Abstract
Although nitrogen has historically limited terrestrial plant productivity in the northern hemisphere, accelerated industrial activity is changing the availability of N, with consequences for ecosystem properties including altered susceptibility to biological invasion. Alliaria petiolata (Bieb.) Cavara & Grande is an increasingly problematic invader in forests of eastern North America. Population growth rate of this species is especially high in N-rich habitats, and it produces a variety of N-based compounds that have been shown to interfere with the growth and reproduction of native plants. To investigate how increases and shifts in forms of N will impact A. petiolata, seedlings were transplanted to the greenhouse from the field and grown in sand culture. We applied three concentrations of N (0.25, 1 and 2 mM) using five different ratios of NH4 + and NO3 − (100/0, 75/25, 50/50, 25/75, 0/100) in a crossed design to yield fifteen different treatments. Plants were measured throughout the growing season and a final harvest yielded measures of biomass and tissue quality. Plant growth increased significantly in response to increased concentration of total N. These increases were similar for all combinations of N. This flexibility in uptake ability may facilitate the invasion of this species, not only by increasing the range of habitats A. petiolata can occupy but also by enhancing N uptake that can lead to the production of secondary compounds disrupting other species’ belowground mutualisms. We suggest that this species’ ability to respond rapidly to changes in N availability, regardless of its form, may modify competitive interactions with natives and intensify its negative impacts.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The availability of nitrogen (N) has historically limited primary production in terrestrial ecosystems of the northern hemisphere (Crawford and Glass 1998). Even though N is abundant in the atmosphere, plants mainly take up N in mineral form (ammonium (NH4 +) and nitrate (NO3 −)), compounds formed during microbial fixation or breakdown of organic materials.
Environmental N availability is escalating dramatically because of human activity. Increased production, application and runoff of synthetic, inorganic fertilizers have led to eutrophication of water ways, shifts in nutrient pools and ecosystem productivity. These modifications can alter the historical patterns of relative nitrate and ammonium abundance, resulting in irreversible changes in biodiversity and function of natural ecosystems mediated by plant uptake patterns (Matson et al. 2002).
It is well understood that plant species differ in their ability to assimilate nitrate and ammonium (Olsson and Falkengren-Grerup 2000; de Graaf et al.1998). For example, when ammonium is the sole N source available, Arabidopsis thaliana plants experience growth retardation as seedlings, before the full onset of photosynthetic activity (Hoffmann et al. 2007). In contrast, 4 year old Picea abies and other coniferous trees show preferential uptake of ammonium by non-mycorrhizal roots (Marschner et al. 1991). N metabolism can also change with plant age; early Allium sp. growth is maximized when ammonium is the sole source of N, but nitrate is required by older plants (Abbès et al. 1995). In Vigna and Glycine sp., nitrate uptake increases throughout vegetative growth, peaks at early reproductive stages and decreases during seed formation (Imsande and Touraine 1994; Imsande and Edwards 1988). Many species grow best with a complement of both nitrate and ammonium (Miller and Bowman 2002). It has been reported that Lycopersicon roots grow optimally in a 3:1 ratio of nitrate to ammonium and growth is inhibited if the ammonium concentration is too high (Crawford and Glass 1998; Bloom et al. 1993). These different optima are the result of natural selection, as enzyme production pathways are genetically determined (Glass et al. 2002). The ecological consequences of such preferences will influence plant distributions and biological diversity at a variety of scales.
If introduced species can respond rapidly to increases in total N availability and/or have a greater or differential affinity for increasingly available forms of N, these changes may open the door for invasions to occur or accelerate existing ones. By monopolizing soil nutrient pools, species that can capitalize on N modifications will directly or indirectly suppress native species (Ehrenfeld 2003; Tilman 1982). This interaction can result in a feedback loop, with changes in species composition within ecosystems altering nutrient availability, leading to further biodiversity changes (Ehrenfeld 2006). Thus, it is important to understand how introduced species respond to both concentration and sources of N.
