Abstract
The objective of this study was to understand the degradation of the organic matter of olive mill wastewater (OMW) and its phytotoxic and water repellent effects in dependence on four different climatic conditions. We hypothesized that warm conditions with sufficient soil moisture ensure optimal biological activity and thus minimize negative effects of the OMW treatment. Therefore, OMW-treated soil was incubated for 60 days under four climatic conditions. During incubation, we monitored pH, contents of nitrate, manganese and phenolic compounds, soil respiration, soil water repellency, and δ13C. Additionally, calorific value and thermal stability of the soil organic matter at the beginning and end of incubation were determined. Soil samples of the wet-cold and moist-warm incubation were tested for phytotoxicity using a seed germination bioassay with Lepidium sativum. As a function of climatic conditions, positive and negative effects, e.g., addition of nutrients, phytotoxicity, and soil water repellency, were observed. Under dry-hot conditions, the soil was still water repellent after 60 days of incubation whereas the wet-hot, moist-warm, and wet-cold incubation show that soil would stay wettable if soil moisture before OMW treatment would be sufficient. Thus, the impact of OMW treatment on soil quality strongly depends on the environmental conditions which should favor an enhancement of microbial activity to minimize negative effects.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Five million tons of oil mill wastewater (OMW) are being generated annually using the 3-phase extraction for the production of olive oil (Niaounakis and Halvadakis 2006). Due to the higher cost, a switch to the 2-phase extraction, which produces significantly less OMW, has not taken place in a number of countries (e.g., Israel and Palestine) (Azbar et al. 2004; Laor et al. 2011). OMW has a low pH as well as a high concentration of salts and organic substances (Cabrera et al. 1996; Sierra et al. 2001). Furthermore, the chemical and biological oxygen demand is 200–400 times that of household wastewater (El Hajouji et al. 2008). On the one hand, the application of OMW can enhance soil fertility (Chaari et al. 2014; Kapellakis et al. 2015) but it contains phenolic compounds, which are known to have phytotoxic and bacteriostatic effects (Barbera et al. 2014). For example, germination rates of cress seeds in OMW-treated soil are reduced (Greco et al. 2006) and bacterial walls are damaged (Carraro et al. 2014). Furthermore, OMW-polluted soils showed increased water repellency (Aranda et al. 2016), decreased hydraulic conductivity (Mohawesh et al. 2014), and decreased sorption capacity (Keren et al. 2015). In addition, OMW treatment increased biological activity as shown by bait-lamina test (Steinmetz et al. 2015), suppressed sensitive species by toxic compounds (Kurtz et al. 2015), and changed the microbiological composition (Di Serio et al. 2008). In a field trial, Karpouzas et al. (2010) treated loamy sand soil weekly with 4% OMW and observed alterations in the composition of the microbial community. Some ammonia-oxidizing bacteria were favored (e.g., Nitrosospira sp.) while the abundance of others was negatively affected (Karpouzas et al. 2010). The authors concluded that the differences in actinobacteria and ammonia-oxidizing bacteria were caused by decreased oxygen availability, strong competition for mineral-N, and selective inhibition by phenolic compounds. Further field experiments showed that OMW treatment increased the absolute number of soil bacteria and fungi as well as the ratio of fungi to bacteria (Laor et al. 2011; Siles et al. 2014), and the nitrifying population was sensitive to OMW (Gargouri et al. 2014). On the basis of results from soil respiration, extractable total phenolic content and C isotope ratio, Buchmann et al. (2015) showed that the degradation of the organic compounds in OMW can be divided into four phases: (i) transformation of phenolic compounds and degradation of organic matter (OM), (ii) decrease of phytotoxicity, (iii) degradation of phytotoxic phenolic compounds, and (iv) immobilization of phytotoxic compounds. The authors concluded that the climatic conditions for the application of OMW need to be optimized to ensure a rapid degradation of OMW as well as minimize the effects of immobilization and phytotoxicity (Buchmann et al. 2015). A study of Chiesura et al. (2005) showed that the porosity of soil increases when OMW was applied in spring whereas it remained unchanged in winter, which has been attributed to variation in biological activity due to changes in soil temperature and moisture (Barbera et al. 2013). Furthermore, negative effects (e.g., acidification, soil water repellency) of OMW application were reduced when the OMW was applied under moist-warm conditions favoring biological activity in the field (Tamimi et al. 2016). Tamimi et al. (2016) investigated the effect of the season in which OMW was applied to soil, on the transport, persistence, and fate of OMW constituents. The results showed that the duration of the hot and dry periods following the application increased persistence of OMW effects, which was attributed to accumulation and abiotic polymerization of OMW. Furthermore, the time under conditions favoring biological activity favored degradation over increase in persistence (Tamimi et al. 2016). Thus, the changes in OM quality directly depended on the climatic conditions. A more detailed understanding on the process controlling the fate of the OMW-derived OM in the soil, however, requires studies under controlled conditions and a more detailed analysis of the OM quality.
Therefore, we combined in this study for the first time simultaneous thermal analysis (thermogravimetry and differential scanning calorimetry) and isotope ratio mass spectrometry with the analysis of hydrophobicity and phytotoxicity of soil as well as common soil parameters to characterize changes of the quality of soil organic matter in dependence of climatic conditions. Experiments under controlled conditions are important to understand how temperature, soil moisture and respiration, hydrophobicity, phytotoxicity, and the organic substances in soil are influenced by OMW application and to draw conclusions applicable to field OMW application. Despite the contribution of biological activity and abiotic CO2 release, soil respiration is commonly used to determine microbial activity (Sparling 1997). In addition to common soil chemical properties (i.e., pH, conductivity, cation and anion content, organic C and UV absorption) soil samples were characterized by determining thermostability (TS), calorific value (CVs), and C isotope ratio which allows an estimation of the degree of degradation of OM added by OMW pollution (Peikert et al. 2015).
The aim of this research was to find out whether the negative effects of OMW treatment (in particular hydrophobicity and phytotoxicity) can be significantly reduced through climate-dependent biological activity and how these effects are related to changes in organic matter quality. In this context, it was hypothesized that warm and wet conditions ensure optimal biological activity and there maximize the degradation of organic components of the OMW and thus minimize its negative effects. In order to test this hypothesis, soil was treated with OMW and incubated under controlled soil temperature and moisture conditions of the Palestinian winter (wet/cold), spring (moist/warm), and summer (dry/hot). In addition, a summer variant simulating irrigation (wet/hot) was included to allow comparison between different soil moisture (dry/hot vs. wet/hot) and soil temperatures (wet/hot vs. wet/cold). These results will contribute to a better understanding of the changes of organic soil components through OMW treatment as well as enable recommendations for optimal timing of OMW application.
Material and methods
Soil and OMW
Soil samples were taken in Bait Reema, which is characterized by the calcareous Rendzina soil (Singer 2007). Nine surface (0-15 cm) soil samples were taken, air-dried, sieved (<2 mm), and combined to form 5 kg of composite clay loam, containing 38 ± 0.6% and 39 ± 0.3% of clay and silt, respectively. Prior to the experiments pH, electrical conductivity, cation and anion concentrations, dissolved organic carbohydrates, specific UV absorption (SUVA), soluble phenolic compounds (SPC), C isotope ratio (δ13C), water retention curve, water drop penetration time, contact angle, and the grain size distribution were determined.
