Abstract
Background and aims
Olive mill wastewater (OMW) generated in Mediterranean countries is partly disposed of on soil. Its underlying fate mechanisms and influences on plant growth are still largely unknown. Our goal was to understand OMW organic matter (OMW-OM) degradation in soil and its phytotoxic effects. We hypothesized that OMW phytotoxicity decreased with degradation of its phenolic components.
Methods
In a 60 day incubation study, we monitored soil respiration, extractable total phenolic content (TPC) and carbon isotope ratio (δ13C) of OMW treated Israeli soil. The soil was extracted using accelerated solvent extraction (ASE) and its extracts were exemplarily analyzed for four phenolic substances by LC/MS. Phytotoxicity of soil and soil extracts were tested using a Lepidium sativum seed germination bioassay.
Results
Soil respiration was 2.5 times higher for OMW treated soil with two respiration maxima and indicated a degradation of up to 27 % of the added OMW-OM. Four phases of OMW-OM degradation were identified: (i) degradation of easily degradable OMW-OM and transformation of phenolic compounds, (ii) intermediate suppression of phytotoxicity, (iii) degradation of phytotoxic phenolic compounds and (iv) significant physical immobilization of phytotoxic compounds.
Conclusion
Environmental conditions during and after OMW disposal on soil ought to favor fast degradation of OMW-OM, minimizing their physical immobilization and phytotoxic effects.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Mediterranean countries such as Israel, Italy and Spain generate large amounts of olive mill wastewater (OMW) during the olive oil production process. This results in 1 – 1.2 m3 OMW per ton of processed olives for three-phase systems (Saadi et al. 2007; Hanifi 2009). OMW from three-phase systems is typically acidic, has high biological and chemical oxygen demands, high organic matter and mineral content and contains growth-inhibiting substances such as lipids and phenols (Saadi et al. 2007; Di Serio et al. 2008). As it is toxic for some microorganisms, OMW disposal by sewage treatment plants is not accepted (Khatib et al. 2009). For that reason, the direct application on soil is one of the most favored treatment methods with up to 1.5 million tons of disposed OMW in the Mediterranean area per year (Arapoglou et al. 2010). To avoid its uncontrolled disposal on soil, the OMW application is limited in several Mediterranean regions. The existing legal framework shows a lack of common policy and guidance for the OMW management among the existing olive-oil producing countries (Kapellakis et al. 2008). The application amount of OMW to soils is limited in several Mediterranean regions. For example, Italian laws permit an annual spreading of 50 – 80 m3 ha−1 a−1, the Ministry of Environmental Protection in Israel recommends up to 100 m3 ha−1 a−1 (Saadi et al. 2007; Laor et al. 2011). In both cases, the quantities of disposed OMW are considered as a low cost method with fertilizing properties and no harmful effects to crops and soil (Chartzoulakis et al. 2010; Saadi et al. 2007). In spite of the restricted OMW disposal on soil, an uncontrolled disposal at even higher quantities than recommended must be assumed (Kavvadias et al. 2014).
Several studies have addressed the effects of OMW on several parameters such as physico-chemical soil characteristics, seed germination and plant growth (see Barbera et al. 2013 for a detailed overview). Most studies concluded that OMW application on soil can have positive effects on soil structure (e .g. increased aggregate stability and reduced run-off). However, negative environmental impacts of OMW spreading have also been reported, although the results of recent studies are sometimes contradictory and depending on soil parameters, test organisms and application rate (Deeb et al. 2012). Negative effects on physico-chemical soil properties, e. g. accumulation of salts and reduced water infiltration (Mahmoud et al. 2010), phytotoxic effects on crops (Di Serio et al. 2008) and potential groundwater pollution (Boukhoubza et al. 2008), are limiting constrains for the application of OMW on soil. OMW is also characterized by a relatively slow degradation and biological mineralization for some of its organic substances (Saviozzi et al. 1991). Nevertheless, organic substances from OMW (OMW organic matter ‘OMW-OM’) can provide energy and act as carbon source for the growth of soil microorganisms (Piotrowska et al. 2006). The increase of microbial activity, commonly measured by soil respiration, is one of the most important and characteristic indicators for soil microbiological metabolism and soil health (Sparling 1997; Nielsen and Winding 2002).
The knowledge of time-dependent degradation dynamics in soil after carbon input can give detailed information on the discrimination and decomposition of certain compound groups (Wlodarczyk et al. 2008). In this context, the changes of δ13C-signatures with time can be used to describe the degradation of organic substances from certain external sources and throughout different environmental compartments (Macko et al. 1987; Staddon 2004). Different δ13C-signatures have also been described for substance groups from olive trees (Bianchi et al. 1993; Angerosa et al. 1999). Additionally, the knowledge of time-dependent OMW (bio-) availability in soil can be useful to estimate effects on plant growth and might give reasonable solutions regarding the appropriate application of OMW on soil. To our best knowledge, only little is known about the interplay between physico-chemical immobilization processes and the degradation of OMW constituents in soil. Soil extractions using selective organic solvents such as ethyl acetate, hexane or methanol are used to mimic or predict the bioavailable part of organic compounds in soil (Kelsey et al. 1997; Tao et al. 2006; Bogolte et al. 2007), but have not been applied on OMW-polluted soils yet. Among different extraction techniques, accelerated solvent extraction (ASE) is one of the most promising tools due to reduced sample preparation time, constant extraction conditions and a fully automated and programmable extraction process (Fisher et al. 1997).
The objective of this incubation study was to obtain first insights into the degradation processes of OMW-OM in soil, their mechanisms and their time-dependent relation with phytotoxic effects. The amount of applied OMW was set higher than the amount recommended by the Ministry of Environmental Protection in Israel for one single application on the field (up to 10 L m−2). With this, simulated a maximum effect expected for controlled OMW application under the consideration that the OMW composition varies between sites and years. We furthermore aimed to be able to account for the expected higher amounts applied through uncontrolled OMW application. We monitored the changes in soil respiration activity, δ13C, extractable total phenolic content (TPC) and concentrations of selected target phenolic compounds of OMW treated soil. Until now, OMW-OM degradation and its phytotoxicity in soil have not been monitored via these parameters. Effects on Lepidium sativum (English garden cress) growth were investigated to assess the bioavailability and phytotoxicity of OMW derived compounds in OMW treated soil and its extracts. If OMW derived, phenolic substances are the main reason for phytotoxic effects on plants, the TPC in OMW treated soil and its extracts should be related with changes in the phytotoxicity. Further assuming that phenolic substances are less easily degradable, they should remain longer times in soil compared to other organic substances from OMW, resulting in a high toxicity level at the beginning of the incubation experiment. If our assumptions were right, the subsequent degradation of those phenolic substances should come along with a decrease of phytotoxicity and TPC in OMW treated soil and its extracts over time.
