Abstract
Background and aims
Alien success has frequently been associated with changes in the concentrations of soil nutrients. We aim to investigate the effects of plant invasion on soil nutrients, potential enzyme activity and litter elemental composition and stoichiometry.
Methods
We compared stands of secondary forest invaded by Ligustrum lucidum and those dominated by natives, and performed litter chemical analyses on 3 native and 2 exotic tree species.
Results
Soils of invaded sites had 20 and 30 % increase in β-glucosidase and alkaline phosphatase activity, higher Olsen-phosphorus (P) and potassium (K) concentrations and lower nitrogen (N) concentration and N:P, N:K and ammonium:Olsen-P ratios. Invaded and non-invaded sites differed in their overall nutrient composition and enzyme activity. Natives and exotics differed in nine of the 16 litter elemental composition and stoichiometry variables analyzed.
Conclusions
The low N:P ratio in litter, the decrease in soil N in invaded stands and the low N concentration of exotics suggest that N is the limiting nutrient and that exotic success is related to higher N uptake and use efficiency. The higher investment in the acquisition of soil resources, higher nutrient uptake and use efficiency of limiting nutrients contribute to the success of exotics in this subtropical forest.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Litter decomposition in terrestrial ecosystems is largely the result of the activity of soil enzymes from communities of bacteria and fungi. This activity is in turn conditioned by physical factors (e.g. temperature, soil humidity and soil pH) and litter characteristics (Sinsabaugh et al. 1993; Kourtev et al. 2002, Tharayil et al. 2013). Vegetational cover modifies both environmental and litter characteristics and consequently the abundance, diversity and activity of microbial communities (van der Putten et al. 2007). Importantly, invasions by exotic species represent a rapid change in community composition and are thus likely to affect the control of litter decomposition and, in turn, the concentrations of soil nutrients and stoichiometric relationships (Ehrenfeld et al. 2001; Ehrenfeld 2003; Allison and Vitousek 2004; Joanisse et al. 2007; Flory and Clay 2010).
β-glucosidases, proteases, ureases and phosphatases are the most important soil enzymes involved in the carbon (C), nitrogen (N) and phosphorus (P) cycles in the soil (Sardans and Peñuelas 2005; Sardans et al. 2008). β-glucosidases break down labile cellulose and related carbohydrates with 1–4 glucosidic bonds, degrading plant cell walls and thus contributing to the firsts phases of plant cell tissues decomposition, which then facilitate the activities of other enzymes such as proteases and phosphatases (Debosz et al. 1999; Sardans et al. 2008; Stege et al. 2010). Proteases are involved in the first phase of N mineralization by hydrolyzing the peptide bonds of amino acids. Ureases regulate the release of N-NH4 by urea hydrolysis, which is essential in the chain of hydrolysis of amino compounds. Phosphatases regulate the hydrolysis of O-P bonds, releasing orthophosphate from organic matter (Sardans and Peñuelas 2005).
Water and resource limitation partly constrain the production and activities of soil enzymes (Criquet et al. 2004; Allison and Vitousek 2005; Sardans and Peñuelas 2010). Plant invasions generate changes in a forest’s capacity to take up and use soil resources (water and nutrients) by both introducing a new species and by modifying the capacities of native species, hence invasions can modify soil nutrient and water contents, that in turn may affect the activities of soil enzymes (Kolb et al. 2002; Ehrenfeld 2003; Allison and Vitousek 2004; Joanisse et al. 2007). Several studies have described the effects of plant invasion on the concentrations of soil nutrients (Evans et al. 2001; Allison and Vitousek 2004) but little is known about its effects on soil stoichiometry and the mechanisms underlying these changes (Sardans and Peñuelas 2012). The objective of the present study was to discern the impacts of plant invasion on the activities of soil enzymes and on soil and litter nutrient concentrations and stoichiometry by studying forest stands in sites heavily invaded by Ligustrum lucidum (Oleaceae) and in sites dominated by native species in a montane forest of northwestern Argentina.
L. lucidum is an evergreen, shade-tolerant tree with high resprouting capacity, survival and growth rates (Easdale et al. 2007). Invasion by Ligustrum modifies soil moisture, light availability, litter depth and plant diversity (Lichstein et al. 2004; Aragón et al. 2014; Ayup et al. 2014). The litter of this invasive species has a significantly higher decomposition rate than those of three of the most common native species in the area (Aragón et al. 2014). Here we analyzed, for the first time to the best of our knowledge, the effects of plant invasion on the elemental composition and stoichiometry of both soil and litter and on the activities of soil enzymes. Given Ligustrum high growth rate, we hypothesize a potentially high demand of resources. In addition to Ligustrum demands, the environmental conditions (i.e., lower soil moisture and light availability) present in invaded stands, could lead to increases in soil-enzyme concentration in order to compensate for the unfavorable conditions. All these in turn, could result in increases in soil and litter nutrient concentrations and in changes in soil and litter stoichiometries.
Methods
Study site
The study was conducted in the lower montane forest of Sierra de San Javier, Tucumán, Argentina (26° 70’ S, 65° 35’ W) at approximately 800 m a.s.l. The area represents the southern-most limit of the subtropical Andean montane forest (also known as Yungas), which extends from Bolivia to the province of Catamarca in Argentina (Cabrera and Willink 1980; Grau and Brown 1995). Average annual precipitation ranges from 1,300 to 1,500 mm distributed in a monsoonal regime with dry winters and wet summers (Bianchi and Yañez, 1992). The mean annual temperature is 18 °C, with frosts occurring from June to August. Most of the Sierra de San Javier piedmont was cleared for crop production and grazing during the early twentieth century (Grau and Brown 1995; Brown et al. 2001), but many cleared areas were abandoned in the last two decades and currently have forests at different stages of regeneration (Grau and Aide 2007; Grau et al. 2008). Many of these secondary forest stands are colonized by several exotic species (Grau and Aragón 2000), but L. lucidum is by far the most abundant exotic species and the only one that forms monodominant forest stands in this area.
Studied species
We included five tree species in this study: two exotics (L. lucidum and Morus sp.) and three natives (Cinnamomum porphyrium, Cupania vernalis and Myrsine laetevirens). L. lucidum is an Asian tree that colonizes areas of varying land-use histories and ages. It is more abundant in secondary forest stands but also grows in openings in old-growth forests (Aragón and Morales 2003). L. lucidum is evergreen, shade tolerant and has a high growth rate (Easdale et al. 2007). Importantly, its distribution is expected to expand in the near future (Grau et al. 2008). Morus sp. is also of Asian origin, but unlike Ligustrum, it is deciduous and shade intolerant (Grau et al. 1997; Easdale et al. 2007). Morus sp. is a fast-growing species as well and is most abundant on the edges of young secondary forest stands that were reclaimed from citrus orchards.