Before incubation, the pF-values of soil were measured to determine the correlation between suction and soil water content (Wedler and Boguslawski (1965). The eight soil samples were placed in centrifuge filter tubes (Maxi-spin filter tubes, PVDF, 0.45 μm, Chrom Tech, USA) and saturated with water. Subsequently, the sample was centrifuged for 45 min at a relative centrifugal force of 0, 0.3, 10, 17, 42, 158, 356, 861, and 2125g correlating to pF-values of 0, 1.6, 1.9, 2.0, 2.6, 3.0, 3.3, 3.7, and 3.8. By measuring the difference in weight, the water content of the sample was determined (Wedler and Boguslawski 1965).
The OMW sample was obtained from an olive mill in Bait Reema (Palestine). After measurements of pH and conductivity (ISO 1997; ISO 2005), the sample was mixed well, aliquoted in 200 mL batches and stored at −18°C. Then, samples were filtered (PTFE, 0.45 μm, Whatman) and concentrations of dissolved organic C (DOC), total phenol content, and SUVA were determined. Using an N/C analyzer (multi N/C analyzer 2100/2100S, Analytik Jena, Germany), total C and dissolved inorganic C contents were determined. SUVA was determined by dividing the absorption at 254 nm by the DOC content. Soluble phenolic compounds were measured using Folin–Ciocalteu reagent (Sigma-Aldrich, Germany) and gallic acid for the titration curve (Box 1983; Buchmann et al. 2015; Li et al. 2007; Prior et al. 2005). Two hundred microliters of OMW were mixed with 10% (v/v) Folin–Ciocalteu reagent, after 4 min 800 μL of carbonic acid were added and an hour later the extinction coefficient at 700 nm was measured. The results are given in gallic acid units (GAU) per gram of dry soil. Furthermore, the dry weight of the OMW was determined at 105°C and OMW droplets were snap frozen in liquid nitrogen. The snap-frozen droplets were freeze-dried at −33°C and 0.28 mbar using a Christ Alpha 1-2 LD Freeze Dryer (Matrin Christ, Germany). The freeze-dried samples were used for isotope-ratio mass spectrometry and thermal gravimetric analysis.
Incubation experiments in the laboratory
Based on climate data (PCBS 2014), the condition of wet-cold moist-warm, wet-hot, and dry-hot soil were simulated as shown in Table 1. The soil moisture in wet-cold and wet-warm conditions corresponded to the field capacity.
The samples were incubated in screw cap glass jars. All samples were pre-incubated for 2 weeks under the respective conditions. Assuming a porosity and penetration depth of 50 % and 3 cm accordingly, the OMW applied (0.31 mL/g) correlated to an application rate of 14 L/m2. In total, 6.2 ml of OMW per sample were applied after the pre-incubation. Distilled water was used as a control. To adjust for the final water content, the samples were dried at 75 mbar in a vacuum desiccator containing phosphorus pentoxide for 0–5 days. It is possible that during this treatment volatile components of OMW were lost. However, this was taken into account to minimize the delay prior to treatment. After the addition of OMW, water content was regularly measured gravimetrically and sample was aerated every 3 days. Furthermore, the temperature was measured every 300 s using a data logger (Dostmann LOG 11). The samples were taken 0.5, 1, 2, 3, 4, 6, 9, 12, 15, 21, 39, and 60 days after OMW treatment. Three replicates were used for OMW-treated samples while only one sample for the control was obtained, in order to reduce the total number of samples to 185. Each sample was air-dried and pH, conductivity, anion and cation content, DOC, SUVA, water drop penetration time (WDPT), contact angle, δ13C, and thermal properties were analyzed.
The soil respiration was determined according to DIN ISO 16072 (2005). The released CO2 was trapped in 20 mL 0.25 M sodium hydroxide. After 72 h, the sodium hydroxide was replaced and 2 mL 0.5 M BaCl2 and 2–3 drops of phenolphthalein were added and the sample titrated with 0.5 M HCl. The samples were corrected using 20 mL of sodium hydroxide as a blank.
Determination of soil properties
General soil parameters
The soil grain size was determined using sedimentation (ISO 2002). Total N and C contents were measured using an elemental analyzer (Vario micro cube, Elementar Analysensysteme GmbH, Germany). The electrical conductivity (ISO 1997) and pH (ISO 2005) were analyzed in a 1:5 (water/soil) suspension. Furthermore, DOC were extracted by adding 25 mL of ultrapure water to 5 g of air-dried soil, followed by shaking overnight. Subsequently, the samples were centrifuged for 15 min at 3717g and membrane filtered (PTFE, 0.45 μm, Whatman). DOC was measured using an N/C analyzer (multi N/C analyzer 2100/2100S, Analytik Jena, Germany). In addition, cation (Ca2+, K+, Mg2+, Mn2+, and Na+), anion (F−, Cl−, NO2 −, NO3 −, PO4 3-, and SO4 2-) concentrations, and soluble phenolic compounds were determined. The analysis of cations was done by inductively coupled plasma MS (Agilent 7500 a); the soil extract was diluted 1:10 and Y, Rh, and Ho were applied as internal standards. Anions were measure in 1:6 diluted samples using ion chromatography (881 Compact IC pro, Metrohm, Switzerland).
Carbon isotope ratio
The natural distribution of the stable C isotopes was determined using isotope-ratio mass spectrometry (Muccioa and Jackson 2009). Soil samples were combusted using an Automatic Elemental Analyzer (Flash HAT 2000, Thermo Scientific). The released H2O was removed using a water trap. The gaseous mixture containing CO2, N2, and SO2 was passed through a Poropack QS column (50/80 mesh, 3 m × 5 mm). Samples were subsequently diluted with helium using a continuous flow interface (Conflo IV, Thermo Scientific) to processing using an Isotope Ratio Mass Spectrometer (Delta V Advantage, Thermo Scientific). The δ13C values were normalized using international references as shown in formula 1 (Weller et al. 2011). Therefore, larger δ13C values correlate to higher 13C concentrations in the soil samples.
Due to the fact that results of thermogravimetry showed no significant decrease of carbonate content in the OMW-treated soil samples, a significant effect on the C isotope ratio because of the decomposition of carbonates was not expected. Therefore, carbonates were not decomposed prior to analysis of the C isotope ratio. This did not influence the process analysis of this study, but makes it difficult to compare the results with literature data. Furthermore, trapped CO2 of some spring and winter samples (days 1, 6, and 60) was precipitated with BaCl2 and analyzed using C isotope mass spectrometry.
Simultaneous thermal analysis
Previous experiments showed that thermal analysis would only be sensitive enough to monitor differences between single OMW-treated samples at the beginning and end of incubation. Therefore, only soil samples 5 and 60 days post-OMW treatment were analyzed as described by Peikert et al. (2015):
Using simultaneous thermal analysis thermogravimetry (TG) as well as differential scanning calorimetry (DSC) can be conducted at the same time. The analysis was carried out using a STA 449 F3 Jupiter (Netzsch, Germany). Sn, Bi, Zn, Al, Ag. and Au standards were used for the calibration of temperature and enthalpy, while the mass change of calcium carbonate was used to calibrate the TG scale.