Materials and methods
Soil and OMW
For the incubation experiment, soil from an olive orchard near Gilat, South Israel, and OMW from a three-phase system near Revivim, Israel was used. After sampling, the soil was dried, sieved to 2 mm and stored in plastic boxes. OMW was stored at – 15 °C until used.
Physico-chemical properties of the soil and OMW were determined in triplicate according to typical standard methods. Soil texture was determined by sieving and pipette method according to DIN ISO 11277 (1998). Soil dry density was measured according to DIN ISO 11272 (2001). Elemental analyses of the soil samples were done using an elemental analyzer (Vario micro cube, Elementar Analysensysteme GmbH, Germany). Organic carbon was analyzed by elemental analyzer after adding hydrochloric acid (4 mol L-1) to the sample and drying it at 70 °C until constant weight (DIN ISO 10694 1996). Carbon isotope ratio of the soil and OMW was determined as described in section 2.5. Soil and OMW pH were determined according to DIN ISO 11265 (1997) and DIN ISO 38404–5 (2009), respectively. TPC was determined using the Folin-Ciocalteu reagent (Sigma-Aldrich, Germany) as described in detail below. Thermogravimetric Analysis (TGA) for both soil and OMW were performed using a STA 449 F3 Jupiter system (Netzsch, Germany). TG balances were verified by mass losses of calcium carbonate. Soil and OMW properties are presented in Table 1.
Incubation experiment
In total, 96 sub-samples (each 10 g) of Israeli soil were transferred into jars with screw caps and spiked with 2.70 mL demineralized water to reach a matrix potential of -60 hPa. The samples were pre-incubated at 15 °C for 14 days to simulate Israeli winter/ early spring conditions during microorganism acclimatization. After pre-incubation, the soil samples were air-dried overnight and 0.31 mL OMW (14 L m−2) or demineralized water per gram dry soil were applied to each soil sample. Afterwards, the samples were incubated at 15 °C and their soil moisture was measured every 72 h. Incubation temperature was monitored every 300 s throughout the whole incubation time using a Dostmann LOG 111 data logger (Dostmann electronic, Wertheim-Reicholzheim, Germany). At defined time steps, two control samples and six OMW treated samples were used for the measurements described below.
Soil respiration was determined according to DIN ISO 16072 (2005). The released CO2 during the incubation was continuously absorbed into 20 mL of 0.05 M NaOH solution. The latest every 72 h, the adsorbed CO2 within the NaOH solution was precipitated with 2 mL of 0.5 M BaCl2 solution and titrated with 0.1 M HCl using 2 – 3 drops of phenolphthalein indicator. All samples were corrected against a blank in triplicate (20 mL NaOH in an empty incubation vessel). The overall soil respiration is given as total CO2-equivalents, the soil respiration rate as daily CO2-equivalents in relation to the mass of dry and untreated soil.
Soil extractions and soil phenolic content determination
For the extraction of organic substances from OMW-OM in soil, organic solvent extractions were performed using a Dionex ASE 350 accelerated solvent extraction system (Thermo Fisher Scientific, Waltham, USA). At each time step, sequential extractions were performed with three OMW treated soil replicates and one control sample using hexane (Hex) at 140 °C, followed by methanol (MeOH) at 140 °C. For each extraction step, a 7 min warm-up was conducted, followed by 5 min static extraction. After the sequential extractions, each extract was concentrated to a volume of 1.5 mL by a slight nitrogen stream.
TPC of each concentrated soil extract was determined by Folin-Ciocalteu (FC) method according to Box (1983) and Li et al. (2007) in a modified way. 200 μL of the concentrated extract were added to 1 mL of 1:10 dH2O-diluted Folin-Ciocalteu reagent. After 4 min, 800 μL of saturated sodium carbonate solution (75 g L−1) were added. Absorbance was measured after 1 h at 760 nm against a matrix blank using a Specord 50 UV/VIS spectrometer (Analytik Jena, Jena, Germany). To evaluate the photosensitivity of the FC reagent towards different phenolic compounds, calibration curves with gallic acid, tyrosol, caffeic acid, coumaric acid and protocatechuric acid were prepared. Gallic acid (0 – 500 mg L−1) was used as standard calibration curve for the TPC calculations. Results are presented in mg gallic acid units (GAU) per gram dry soil.
For semi-quantitative target analysis of phenolic compounds, soil extracts were exemplarily analyzed for four substances, typical representatives of phenolic compounds in OMW: tyrosol, caffeic acid, coumaric acid and protocatechuic acid (Colarieti et al. 2006; Greco et al. 2006). Analysis were carried out in a LC-HRMS (High resolution mass spectrometry detection) Thermo Scientific® system conformed by a quaternary Accela® pump and an Exactive® Orbitrap MS detector (Thermo Fisher Scientific, Waltham, USA). Hypersil Gold column 50 × 2.1 mm × 1.9 μm at room temperature was utilized for the chromatographic separation. The mobile phase was constituted by acetonitrile (solvent A) and milli-Q water (Solvent B) in the following gradient: 0–2 min 10 % A, 2–4.5 min 10 – 100 % A, 4.5–6 min 100 % A, 6.5–9 min: 10 % A (re-equilibration). The flow rate was 0.2 mL min−1 and the injection volume 20 μL of undiluted soil extract. ESI-MS was executed in negative ion mode, the specific transition mass (m/z) of precursor ions and retention times (rt) were as follows: Tyrosol: 137.0608 (2.5 min); Caffeic acid: 179.0350 (2.8 min); Protocatechuic acid: 153.0193 (1.6 min); Coumaric acid: 163.0401 (4.5 min). Caffeic acid, protocatechuic acid and coumaric acid showed a linear calibration curve in a range from 50 – 750 ng mL−1, Tyrosol from 250 – 5000 ng mL−1. Matrix effects were estimated by post-extraction spike method at two concentrations: 100 and 1000 ng mL−1. Strong ion enhance was observed for caffeic acid and protocatechuic acid (>100 %), meanwhile a weak ion suppression was determined for tyrosol and coumaric acid. To compensate those matrix effects, the samples were quantified using a matrix-matched calibration method.