The native species in this study are all late successional and bird dispersed, with relatively high growth rates compared to the other native species in this area (these species are among the 10 fastest-growing species among a set of 29 species studied by Easdale et al. (2007)). They are also among the most abundant in the canopy or subcanopy strata (Grau et al. 1997; Easdale et al. 2007). C. porphyrium is a semi-deciduous, tall, shade-tolerant tree abundant in the canopies of secondary and old-growth forests (Grau et al. 1997; Easdale et al. 2007). C. vernalis has similar life-history characteristics, but it integrates into the subcanopy stratum. Its saplings account for approximately 70 % of the saplings in native and invaded forest understories (Grau et al. 1997; Lichstein et al. 2004). M. laetevirens is an evergreen tree, of intermediate height (it sometimes integrates into the canopy) and has the highest growth rate among the three native species studied (Easdale et al. 2007). Unlike the other species, Myrsine bears fruit during the winter, partially coinciding with L. lucidum fructification. The morphological and demographic characteristics of Myrsine are also more similar to those of Ligustrum (e.g. maximum growth rate, growth in well-lit conditions and density in secondary forests) (Easdale et al. 2007).
Experimental design and sampling
To evaluate the activities of soil enzymes in native and invaded forests, we used a paired design with five invaded-native stand pairs .We considered as invaded those stands which had L. lucidum as the dominant species in the canopy and occurring at densities higher than 500 ind./ha. Native stands were dominated by C. porphyrium, Blepharocalix salicifolius and C. vernalis among others, and even though some individuals of L. lucidum were present, especially as saplings, they could be considered rare. Even though Morus sp is abundant in many secondary patches, it does not form mono-dominant stands. For this reason, we only considered two forest types: stands invaded by Ligustrum, and stands dominated by native species. Importantly, native and exotic species co-occur in the different stands but at very dis-similar abundance. For more details about species composition and stands characteristics see Grau et al. 1997; Aragón and Morales 2003 and Easdale et al. 2007. Within each pair, the stands were similar in age (between 30 and 50 years of succession), altitude (between 550 and 700 m), slope and soil type (typically hapludoll with loam sandy texture with 50–30–20 % of loam, sand and clay respectively) and were larger than 2 ha. Pairs were selected based on the greatest similarity in age and the smallest geographic separation (from 200 to 500 m between the members of each pair). For more details about the location of the stands in the field see Aragón et al. (2014). We established a 3 x 3 m plot in each stand, avoiding edges and gaps in the canopy.
Three soil cores (diameter 6 cm) from the top 10 cm of the soil profile were collected from each plot of the five invaded/native pairs in April 2012. Each soil sample was kept at approximately 5 °C until analyzed. In the laboratory, we first sieved the soil through a 2-mm mesh and then analyzed the two fractions for soil-enzyme activity and concentrations of C and N and the main nutrients Ca, Fe, Mg, Mn, and Na. For the soil analyses (i.e., enzymes and nutrients) five pairs of stands (invaded and non-invaded forests in each pair) in 5 different sites were considered to account for the potential site to site variability. Each pair was taken as a block (5 repetitions), and forest types (two levels) as treatment (fixed factor).
The litterfall of 3–8 individuals of each of the five studied species was collected in invaded and native sites between May and September 2011 into plastic bags suspended underneath each plant. Leaves were air-dried for 3–5 days and stored in open paper bags until further analysis.
Chemical analyses
Activities of soil enzymes
To determine β-glucosidase activity, we incubated 5 g of soil for 3 h at 37 °C with acetate buffer (2 M, pH 6.2 diluted in 1,000 ml of distilled water) and with salicin (β-glucosido-saligenin) as a substrate (Tabatabai 1994). The solutions were filtered (Millipore 0.45-μm HA nitrocellulose filter) and the saligenin released from the substrate was determined colorimetrically after coloring with 2,6-dibromomchinon-4-chloroimide in a borate buffer (0.2 M, pH 10). At pHs above 9, saligenin forms a blue indophenol dye with 2,6-dibromchinon-4-chloroimide, which was then measured at 578 nm with a Helios α spectrophotometer (Thermo Scientific, Waltham, MA, USA) against the reagent blank. We calculated the saligenin content by referring to a calibration curve obtained with standards containing 0, 10, 20, 50 and 100 μg of saligenin per ml. β-glucosidase activity was expressed as μg of saligenin released per gram of soil per hour. For the analyses of all enzymes we first dried the soil samples by freezing in order to prevent protein damage by heat.
To determine protease activity, we used the method of Ladd et al. (1976) using casein as substrate. Briefly, 5 mL of substrate solution (casein 2 %, w/w) was added to 1 g of soil sample. We added 5 mL of Tris (Tris-hydroxymethyl-aminomethane) buffer (0.05 M, pH 8.1) and then incubated for 2 h at 50 °C. After incubation, the remaining substrate was precipitated with trichloroacetic acid. Thereafter, samples and controls were filtered immediately. For photometric analysis, 5 mL of the filtrate was added to 7.5 mL of alkali reagent in a test tube, mixed well, 5 mL of Folin-Ciocalteu’s phenol reagent were added, and mixed again. Alkali reagent is a mix of three solutions: a) 50 g of sodium carbonate, 60 mL of 0.1 M NaOH in 600 mL of distilled water (1,000 mL); b) 5 g of copper sulfate pentahydrate in 1,000 mL of distilled water (20 mL); and c) 10 g of sodium potassium tartate in 1,000 mL of distilled water (20 mL). Before colorimetric measurement, the samples, controls and standards were filtered (Millipore 0.45-μm HA nitrocellulose filter) to prevent interference from the precipitates formed by the casein reaction products. The solutions were then allowed to stand at room temperature for exactly 90 min for color development. We measured the extinction at 700 nm with the spectrophotometer against the reagent blank and calculated the tyrosine content by referring to a calibration curve obtained with standards containing 0, 100, 250, 1,000 and 1,500 μg of tyrosine per ml. Protease activity was expressed as μg tyrosine per gram of soil per hour.
We used the Kandeler and Gerber (1988) method for determining urease activity. An aqueous (controls) or a buffered urea solution (samples) was added to 5 g of soil sample and incubated for 2 h at 37 °C. Released ammonium (NH4 +) was extracted with 2 mol L−1 KCl and quantified by a modified Berthelot reaction (Schinner et al. 1996). The solutions were shaken for 30 min and filtered (Millipore 0.45-μm HA nitrocellulose filter) to prevent interference from possible precipitates. The determination was based on the reaction of sodium salicylate with NH3 in the presence of sodium dichloroisocyanurate, which forms a green complex under alkaline pH conditions. The extinction was measured at 690 nm against the reagent blank. Sodium nitroprusside was used as a catalyst to increase the sensitivity of the method approximately 10-fold. We calculated the NH4 + content by referring to a calibration curve obtained with standards containing 0, 1, 1.5, 2, and 2.5 mg NH4 + L−1. Urease activity was expressed as μg NH4 + released per gram of soil per hour.