Two alumina crucible were measured, one was loaded with 20 mg air-dried soil sample (grain size <1 mm) while the other was used as a reference. The measurement was conducted in a temperature range of 30 to 1000°C increasing at 10°C/min. Synthetic air (flow rate 50 mL/min) was used to aid combustion. The capillary inlet was heated to 150°C in order to prevent condensation of the sample between the STA and mass spectrometer (MS 403 Aëolos II, Netzsch, Germany). Seventy eV were used to ionize the sample and mass spectrum was recorded between 0 and 150 amu in 1-min intervals.
The results were analyzed using the Netzsch Proteus Software 5.2.1. Following the recommendation by the manufacturer, the TG signal was smoothened (eightfold) to simplify the analysis of the start and end of the mass steps. In addition, the mass spectrometric signal of 18 u (H2O) was analyzed to determine the starting and endpoint of the combustion. Based on previous observation (Dell’Abate et al. 2003; Lopez-Capel et al. 2005; Manning et al. 2005; Plante et al. 2005), the thermogram was subdivided into four temperature ranges. In the first region (0-200°C), decarboxylation and dehydration reactions occur while the regions above 200°C (regions II-IV) are less well defined and are under current scientific debate. The following sub-divisions have been previously used (Dell’Abate et al. 2003; Lopez-Capel et al. 2005; Manning et al. 2005; Plante et al. 2005): labile organic matter (region II; 220-400°C), recalcitrant organic matter (region III; 400-580°C), and carbonate-derived materials (region IV; 580-850 °C).
As the focus of the analysis was on soil organic matter quality, regions I and IV are not discussed in detail. The sum of the mass loss of temperature regions II and III served for the determination of loss on ignition (LOI). The thermostability index (TS) according to Lopez-Capel et al. (2005) was calculated by dividing the mass loss of recalcitrant organic matter (region III) by LOI. Additionally, the calorific value of the labile (CVL) and recalcitrant organic matter fraction (CVs) was calculated. Therefore, we determined the area under the curve of the DSC signal, using the same interval as for the TG analysis and assuming a linear starting slope. The amount of heat released was divided by the mass loss over the same interval to evaluate the calorific value. The calorific value is given in kilojoule per gram mass loss of this organic matter fraction.
Phytotoxicity and phenol concentration
Because not enough OMW was left to run phytotoxicity tests for all incubation conditions, we needed to shorten the setup: as OMW is predominantly produced during winter and spring, only these two climatic conditions were investigated and the number of sampling days (5, 7, 9, 15, 20, 26, and 31) was reduced. After incubation, the soil samples were transferred to a petri dish and phytotoxicity was determined using seed germination of water cress (Lepidium sarivum) (Komilis et al. 2005). In each sample, 10 water cress seeds were planted in equal depth. After 96 h at 15° and daily ventilation, the root length of the OMW-treated samples (RLx) and control samples (RLc) was determined to obtain the relative root growth (RRG) (Hoekstra et al. 2002)
where RLx is the root length of OMW-treated samples and RLc is the root length of control samples. In addition, soil extracts were obtained (see General soil parameters) and soluble phenolic compounds were measured (see Soil and OMW).
Wettability
To determine the hydrophobicity of the soil, the contact angle and water drop penetration time were determined. The dynamic Wilhelmy Plate Method (WPM) (Diehl et al. 2010; Wilhelmy 1863) was used for the contact angle measurement whereas the water drop penetration time was determined as described by Diehl and Schaumann (2007). Each analysis was repeated five times.
Conditioning of samples in previous experiments resulted in a small standard deviation and thus samples were incubated for 24 h at 30°C in a drying cabinet. All samples were placed in small plastic containers and the surface was carefully leveled. Subsequently, 100 μL of deionized water were added using a micropipette (Finnpipette F2, Thermo Scientific) and the time until complete penetration was determined. When the water drop penetration times were larger than a minute, automatic pictures were taken in 12-s intervals up to 10 min. Using this method, the relative error of the WDPT test remained <5%. The soil samples assigned to the following repellency classes (Bisdom et al. 1993): wettable (<5 s), slight (6-60 s), and moderate (61-600 s) water repellent.
The contact angle was determined using the variation of the Wilhelmy Plate Method. The conditioned soil samples (grain size <1 mm) were fixed onto microscope glass slides (76 × 26 × 1 mm) using double-sided tape. Subsequently, the samples were mounted in the dynamic contact angle meter (DCAT 21, DataPhysics, Filderstadt, Germany) and immersed in distilled water (20°C). The force generated when the sample is immersed in water was used to calculate the advancing contact angle.
Statistical analysis
All results are presented as mean ± SD. Data was assessed using Excel 2007 (MS Office) and the statistical analysis was done with SPSS Statistics 21 (IBM). Significance was determined using Kruskal-Wallis ANOVA; p < 0.05 and p < 0.01 were regarded significant and highly significant, respectively. Pearson’s correlation coefficient was used to assess dependency.
Results
Characteristics of untreated soil and OMW
The chemical and physical properties of soil and OMW are shown in Table 2. The untreated soil had an organic C content of 31 ± mg/g; a pH of 8.3 ± 0.1, due to the buffering effect of carbonates; and its aqueous extract revealed an electrical conductivity of 0.3 ± 0.01 mS/cm. The soil was slightly hydrophobic with respect to its contact angle (92°), but appeared readily wettable with respect to its WDPT (1 s). In addition, the soil sample contained a delta C value of -9.2 ± 0.4‰ both organic and inorganic C. The thermal stability of the organic matter was 526 mg/g LOI and the calorific value of the stable C fraction was 4.5 kJ/g LOI.
The OMW was acidic (pH = 4.7) and showed a high electrical conductivity (10.1 ± 0.1 mS/cm) as well as high concentrations of dissolved organic C (20.7 ± 0.1 g/L) and soluble phenolic compounds (3.5 ± 0.1 g/L). The calorific value of the labile C fraction (2.9 kJ/g) was lower than of the stable C fraction (26 kJ/g). In comparison to soil, OMW was characterized by a low delta C value (-25.6‰) and a low thermal stability (461 mg/g LOI).