Carbon isotope ratio
Incubated soil samples were characterized for their δ13C before and after each sequential extraction step using a Flash 2000 HT Elemental Analyzer (Thermo Fisher Scientific, Waltham, USA). Soil samples were combusted in an oxygen atmosphere at 1020 °C with element separation using a packed column (Poropack QS 50/80 mesh, 3 m × 5 mm) at 40 °C. Results were expressed as δ13C according to equation (1) as the differences of 13C:12C ratio of the soil sample (RSample) in relation to the defined reference standard (RStandard; Vienna-PeeDee belemnite, ‘V-PDB’ with a 13C:12C ratio of 1.1237 × 10−2) (Staddon 2004).
IRMS Reference material was IAEA-CH-3 (δ13C = -24.724 ‰ V-PDB) of the International Atomic Agency (IAEA, Vienna, Austria).
Phytotoxicity tests
Phytotoxicity was tested for OMW treated soil, its sequential solvent extracts and the respective controls using a L. sativum seed germination bioassay. Tests were conducted in 60 mm petri dishes directly on OMW treated soil and control soil, the respective soil extracts were tested on Whatman Grade 41 filter paper. 1 mL of the respective extract was added onto the filter paper and the solvent was evaporated for 24 h under the fume hood. Afterwards, the filter paper was rewetted with 1 mL dH2O and 10 English cress seeds were placed on it. Petri dishes were incubated at 15 °C for 96 h with daily aeration. After 96 h, relative seed germination (RSG), relative root growth (RRG) and germination index (GI) were determined according to Hoekstra et al. (2002) (Eqs. 2–4):
SX and SC are the number of germinated seeds (radicle protruding from seed), RLX and RLC the mean root length of the cress plants for treated (X) and control samples (C).
Data analysis
Results were statistically analyzed using One-way analyses of variances (ANOVA) and Tukey’s multiple comparison test at p < 0.05 to find significant differences between OMW treated samples and untreated control samples. If necessary, data were \( y=\sqrt{y+1} \) transformed to fulfill the assumptions of ANOVA. Variability within the replicates was presented as standard errors of the mean values. Linear regression and correlation analyses (Pearson correlation) were performed to determine relationships between the different parameters.
Results
Table 1 summarizes the physico-chemical properties of the Israeli orchard soil and the OMW used for the incubation experiments. The soil texture was classified as sandy clay loam (130 g Kg−1 silt, 560 g Kg−1 sand, 310 g Kg−1 clay). The soil has a low organic carbon content of 17.8 mg g−1 dry soil and a low TPC < 0.01 mg GAU g−1 dry soil. It is slightly alkaline, with a pH of 8.8. δ13C is -8.5 ‰. In contrast, the OMW has a much higher organic carbon content of 473 mg g−1 dry OMW and a high TPC of 393 mg GAU g−1 moist OMW. It has an acidic character, with a pH of 4.7. Its δ13C of −27.4 ‰ shows a strong contrast to the soil.
The soil respiration activity of OMW treated soil and control soil is presented as cumulative soil respiration in Fig. 1 and as daily soil respiration rate in Fig. 2a, respectively. The OMW treated soil released 16 mg CO2 g−1 during incubation, the untreated control soil 6 mg CO2 g−1. Thus, the cumulative respired CO2 of the OMW treated soil was more than 2.5 times higher than for the untreated control soil, resulting in clearly increased daily respiration rates. Assuming that the release of carbonate C can be neglected and the difference between the respiration of OMW treated soil and untreated control soil indicates the mineralization of OMW-OM, the higher release of 10 mg CO2 g−1 for OMW treated soil can be attributed to the mineralization of OMW-OM. This suggests a mineralization of up to 27 % of OMW derived carbon during the 60 days of incubation.
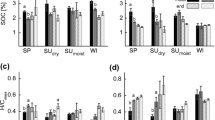
a daily soil respiration rate per g of dry control soil and OMW treated soil during 60d incubation with OMW at 23 % soil moisture and 15 °C. b TPC per g of dry control soil, OMW treated soil and its two organic solvent fractions. c changes of δ13C in control soils and OMW treated soil, before and after methanol (MeOH) extraction. d germination index (GI) for L. sativum exposed to OMW treated soil and its methanol extract in relation to untreated control soil and extract. Asterisks: significant at p < 0.05 compared to untreated control (One-way ANOVA with Tukey’s multiple comparison test). Error bars represent standard error of means. The dotted connecting lines between the figures are plotted in order to visualize the different phases of development I-IV for the determined parameter
Based on the development of the daily soil respiration, the incubation period was subdivided into four phases. In phase I (0–5 days), the respiration rate of both OMW treated soil and untreated control soil reached its respiration maximum of 0.8 (±0.1) mg d-1 g−1 and 0.43 (±0.01) mg g−1 d−1. In phase II (5–12 days), the respiration decreased in both soils, but re-increased again after day 12 in OMW treated soil to reach a second respiration maximum (0.6 (±0.1) mg g−1 d−1) at the beginning of phase III (day 13). The soil respiration rate of OMW treated soil steeply declined during phase III (13–22 days) and reached a constant, non-significant level in phase IV (>22 days).
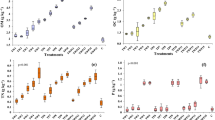
Figure 2b shows the concentration of extractable TPC as a function of extraction solvent and incubation time. The TPC of the untreated control soil was 0.18 (±0.02) mg GAU g−1 and remained constant over the whole incubation time. The TPC of the OMW treated soil was 5.0 (±0.1) mg GAU g−1 dry soil directly after OMW addition. With time, the TPC of the soil extracts declined to a final TPC of 1.5 (±0.1) mg GAU g−1 at the end of phase IV. In phase I, the TPC decreased and re-increased again to reach a flat maximum in phase II. Although the TPC of the OMW treated soil declined with incubation time, it remained significantly higher than the untreated control soil for the complete incubation period. The highest TPC was extracted using methanol as extraction solvent. Its averaged share was 84.8 (±2.3) % of the overall extracted TPC. The TPC of the hexane extracts decreased during phase I and increased again from phase III to a final concentration of 0.3 (±0.1) mg GAU g−1. In total, 70 % of the OMW derived TPC were degraded during the 60 days of incubation, indicating certain persistence of phenolic compounds. In this context, it is important to consider that the photosensitivity of the Folin-Ciocalteu reaction differs for different phenolic compounds as demonstrated for four phenolic compounds in comparison to gallic acid (Fig. 3). The sensitivity of the Folin reaction towards the four compounds is 1.4-fold and 1.2-fold for protocatecuic acid and caffeic acid, respectively, and 0.7-fold for both coumaric acid and tyrosol.