Alliaria petiolata (Bieb.) Cavara & Grande is a prominent invader in deciduous forests of North America. Its success has been attributed to its resource use patterns, its phenology and its chemical weaponry. It has been classified as a nitrophilic species due to its accumulation of nitrate in vacuoles enhancing osmotic regulation (Marschner 2002). It germinates in late February and early March while native species are dormant, and lives as a rosette for a full calendar year before reproducing in early summer (Nuzzo 2000; Roberts and Boddrell 1983; Cavers et al. 1979). Alliaria petiolata has been demonstrated to alter the microbial diversity of soils by releasing a host of secondary metabolites from root and leaf tissues (Cipollini and Gruner 2007; Stinson et al. 2006). These compounds disrupt mycorrhizal relationships, preventing native plant species from recolonizing invaded soils (Cipollini and Gruner 2007; Stinson et al. 2006).
In the field, A. petiolata responds to total N availability, but its form preferences have not yet been investigated. Does it perform best using only one form of N or can it use all forms interchangeably? To what extent does it respond to increasing N availability? Field observations and models based thereon have revealed that A. petiolata population growth responds in a significant and nonlinear way to ammonium availability, while estimated plant chlorophyll content in the field increases with increasing soil nitrate (Hyatt, unpublished data). These observations suggest that A. petiolata survival, growth and reproduction is likely to be dependent on the availability of both nitrate and ammonium. If so, those environmental responses, mediated through demographic processes, might explain some of the wide variability in invasion processes observed throughout the introduced range (Meekins and McCarthy 2000).We investigated the growth of first year A. petiolata plants in response to the source and concentration of available N in a greenhouse experiment.
Methods
Design
Two-month old Alliaria petiolata (garlic mustard) plants used in the experiment were collected from a wooded area on the southern border of the Rider University campus on June 6, 2006. A. petiolata is somewhat difficult to germinate in the greenhouse so we chose uniformly sized field-germinated plants that were in abundance in this location. All plants had two leaves, were under 2 cm in height, and were shallowly rooted. To minimize transplantation effects, plants were collected from higher-light environments with low canopy density. The root systems were washed with distilled water to limit residual soil effects.
The seedlings were planted in a 1:1:1 sand culture of perlite, vermiculite and sand. This growing medium allowed experimental control of all soil nutrient availability (Piccini and Azcon 1987). The growing medium was moistened and mixed thoroughly. Forty-five 15 cm diameter 20 cm tall pots were then filled loosely. Three seedlings were planted in each pot and the pots were assigned a random location on greenhouse benches. Climate within the greenhouse was set to a constant 26.6°C day and night with ambient midday light averaging 300 μmol m−2 s−1.
To investigate the effects of N source and concentration, we used five different NO3 −/NH4 + ratios (100/0, 75/25, 50/50, 25/75, 0/100) crossed with three different total N concentrations (0.25, 1, 2 mM). These concentrations were selected based on the range of total available N as measured by field resin ion-exchange bags placed in permanent A. petiolata study plots within our region (mean = 1058 μm, max = 4072.6 μm, min = 155.7 μm). The 45 pots were each assigned one of 15 treatments, with three replicates each, a total of 135 plants.
Plants were watered twice weekly with a modified Johnson’s complete nutrient solution (Fichtner and Schulze 1992). The pH of the bulk solution was normalized to 5.8 using phosphoric acid stock and then N ratios and availabilities were manipulated by adding combinations of 1 M sodium nitrate and 1 M ammonium sulfate. These additions did not change the pH. The ammonium sulfate additions added a negligible amount of sulfate to the NH4 + solutions because the stock solution was made with an already high S concentration. We were unable to control for the added sodium in the NO3 − treatments, but the amount added was very small (less than 2.5 mg Na/L for high NO3 − solutions, less for the other ratios).
Responses
To measure plant responses to our nutrient treatments, several approaches were used to measure plant performance. The measurements served as a proxy for assimilation. To estimate plant size and growth rate we counted leaf number and measured the width of the largest leaf biweekly. These were nondestructive measurements that together estimate aboveground biomass well for first year plants (r 2 = 0.806, N = 72, unpublished data). We also measured chlorophyll content using a Minolta SPAD meter (Hoel and Solhaug 1998; Markwell et al. 1995). SPAD is a moderately good predictor of leaf C:N in Alliaria petiolata (R 2 = 0.55 for logarithmic regression, N = 44, also see Spencer et al. 2007). Thus, we assume that leaves with higher measures of SPAD also contain more N within their leaf tissues. Because there was no variation in leaf number until 1 month after planting, we only measured leaf number three times during the experiment.