Development of soil properties during the incubation
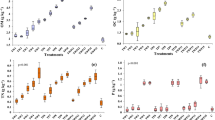
Over the whole incubation period, control samples were characterized by the following average values: 73 ± 74 μg/kg of water-soluble Mn2+, a δ13C-value of −8.7 ± 0.6‰, a loss on ignition of 65 ± 2 mg/g, and a soil respiration rate of 4.5 ± 4.1 μg CO2 g−1 h−1. In addition, the nitrate concentration in the control samples increased in wet-warm, dry-hot, and wet-cold conditions continuously, while the nitrite content increased for the first 3 days and then started to decrease. Only under wet-hot conditions, the nitrite concentrations increased substantially between days 39 and 60. Nitrite and nitrate concentrations in the wet-cold incubation were below the detection limit with the exception of the period between days 39 and day 60. The addition of OMW resulted in an increase of the brown coloration which remained visible until the end of the incubation (day 60). In addition, 10-12 days after OMW treatment fungi growth were detected in all soil samples throughout the incubation independently of the climatic conditions. After OMW treatment, water-soluble Mn2+ increased, while the delta C value decreased. The nitrite and nitrate concentrations remained below the detection limit of 15 mg/kg (Fig. 1a-d). During the incubation period, 27 ± 5 mg CO2 g−1 (dry-hot), 35 ± 6 mg CO2 g−1 (wet-hot), 13 ± 4 2 mg CO2/g (wet-cold), and 15 ± 2 mg CO2/g (wet-warm) were released. The highest maximal soil respiration rates were measured in moist-warm conditions followed by wet-cold, dry-hot, and wet-hot (Fig. 1a). Under wet-hot conditions, the maximal soil respiration was 122 μg CO2 g−1 h−1 on day four. Mn2+ concentration in soil was the highest in wet-hot, followed by dry-hot, wet-cold, and moist-warm, thus inversely correlated to the values for soil respiration (Fig. 1b). Following an initial steep increase in Mn2+ and soil respiration, the values for both declined with time showing a large fluctuation. In contrast to these values, the C isotope ratio increased with the duration of the incubation. In addition, the nitrite and nitrate values increased under hot incubation conditions moderately towards the end of the incubation. The least negative delta C value was measured under wet-hot (-9.0 ± 0.8‰) and dry-hot (-9.9 ± 0.4‰) conditions. The difference between wet-hot and moist-warm (-11.5 ± 0.2‰) conditions was significant. At the same time, the loss on ignition was lowest under hot conditions (wet-hot 71.2 ± 2.3 mg/g; dry-hot 65.8 ± 5.8 mg/g), whereas a greater loss on ignition of 80.7 ± 3.7 mg/g and 76.9 ± 2.9 mg/g was determined under moist-warm and wet-cold conditions, respectively. In relation to the loss on ignition, 6% for wet-hot and 9% for dry-hot conditions of the organic matter added by the OMW treatment remained in the soil. These values were 18 and 20 % for the wet-cold and moist-warm incubation, respectively. The C isotope ratio and cumulative soil respiration yielded similar results.
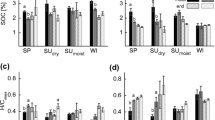
The LOI is derived from the sum of the mass loss of the labile and stable C fraction (Fig. 2a). The mass loss of the stable C fraction in relation to loss on ignition reflects the thermal stability (TS) of the organic compounds. TS of the control samples hardly changed over the incubation period with an average LOI of 532 mg/g (Fig. 2b). In contrast to that, TS under wet-hot conditions decreased to 489 mg/g LOI during the incubation. In addition, under wet-cold conditions, the calorific values of the labile (CVL) and stable C fraction (CVS) were reduced after the incubation while no noticeable difference was detected under the other conditions (Fig. 2c-d). Through the OMW treatment, the concentrations of both C fractions increased, in particular of the labile C fraction. Towards the end, TS increased most substantially for the wet-hot incubation, followed by wet-cold, moist-warm, and dry-hot, but these changes were not significantly different. Mass loss of the stable C fraction of the OMW-treated samples decreased under dry-hot and wet-hot conditions. In contrast, under moist-warm and wet-cold conditions, the mass loss of the stable C fraction remained constant. CVL of OMW was lower than that of the soil samples (Table 2). Throughout the incubation, CVL decreased under all conditions with the exception of the wet-hot samples. The most pronounced decrease was observed under moist-warm conditions. Similarly, CVS of the soil samples increased after OMW treatment. The CVs in wet-hot and moist-warm conditions was comparable before and after the incubation period while the CVs for dry-hot and wet-cold increased and decreased, respectively.
Loss on ignition (a) and thermal stability (b) ± SD as well as calorific value of the labile (c) and stable (d) C fraction of control (left) and OMW-treated (right) at beginning B and end E of the wet-cold (checkered), moist-warm (hatched), dry-hot (black dotted), and wet-cold (white dotted) incubation
Relative root growth and phenol concentration
Figure 3 shows the content of soluble phenolic compounds (a) and root growth (b) during the incubation period under wet-cold and moist-warm conditions. While the concentration of soluble phenolic compounds in the control samples throughout the incubation was constant at around 0.2 ± 0.4 mg GAE/L (not shown), soluble phenolic compounds values reached up to 63 mg GAE/L after the OMW treatment. Immediately after addition of OMW, no root growth was measured for the wet-cold incubation whereas an increase of 14 ± 8% was measured under moist-warm conditions. Soluble phenolic compounds decreased after 7 days under both conditions, although more rapidly for the moist-warm incubation. Despite the decrease of soluble phenolic compounds, the relative root growth decreased (moist-warm) or remained low (wet-cold) until 15 days after OMW treatment. After day 15, the relative root growth increased in both samples, although more pronounced in the moist-warm treatment. This difference is reflected in the lower concentration of soluble phenolic compounds under moist-warm conditions (10.3 ± 0.6 mg GAE/L) compared to the wet-cold incubation (19.5 ± 0.1 mg GAE/L) as well as increased relative root growth under moist-warm conditions (86 ± 32%) compared to wet-cold (54 ± 20%).
Contact angle and water drop penetration time
The water drop penetration time was below 5 s for all controls (Fig. 4a), whereas the contact angle varied significantly between the different incubations. Contact angles of the hot incubated control (84 ± 5°) and the pooled sample prior to incubation (91 ± 6°) were comparable. In contrast to that, contact angles of the moist-warm (6 ± 13°) and wet-cold control (10 ± 8°) were significantly lower and decreased throughout the incubation period. OMW-treated samples of the dry-hot incubation between days 5 and 7 and days 38 and 155 could be classified as slightly and moderately water repellent, respectively (Bisdom et al. 1993), whereas all other samples under dry-hot conditions were hydrophilic. Looking at the other OMW-treated samples, the water drop penetration time was only twice above 5 s namely under moist-wet conditions on day 44 (6 s) and wet-cold conditions on day 9 (7 s). Further, the contact angle between day 10 and day 15 ranged between 50° and 100° (Fig. 4b). Subsequently, the contact angle rose above 100° for the wet-cold and dry-hot samples. In contrast to that, contact angles of the moist-warm and wet-hot incubation remained below 100°. The water drop penetration time decreased towards the end of the incubation under dry-hot conditions but never reached values below 14 s and the contact angle was larger than 100°. At the end of the incubation, the contact angle increased in the following order wet-cold < moist-warm < wet-hot < dry-hot.
Discussion
Degradation and qualitative changes of the OMW OM
Overall, the results of soil and OMW were comparable to earlier findings (Peikert et al. 2015) with the exception of C content of the soil which was increased in this study. The observed reduction of the pH and the increase of water-soluble ions and electrical conductivity after the addition of OMW are furthermore comparable with previous studies dealing with OMW-treated soils (Buchmann et al. 2015; Piotrowska et al. 2006). After OMW treatment soil respiration increased, which is related to bacterial processes (Di Serio et al. 2008; Piotrowska et al. 2006; Saadi et al. 2007). The increase of water-soluble Mn2+, however, can be explained both by biological or abiotic processes.
According to reaction 1, the balance between oxides and bioavailable Mn2+ is largely dependent on the pH value and the redox potential (Blume et al. 2002).
A decrease of the pH and the redox potential shifts the balance towards the formation of Mn2+. However, the decreased pH following the OMW treatment alone does not account for the increase of Mn2+ under moist-warm conditions as the pH was substantially lower under wet-cold conditions. As depicted in reaction equation 1, more Mn2+ is formed when the redox potential is lower. In addition, MnO2 is reduced by anaerobic bacteria which in turn under aerobic conditions can be oxidized to polyvalent manganese oxides (Blume et al. 2002).