The target analysis of the methanol extracts of OMW treated soil showed a high tyrosol concentration of 1.2 (±0.1) mg g−1 directly after OMW addition (Fig. 4). Its concentration declined during phase I towards a constant level of 0.049 (±0.005) mg g−1 until the end of phase IV. The initial concentrations of caffeic acid (4.1 ± 0.2 μg g−1), protocatechuic acid (9 ± 1 μg g−1) and coumaric acid (6.9 ± 0.6 μg g-1) were several times lower than for tyrosol. Secondly, the concentration of both protocatechuic acid and caffeic acid increased to the highest measured concentrations at the beginning of phase II (0.66 (±0.2) mg g−1 and 0.15 (±0.02) mg g-1, respectively). From this point on, their concentrations declined to 2.0 (±0.3) μg g-1 for protocatechuic acid and a non-detectable limit for caffeic acid at the end of phase IV. The concentration of coumaric acid continuously declined from the end of phase I to a final concentration of 0.50 (±0.01) μg g-1. Considering the different sensitivities towards the Folin reaction (Fig. 4) and the extract:soil ratio, tyrosol could explain 20.3 % of the TPC at the beginning of phase I and 3.5 % of the TPC after 60 days; coumaric acid accounts for max. 0.1 % of the TPC.
The development of δ13C is illustrated in Fig. 2c as function of extraction solvent and incubation time before and after each sequential extraction step. The untreated control soil showed a stable δ13C of −8.5 (±0.1) ‰ during the whole incubation. OMW application resulted in a significantly decreased δ13C = -14.0 (±0.6) ‰. δ13C further decreased during the phases I and II towards a minimum of -15.2 (±0.7) ‰. Afterwards, an increase of δ13C until the end of phase III was observed. From phase IV on, δ13C remained stable (-12.5 (±0.9) ‰).
For the untreated control soil, no performed solvent extraction significantly changed δ13C (Table 1 in the SI). Extractions of OMW treated soil with hexane did not change its δ13C, whereby methanol extraction resulted in a significantly increased δ13C (-11.3 (±0.2) ‰). Despite the solvent extraction, the δ13C of OMW treated soils did not reach the original δ13C of the untreated control soil, suggesting that a large part of the OMW-OM was not extractable with the chosen solvents.
L. sativum growth parameters are shown in Fig. 5a as a function of the methanol-extractable phenolic content (MPC). Only GI and RRG changed with time while RSG was 90 % throughout the incubation for both the OMW treated soil and its extracts. The only exception was the reduction of RSG by 30 % for OMW treated soil directly after OMW addition (day 0). GI and RRG correlated significantly (p < 0.001) with a clear dependence on the MPC (p < 0.001) (Table 2 in the SI). Thus, phytotoxicity expresses mainly in the growth period. Neither the solvent extracts of the control soil nor the hexane extracts of the OMW treated soil showed significant effects on L. sativum growth. Therefore, we will only discuss the GI for the soil samples and its methanol extracts (Tables 3 – 7 in the SI for the other growth indices).
a correlation of L. sativum growth parameter with methanol-extractable phenolic content (MPC) of OMW treated soil; b correlation of growth indices from methanol (MeOH) soil extracts of OMW treated soil with the respective growth indices from OMW treated soil. Dotted line represents 1:1 line where growth index of OMW treated soil = growth index of MeOH extract. RSG: relative seed germination, RRG: relative root growth, GI: germination index
Figure 2d shows the GI for each methanol extract and OMW treated soil as function of incubation time. The growth of L. sativum on the methanol extracts of OMW treated soil was reduced to 2.2 (±0.3) % during phase I and II. In phase III, a steep increase of GI was observed, followed by a continuous increase to 66 (±8) % in phase IV. The GI on OMW treated soil was comparable to that on the methanol extracts for phases I and III, but was significantly higher in phase II. During phase II, GI decreased from 36 (±6) to 17 (±8) %, and increased again during phase IV to a final, non-significant level of 104 (±7) %.
To that point, OMW treated soil and its methanol extracts showed similar GI only in phases I and III. In phases II and IV, the toxicity of the methanol extracts was higher than for the OMW treated soil as indicated by their positions below the 1:1 line in Fig. 5b.
Discussion
Our objective was to obtain first insights into the degradation of OMW-OM in soil and its time-dependent relation to phytotoxic effects under controlled, laboratory conditions. The presented results showed characteristic changes and responses of the soil on OMW addition. From phase I on, a stimulation of the soil respiration activity in OMW treated soil took place due to the addition of easily available carbon sources. These include especially proteins, amino acids and sugars which are easily degradable and transformable by soil microorganisms (Brant et al. 2006; Hoorman and Rafiq 2010). The decrease of δ13C during phase I and at the beginning of phase II further supports a predominant degradation of those easily degradable substances as they are typically 13C-enriched compared to aromatic substances such as phenolic compounds, lignins and sterols (Bianchi et al. 1993; Santruckova et al. 2000; Werth and Kuzyakov 2010). During the phases I and II, TPC remained at a high level, further supporting the assumption of variable decomposition rates for OMW-OM in soil. Similar observations were already stated by Saviozzi et al. (1990) for OMW-OM in soil. They distinguished between a fast primary phase with a decomposition of rapidly degradable substances such as sugars and a slower secondary phase with a degradation of more stable substances such as lignins. Martin and Haider (1986) reported a mineralization of 73 % for glucose in sandy soil after 1 week. In comparison, the mineralization of catechol and caffeic acid, two well-known phytotoxic phenols in OMW (Isidori et al. 2005), were 11 % and 40 % after the same time span. Even after 28 weeks, 74 % of the catechol and 32 % of the caffeic acid were still present as they accumulated during the initial phase of plant litter decomposition (Sollins et al. 1996). Thus, the subsequent changes in degradation processes as a result of altered carbon sources most probably accounts for the transient decrease of the soil respiration in phase II. It may also be linked to changes in active microbial communities (Mekki et al. 2006; Piotrowska et al. 2006).
