatio-temporal variations determine plant-microbe competition for inorganic nitrogen in an alpine meadow. J Ecol 99:563–571. https://doi.org/10.1111/j.1365-2745.2010.01789.x
Xu X, Wanek W, Zhou C, Richter A, Song M, Cao G, Ouyang H, Kuzyakov Y (2014) Nutrient limitation of alpine plants: implications from leaf N: P stoichiometry and leaf δ15N. J Plant Nutr Soil Sci 177:378–387. https://doi.org/10.1002/jpln.201200061
Yan L, Zhou G, Zhang F (2013) Effects of different grazing intensities on grassland production in China: a meta-analysis. PLoS One 8. https://doi.org/10.1371/journal.pone.0081466
Yang Y, Fang J, Tang Y, Ji C, Zheng C, He J, Zhu B (2008) Storage, patterns and controls of soil organic carbon in the Tibetan grasslands. Glob Chang Biol 14:1592–1599. https://doi.org/10.1111/j.1365-2486.2008.01591.x
Yu C, Zhang X, Zhang J, Li S, Song C, Fang Y, Wurst S, Wu J (2016) Grazing exclusion to recover degraded alpine pastures needs scientific assessments across the northern Tibetan plateau. Sustainability 8:1162. https://doi.org/10.3390/su8111162
Zhang T, Zhang Y, Xu M, Zhu J, Wimberly MC, Yu G, Niu S, Xi Y, Zhang X, Wang J (2015) Light-intensity grazing improves alpine meadow productivity and adaption to climate change on the Tibetan Plateau. Sci Rep 5:15949. https://doi.org/10.1038/srep15949
Zhong L, Zhou X, Wang Y, Li F, Zhou S, Bai Y, Rui Y (2017) Mixed grazing and clipping is beneficial to ecosystem recovery but may increase potential N2O emissions in a semi-arid grassland. Soil Biol Biochem 114:42–51. https://doi.org/10.1016/j.soilbio.2017.07.002
Zhou H, Zhao X, Tang Y, Gu S, Zhou L (2005) Alpine grassland degradation and its control in the source region of the Yangtze and Yellow Rivers, China. Jpn Soc Grassl Sci 51:191–203. https://doi.org/10.1111/j.1744-697x.2005.00028.x
Zhou G, Zhou X, He Y, Shao J, Hu Z, Liu R, Zhou H, Hosseinibai S (2016) Grazing intensity significantly affects belowground carbon and nitrogen cycling in grassland ecosystems: a meta-analysis. Glob Chang Biol 23:1167–1179. https://doi.org/10.1111/gcb.13431
Zong N, Shi P, Song M, Zhang X, Jiang J, Chai X (2016) Nitrogen critical loads for an alpine meadow ecosystem on the Tibetan Plateau. Environ Manag 57:531–542. https://doi.org/10.1007/s00267-015-0626-6
Acknowledgements
We thank the German KEMA research team and Chinese colleagues from the Institute of Tibetan Plateau Research (ITP) for their help in the field. We also thank Jingrong Yang, Jingfeng Bu, and Qianru Li for their help in the laboratory and Kyle Mason-Jones, Yun Wang, and Jiarui Gan for their helpful suggestions on this manuscript.
Funding
This study was supported by the National Key Research and Development Program of China (Grant No. 2016YFC0501802), the National Natural Science Foundation of China (31470560; 41671253), and the German Research Council (DFG) DFG KU 1184/14 within the Priority Program 1372 “Tibetan Plateau: Formation, Climate, Ecosystems” (TiP) and the China Scholarship Council (CSC) to Yue Sun.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
ESM 1
(DOCX 3.14 mb)
Rights and permissions
About this article
Cite this article
Sun, Y., Schleuss, PM., Pausch, J. et al. Nitrogen pools and cycles in Tibetan Kobresia pastures depending on grazing. Biol Fertil Soils 54, 569–581 (2018). https://doi.org/10.1007/s00374-018-1280-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00374-018-1280-y