In general, the wetness of the soil is not the only important factor for an increased reduction of Mn2+. A water content as low as 60–70 % in nutrient-rich, small-pored soil can be sufficient to deplete oxygen because the consumption of microorganisms will be faster than the supply by diffusion. These oxygen-lacking areas favor the activity of denitrifying bacteria (Tsiknia et al. 2014). The increased concentration of water-soluble Mn2+ after OMW treatment together with the reduction of nitrate and nitrite may be due to reducing conditions. By measuring the activity of different enzymes following OMW treatment, Piotrowska et al. (2006) concluded that the oxygen concentrations were limited due to the oxidation of phenolic compounds. In accordance with this, the highest Mn2+ concentrations were expected under wet-cold and wet-hot conditions. However, the Mn2+ concentrations were highest under moist-warm conditions. A combination of abiotic and biotic processes and their varying dependency on pH and temperature could thus be responsible for the increased formation of Mn2+ under moist-warm conditions. The key factor could be the manganese-catalyzed polymerization of monomeric phenols (Huang 1990; Piotrowska et al. 2006). Metal-phenol-complexes are formed during the polymerization which in the presence of oxygen are broken-up and release radicals. Through the combination of two radicals, methanol and carbon dioxide can be released (Gianfreda et al. 2006). In addition, carbon dioxide can be released during the Mn-catalyzed decarboxylation of phenolic compounds (Dec et al. 2003). Both a recombination of radicals and a decarboxylation would lead to a deoxidation of the soil organic matter, which could explain the increased CVL after the addition of OMW. The isotope ratio of the precipitated CO2 in wet-cold and moist-warm incubation conditions was more negative (mean −11.4 ± 0.6‰) than in the control samples (mean −9.2 ± 0.4‰), but more positive than in the OMW (−25.6‰). Further, a decomposition of carbonate and subsequent release of carbon dioxide should also be considered, but the thermal analysis did not show a significant mass loss in region IV (carbonate) following OMW treatment. However, the organic components of the OMW were not the only sources of the released CO2 but also the soil-borne organic substances (and carbonates) probably contributed.
Independently of these (a)biotic processes, it should be noted that the nitrate concentrations decreased following the OMW treatment. It is likely that the lower pH, the crust formation, bacteriostatic and phenolic compounds, and increased salt concentrations in addition to increased substrate availability reduced the activity of nitrifying microorganisms (Sindhu and Cornfield 1967). The low increase in nitrate towards the end of the incubation showed that this is only a temporary effect of the OMW treatment. Similarly, nitrification was reduced in a field experiment a few days after treatment with 30, 180, and 360 m3/ha OMW (Sierra et al. 2007). After 40 days, the levels of nitrate were comparable to the levels before OMW treatment and continued to increase continuously until 80 days post-OMW treatment. The authors emphasized in that study that the reduced nitrate levels following OMW treatment may negatively affect plant growth temporarily but will increase fertility of the soil in the long term (Sierra et al. 2007). Overall, soil respiration, C isotope ratio, and thermal analysis showed degradation of organic substances depended on the temperature and soil wetness as also reported by Shi and Marschner (2015), who showed that the number of moist days determines respiration in drying and rewetting cycles. The added organic matter was almost completely converted during hot incubations and this effect was further accelerated under wet-hot conditions. In comparison, substantially less organic substances were degraded under wet-cold and moist-warm conditions. Thus, it can be concluded that OMW treatment should be performed under warm conditions with sufficient soil moisture to ensure a quick degradation of the added organic substances.
The different incubation conditions did not only affect the quantity of the organic matter but also its quality because the degradation of the labile C fraction, which in particular increased after the OMW treatment depended on the incubation conditions. With the exception of the wet-hot incubation, the soil samples contained more compounds of the labile C fraction after the incubation. Thus, TS of the samples decreased in comparison with the untreated soil. Under dry-hot conditions, the labile C fraction was further increased by the predominant degradation of the stable C fraction. A part of this fraction is represented by substances of high molecular weight (Foth 1990) with mainly aromatic structure (Leinweber and Schulten 1992; Leinweber et al. 1992; Lopez-Capel et al. 2005). Interestingly, the calorific value of this C fraction increased under dry-hot conditions which could be explained by the growth of fungi. Laor et al. (2011) reported a higher number of spores and a lower bacterial count 3 months after the OMW treatment compared to the samples taken 9 days after application. The authors concluded that the bacteria were responsible for the initial reduction of easily degradable compounds and the fungi for the subsequent degradation of the stable C fraction (Borken et al. 2002; Scheu and Parkinson 1994). Therefore, the formation of hyphae and thereby formation of energy-rich chitin (21 kJ/g) could account for the increase in CVS (Groot et al. 1995). An alternative explanation for the increase in CVS under dry-hot conditions is the presence of long-chain fatty acids in the OMW (Peikert et al. 2015). These are usually accounted to the labile C fraction having a combustion temperature of 200-300°C (Santos et al. 2007). However, the temperature can be raised by modifications such as esterification with starch and amylose (Aburto et al. 1999). Furthermore, potentially strong interactions between long-chain fatty acids and, e.g., soil particles, could account for the increase of CVS.
In contrast to the dry-hot incubation, the mass loss of the stable C fraction remained stable under the wet-cold condition while CVS was reduced. This indicates a conversion to compounds with a low energy value rather than degradation of the stable C fraction. This could be attributed to a temperature-dependent incorporation of unsaturated (Russell et al. 1995) or shortened fatty acids (McGibbon and Russell 1983) into the cell wall as CVL decreased. This adaptation occurs to prevent the crystallization of the membrane (Beales 2004) under cold conditions.
Over the whole incubation period, soil respiration, C isotope ratio, and Mn2+ concentration varied considerably. Although it should be noted that the soil respiration and the concentration of Mn2+ under all incubation conditions correlated with a high significance, the correlation between soil respiration and C isotope ratio was only significant under wet-hot conditions. It seems likely that the δ13C values under the different conditions were influenced by other factors. For example, Šantrůčková et al. (2000) showed a relationship between the C isotope ratio of respired CO2 and microbial biomass depended on the duration of incubation. They observed a depletion of 13C in the microbiotic mass and explained this by a change in the composition of the microbial community and the isotope ratio of the substrate. The correlation between soil respiration and C isotope ratio (local maxima on days 3, 5, and 7) is in accordance with the results (Buchmann et al. 2015), who observed a temporary increase in soil respiration on days 5 and 13 during the incubation of sandy clay under moist-warm conditions. From these observations and the analysis of phenolic compounds, C isotope ratio, and phytotoxicity, the authors defined four phases of OMW degradation that could be accompanied by different microorganisms. A similar correlation was not observed under wet-cold, moist-warm, and dry-hot conditions. Thus, it can be concluded that the phases proposed by Buchmann et al. (2015) can be applied to a loamy clay soil under optimal conditions (wet-hot) but not under the other.