Phosphatase activity was determined by adding 4 mL of THAM solution (Tris-hydroxymethyl-aminomethane with citric, maleic and boric acids), buffer (tris hydroxymethil aminomethane, maleic acid, citric acid monohydrate and boric acid in 500 mL of 1 M NaOH, at pH 6.5 for acid phosphatase assays or pH 11 for alkaline phosphatase assays) and 1 mL of p-nitrophenyl phosphate solution (as a substrate) prepared in the same buffer to 1 g of soil in a flask. We then swirled the flask for a few seconds to mix the contents. The stoppered flask was incubated at 37 °C for 1 h, and then1 mL of 0.5 M CaCl2 and 4 mL of 0.5 M NaOH were added. The flask was again swirled for a few seconds to stop the reaction. The solution was filtered as above to prevent the appearance of possible precipitates. The fading of the intensity of the yellow color in the calibration standards, samples and controls was measured at 398 nm against the reagent blank. We calculated the p-nitrophenol content by referring to a calibration curve obtained with standards containing 0, 10, 20, 30, 40 and 50 ppm of p-nitrophenol. Phosphatase activity was expressed as μg p-nitrophenol per gram of soil per hour.
Chemical analyses of litter and soil
For chemical analyses of foliar tissue, leaves were dried in an oven at 60 °C to a constant weight and then ground in a CYCLOTEC 1093 (Foss Tecator, Hoganas, Sweden) and stored in desiccators until analysis. C and N contents were determined from 0.7 mg of pulverized dried sample by combustion coupled to gas chromatography in an Elemental Analyzer CHNS Eurovector 3011 Thermo Electron Gas Chromatograph model NA 2100 (C.E. Instruments-Thermo Electron, Milan, Italy). For the other nutrients (Ca, Fe, Mg, Mn, S, P, K and Na), 0.25 g of pulverized dried sample was diluted with the acid mixture HNO3 (60 %) and H2O2 (30 % w/w) and digested in a MARSXpress microwave system (CEM, Matthews, NC, USA). The digested solutions were brought to a final volume of 50 mL with ultra pure water and at 1 % HNO3. Blank solutions (5 mL of HNO3 with 2 mL H2O2 without any sample biomass) were regularly analyzed. After digestion, the concentrations of Ca, Fe, Mg, Mn, S, P, K and Na were analyzed with an Optima 4300DV ICP-OES (Optical Emission Spectrometer for Inductively Coupled Plasma, Perkin-Elmer, Waltham, MA, USA). To assess the accuracy of the biomass digestion and analytical procedures, we used certified biomass NIST 1573a (tomato leaf) standards. To analyze the soil samples, we followed the same protocols used for the foliar tissues, but we filtered the samples with a 0.45 μm microfilter.
In addition to C, N and the other nutrients, we also determined immediately available P (Olsen P) and ammonium. The P available to plants in the soil was determined by Olsen’s method (Olsen et al. 1954). This method measures the relative availability of orthophosphate (PO4-P) extracted in 0.5 M NaHCO3 adjusted to pH 8.5. Phosphorus content in 1 g of soil was determined spectrophotometrically at 882 nm in an acidic medium of 0.24 M H2SO4 by reacting with ammonium molybdate using ascorbic acid as a reductant in the presence of antimony potassium tartrate. Phosphorus concentration was determined using a calibration curve built with seven solutions containing 0.0, 0.25, 0.50, 0.75, 1, 2 and 3 mg L−1 PO4-P.
To determine the ammonium concentration in the soil, we used a procedure similar to that described for urea. Released ammonium (NH4 +) was extracted with a 2 mol L−1 KCl solution added to 1 g of dried soil. The determination was based on the reaction of sodium salicylate with NH3 in the presence of sodium dichloroisocyanurate and using sodium nitroprusside as a catalyst. The measurement was performed at 690 nm using a calibration curve similar to that described for urea.
Statistical analyses
We analyzed soil-enzyme activities, ammonium and Olsen-P concentrations and ammonium:Olsen-P ratios in the soil with two-way ANOVAs, with forest types as treatments (invaded and native) and the five pairs of sites as blocks (repetitions). In the case of leaf litter, we first considered two sources of variation: forest types (litter collected from individuals occurring in invaded or in native stands: two levels) and species (five levels). Secondly, we explored the differences only among species, and lastly we grouped species in native (three species) and exotics (two species) (i.e., two levels).
We performed principal component analyses (PCA) using correlation matrices with 20 variables for the soil (five enzymes, 12 nutrients including ammonium and Olsen P and three ratios) and 13 variables for the litter (10 nutrients and three ratios). The differences among treatments (invaded and native for the soil and five species for the litter) in the variable distribution in the multidimensional space defined by the PCA were tested using an ANOVA for the scores of sites (n = 10) or individuals (10 of each species). In addition, to specifically evaluate if the invasion of L. lucidum changed over all studied soil variables, nutrient compositions and potential enzyme activities in the soil and nutrient composition in the litter, we used a multi-response permutation procedure (MRPP) based on Euclidean distance (Biondini et al. 1985). MRPP is a non-parametric procedure for testing the null hypothesis of differences between groups or entities. It provides a statistic, δ, that is the weighted mean of within-group distances and is associated with a p-value that indicates the likelihood of δ being equal to or smaller than that observed by chance (McCune and Mefford 1999). For the soils, we used a block-MRPP considering the five pairs of invaded/native sites, and for the litter, we grouped data from all species and considered forest type as the only source of variation. All the multivariate analyses were performed with the PC-ORD 5 program (McCune and Mefford 1999). In addition, for the litter, we performed univariate ANOVAs with 16 variables (10 nutrients and six ratios) and with species status (native or exotic) as the classification factor. We also made multiple comparisons among species (five levels).
Results
Enzymes and nutrients in the soil
Invaded sites had an approximately 20 % increase in β-glucosidase activity and a 30 % increase in alkaline phosphatase activity. The soil of invaded sites had a lower N concentration (both as NH4 + and total N), higher Olsen-P concentration (40 %) and lower ammonium:Olsen-P ratio (67 %) (Table 1). Invaded stands also had a higher K concentration (13 %) and lower N:P and N:K ratios (25 and 39 %, respectively) (Table 2). The first two axes of the PCA ordination that considered 20 variables explained 66 % of the total variance (Fig. 1). The first axis (explaining 42.8 % of the total variance) was related to the variability among blocks, and the second axis (explaining 23.4 % of the total variance) separated invaded from native stands (ANOVA with PCA scores: Axis I: F for blocks =7.1, p = 0.04; Axis II: F for forest type =17.27, p = 0.01). Invaded sites were associated with higher enzyme activities and Olsen-P and K concentrations, while the ammonium:Olsen-P ratio and Mg and Fe concentrations were higher in the native sites (Fig. 1). This pattern was reinforced by the MRPP results. Overall, the distances within invaded and native stands were smaller than expected by chance (chance-corrected within group agreement =0.19, p = 0.03), so we can conclude that the invaded and native sites significantly differed in their nutrient compositions and enzyme activities.