After 17 weeks (5 October 2006) the leaves were counted and removed from each plant. Leaf area, excluding petioles, was measured using an Epson Perfection 1250 scanner (Seiko Epson Corp.) and Image J software (National Institutes of Health, USA, see Shimoji et al. 2006). To examine the effects of treatments on specific leaf area, one leaf from each plant was sampled with a 5 mm diameter punch and dried and weighed. The remaining leaves and stems were then dried for 72 h in a 60°C oven and weighed to measure above ground biomass. Roots were washed, separated and extracted from the soil using water. These were also dried and weighed. Afterwards leaves and stems were ground in a Wiley Mill (40 mesh) for C:N elemental analysis using a Carlo Erba C:H:N elemental analyzer. We only analyzed C:N for plants supplied with pure NH4 +, pure NO3 − and a 50:50 ratio for plants grown at each N concentration.
Analysis
Repeated measures effects of N concentration and source ratios on dependent variables were examined using repeated measures 2-way ANOVA tests (SPSS, SPSS Inc, Chicago, IL, USA).
The effects of concentration and source ratio on final width of largest leaf, SPAD, leaf number, specific leaf area, above and below ground biomass, and C:N ratio were examined using two-way ANOVA tests with post-hoc comparisons carried out using Bonferroni significance corrections (STATA, StataCorp, College Station, TX, USA and R statistical software Version 2.9.2 (2009-08-24)).
Results
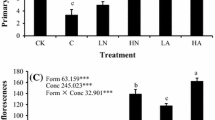
Nondestructive measures of plant size revealed that N concentration significantly increased width of largest leaf, leaf number, and SPAD (Table 1; Fig. 1). The significant interaction between time and concentration for width of largest leaf and SPAD indicates that the rate at which leaves grew and N accumulated in the plants changed with solution concentration (Fig. 1). There were no significant effects of N source or source × time interaction on these measures over the growing season (Table 1), thus post hoc tests were not carried out.
Effect of N concentration on plant leaf number, leaf size and SPAD measurements over time. Solid circles/solid lines: Low N concentration (0.25 mM), Hollow triangles and dotted lines: Medium N concentration (1 mM), Hollow squares and dashed lines: High N concentration (2 mM). Error bars indicate one standard error at each time period
At harvest, a final series of nondestructive measures were taken to measure experimental plants’ response to N treatments. N concentration had a significant effect on A. petiolata size, reflecting the results of the repeated measures tests throughout the growing season. With increasing N concentration, plants had more and wider leaves (Table 2). Further, increasing N concentration was also associated with an increase in SPAD measurements (Table 2). N source ratio had no effects on plant leaf number, leaf width or SPAD leaf measurements (Table 2).
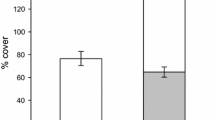
Destructive measures of plants yielded similar results. The concentration of N had a significant effect on plant size as measured by aboveground biomass, belowground biomass, leaf area and SPAD. Source ratios had no significant effects on any of these plant growth measures (Table 2; Fig. 2 shows stereotypical patterns for dependent variables, using dry shoot biomass as an example). Significant interactions between source and concentration for SPAD measurements arose due to low SPAD readings under medium nitrogen conditions for plants growing in 75% or more nitrate. Other source treatments showed consistently increasing SPAD readings from low to high overall nitrogen concentrations. Specific leaf area (Table 2) and leaf C:N ratio (F(source)2,25 = 2.63, F(concentration)2,25 = 3.31, F(source × concentration)4,25 = 0.46 and Fig. 3) were not statistically significantly affected by N source or concentration although there was a trend for both measures to decrease with increasing N concentration.