Phytotoxicity
Phenolic compounds added to the soil samples through the OMW treatment were degraded during the incubation period. The highly significant correlation of the soil respiration rate with the concentration of soluble phenolic compounds could be explained either by a decreased microbiotic activity caused by a depletion of soluble phenolic compounds or by a lower CO2 release due to a decrease in abiotic oxidation of soluble phenolic compounds. While soluble phenolic compounds were degraded/converted during the incubation, the root growth increased, which could be related to a lower toxicity of polymeric phenol to plants and bacteria as suggested by Iamarino et al. (2009) or a reduction of phytotoxic Mn2+ (Millaleo et al. 2010). In addition to the duration of the incubation, the root growth also depended on soil temperature and wetness. The minimum root growth on day 15 was present under both conditions but stronger reduced under wet-cold conditions. The subsequent increase in root growth and soil respiration was also observed by Buchmann et al. (2015). The authors suggested a positive effect of microorganisms on root growth either by the supply of growth-promoting substances or protection from phytotoxic compounds. Overall, the OMW-treated samples incubated under moist-warm conditions finally showed a similar root growth as the control samples and a reduction of soluble phenolic compounds by 83%. In contrast to this, the concentration of soluble phenolic compounds under wet-cold conditions was only decreased by 67% and the soil was more phytotoxic.
Soil water repellency
Only under dry-hot conditions the OMW treatment resulted in a water repellent soil. Interestingly, under these conditions, the soil did not become directly hydrophobic, but after 12–15 days of incubation. This suggests that not a single substance added by the OMW treatment but processes within the first days of incubation account for the hydrophobicity. During this initial incubation period, fungal growth was detected which has been reported to form stable aggregates with soil particles (Ingham et al. 2000). A study from Unestam (1991) found that water drops did not penetrate into the majority of forest soil samples with fungal mycelium (20 out of 24) 1 h after the incubation. Thereby, the mycelium retained aerobic conditions that are essential for growth. Another possibility for the reduced water uptake is presented in a study of petroleum induced hydrophobicity from Todoruk et al. (2003). The authors concluded that water was present between partially oxidized hydrocarbons. Through dehydration, this water layer was probably removed and the relevant compounds could directly interact with the soil particles (Todoruk et al. 2003). The already discussed deoxidation of the added organic matter or the time the rearrangement of soil particles to prevent penetration of water takes could explain the delayed hydrophobicity (Doerr et al. 2000). The necessity for the removal of the water layer would not only explain the hydrophobicity under dry-hot condition but also why the wet-cold and moist-warm samples were hydrophilic despite the low degradation of organic substances. It cannot be excluded that the soil will become water repellent as soon as the soil dries because the water interlayer prevents the direct contact with the soil surface. In contrast to the dry-hot incubation, the added organic matter of the OMW was almost completely degraded under wet-hot conditions and thereby the soil remained hydrophilic. Particularly well suited for the degradation of hydrophobic substances are certain species of actinobacteria (Muller and Deurer 2011), such as Streptomyces ssp., Rhodococcus ssp., and Mycobacterium ssp. (Roper 2004). At the incubation end, the contact angle decreased with temperature, suggesting that the temperature additionally influences the penetration of water into the soil. Based on these findings, the temperature and moisture of the soil should be carefully considered to prevent the formation of water-repellent soils.
Conclusions
The incubation experiments show that OMW treatment can result in a temporary increase in organic and inorganic nutrients in soil. From an agricultural standpoint, this is a positive effect. Furthermore, the treatment with OMW stimulated the soil respiration which promoted the conversion of the organic substances. However, in the experimental setup, it could not be determined whether the carbon dioxide release originated from a biotic or abiotic source but the increase in Mn2+ directly after the OMW treatment suggests an abiotic process. During the incubation (all treatments), the labile C fraction was degraded. In addition, a degradation of the stable C fraction was observed under dry-hot conditions, which is likely to be caused by fungi. The C isotope ratio analysis showed that under wet-cold and moist-warm conditions, even after 60 days of incubation, approximately one fifth of the organic substances of the OMW remained in the soil, while under wet-hot and dry-hot conditions an almost complete degradation occurred.
Negative consequences of the OMW treatment are the decrease in pH, the reduction of nitrate, phytotoxicity, and, under dry conditions, water repellency. Nitrate content and pH increased and the phytotoxicity decreased towards the end of the incubation period but after 60 days under dry-hot conditions the soil was still water repellent. The hydrophobicity could either be caused by mycelium growth or the interaction of long-chain fatty acids with soil particles. Both are in accordance with an increase of the calorific value of the stable C fraction.
As expected, the effect of OMW treatment under wet-hot conditions had the most positive effect on soil quality. However, it is surprising that the effects of OMW treatment under wet-cold and moist-warm conditions are rather similar. The excess of substrate and the start of the phenol oxidation under these conditions are probably resulting in reducing conditions and thereby delaying a quick conversion of organic matter. However, it should be noted that in a laboratory setup not all factors are accounted for such as root respiration and faunal activity. In field experiments, these factors can influence the results especially in the beginning of spring when the biological activity increases. Despite the poor degradation of the organic matter during the wet-cold and moist-warm conditions, the soil remained hydrophilic. Probably a fine water layer around the soil particle prevented direct interactions of the OMW organic matter with the mineral soil surface. To avoid a poor water penetration into the soil, OMW treatments should only occur on wet soil. In addition, a high microbiological activity enhanced through tillage (as commonly practiced in Israel and Palestine) could have a positive effect on the degradation of the added organic matter after.
References
Aburto J, Alric I, Thiebaud S, Borredon E, Bikiaris D, Prinos J, Panayiotou C (1999) Synthesis, characterization, and biodegradability of fatty-acid esters of amylose and starch. J Appl Polym Sci 74:1440–1451. doi:10.1002/(SICI)1097-4628(19991107)74:6<1440::AID-APP17>3.0.CO;2-V