The unexpected fluctuation of the TPC in OMW treated soil during phase I can be attributed to the transformation between phenolic compounds with different sensitivities towards the FC-reagent (Fig. 4). These assumptions are further supported by the conducted LC/MS measurements. In phase I, the concentration of tyrosol, a dominant phenolic compound in OMW (Shadabi et al. 2013), strongly decreased within the first 24 h and likely explains the initial decrease of the TPC (Fig. 2b). The subsequent re-increase of the TPC coincides with the intermediate formation of protocatechuic acid and caffeic acid. Both phenolic compounds have a higher sensitivity towards the FC-reagent than tyrosol (Fig. 4). This exemplarily supports the assumption of partly degraded, polymerized or transformed phenolic compounds with an altered reactivity towards the FC-reagent (Wang et al. 1986; Greco et al. 2006; Negi and Dey 2009). Although the investigated phenolic compounds for the LC/MS measurements are of great relevance for OMW (Colarieti et al. 2006; Greco et al. 2006), more phenolic substances has to be expected in the soil extracts which might have different reactivities towards the FC-reagent and therefore an influence on the measured TPC.
Both OMW treated soil and its methanol extracts showed high phytotoxic effects on L. sativum during the phases I-III. The clear relation of phytotoxicity with the MPC supports our hypothesis of a relationship between OMW derived phenolic compounds and phytotoxic effects on plants such as L. sativum. Methanol is a well-known, typically used extraction solvent for phenolic compounds (Casazza et al. 2010; Mussatto et al. 2011) as its high polarity and ability to penetrate into smaller soil pores enhances the dissolution of polar compounds (Jonker and Koelmans 2002). Methanol extracts of several plants and soils have shown comparable phytotoxic effects on different plants such as wheat, sugarcane and soybean (Zhang and Fu 2010; Khan et al. 2012).
The steep decline of tyrosol during phase I did not reduce the phytotoxicity on L. sativum. This is in line with the frequent observation that tyrosol has minor or even no phytotoxic effects (Colarieti et al. 2006; Reigosa and Pazos-Malvido 2007). Colarieti et al. (2006) stated an effect of 10 % on the GI of L. sativum at high tyrosol concentrations of 221 mg L−1. Furthermore, the authors showed much higher phytotoxic effects for coumaric acid and caffeic acid on L. sativum. In our case, the low concentrations and the partially degradation of both coumaric acid and caffeic acid during phases I and II did not reduce the phytotoxicity of the methanol extracts. This suggests either a concentration level substantially higher than the toxicity threshold or their subordinate role on L. sativum growth compared to other phenolic compounds. The small selection of target compounds in our study, accounting for max 6 % of the remaining TPC after 60 days, underlines that large parts of the observed phytotoxic effects has to be attributed to other phenolic compounds. Furthermore, it is well-known that mixtures of phenolic compounds can lead to an enhanced phytotoxicity compared to a single compound exposure (Blum 1996). Although we cannot give a final answer on the toxic effect of single phenolic compounds on L. sativum in a complex mixture such as the methanol extract or the soil solution of OMW treated soil, our results indicate that the observed phytotoxic effects are likely the result of a complex interplay between different phenolic substances from OMW in dependence on their concentrations, mixture composition and toxicity mechanisms.
Contrary to the methanol extracts, the phytotoxicity of OMW treated soil decreased from phase I to II. For both respiration maxima of the OMW treated soil, the growth of L. sativum was strongly reduced with a clearly lower phytotoxicity of the soil before and after them. This effect was not observed for the methanol extract which had a constantly high phytotoxicity until the end of phase II. This suggests additional processes in OMW treated soil during the germination period which could not take place in the absence of soil. Greco et al. (2006) showed that the phytotoxicity of OMW on L. sativum can be reduced due to the oxidative polymerization of monophenolic compounds. The synchronicity of the high phytotoxicity and the transient minimum in soil respiration during phase II suggests a significant influence of microbial activity on plant growth. In this context, the activity of microbial communities may support germination and/or suppress parts of the phytotoxicity, for example by processes promoting germination or protecting seeds from the toxic phenolic compounds. Gangwar et al. (2011) showed that indole acetic acid (IAA) protected pea seedlings during the early growth period against Cr phytotoxicity by regulating Cr accumulation and oxidative damage. IAA and other plant growth promoting chemicals can be produced by soil and rhizobacteria (Ambardar and Vakhlu 2013). Thus, the production of such compounds could be responsible for the protection of germinating seeds against toxic phenolic compounds during phase II.
Phase III describes a new degradation step of OMW-OM in soil. This is further underlined by the second respiration maximum and the prospective decrease of phenolic compounds in methanol soil extracts and the increase of δ13C. After the predominant degradation of easily degradable OMW-OM, shifts in the microbial community structure might have led to the enhanced degradation and mineralization of more persistent substances such as (poly-) phenols or lignin and thus an enriched δ13C in soil. Plante and McGill (2002) observed comparable changes in δ13C for respired CO2 after the second week of incubation. During this time span, the δ13C of respired CO2 changed from depleted to enriched. Santruckova et al. (2000) found a relationship between changes in soil δ13C and microbial activity, depending on the stage of incubation. At the beginning of the incubation, they observed 13C-enriched microbial biomass, suggesting a preference of 13C-rich compounds for building up microbial structures. During prolonged incubation, microbial biomass became 13C-depleted compared to earlier measure points, most likely due to changes in microbial community and degraded substrate.
The higher TPC in hexane extracts from phase III on might be the result of emerging, less polar transformation products or microbial exudates during OMW-OM degradation. Several researchers have shown that soil microorganisms can produce hydrophobic exudates and by-products over time which can have water-repellent effects in soil (Hallett and Young 1999; Tarchitzky et al. 2007). Water repellency was also observed for OMW treated soils and could be related to original and partly decomposed hydrophobic organic substances from OMW in the top soil horizons (Travis et al. 2008; Mahmoud et al. 2010). Hence, the occurrence of less polar substances in hexane extracts from phase III on might indicate an increasing potential of OMW treated soil to become water repellent.