Strong response of biomass to N concentration but no response to source. Bars show mean dry biomass of A. petiolata under different source combinations: white bars: 100% NH4 +, grey bars: 75% NH4 +:25% NO3 −, hatched bars: 50% NH4 +:50% NO3 −, dotted bars: 25% NH4 +:75% NO3 −, solid bars: 100% NO3 −. Error bars show one standard error
C:N Ratio decreases with increasing N concentration but there is no effect of ratio on tissue C:N. Symbols as in Fig. 2. No data shown for 75% NH4 +:25% NO3 − or 25% NH4 +:75% NO3 − ratios
Discussion
Alliaria petiolata performed equally well under all ammonium/nitrate combinations despite the fact that many members of the Brassicaceae are known to perform better under nitrate-enriched conditions (Hoffmann et al. 2007; Zhang et al. 2007; Glass et al. 2002; von Wirén et al. 2000). Increasing N concentrations led to significant increases in growth and vigor, underscoring A. petiolata’s nitrophilic metabolism. These two results may help explain not only the ability of A. petiolata to invade a broad range of soils in North America but also the wide variability of the invasion process throughout the region.
The ability to take up N in multiple forms may intensify negative interactions between A. petiolata and native vegetation. Field observations suggest that overwinter survival of plants is positively associated with total soil N and also has a strong influence on per capita population growth rate (Hyatt, unpublished data). Since reproduction occurs only in the second year of life, soil N plays a significant role in sustaining and increasing population size, a major factor contributing to A. petiolata invasion. In our study region, this species most frequently occurs in soils characterized by an approximately 9:1 ratio of NO3 − to NH4 + (unpublished data). If the natives are unable to take up any of the NH4 +, this gives a generalist access to 10% more total N. It is possible that natives can take up some of both types of N, but generalists can still have access to more total N although their ability to assimilate it may be different. Native species in these populations during the first summer season include vines like Parthenocissis quinquefolium, Rubus hispidus, and Toxicodendron radicans, as well as herbaceous and woody species such as Circaea lutetiana, Eurybia divaricata, Impatiens capensis, and Polygonum virginianum. There are also many exotics in these communities, including Celastrus orbiculatus, Galinsoga parviflora, Lonicera japonica and Microstegium vimineum (Hyatt, pers. obs.). These coexisting species likely vary in their associations with nitrate and ammonium. Clearly, more experimental evidence concerning competitive interactions between A. petiolata and native as well as exotic vegetation with different types of N supply is needed (Rickey and Anderson 2004; Hyatt, in prep).
The synthesis of N-based secondary compounds involved in defense against herbivory and allelopathic interactions is limited by N availability. The production and release of glucosinolates and other secondary metabolites negatively impacts mutualistic relationships between native plants and arbuscular mycorrhizal fungi (Cipollini and Gruner 2007; Stinson et al. 2006). These fungi are known to provide other plants with N and phosphorous in exchange for carbon in the form of sugar molecules (Govindarajulu et al. 2005). In the absence of these fungi, native plant species cannot form mutualistic relationships that help them acquire needed nutrients and could eventually become locally extinct. Thus, increased N could magnify the suppressive interactions between A. petiolata and native vegetation.
As fossil fuel use and agricultural application of N increases, deposition of N in relatively undisturbed habitats may render those ecosystems more susceptible to invasion by nitrophilic species like A. petiolata. This effect will be even more magnified in disturbed habitats where the native vegetation may be compromised already. Because of its ability to take up multiple forms of nitrogen and respond vigorously to increases in N supply, A. petiolata will be presented with increased colonizing opportunities as a consequence of global changes.
Uptake mechanisms
Although there are no data about allocation of these two forms of N, this invader clearly is able to take up and use both forms equally. Because of the broad nature of its N metabolism, during the first year of its growth A. petiolata is not limited by N toxicity or nitrate reductase deficiency, a problem that many species experience under suboptimal N conditions (Glass et al. 2002; von Wirén et al. 2000; Crawford and Glass 1998; Imsande and Touraine 1994). Nitrogen preferences of other members of the Brassicaceae including Arabadopsis thaliana and Brassica campestris keep them from performing well when ammonium is the sole source of N (Zhang et al. 2007; Hoffmann et al. 2007).
Differences in N source preferences arise from energetic costs of uptake and assimilation, rhizosphere and root cell pH, and evolutionary history (Marschner 2002). Ammonium uptake requires the exchange of cations through proton pumping and reduction into amino groups before moving into the vascular bundle. This process can alter soil pH by as many as two units (Marschner et al. 1991). Nitrate, on the other hand, is more mobile and can move more easily into vascular tissue. However, more energy is required to reduce nitrate-N with nitrate reductase before it can be incorporated into amino acids (Gurevitch et al. 2002). When a mixture of nitrate and ammonium is available, it facilitates the permeation of other important cations like calcium (Glass et al. 2002). Although ammonium is more energetically favorable because it takes less energy to reduce than nitrate, when it is the lone N source some plants suffer N toxicity and growth inhibition because of reduced cation availability (Hoffmann et al. 2007; von Wirén et al. 2000).