Aranda V, Calero J, Plaza I, Ontiveros-Ortega A (2016) Long-term effects of olive mill pomace co-compost on wettability and soil quality in olive groves. Geoderma 267:185–195. doi:10.1016/j.geoderma.2015.12.027
Azbar N, Bayram A, Filibeli A, Muezzinoglu A, Sengul F, Ozer A (2004) A review of waste management options in olive oil production. Crit Rev Environ Sci Technol 34:209–247
Barbera AC, Maucieri C, Cavallaro V, Ioppolo A, Spagna G (2013) Effects of spreading olive mill wastewater on soil properties and crops: a review. Agr Water Manage 119:43–53
Barbera AC, Maucieri C, Ioppolo A, Milani M, Cauallaro V (2014) Effects of olive mill wastewater physico-chemical treatments on polyphenol abatement and Italian ryegrass (Lolium multiflorum Lam.) germinability. Water Res 52:275–281. doi:10.1016/j.watres.2013.11.004
Beales N (2004) Adaptation of microorganisms to cold temperatures, weak acid preservatives, low pH, and osmotic stress: a review. Compr Rev Food Sci Food Saf 3:1–20
Bisdom EBA, Dekker LW, Schoute JFT (1993) Water repellency of sieve fractions from sandy soils and relationships with organic material on soil structure. Geoderma 56:105–118
Blume HP, Bruemmer G, Schwertmann U, Horn R, Koegel-Knabner I, Stahr K, Auerswald K, Beyer L, Hartmann A, Litz N, Scheinost A, Stanjek H, Welp G (2002) Scheffer/Schachtschabel: Lehrbuch der Bodenkunde, 15. Auflage, Enke Stuttgart
Borken W, Muhs A, Beese F (2002) Changes in microbial and soil properties following compost treatment of degraded temperate forest soils. Soil Biol Biochem 34:403–412
Box JD (1983) Investigation of the Folin-Ciocalteau phenol reagent for the determination of polyphenolic substances in natural waters. Water Resour 17:511–525
Buchmann C, Felten A, Peikert B, Muñoz K, Bandow N, Dag A, Schaumann GE (2015) Development of phytotoxicity and composition of a soil treated with olive mill wastewater (OMW): an incubation study. Plant Soil 386:99–112. doi:10.1007/s11104-014-2241-3
Cabrera F, López R, Martinez-Bordiú A, de Lome ED, Murillo JM (1996) Land treatment of olive oil mill wastewater. Int Biodeterior Biodegradation 38:215–225
Carraro L, Fasolato L, Montemurro F, Martino ME, Balzan S, Servili M, Novelli E, Cardazzo B (2014) Polyphenols from olive mill waste affect biofilm formation and motility in Escherichia coli K-12. Microb Biotechnol 7:265–275. doi:10.1111/1751-7915.12119
Chaari L, Elloumi N, Gargouri K, Bourouina B, Michichi T, Kallel M (2014) Evolution of several soil properties following amendment with olive mill wastewater. Desal Water Treat 52:2180–2186. doi:10.1080/19443994.2013.821030
Chiesura A, Marano V, De Francesco P, Maraglino A (2005) Verso la sostenibilità della filiera olivicola: trattamento, recupero e valorizzazione dei sottoprodotti oleari. UNASCO, Roma
Dec J, Haider K, Bollag J-M (2003) Release of substituents from phenolic compounds during oxidative coupling reactions. Chemosphere 52:549–556. doi:10.1016/S0045-6535(03)00236-4
Dell’Abate MT, Benedetti A, Brookes PC (2003) Hyphenated techniques of thermal analysis for characterisation of soil humic substances. J Sep Sci 26:433–440
Di Serio MG, Lanza B, Mucciarella MR, Russi F, Lannucci E, Marfisi P, Madeo A (2008) Effects of olive mill wastewater spreading on the physico-chemical and microbiological characteristics of soil. Int Biodeterior Biodegradation 62:403–407
Diehl D, Schaumann GE (2007) The nature of wetting on urban soil samples: wetting kinetics and evaporation assessed from sessile drop shape. Hydrol Process 21:2255–2265. doi:10.1002/hyp.6745
Diehl D, Bayer JV, Woche SK, Bryant R, Doerr SH, Schaumann GE (2010) Reaction of soil water repellency on artificially induced changes in soil pH. Geoderma 158:373–384
Doerr SH, Shakesby SH, Walsh RPD (2000) Soil water repellency: its causes, characteristics and hydro-geomorphological significance. Earth-Sci Rev 51:33–65. doi:10.1016/S0012-8252(00)00011-8
El Hajouji H, Barje F, Pinelli E, Bailly JR, Richard C, Winterton P, Revel JC, Hafidi M (2008) Photochemical UV/TiO2 treatment of olive mill wastewater (OMW). Bioresour Technol 99:7264–7269. doi:10.1016/j.biortech.2007.12.054
Foth HD (1990) Fundamentals of soil science. John Wiley and Sons, Inc., New York
Gargouri K, Masmoudi M, Rhouma A (2014) Influence of olive mill wastewater (OMW) spread on carbon and nitrogen dynamics and biology of an arid sandy soil. Commun Soil Sci Plant Anal 45:1–14. doi:10.1080/00103624.2013.849727
Gianfreda L, Iamarino G, Scelza R, Rao MA (2006) Oxidative catalysts for the transformation of phenolic pollutants: a brief review. Biocatal Biotransformation 24:177–187. doi:10.1080/10242420500491938
Greco G, Colarieti ML, Toscano G, Iamarino G, Rao MA, Gianfreda L (2006) Mitigation of olive mill wastewater toxicity. J Agric Food Chem 54:6776–6782
Groot C, Margolis L, Clark W (Eds) (1995) Physiological Ecology of Pacific Salmon in UBC Press, Vancouver, p 213
Hoekstra N, Bosker T, Lantinga E (2002) Effects of cattle dung from farms with different feeding strategies on germination and initial root growth of cress (Lepidium sativum L.). Agric Ecosyst Environ 93:189–196
Huang P (1990) Role of soil minerals in transformations of natural organics and xenobiotics in soil. In: Bollag JM, Stotzky G (eds) Soil biochemistry vol 6. Marcel Dekker, New York, pp 29–115
Iamarino G, Rao MA, Gianfreda L (2009) Dephenolization and detoxification of olive-mill wastewater (OMW) by purified biotic and abiotic oxidative catalysts. Chemosphere 74:216–223. doi:10.1016/j.chemosphere.2008.09.061
Ingham ER, Moldenke AR, Edwards CV (2000) Soil Biology Primer. Soil and Water Conservation Society, Ankeny, Iowa
Kapellakis I, Tzanakakis VA, Angelakis AN (2015) Land application-based olive mill wastewater management. Water 7:362–376. doi:10.3390/w7020362
Karpouzas DG, Ntougias S, Iskidou E, Rousidou C, Papadopoulou KK, Zervakis GI, Ehaliotis C (2010) Olive mill wastewater affects the structure of soil bacterial communities. Appl Soil Ecol 45:101–111
Keren Y, Borisover M, Bukhanovsky N (2015) Sorption interactions of organic compounds with soils affected by agricultural olive mill wastewater. Chemosphere 138:462–468. doi:10.1016/j.chemosphere.2015.06.085
Komilis DP, Karatzas E, Halvadakis CP (2005) The effect of olive mill wastewater on seed germination after various pretreatment techniques. J Environ Manage 74:339–348
Kurtz MP, Peikert B, Brühl C, Dag A, Zipori I, Shoqeir Hasan J, Schaumann GE (2015) Effects of olive mill wastewater on soil microarthropods and soil chemistry in two different cultivation scenarios in Israel and Palestinian Territories. Agr 5:857
Laor Y, Saadi I, Raviv M, Medina S, Erez-Reifen D, Eizenbergc H (2011) Land spreading of olive mill wastewater in Israel: current knowledge, practical experience, and future research needs Israel. Israel J Plant Sci 59:39–51
Leinweber P, Schulten HR (1992) Differential thermal-analysis, thermogravimetry and in-source pyrolysis-mass spectrometry studies on the formation of soil organic-matter. Thermochim Acta 200:151–167. doi:10.1016/0040-6031(92)85112-9
Leinweber P, Schulten HR, Horte C (1992) Differential thermal analysis, thermogravimetry and pyrolysis-field ionization mass spectrometry of soil organic matter in particle-size fractions and bulk soil samples. Thermochim Acta 194:175–187