Principal component analysis with three sets of variables: enzymes, nutrients and nutrient ratios (20 variables) measured in invaded (red) and native (light blue) stands. The symbols indicate the corresponding score group means ± SE. and the arrows represent variable eigenvectors in the space plotted by the first two PCA axes. The projection of the lines along each axis indicates their relative importance
Chemical analyses of leaf litter
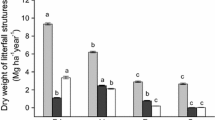
The litter of the different species had similar elemental compositions in the highly invaded stands and the native stands (Table 4, appendix). This pattern was evident from both the univariate and the MRPP analyses (chance-corrected within group agreement =0.01, p = 0.15). In contrast, the litter of the different species had several differences in elemental composition and stoichiometry. The native and exotic species as groups differed in nine of the 16 studied variables (Table 3). Exotics had higher concentrations of Ca and K and lower concentrations of Mn, P, C and N. Differences among species were also evident through the PCA ordination, especially between the natives Cinnamomum and Cupania and the other studied species, whereas the two exotics and the native Myrsine all shared some characteristics (Fig. 2a). The first three axes of the PCA ordination explained 63 % of the total variability. Two groups could be distinguished along the first axis (29.7 % of the variability) (Fig. 2a, letters on the top): exotics and Myrsine on the positive side and Cinnamomum and Cupania on the negative side. Exotics and Myrsine had higher concentrations of Ca and K and lower N:K and P:K ratios compared to the remaining two species (Fig. 2b). The second axis (20.4 % of the variability) separated three groups: Cupania with litter concentrations of Mg, Fe, S, Na, P and Mn higher than those for Cinnamomum and exotics and Myrsine with intermediate characteristics (Fig. 2a letters on the right). The third axis (13.6 % of the variability) separated, although to a minor degree, exotics (Ligustrum and Morus) from Myrsine (Fig. 3a letters on the right), and overall, the invasive from the native species as groups (Fig. 3 a, right arrows) Exotics had lower N concentrations and higher Ca concentrations (Fig. 3b) (Table 3).
a Principal component analysis with 13 variables (nutrients and nutrient ratios) of the five species studied. C (Cinnamomum), Cu (Cupania) and My (Myrsine) are native species. L (Ligustrum) and M (Morus) are exotic species (axes 1 and 2). The symbols correspond to the score group means ± SE. Different letters indicate significant differences among species at each axis at p < 0.05. b Variable eigenvectors in the space plotted by the first two PCA axes. The projection of the lines along each axis indicates their relative importance
a Principal component analysis with 13 variables (nutrients and nutrient ratios) of the five species studied. Codes as in Fig. 2a (axes 1 and 3). The symbols correspond to the score group means ± SE. Different letters indicate significant differences among species at each axis at p < 0.05. When big arrows are present they indicate significant differences between invasive and exotic species. b Variable eigenvectors in the space plotted by the first and third PCA axes. The projection of the lines along each axis indicates their relative importance
Discussion
Effects of invasion on soil and litter elemental composition and stoichiometry
The soils of invaded stands had lower N concentrations (both as NH4 + and total N), higher extractable K concentrations, higher plant-available P concentrations and a general trend toward higher soil-enzyme activities. All these data suggest higher rates of nutrient cycling, mainly of N, in the invaded stands. These results are in agreement with several studies reporting significant impacts of alien plants on the availability of soil nutrients, decomposition of organic matter, nutrient cycling and soil stoichiometry (Sardans and Peñuelas 2012, Tharayil et al. 2013). In a recent review of the effect of invasive plants on N and P availability, C:N:P ratios of soils and rates of soil decomposition, mineralization and nutrient cycling from 65 studies conducted in environments with unclear limitations of nutrients (except some conducted mainly in arid and semiarid areas of the USA), 48 studies reported increases in the availability of soil nutrients, 14 reported decreases and three were inconclusive (Sardans and Peñuelas 2012). Most of the 14 studies reporting decreases in soil nutrients were studies with Bromus tectorum, an invasive grass of semiarid areas of the USA (but see Castro- Díez et al. 2013), indicating that most invasions in nutrient-rich ecosystems tend to increase the availability of soil nutrients and hence to increase nutrient cycling. In forests invaded by Ligustrum, however, we found a decrease in nutrients, particularly N. Importantly, less is known of the effect of plant invasions on nutrient imbalances. By investigating litter and soil stoichiometry and soil-enzyme activity in Argentine subtropical forests, we have shown that the success of invasive plants is associated with an overall change in soil nutrient composition and function, mainly by a decrease in the most limiting nutrient, here N, increasing the imbalances with other nutrients such as P, which tends to increase its availability in the soil (i.e. decreasing N:P ratio). This result sheds light on the role of N:P ratios in plant invasions, which has remained inconclusive (Sardans and Peñuelas 2012).
Even though we cannot effectively discriminate plant available N with our data, there are several indications that N appears to be the most limiting element in this area. The global litter N:P ratio is 46:1 (±3:1) (McGroddy et al. 2004) but was 7.4:1 (±1:1) in our study. Moreover, the lower soil water content in invaded stands was associated with lower total N concentrations, suggesting that invasive success is related to higher N uptake. In contrast, P in these soils did not appear to be limiting for plant growth. Invaded stands had higher Olsen-P concentrations related to higher soil-enzyme activity and generally faster cycling of water and elements. This area receives an annual precipitation above 1,000 mm, so water is not likely an important limiting factor. All these data thus strongly suggest that N is the limiting nutrient and that invasive plant success depends on a large capacity of N uptake, reduced soil N availability and a higher limitation of N. The current literature suggests that alien invasion in nutrient-rich environments frequently favors plant species with high rates of photosynthesis and growth (Baruch and Goldstein 1999; Leishman et al. 2007; Mozder and Zieman 2010; Feng et al. 2011), low costs of foliar construction (Nagel and Griffin 2001; Feng et al. 2007; González et al. 2010), large investments of N in photosynthetic production (Ehrenfeld 2003; Xu et al. 2007; Feng 2008; Shen et al. 2011), higher capacities of nutrient uptake (Zabinsky et al. 2002; Harrington et al. 2004; Blank and Sforza 2007; Feng 2008; Blank 2010; Hewins and Hyatt 2010; Leffler et al. 2011; Peng et al. 2011) and high levels of plasticity in the acquisition of resources as a function of pulses in nutrient availability (Leffler et al. 2011). These factors indicate that higher efficiency in nutrient uptake and foliar traits enabling rapid rates of growth (Leishman et al. 2007; Zabinsky et al. 2002) will help invading species to succeed when resources are not limited (Bray et al. 2003; Funk and Vitousek 2007; Shah et al. 2009).