As for the TPC, the original δ13C of the untreated control soil was not reached by the OMW treated soil at the end of phase IV, neither for non-extracted nor extracted soil. Even three out of the four phenolic substances measured by LC/MS did not completely disappear at the end of phase IV (23 % protocatechuic acid, 7 % coumaric acid and 5 % tyrosol of the initial concentrations were still detected). Although this implies a larger degree of degradation than reported by Isidori et al. (2005) and Sollins et al. (1996), our results indicate an incomplete degradation of OMW-OM in soil. The fact that phytotoxic effects were still observed for methanol soil extracts but not for OMW treated soil at the end of phase IV shows that phytotoxic phenolic substances in OMW treated soil were still existing but not available for the plant anymore. This supports the assumption that at least parts of the OMW-OM and TPC are immobilized and not fully bioavailable, most likely due to soil-interaction processes such as adsorption to soil particles or physical immobilization in SOM (Greco et al. 2006; Sierra et al. 2007). Several authors stated strong adsorptions of phenolic compounds on soil particles up to 100 % for some compounds. Vidal and Bauman (1997) reported adsorption of 10–40 % of phenolic compounds such as caffeic acid or catechol to soil particles which led to a reduced phytotoxicity in soil compared to petri-dish experiments. According to Dalton et al. (1989), ferulic acid can be immobilized even up to 100 % by a histosol.
To which extent this immobilization is irreversible for contact times longer than 60 days cannot be decided from the current data. Although no further phytotoxic effects for OMW treated soil were observed at the end of phase IV, Oleszczuk et al. (2012) showed that phytotoxicity of applied wastewater can suddenly increase again after several months due to the remobilization of toxic compounds during organic matter breakdown or weathering processes. In this context, the desorption of adsorbed or sequestered toxic phenolic substances has to be taken into account, potentially leading to a reoccurring phytotoxicity with time.
Throughout all phases, RSG was the only growth parameter that showed no negative effects of OMW exposure, neither for OMW treated soil nor for its methanol extracts. In both cases, the cress plants were able to germinate but not to grow at higher TPC. Saadi et al. (2007) showed that seed germination of L. sativum was still 100 % for OMW concentrations up to 10 %. In the opposite, Ben Sassi et al. (2006) showed an inhibition of 60 % for H. vulgare seed germination in 1:16 diluted OMW. As stated by Montemurro et al. (2011), OMW can have a variable sensitivity on living organisms, depending on several parameters such as species, dose, extraction process and soil properties. Our results are in accordance with Hanifi (2009) who concluded that the inhibition of plant development rather takes place during the first development stage than during the germination process.
Conclusion
Phytotoxicity of OMW was clearly attributed to phenolic substances, affecting the growth but not the germination of L. sativum. During the 60 day incubation period, only 27 % of the added OM and 68 % of the TPC was degraded. Four phases in OMW degradation were distinguished: Phase I (0–5 days) is characterized by degradation of labile organic matter and transformation of polyphenolic compounds, while during phase II (5–12 days) the microbial metabolism changes together with the gradual change of carbon source towards more stable compounds including phenolic substances. The microbial community likely produces germination-promoting substances or substances protecting the seedlings from phenolic compounds. Phase III (13–22 days) is largely characterized by the degradation and potentially immobilization of phenolic compounds, linked to a decrease in phytotoxicity, while in phase IV (>22 days), the physical immobilization of phenolic compounds overbalances their degradation. Only the initial reduction of phytotoxicity (phases I-III) is a result of degradation processes, while the finally observed phytotoxicity is mainly dominated by physical immobilization. Our results show that phytotoxic effects may rather be the result of a complex interplay between different phenolic substances than a predominant effect of a single compound. A further relevant finding of our study is that the immobilization of phenolic compounds represents a process proceeding slower or starting later than their degradation. Phenolic compounds which are not degraded within the first 2–3 weeks of OMW-soil contact are preferentially immobilized with the risk of remobilization under appropriate conditions. Therefore, non-degraded phenolic compounds might represent a risk of reoccurring phytotoxicity during further organic matter breakdown. The interplay between degradation and immobilization will be controlled by environmental conditions. Environmental conditions favoring biological activity during the first weeks of OMW-soil contact are decisive for reaching a sufficient degree of sustainable completeness of detoxification. As the phytotoxicity of OMW differs for different growth states, the application of OMW under restricted and controlled conditions (e.g. some weeks before or after germination) might further reduce the potential of negative effects on plant growth. The findings of this study will help to understand mechanisms of OMW transformation and effects of OMW application on soil and plant growth. The mechanistic information gained in this study should be evaluated in targeted field studies in order to verify their relevance under field conditions.
References
Ambardar S, Vakhlu J (2013) Plant growth promoting bacteria from crocus sativus rhizosphere. World J Microbiol Biotechnol 29:2271–2279. doi:10.1007/s11274-013-1393-2
Angerosa F, Bréas O, Contento S, Guillou C, Reniero F, Sada E (1999) Application of stable isotope ratio analysis to the characterization of the geographical origin of olive oils. J Agric Food Chem 47:1013–1017. doi:10.1021/jf9809129
Arapoglou D, Doula M, Kavvadias V, Iconomou D, Theocharopoulos S, Tountas P (2010) Monitoring of phenols concentration in soil of olive oil mill waste disposal site. Proceedings of the 2nd international conference on hazardous and industrial waste management: 477–479