Li H-B, Cheng K-W, Wong C-C, Fan K-W, Chen F, Jiang Y (2007) Evaluation of antioxidant capacity and total phenolic content of different fractions of selected microalgae. Food Chem 102:771–776
Lopez-Capel E, Sohi SP, Gaunt JL, Manning DAC (2005) Use of thermogravimetry-differential scanning calorimetry to characterize modelable soil organic matter fractions. Soil Sci Soc Am J 69:136–140
Manning DAC, Lopez-Capel E, Barker S (2005) Seeing soil carbon: use of thermal analysis in the characterization of soil C reservoirs of differing stability. Mineralogical Mag 69:425–435. doi:10.1180/0026461056940260
McGibbon L, Russell NJ (1983) Fatty acid positional distribution in phospholipids of a psychrophilic bacterium during changes in growth temperature. Curr Microbiol 9:241–244. doi:10.1007/bf01567194
Millaleo R, Reyes-Diaz M, Ivanov AG, Mora ML, Alberdi M (2010) Manganese as essential and toxic element for plants: transport, accumulation and resistance mechanisms. J Soil Sci Plant Nutr 10:470–481. doi:10.4067/S0718-95162010000200008
Mohawesh O, Mahmoud M, Janssen M, Lennartz B (2014) Effect of irrigation with olive mill wastewater on soil hydraulic and solute transport properties. Int J Environ Sci Technol 11:927–934. doi:10.1007/s13762-013-0285-1
Muccioa Z, Jackson GP (2009) Isotope ratio mass spectrometry. Analyst 134:213–222. doi:10.1039/B808232D
Muller K, Deurer M (2011) Review of the remediation strategies for soil water repellency. Agric Ecosyst Environ 144:208–221. doi:10.1016/j.agee.2011.08.008
Niaounakis M, Halvadakis CP (2006) Olive processing waste management. Elsevier, Amsterdam
Peikert B, Schaumann GE, Keren Y, Bukhanovsky N, Borisover M, Garfha MA, Shoqueir JH, Dag A (2015) Characterization of topsoils subjected to poorly controlled olive oil mill wastewater pollution in West Bank and Israel. Agric Ecosyst Environ 199:176–189. doi:10.1016/j.agee.2014.08.025
Piotrowska A, Iamarino G, Rao MA, Gianfreda L (2006) Short-term effects of olive mill waste water (OMW) on chemical and biochemical properties of a semiarid Mediterranean soil. Soil Biol Biochem 38:600–610
Plante AF, Pernes M, Chenu C (2005) Changes in clay-associated organic matter quality in a C depletion sequence as measured by differential thermal analyses. Geoderma 129:186–199. doi:10.1016/j.geoderma.2004.12.043
Prior RL, Wu X, Schaich K (2005) Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J Agric Food Chem 53:4290–4302
Roper MM (2004) The isolation and characterisation of bacteria with the potential to degrade waxes that cause water repellency in sandy soils. Aust J Soil Res 43:801–810. doi:10.1071/SR03153
Russell N, Evans R, Ter Steeg P, Hellemons J, Verheul A, Abee T (1995) Membranes as a target for stress adaptation. Int J Food Microbiol 28:255–261
Saadi I, Laor Y, Raviv M, Medina S (2007) Land spreading of olive mill wastewater: effects on soil microbial activity and potential phytotoxicity. Chemosphere 66:75–83
Santos NA, Tavares MLA, Rosenhaim R, Silva FC, Fernandes VJ Jr, Santos IMG, Souza AG (2007) Thermogravimetricand calorimetric evaluation of babassu biodiesel obtained by the methanolroute. J Therm Anal Calorim 87:649–652. doi:10.1007/s10973-006-7765-1
Šantrůčková H, Bird M, Lloyd J (2000) Microbial processes and carbon‐isotope fractionation in tropical and temperate grassland soils. Funct Ecol 14:108–114
Scheu S, Parkinson D (1994) Changes in bacterial and fungal biomass C, bacterial and fungal biovolume and ergosterol content after drying, remoistening and incubation of different layers of cool temperate forest soils. Soil Biol Biochem 26:1515–1525
Shi A, Marschner P (2015) The number of moist days determines respiration in drying and rewetting cycles. Biol Fertil Soils 51:33–41. doi:10.1007/s00374-014-0947-2
Sierra J, Marti E, Montserrat G, Cruanas R, Garau MA (2001) Characterisation and evolution of a soil affected by olive oil mill wastewater disposal. Sci Total Environ 279:207–214
Sierra J, Marti E, Garau MA, Cruanas R (2007) Effects of the agronomic use of olive oil mill wastewater: field experiment. Sci Total Environ 378:90–94. doi:10.1016/j.scitotenv.2007.01.009
Siles J, Cajthaml T, Hernández P, Pérez-Mendoza D, García-Romera I, Sampedro I (2014) Shifts in soil chemical properties and bacterial communities responding to biotransformed dry olive residue used as organic amendment. Microb Ecol 70:231–243. doi:10.1007/s00248-014-0552-9
Sindhu M, Cornfield A (1967) Effect of sodium chloride and moisture content on ammonification and nitrification in incubated soil. J Sci Food Agr 18:505–506
Singer A (2007) The soils of Israel. Springer, Berlin
Sparling GP (1997) Soil microbial biomass, activity and nutrient cycling as indicators of soil health. In: Pankhurst C, Doube BM, Gupta VVSR (eds) Biological indicators of soil health. CAB International Publishing, Adelaide, pp 97–117
Steinmetz Z, Kurtz MP, Dag A, Zipori I, Schaumann GE (2015) The seasonal influence of olive mill wastewater applications on an orchard soil under semi-arid conditions. J Plant Nutr Soil Sci 178:641–648. doi:10.1002/jpln.201400658
Tamimi N, Diehl D, Njoum M, Marei Sawalha A, Schaumann GE (2016) Effects of olive mill wastewater disposal on soil: interaction mechanisms during different seasons. J Hydrol Hydromech 64:176–195. doi:10.1515/johh-2016-0017
Todoruk TR, Litvina M, Kantzas A, Langford CH (2003) Low-field NMR relaxometry: a study of interactions of water with water-repellant soils. Environ Sci Technol 37:2878–2882
Tsiknia M, Tzanakakis VA, Oikonomidis D, Paranychianakis NV, Nikolaidis NP (2014) Effects of olive mill wastewater on soil carbon and nitrogen cycling. Appl Microbiol Biotechnol 98:2739–2749. doi:10.1007/s00253-013-5272-4
Unestam T (1991) Water repellency, mat formation, and leaf-stimulated growth of some ectomycorrhizal fungi. Mycorrhiza 1:13–20
Wedler W, Boguslawski EV (1965) Zur Methodik der pF-Wert-Bestimmung mit der Zentrifuge Zeitschrift für Pflanzenernährung. Düngung, Bodenkunde 109:249–260
Weller P, Boner M, Foerstel H, Becker H, Peikert B, Dreher W (2011) Isotopic fingerprinting for the authenticity control of crop protection active compounds using the representative insecticide Fipronil. J Agr Food Chem 59:4365–4370
Wilhelmy L (1863) Ueber die Abhängigkeit der Capillaritäts - Constante des Alkohols von Substanz und Gestalt des benetzten festen Körpers. Annalen der Physik und Chemie 119:177
Acknowledgments
This research was conducted within the trilateral project “OLIVEOIL” funded by the DFG (SCHA849/13). We would like to thank Dr. Alexander von Wilamovitz-Moellendorff for his encouraged support and Dr. Jawad Hasan Shoqueir for his help with sampling. Special thanks to Eugenia Podolskaja, Felix Thelen, and Andreas Hirsch for their help in the laboratory.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
Rights and permissions
About this article
Cite this article
Peikert, B., Schaumann, G.E., Bibus, D. et al. Effects of olive oil mill wastewater on chemical, microbiological, and physical properties of soil incubated under four different climatic conditions. Biol Fertil Soils 53, 89–102 (2017). https://doi.org/10.1007/s00374-016-1157-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00374-016-1157-x