Similar to our findings, Lichstein et al. (2004) reported that the percentage of soil organic matter was negatively correlated with Ligustrum basal area. That study hypothesized that this pattern could be associated with litter quality or with the rapid growth rate in Ligustrum and presumably with rapid nutrient uptake. Our results support this hypothesis, because Ligustrum has rather low concentrations of N and C in its leaf litter.
Higher soil concentrations of extractable K were associated with higher K concentrations in the leaf litter of invasive species and of Myrsine. Previous studies have demonstrated that invaded stands have less soil moisture (Aragón et al. 2014), suggesting a higher water uptake with the increasing abundance of invasive species (Gerlach 2000; Levine et al. 2003; Holmes et al. 2005; van Wilgen et al. 2008) that in turn decreases runoff (Dye and Jarmain 2004; Gorgens and Van Wilgen et al. 2008; Holmes et al. 2005; van Wilgen et al. 2008). Invasive plants can thus prevent the loss of K by increasing water uptake, decreasing runoff and taking up more K. Higher K uptake should be correlated with higher concentrations of K in the litter and, in general, with faster plant-soil-plant K cycling. We have observed a marginally significant higher K concentration in the litter of invaded stands, especially in L. lucidum litter, coinciding with the lower soil moisture observed in these stands (Aragón et al. 2014). Importantly, ecosystem-level impacts of an invasive species depend on the combination of traits that determine its per capita effect, together with its abundance (Drenovsky et al. 2012). Ligustrum dominance must be taken into account when assessing its potential impacts at community scale (Aragón et al. 2014).
Effect of invasion on potential soil enzyme activity
The observed increases in phosphatase and β-glucosidase activities reinforce the general results of soil and litter composition, suggesting that plant invasion accelerates nutrient uptake and nutrient-cycling rates. The success of invasive plants in this semi-wet, sub-tropical ecosystem is thus associated with faster water and nutrient cycles, higher soil-enzyme activities, lower concentrations of some nutrients in the soil and with higher levels of nutrients in stand biomasses and faster growth. These results also suggest cascade effects, because higher soil-enzyme activity and higher N uptake can be related to low ammonium:Olsen-P ratios in soils. Lower soil N:P ratios impact soil trophic webs, increasing the abundance of rapidly growing microbial groups (Elser et al. 2003; Fierer et al. 2007), and may be associated with the observed increase in the potential activity of some soil enzymes. Microbes adjust their extracellular release of soil enzymes to maximize the mobilization of substrates rich in their limiting element (Wallenstein and Weintraub 2008: Burns et al. 2013). The higher C:N ratio of the soil in invaded stands is related to the higher levels of potential soil β-glucosidase activity. This enzyme catalyzes the first steps of the hydrolysis of large C-chains and is critical for the further action of enzymes linked to N and P mineralization (Debosz et al. 1999; Stege et al. 2010). Moreover, since β-glucosidase is involved in cellulose catabolism, more abundant and extended litter production in invaded stands (Ligustrum is a perennial species which has an extended period of litter fall) (Grau and Aragón 2000; Easdale 2006) may also explain the higher activity of this enzyme in this type of forest.
The maximum potential enzyme activity depends of the density of active enzymes present in soil. Our results thus show a higher density of soil enzymes, consistent with the idea that invaded stands have a higher investment in the production of soil enzymes. Our findings are thus an indication of enzyme concentration and capacity in the soil but not necessarily of their actual activity in the field. As stated above, soil moisture in stands dominated by Ligustrum is lower than in native stands (Lichstein et al. 2004; Aragón et al. 2014). Humidity directly affects enzyme activity by affecting hydrolysis and hence subsequently determines nutrient mineralization. Litter decomposition in four species (two natives and two exotics) tended to be lower in stands dominated by Ligustrum (Aragón et al. 2014), and the same was true for a standard substrate (leaves of Populus sp.) (Fernandez 2012). Consequently, invaded stands appear to invest more in the production of enzymes (that accumulate in the soil) to compensate for the unfavorable environmental conditions. This is particularly important given the higher growth rate of Ligustrum and hence its potentially higher demand for resources.
Species-specific effects
We have observed clear differences between the native and invasive species and also some differences among the natives. Whereas the litter traits of Cinnamomum and Cupania clearly differed from those of the invasive species, Myrsine shared several characteristics with them. This observation supports previous studies that found some morphological and demographic similarities among Myrsine, Ligustrum and Morus (Easdale et al. 2007; Easdale and Healey 2009). Easdale et al. (2007) measured 19 demographic variables in 29 montane tree species of northwestern Argentina, and Myrsine was closer in multidimensional space to Ligustrum and Morus than to Cinnamomum and Cupania. Myrsine and the two exotic species also shared ecomorphological features (structural, biochemical and morphological) such as seed mass, maximum growth rate and foliar P concentration (Easdale 2006). The similarities were especially evident with Ligustrum, which also has an overlapping fructification phenology. All these similarities indicate that the morphological and life-history characteristics of Myrsine resemble those of the exotics in this study. The invasion of Ligustrum in the study area has caused changes in species cover, dominance, diversity and sapling recruitment (Lichstein et al. 2004; Aragón and Morales 2003). Importantly, native species may be affected in different ways. Exotics may specially affect species that share attributes with them and hence potentially have similar requirements (Drenovsky et al. 2012), as perhaps happens with Myrsine, whose recruitment and growth is particularly reduced in stands dominated by Ligustrum (Bartolucci 2011). In a comparison of native and invaded stands of similar age, Myrsine showed a reduction in sapling recruitment of approximately 85 % in Ligustrum stands, while Cinnamomum and Cupania were less affected (between 3.5 and 50 %). The same trend was found for changes in basal area of adults (an average reduction of 80 % in Myrsine) (Bartolucci 2011). Myrsine has more similar elemental composition and stoichiometry with invasive species than Cinnamonium and Cupania, which seem to explain the more negative impact over Myrsine. The native Myrsine would tend to use the resources in the same way than the invasive species and should compete stronger with it.
These results agree with those expected under the biogeochemical niche hypothesis (Peñuelas et al. 2008; 2010; Sardans and Peñuelas 2013), which claims that elemental composition (nutrient concentrations and their stoichiometric relationships) varies among plant species as a consequence of differential genotypic expression and functioning. This hypothesis predicts that different plant species growing in the same community would tend to have different elemental compositions to reduce the overlap in the use of soil resources and consequently would reduce direct competition. These results are consistent with the “competitive niche exclusion” a basic paradigm of the ecological niche theory (Bonsall et al. 2004; Phillips et al. 2004; Levine and HilleRisLambers 2009; Alder et al. 2010). In this case the native species with more similar biogeochemical niche to the invasive species was the species first affected by the success of the invasive species. The biogeochemical niche thus appears as a useful tool to detect niche overlap intensity in the study of invasive species, frequently very difficult to be investigated in field conditions (Mooney and Cleland 2001; Davies et al. 2007). Moreover, it can provide clues of which native species would present the highest sensitivity to invasive species spread.