Barbera AC, Maucieri C, Cavallaro V, Ioppolo A, Spagna G (2013) Effects of spreading olive mill wastewater on soil properties and crops, a review. Agric Water Manag 119:43–53
Ben Sassi A, Boularbah A, Jaouad A, Walker G, Boussaid A (2006) A comparison of olive oil mill wastewaters (OMW) from three different processes in morocco. Process Biochem 41:74–78
Bianchi G, Angerosa F, Camera L, Reniero F, Anglani C (1993) Stable carbon isotope ratios (carbon-13/carbon-12) of olive oil components. J Agric Food Chem 41:1936–1940
Blum U (1996) Allelopathic interactions involving phenolic acids. J Nematol 28:259
Bogolte BT, Ehlers GAC, Braun R, Loibner AP (2007) Estimation of PAH bioavailability to lepidium sativum using sequential supercritical fluid extraction - a case study with industrial contaminated soils. Eur J Soil Biol 43:242–250. doi:10.1016/j.ejsobi.2007.02.007
Boukhoubza F, Ait Boughrous A, Yacoubi-Khebiza M, Jail A, Hassani L, Loukili Idrissi L, Nejmeddine A (2008) Impact of olive oil wastewater on the physicochemical and biological quality of groundwater in the haouz plain, south of marrakesh (morocco). Environ Technol 29:959–974. doi:10.1080/09593330802131669
Box JD (1983) Investigation of the folin-ciocalteau phenol reagent for the determination of polyphenolic substances in natural waters. Water Resour 17:511–525
Brant JB, Sulzman EW, Myrold DD (2006) Microbial community utilization of added carbon substrates in response to long-term carbon input manipulation. Soil Biol Biochem 38:2219–2232
Casazza AA, Aliakbarian B, Mantegna S, Cravotto G, Perego P (2010) Extraction of phenolics from vitis vinifera wastes using non-conventional techniques. J Food Eng 100:50–55. doi:10.1016/j.jfoodeng.2010.03.026
Chartzoulakis K, Psarras G, Moutsopoulou M, Stefanoudaki E (2010) Application of olive mill wastewater to a cretan olive orchard: effects on soil properties, plant performance and the environment. Agric Ecosyst Environ 138:293–298
Colarieti M, Toscano G, Greco G (2006) Toxicity attenuation of olive mill wastewater in soil slurries. Environ Chem Lett 4:115–118
Dalton BR, Blum U, Weed SB (1989) Plant phenolic acids in soils: Sorption of ferulic acid by soil and soil components sterilized by different techniques. Soil Biol Biochem 21:1011–1018. doi:10.1016/0038-0717(89)90038-2
Deeb AA, Fayyad MK, Alawi MA (2012) Separation of polyphenols from jordanian olive oil mill wastewater. Chromatogr Res Int 2012:8. doi:10.1155/2012/812127
Di Serio MG, Lanza B, Mucciarella MR, Russi F, Iannucci E, Marfisi P, Madeo A (2008) Effects of olive mill wastewater spreading on the physico-chemical and microbiological characteristics of soil. Int Biodeterior Biodegrad 62:403–407
DIN ISO 10694 (1996) Bodenbeschaffenheit-Bestimmung von organischem Kohlenstoff und Gesamtkohlenstoff nach trockener Verbrennung (Elementaranalyse). Beuth, Berlin
DIN ISO 11265 (1997) Bodenbeschaffenheit-Bestimmung der spezifischen elektrischen Leitfähigkeit. Beuth, Berlin
DIN ISO 11272 (2001) Bodenbeschaffenheit–Bestimmung der Trockenrohdichte. Beuth, Berlin
DIN ISO 11277 (1998) Bodenbeschaffenheit-Bestimmung der Partikelgrößenverteilung in Mineralböden. Verfahren mittels Siebung und Sedimentation. Beuth, Berlin
DIN ISO 16072 (2005) Soil quality – laboratory methods for determination of microbial soil respiration. Beuth, Berlin
DIN ISO 38404–5 (2009) Deutsche einheitsverfahren zur wasser-, abwasser- und schlammuntersuchung - physikalische und physikalisch-chemische kenngrößen (gruppe c) - teil 5: Bestimmung des ph-werts (c 5). Beuth, Berlin
Fisher JA, Scarlett MJ, Stott AD (1997) Accelerated solvent extraction: an evaluation for screening of soils for selected u.S. Epa semivolatile organic priority pollutants. Environ Sci Technol 31:1120–1127. doi:10.1021/es9606283
Gangwar S, Singh VP, Maurya JN (2011) Responses of pisum sativum l. To exogenous indole acetic acid application under manganese toxicity. Bull Environ Contam Toxicol 86:605–609. doi:10.1007/s00128-011-0278-z
Greco G, Colarieti ML, Toscano G, Iamarino G, Rao MA, Gianfreda L (2006) Mitigation of olive mill wastewater toxicity. J Agric Food Chem 54:6776–6782
Hallett PD, Young IM (1999) Changes to water repellence of soil aggregates caused by substrate-induced microbial activity. Eur J Soil Sci 50:35–40
Hanifi S, El Hadrami I (2009) Olive mill wastewaters: Diversity of the fatal product in olive oil industry and its valorisation as agronomical amendment of poor soils: A review. J Agron 8:1–13
Hoekstra N, Bosker T, Lantinga E (2002) Effects of cattle dung from farms with different feeding strategies on germination and initial root growth of cress (lepidium sativum l.). Agric Ecosyst Environ 93:189–196
Hoorman J, Rafiq I (2010) Understanding soil microbes and nutrient recycling. fact sheet of agriculture and natural. The Ohio State University, Resources
Isidori M, Lavorgna M, Nardelli A, Parrella A (2005) Model study on the effect of 15 phenolic olive mill wastewater constituents on seed germination and vibrio fischeri metabolism. J Agric Food Chem 53:8414–8417. doi:10.1021/jf0511695
Jonker MTO, Koelmans AA (2002) Extraction of polycyclic aromatic hydrocarbons from soot and sediment: solvent evaluation and implications for sorption mechanism. Environ Sci Technol 36:4107–4113. doi:10.1021/es0103290
Kapellakis, IE, Tsagarakis, KP, Crowther, JC (2008) Olive oil history, production and by-product management. Rev. Environ. Sci. Biotechnol. 7:1–26
Kavvadias V, Doula M, Theocharopoulos S (2014) Long-term effects on soil of the disposal of olive mill waste waters (omw). Environ Forensic 15:37–51
Kelsey JW, Kottler BD, Alexander M (1997) Selective chemical extractants to predict bioavailability of soil-aged organic chemicals. Environ Sci Technol 31:214–217
Khan RA, Khan NA, Ahmed M, Khan MR, Khan FU, Shah AS, Shah MS (2012) Phytotoxic characterization of crude methanolic extract of periploca aphylla. Afr J Biotechnol 11:11575–11579
Khatib A, Aqra F, Yaghi N, Subuh Y, Hayeek B, Musa M, Basheer S, Sabbah I (2009) Reducing the environmental impact of olive mill wastewater. Am J Environ Sci 5:1–6
Laor Y, Saadi I, Raviv M, Medina S, Erez-Reifen D, Eizenbergc H (2011) Land spreading of olive mill wastewater in israel: current knowledge, practical experience, and future research needs. Isr J Plant Sci 59:39–51