The presence of high and low-affinity transporter systems (HATS and LATS) for both nitrate and ammonium is especially well documented in A. thaliana (Orsel et al. 2002). These genes regulate uptake of ammonium and nitrate in plants and their expression is subject to up and down regulation by glutamine, and feedback inhibition by the presence of ammonium and nitrate (Glass et al. 2002). It is not known if A. petiolata has HATS similar to those found in Arabidopsis, but the results of this experiment do suggest that A. petiolata has an efficient series of uptake pathways as well as a metabolism unlike other well studied members of the Brassicaceae. This metabolism allows A. petiolata to take up high concentrations of ammonium and not suffer relatively low biomass production and growth retardation caused by ammonium toxicity in Brassica campestris and A. thaliana (Zhang et al. 2007; Hoffmann et al. 2007; von Wirén et al. 2000).
It is entirely possible that the preferred form of N and uptake mechanisms change over the lifetime of A. petiolata; we only studied plants up to 6 months of age. In its second year of life, this species reallocates energy to reproduction and may have different resource needs. Since A. petiolata is biologically active for more than 12 months in temperate deciduous forests (Dhillion and Anderson 1999), it encounters a wide range of water availabilities, temperatures and mineral resource concentrations. Dual uptake mechanisms are useful in environments where the availability of N changes throughout the calendar year. Studies with stable isotopes may help to reveal how nitrate and ammonium are allocated within a growing plant over time.
Further examinations of nutrient use by exotic invaders might lead to a greater understanding of the mechanisms whereby invaders alter recipient communities. Few studies explicitly examine N preferences of species; even fewer examine nutrient uptake by exotic invaders. However, invasive Phragmites australis has been shown to be competitively superior to native Spartina pectinata under high N treatments (Rickey and Anderson 2004), and we have observed Lythrum salicaria to grow better in mixed Nitrate-Ammonium solutions than in single N-source ones (Hyatt, unpublished data). It may be the case that exotic species that become invasive are, like A. petiolata, exceptionally flexible when it comes to taking up mineral resources, but further explicit examinations of N acquisition and allocation are required.
If broad resource uptake abilities turn out to characterize successful invasive species, focusing remediation in regions of increased nutrient availability might prove to be especially effective. As environmental availability of nutrients like N and P increase with human activity, it is especially urgent that this approach be explored. Clearly, one of the main mechanisms whereby invasive species alter recipient communities is through their acquisition of resources. Excess exploitation of nutrient resources by exotic invasive species can ultimately change historical resource flows, which may feed back on community biodiversity and ecosystem function resources (Funk and Vitousek 2007; Gross et al. 2005; Pickart et al. 1998).
The conclusions drawn from this study suggest that A. petiolata does have a high affinity for mineral N, but can survive in low N environments as well. In high nitrogen environments, it also showed greater growth and tissue quality. The ability of A. petiolata to take up and use both nitrate-N and ammonium-N may expand its potential habitat range and enhance its competitive advantage over native species. This flexible uptake of N, increased growth and allocation of resources towards defensive chemistry may help to the explain in the invasion of A. petiolata in North America.