In addition, we would like to acknowledge that even though our design intended to control the potential inherent variability between invaded and native stands, we cannot unequivocally assigned the differences in soil enzyme or nutrients to the treatment effect. This is a common and recognized limitation in studies at landscape scale such as most of the studies related to invasive species ecology (van Kleunen et al. 2010). The comparative approach remains, so far, as one of the most commonly used in the field (e.g., Leishman et al. 2010; Tecco et al. 2010). However, these limitations have to be taken into account when interpreting the results.
Final remarks and conclusions
Even though several studies have highlighted the role of biological invasions at global scale, invasion ecology still lacks general influential hypotheses (Strayer 2012). Several attempts that intended to identify traits associated with invasion impacts have yield mixed results mainly because successful invasions are closely linked to native community assembly and environmental filters (Drenovsky et al. 2012). In the context of invasion ecology, understanding the link between invasion and ecosystem functioning is crucial to fully evaluate the effect of invasive species. In this study, even though we focused on one particular exotic species, we intended to provide general understanding of the potential effect of this species on the functioning of the ecosystem.
Our results indicated that invasion by Ligustrum increased the maximum potential of alkaline phosphatase and β-glucosidase activities, probably by compensating for the lower soil water content of the invaded soil, as compared to native forest soil. These observations are supported by previously documented increases in water and plant nutrient uptake in invaded stands (Ayup et al. 2014; Aragón et al. 2014). Plant invasion decreased the availability of soil N, likely the limiting nutrient (litter N:P ratio of 7.4, based on mass) in these soils, whereas it increased the availability of soil P. The lower soil water content and the higher growth capacity of alien plants, coinciding with higher plant nutrient uptake, the large investment in soil-enzyme activity and the lower N and P concentrations in litter, thus suggesting a link between the success of alien plants and a higher capacity to take up nutrients and retain them in the biomass and generally in the ecosystem. Our results showed that the species most sensitive to invasion, Myrsine, had a litter composition more similar to that of the invasive plants than to other native plants. This result strongly suggests that this native species and the exotics Ligustrum and Morus use resources very similarly, and thus Myrsine is most directly affected by competitive pressure from invasive species.
References
Alder PB, Ellner SP, Levine JM (2010) Coexistence of perennial plants: an embarrassment of niches. Ecol Lett 13:1019–1029
Allison SD, Vitousek PM (2004) Rapid nutrient cycling in leaf litter from invasive plants in Hawai’i. Oecologia 141:162–619
Allison SD, Vitousek PM (2005) Responses of extracellular enzymes to simple and complex nutrient inputs. Soil Biol Biochem 37:937–944
Aragón R, Morales JM (2003) Species composition and invasion in NW Argentinean secondary forests: Effects of land use history, environment and landscape. J Veg Sci 14:195–204
Aragón R, Montti L, Ayup MM, Fernández R (2014) Exotic species as modifiers of ecosystem processes: Litter decomposition in native and invaded secondary forests of NWArgentina. Acta Oecol 54:21–28
Ayup MM, Montti L, Aragón R, Grau HR (2014) Invasion of Ligustrum lucidum (Oleaceae) in the southern Yungas: Changes in habitat properties and decline in bird diversity. Acta Oecol 54:72–81
Bartolucci G (2011) Descripción de los bosques de Ligustrum lucidum (Oleácea) en la selva de Yungas en la provincia de Tucumán y su influencia sobre la comunidad de renovales. Undergraduate dissertation. University of Tucumán. Argentina
Baruch Z, Goldstein G (1999) Leaf construction cost, nutrient concentration, and net CO2 assimilation of native and invasive species in Hawaii. Oecologia 121:183–192
Bianchi AR, Yañez C (1992) Las precipitaciones del Noroeste Argentino. Instituto de Tecnología Agropecuaria, Argentina
Biondini ME, Bonham CD, Redente EF (1985) Secondary successional patterns in a sagebrush (Artemisia tridentata) community as they relate to soil disturbance and soil biological activity. Vegetatio 60:25–36
Blank RR (2010) Intraspecific and interspecific pair-wise seedling competition between exotic annual grasses and native perennials: plant-soil relationships. Plant Soil 326:331–343
Blank RR, Sforza R (2007) Plant-soil relationships of the invasive annual grass Taeniatherum caput-medusae: a reciprocal transplant experiment. Plant Soil 298:7–19
Bonsall MB, Jansen VAA, Hassell MP (2004) Life history trade-offs assemble ecological guilds. Science 306:111–114
Bray SR, Kitayima K, Sylvia DM (2003) Mycorrhizae differentially alter growth physiology, and competitive ability of an invasive shrub. Ecol Appl 13:565–574
Brown AD, Grau HR, Malizia LR, Grau A (2001). Argentina. In: Kappelle M and Brown AD (Eds.), Bosques nublados del Neotrópico. Instituto Nacional de Biodiversidad (INBio), Santo Domingo de Heredia, Costa Rica, 623-659
Burns RG, DeForest JL, Marxsen J, Sinsabaugh RL, Stromberger ME, Wallenstein MD, Weintraub MN, Zoppini A (2013) Soil enzymes in a changing environment: Current knowledge and future directions. Soil Biol Biochem 58:216–234
Cabrera AL, Willink A (1980) Biogeografía de América Latina. Organización para Estados Americanos (OEA), Washington
Castro- Díez P, Godoy O, Alonso A, Gallardo A, Saldaña A (2013) What explains variation in the impacts of exotic plant invasions on the nitrogen cycle? A meta-analysis. Ecol Lett. doi:10.1111/ele.12197
Criquet S, Ferre E, Farnet AM, Le Petit J (2004) Annual dynamics of phosphatase activities in an evergreen oak litter: influence of biotic and abiotic factors. Soil Biol Biochem 36:1111–1118
Davies KF, Harrison S, Safford HD, Viers JH (2007) Productivity alters the scale dependence of the diversity-invasibility relationship. Ecology 88:1940–1947
Debosz K, Rasmussen PH, Pedersen AR (1999) Temporal variations in microbial biomass C abd cellulolytic enzyme activity in arable soils: effects of organic matter input. Appl Soil Ecol 13:209–218