Li H-B, Cheng K-W, Wong C-C, Fan K-W, Chen F, Jiang Y (2007) Evaluation of antioxidant capacity and total phenolic content of different fractions of selected microalgae. Food Chem 102:771–776
Macko SA, Fogel ML, Hare PE, Hoering TC (1987) Isotopic fractionation of nitrogen and carbon in the synthesis of amino acids by microorganisms. Chem Geol Isot Geosci Sect 65:79–92. doi:10.1016/0168-9622(87)90064-9
Mahmoud M, Janssen M, Haboub N, Nassour A, Lennartz B (2010) The impact of olive mill wastewater application on flow and transport properties in soils. Soil Till Res 107:36–41. doi:10.1016/j.still.2010.01.002
Martin JP, Haider K (1986) Influencs of mineral colloids on turnover rates of organic soil carbon. Soil Sci Soc Am Spec Publ 17:283–304
Mekki A, Dhouib A, Sayadi S (2006) Changes in microbial and soil properties following amendment with treated and untreated olive mill wastewater. Microbiol Res 161:93–101
Montemurro F, Diacono M, Vitti C, Ferri D (2011) Potential use of olive mill wastewater as amendment: crops yield and soil properties assessment. Commun Soil Sci Plant Anal 42:2594–2603. doi:10.1080/00103624.2011.614035
Mussatto SI, Ballesteros LF, Martins S, Teixeira JA (2011) Extraction of antioxidant phenolic compounds from spent coffee grounds. Sep Purif Technol 83:173–179
Negi B, Dey G (2009) Comparative analysis of total phenolic content in sea buckthorn wine and other selected fruit wines. World Acad Sci Eng Technol 54:99–102
Nielsen MN, Winding A (2002) Microorganisms as indicators of soil health. Ministry of the Environment, National Environmental Research Institute, Roskilde
Oleszczuk P, Malara A, Jośko I, Lesiuk A (2012) The phytotoxicity changes of sewage sludge-amended soils. Water Air Soil Pollut 223:4937–4948. doi:10.1007/s11270-012-1248-8
Piotrowska A, Iamarino G, Rao MA, Gianfreda L (2006) Short-term effects of olive mill waste water (omw) on chemical and biochemical properties of a semiarid mediterranean soil. Soil Biol Biochem 38:600–610
Plante AF, McGill WB (2002) Soil aggregate dynamics and the retention of organic matter in laboratory-incubated soil with differing simulated tillage frequencies. Soil Till Res 66:79–92. doi:10.1016/S0167-1987(02)00015-6
Reigosa MJ, Pazos-Malvido E (2007) Phytotoxic effects of 21 plant secondary metabolites on Arabidopsis thaliana germination and root growth. J. Chem. Ecol. 33: 1456–1466
Saadi I, Laor Y, Raviv M, Medina S (2007) Land spreading of olive mill wastewater: effects on soil microbial activity and potential phytotoxicity. Chemosphere 66:75–83
Santruckova H, Bird M, Lloyd J (2000) Microbial processes and carbon-isotope fractionation in tropical and temperate grassland soils. Funct Ecol 14:108–114
Saviozzi A, Levi-Minzi R, Riffaldi R (1990) Cinetica della decomposizione nel terreno del carbonio organico delle acque di vegetazione. Agrochimica 34:157–164
Saviozzi A, Levi-Minz IR, Riffaldi R, Lupetti A (1991) Effetti dello spandimento di acque di vegetazione sul terreno agrario. Agrochimica 35:135–148
Shadabi S, Ghiasvand A, Hashemi P (2013) Selective separation of essential phenolic compounds from olive oil mill wastewater using a bulk liquid membrane. Chem Pap 67:730–736. doi:10.2478/s11696-013-0373-1
Sierra J, Marti E, Garau MA, Cruanas R (2007) Effects of the agronomic use of olive oil mill wastewater: field experiment. Sci Total Environ 378:90–94. doi:10.1016/j.scitotenv.2007.01.009
Sollins P, Homann P, Caldwell BA (1996) Stabilization and destabilization of soil organic matter: mechanisms and controls. Geoderma 74:65–105
Sparling GP (1997) Soil microbial biomass, activity and nutrient cycling as indicators of soil health. In: Doube BM, Gupta VVSR (eds) C Pankhurst. CABI Publishing Adelaide, Biological indicators of soil health, pp 97–117
Staddon PL (2004) Carbon isotopes in functional soil ecology. Trends Ecol Evol 19:148–154. doi:10.1016/j.tree.2003.12.003
Tao S, Xu F, Liu W, Cui Y, Coveney RM Jr (2006) A chemical extraction method for mimicking bioavailability of polycyclic aromatic hydrocarbons to wheat grown in soils containing various amounts of organic matter. Environ Sci Technol 40:2219–2224
Tarchitzky J, Lerner O, Shani U, Arye G, Lowengart-Aycicegi A, Brener A, Chen Y (2007) Water distribution pattern in treated wastewater irrigated soils: hydrophobicity effect. Eur J Soil Sci 58:573–588. doi:10.1111/j.1365-2389.2006.00845.x
Travis MJ, Weisbrod N, Gross A (2008) Accumulation of oil and grease in soils irrigated with greywater and their potential role in soil water repellency. Sci Total Environ 394:68–74. doi:10.1016/j.scitotenv.2008.01.004
Vidal RA, Bauman TT (1997) Fate of allelochemicals in the soil. Ciênc Rural 27:351–357
Wang TSC, Huang PM, Chou C-H, Chen J-H (1986) The role of soil minerals in the abiotic polymerization of phenolic compounds and formation of humic substances. In: PM Huang, M Schnitzer (eds) Interactions of soil minerals with natural organics and microbes. Soil Science Society of America251-281.
Werth M, Kuzyakov Y (2010) (13)c fractionation at the root-microorganisms-soil interface: a review and outlook for partitioning studies. Soil Biol Biochem 42:1372–1384. doi:10.1016/j.soilbio.2010.04.009
Wlodarczyk T, Ksiezopolska A, Glinski J (2008) New aspect of soil respiration activity measuring. Teka Kom Ochr Kszt Środ Przyr–OL PAN 5:153–163
Zhang C, Fu S (2010) Allelopathic effects of leaf litter and live roots exudates of eucalyptus species on crops. Allelopathy J 26:91–100
Acknowledgments
This research was conducted within the trilateral project “OLIVEOIL” funded by the DFG (SCHA849/13). The authors thank all members of the researchers group for fruitful discussions. Furthermore, we thank Andreas Hirsch, Silvia Eichhöfer and Eugenia Podolskaja for their help during the measurements.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Yong Chao Liang..
Electronic supplementary material
Below is the link to the electronic supplementary material.
Table 1
(pdf 446 kb)
Rights and permissions
About this article
Cite this article
Buchmann, C., Felten, A., Peikert, B. et al. Development of phytotoxicity and composition of a soil treated with olive mill wastewater (OMW): an incubation study. Plant Soil 386, 99–112 (2015). https://doi.org/10.1007/s11104-014-2241-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-014-2241-3