Drenovsky RE, Grewell BJ, D’Antonio CM, Funk JL, James JJ, Molinari N, Parker IM, Richards CM (2012) A functional trait perspective on plant invasion. Ann Bot 110:141–153
Dye P, Jarmain C (2004) Water use by black wattle (Acacia mearnsii): implications for the link between removal of invaded trees and catchment streamflow response. S Afr J Sci 100:40–44
Easdale TA (2006) Comparative morphologies and life histories amongst montane tree species in North-Western Argentina. PhD Dissertation. University of Wales, Bangor, United Kingdom
Easdale TA, Healey JR (2009) Resource-use-related traits correlate with population turnover rates, but not stem diameter growth rates, in 29 subtropical montane tree species. Perspect Plant Ecol Evol Syst 11:203–218
Easdale TA, Healey JR, Grau HR, Malizia A (2007) Tree life histories in a montane subtropical forest: species differ independently by shade-tolerance, turnover rate and substrate preference. J Ecol 95:1234–1239
Ehrenfeld JG (2003) Effects of Exotic Plant Invasions on Soil Nutrient Cycling Processes. Ecosystems 6:503–523
Ehrenfeld JG, Kourtev P, Huang W (2001) Changes in soil functions following invasions of exotic understory plants in deciduous forests. Ecol Appl 11:1287–1300
Elser JJ, Acharya K, Kyle M, Cotner J, Makino W, Markow T, Watts T, Hobbie S, Fagan W, Schade J, Hood J, Sterner RW (2003) Growth rate-stoichiometry couplings in diverse biota. Ecol Lett 6:936–943
Evans RD, Rimer R, Sperry L, Belnap J (2001) Exotic plant invasion alters nitrogen dynamics In an arid grassland. Ecol Appl 11:1301–1310
Feng YL (2008) Photosynthesis, Nitrogen allocation and specific leaf area in invasive Eupatorium adenophorum and native Eupatorium japonicum grown at different irradiances. Physiol Plant 133:318–326
Feng YL, Auge H, Ebeling SK (2007) Invasive Buddleja davidii allocates more nitrogen to its photosynthetic machinery than five native woody species. Oecologia 153:501–510
Feng YL, Li YP, Wang RF, Callaway RM, Valiente-Banuet A, Inderjit (2011) A quicker return energy-use strategy by populations of a subtropical invader in the non-native range: a potential mechanism for the evolution of increased competitive ability. J Ecol 99:1116–1123
Fernandez RD (2012) Descomposición de hojarasca en Bosques secundarios de la Sierra de San Javier: efecto de las especies exóticas. Undergraduate dissertation. University of Tucumán. Argentina
Fierer N, Bradford MA, Jackson RB (2007) Towards an ecological classification of soil bacteria. Ecology 88:1354–1364
Flory LS, Clay K (2010) Non-native grass invasion alters native plant composition in experimental communities. Biol Inv 12:1285–1294
Funk JL, Vitousek PM (2007) Resource use efficiency and plant invasion in low-resource systems. Nature 446:1079–1081
Gerlach Jr. JD (2000) A model experimental system for predicting the invasion success and ecosystem impacts of non-indigenuous summer-flowering annual plants in California’s Central Valley grasslands and oak woodlands. PhD dissertation, University of California, Davis, CA
González AL, Kominoski JS, Danger M, Ishida S, Iwai N, Rubach A (2010) Can ecological stoichiometry help explain patterns of biological invasion? Oikos 119:779–790
Grau HR, Aide TM (2007) Are Rural–Urban Migration and Sustainable Development Compatible in Mountain Systems? Mt Res Dev 27:119–123
Grau HR, Aragón R (2000) Arboles invasores de la Sierra de San Javier. In: Grau HR, Aragón R (eds) Arboles Exóticos de las Yungas Argentinas. LIEY-Universidad Nacional de Tucumán, Tucumán, pp 5–20
Grau HR, Brown AD (1995) Patterns of tree species diversity along latitudinal and altitudinal gradients in the Argentinean subtropical montane forests. In: Churchill SP, Balslev H, Forero E, Luteyn J (eds) Biodiversity and conservation of Neotropical montane forest. New York Botanical Garden, Bronx, pp 295–300
Grau HR, Arturi MF, Brown AD, Aceñolaza PG (1997) Floristic and structural patterns along a chronosequence of secondary forest succession in Argentinean subtropical montane forests. Forest Ecol Manag 95:161–171
Grau HR, Hernández ME, Gutierrez J, Gasparri NI, Casavecchia MC, Flores EE, Paolini L (2008) A peri-urban neotropical forest transition and its consequences for environmental services. Ecol Soc 13(1): 35 [online] URL: http://www.ecologyandsociety.org/vol13/iss1/art35/
Harrington RA, Fownes JH, Cassidy TM (2004) Japanese Barberry (Berberis thunbergii) in forest understory: leaf and whole plant reponses to nitrogen availability. Am Midl Nat 151:206–216
Hewins DB, Hyatt LA (2010) Flexible N uptake and assimilation mechanisms may assist biological invasion by Alliaria petiolata. Biol Inv 12:2639–2647
Holmes PM, Richardson DM, Elser KJ, Witkowski ETF, Fourie S (2005) A decision-making framework for restoring riparian zones degraded by invasive alien plants in South Africa. S Afr J Sci 101:553–564
Joanisse GD, Bradley RL, Preston CM, Munson AD (2007) Soil enzyme inhibition by condensed litter tannins may drive ecosystem structure and processes: the case of Kalmia angustifolia. New Phytol 175:535–546
Kandeler E, Gerber H (1988) Short-term assay of soil urease activity using colorimetric determination of ammonium. Biol Fert Soils 6:68–72
Kolb A, Alpert P, Enters D, Holzapfe C (2002) Patterns of invasion within a grassland community. J Ecol 90:871–881
Kourtev PS, Ehrenfeld JG, Huang WZ (2002) Enzyme activities during litter decomposition of two exotic and two native plant species in hardwood forests of New Jersey. Soil Biol Biochem 34:1207–1218
Ladd JM, Brisbane PG, Butler JHA (1976) Studies on soil fumigation. 3. Effects on enzyme-actrivities, bacterial numbers and extractable ninhydrin reactive compounds. Soil Biol Biochem 8:255–260
Leffler AJ, Monaco TA, James JJ (2011) Nitrogen acquisition by annual and perennial grass seedlings: testing the roles of performance and plasticity to explain plant invasion. Plant Ecol 212:1601–1611
Leishman MR, Haslchurst T, Ares A, Baruch Z (2007) Leaf trait relationships of native and invasive plants: community-and global-scale comparisons. New Phytol 176:635–643
Leishman MR, Thomson VP, Cooke J (2010) Native and exotic invasive plants have fundamentally similar carbon capture strategies. J Ecol 98:28–42
Levine JM, HilleRisLambers J (2009) The importance for the maintenance of species diversity. Nature 461:254–U130
Levine JM, Vilà M, D’Antonio CM, Dukes JS, Grigulis K, Lavorel S (2003) Mechanisms underlying the impacts of exotic plants. Proc R Soc Lond B 270:775–781
Lichstein JW, Grau HR, Aragón R (2004) Recruitment limitation in secondary forests dominated by an exotic tree. J Veg Sci 15:721–728
McCune B, Mefford MJ (1999) PC-ORD Multivariate analysis of ecological data. Version 3. 0. MjM Software Design, Gleneden Beach, OR
McGroddy ME, Daufresne T, Hedin LO (2004) Scaling of C : N : P stoichiometry in forests worldwide: Implications of terrestrial redfield-type ratios. Ecology 85:2390–2401
Mooney HA, Cleland EE (2001) The evolutionary impact of invasive species. Proc Natl Acad Sci U S A 98:5446–5451
Mozder TJ, Zieman JC (2010) Ecophysiological differences between genetic lineages facilitate the invasion of non-native Phragmites australis in North American Atlantic coast wetlands. J Ecol 98:451–458
Nagel JM, Griffin KL (2001) Construction cost and invasive potential: comparing Lythrum salicaria (Lythraceae) with co-occurring native species along pond banks. Am J Bot 88:2252–2258
Olsen SR, Cole CV, Watanabe FS, Dean LA (1954) Estimation of available phosphorus in soils by extraction with sodium bicarbonate. USDA. Cir. n 939
Peng RH, Fang CM, Li B, Chen JK (2011) Spartina alterniflora invasion inceases soil inorganic nitrogen pools through interactions with tidal subsides in the Yangtze Estuary, China. Oecologia 165:797–807
Peñuelas J, Sardans J, Ogaya R, Estiarte M (2008) Nutrient stoichiometric relations and biogeochemical niche in coesxisting plant species: effect of simulated climatic change. Pol J Ecol 56(4):613–622
Peñuelas J, Sardans J, Llusia Owen S, Carnicer J, Giambelluca TW, Rezende EL, Waite M, Niinemets Ü (2010) Faster returns on leaf economics and different biogeochemical niche in invasive compared with native plant species. Glob Chang Biol 16:2171–2185
Phillips RA, Silk JRD, Phalan B, Catry P, Croxall JP (2004) Seasonal sexual segregation in two Thalassarche species: competitive exclusion, reproductive role specialization or foraging niche divergence? Proc R Soc B Biol Sci 271:1283–1291
Sardans J, Peñuelas J (2005) Drought decreases soil enzyme activity in a Mediterranean Quercus ilex L. forest. Soil Biol Biochem 37:455–461
Sardans J, Peñuelas J (2010) Soil enzyme activity in a Mediterranean forest after six Years of drought. Soil Sci Soc Am J 74:838–851
Sardans J, Peñuelas J (2012) The role of plants in the effects of Global Change on nutrient availability and stoichiometry in the plant-soil system. Plant Physiol 160:1741–1761
Sardans J, Peñuelas J (2013) Climate and taxonomy underlie different elemental concentrations and stoichiometries of forest species: the optimum “biogeochemical niche”. Plant Ecol 215:441–455
Sardans J, Peñuelas J, Ogaya R (2008) Experimental drought reduced acid and alkaline phosphatase activity and increased organic extractable P in soil in a Quercus ilex Mediterranean forest. Eur J Soil Biol 44:509–520
Schinner F, Ohlinger R, Kandeler E, Margesin R (1996) Methods in Soil Biology. Springer Lab Manual, Berlin
Shah MA, Reshi ZA, Khasa DP (2009) Arbuscular mycorryzhas: drivers or passengers of alien plant invasion. Bot Rev 75:397–417
Shen XY, Peng SL, Chen BM, Pang JX, Chen LY, Xu HM, Hou YP (2011) Do higher resource capture ability and utilization efficiency facilitate the successful invasion of native plants? Bio Inv 13:869–881
Sinsabaugh RL, Antibus RK, Linkins AE, McClaugherty CA, Rayburn L, Repert D, Weiland T (1993) Wood decomposition: Nitrogen and phosphorus dynamics in relation to extracellular enzyme activity. Ecology 74:1586–1593
Stege PW, Messina GA, Bianchi G, Olsina RA, Raba J (2010) Determination of b-glucosidase activity in soils with a bioanalytical sensor modified with multiwalled carbon nanotubes. Anal Bioanal Chem 397:1347–1353
Strayer DL (2012) Eight questions about invasions and ecosystem functioning. Ecol Lett 17:1199–1210
Tabatabai MA (1994) Soil enzymes. In: Bottomley PS, Angle JS, Weaver RW (eds) Methods of Soil Analysis: Part 2- Microbiological and Biochemical Properties. Soil Science Society of America, Madison, pp 775–833
Tecco P, Diaz S, Cabido M, Urcelay C (2010) Functional traits of alien plants across contrasting climatic and land-use regimes: do aliens join the locals or try harder than them? J Ecol 98:17–27
Tharayil N, Alpert P, Bhowmik PC, Gerard P (2013) Phenolic inputs by invasive species could impart seasonal variations in nitrogen pools in the introduced soils: a case study with Polygonum cuspidatum. Soil Biol Bioche 57:858–867
Van der Putten WH, Klironomos JN, Wardle DA (2007) Microbial ecology of biological invasions. ISME J 1:28–37
Van Kleunen M, Weber E, Fischer M (2010) A meta‐analysis of trait differences between invasive and non‐invasive plant species. Ecol Lett 13:235–245
Van Wilgen BW, Reyers B, Le Maitre DC, Richardson DM, Schonegevel L (2008) A biome-scale assessment of the impact of invasive alien plants on ecosystems services in South Africa. J Environ Manag 89:336–349
Wallenstein MD, Weintraub MN (2008) Emerging tools for measuring and modeling the in situ activity of soil extracellular enzymes. Soil Biol Biochem 40:2098–2106
Xu CY, Griffin KL, Schuster WSF (2007) Leaf phenology and seasonal variation of photosynthesis of invasive Berberis thunbergii (Japanese barberry) and two co-occuring native understory shrubs in a northeastern United States deciduous forest. Oecologia 154:11–21
Zabinsky CA, Quinn L, Callaway RM (2002) Phosphorus uptake, not carbon transfer, explains arbuscular mycorrhizal enhancement of Centaurea maculosa in the presence of native grassland species. Funct Ecol 16:758–765
Acknowledgments
This research was supported by the Spanish Government grant CGL2013-48074-P, the Catalan Government project SGR 2014- 274 and the European Research Council Synergy grant ERC-2013-SyG-610028 IMBALANCE-P. R. Aragón’s stay at CREAF was supported by an external grant from CONICET (National Research Council, Argentina).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Harry Olde Venterink.
Appendix 1
Appendix 1
Rights and permissions
About this article
Cite this article
Aragón, R., Sardans, J. & Peñuelas, J. Soil enzymes associated with carbon and nitrogen cycling in invaded and native secondary forests of northwestern Argentina. Plant Soil 384, 169–183 (2014). https://doi.org/10.1007/s11104-014-2192-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-014-2192-8