References
Abbès C, Parent L, Karam A, Isfan D (1995) Effect of NH4 +:NO3 − ratios on growth and nitrogen uptake by onions. Plant Soil 171:289–296
Bloom AJ, Jackson LE, Smart DR (1993) Root growth as a function of ammonium and nitrate in the root zone. Plant Cell Environ 16:199–206
Cavers PB, Heagy MI, Kokron RF (1979) The biology of Canadian weeds 35 Alliaria petiolata M Bieb Cavara and Grande. Can J Plant Sci 59:217–229
Cipollini D, Gruner B (2007) Cyanide in the chemical arsenal of garlic mustard Alliaria petiolata. J Chem Ecol 33:85–94
Crawford NM, Glass ADM (1998) Molecular and physiological aspects of nitrate uptake in plants. Trends Plant Sci 3:389–395
de Graaf MCC, Bobbink R, Roelofs JGM, Verbeek PJM (1998) Differential effects of ammonium and nitrate on three heathland species. Plant Ecol 135:185–196
Dhillion SS, Anderson RC (1999) Growth and photosynthetic response of first-year garlic mustard Alliaria petiolata to varied irradiance. J Torrey Bot Soc 126:9–14
Ehrenfeld J (2003) Effects of exotic plant invasions on soil nutrient cycling processes. Ecosystems 6:503–523
Ehrenfeld J (2006) A potential novel source of information for screening and monitoring the impact of exotic plants on ecosystems. Biol Invasions 87:1511–1521
Fichtner K, Schulze ED (1992) The effect of nitrogen nutrition on growth and biomass partitioning of annual plants originating from habitats of different nitrogen availability. Oecologia 92:236–241
Funk JL, Vitousek PM (2007) Resource-use efficiency and plant invasion in low-resource systems. Nature 446:1079–1081
Glass ADM, Glass Anthony DM, Britto DT, Kaiser BN, Kinghorn JR, Kronzucker HJ, Kumar A, Okamoto M, Rawat S, Siddiqi MY, Unkles SE, Vidmar JJ (2002) The regulation of nitrate and ammonium transport systems in plants. J Exp Bot 53:855–864
Govindarajulu M, Pfeffer PE, Jin H, Douds DD, Allen JW, Bücking H, Lammers PJ, Shachar-Hill Y (2005) Nitrogen transfer in the arbuscular mycorrhizal symbiosis. Nature 435:819–823
Gross KL, Mittelbach GG, Reynolds HL (2005) Grassland invasibility and diversity: responses to nutrients, seed input, and disturbance. Ecology 86:476–486
Gurevitch J, Scheiner S, Fox G (2002) The ecology of plants. Sinauer Associates Incorporated, Sunderland, 518 pp
Hoel BO, Solhaug KA (1998) Effect of irradiance on chlorophyll estimation with the Minolta SPAD-502 Leaf Chlorophyll Meter. Ann Bot London 82:389–392
Hoffmann A, Milde S, Desel C, Hümpel A, Kaiser H, Hammes E, Piippo M, Soitamo A, Aro E, Gerendás J, Sattelmacher B, Hansen U (2007) N form-dependent growth retardation of Arabidopsis thaliana seedlings as revealed from physiological and microarray studies. J Plant Nutr Soil Sci 170:87–97
Imsande J, Edwards G (1988) Decreased rates of nitrate uptake during pod fill by cowpea green gram and soybean. Agron J 80:789–793
Imsande J, Touraine B (1994) N demand and the regulation of nitrate uptake. Plant Physiol 105:3–7
Markwell J, Osterman JC, Mitchell JL (1995) Calibration of the Minolta SPAD-502 leaf chlorophyll meter. Photosynth Res 46:467–472
Marschner H (2002) Mineral nutrition of higher plants. Academic Press, London, 889 pp
Marschner H, Haussling M, Eckhard G (1991) Ammonium and nitrate uptake rates and rhizosphere pH in non-mycorrhizal roots of Norway spruce [Picea abies L Karst]. Trees Struct Funct 5:14–21
Matson P, Lohse KA, Hall SJ (2002) The globalization of nitrogen deposition: consequences for terrestrial ecosystems. Ambio 322:113–119
Meekins JF, McCarthy BC (2000) Responses of the biennial forest herb Alliaria petiolata to variation in population density, nutrient addition and light availability. J Ecol 88:447–463
Miller A, Bowman W (2002) Variation in nitrogen-15 natural abundance and nitrogen uptake traits among co-occurring alpine species, do species partition by nitrogen form? Oecologia 130:609–616
Nuzzo V (2000) Element stewardship abstract for Alliaria petiolata, Alliaria officinalis Garlic mustard. The Nature Conservancy, Arlington, 19 p
Olsson MO, Falkengren-Grerup U (2000) Potential nitrification as an indicator of preferential uptake of ammonium or nitrate by plants in an oak woodland understorey. Ann Bot 85:299–305
Orsel M, Filleur S, Fraisier V, Daniel-Vedele F (2002) Nitrate transport in plants: which gene and which control? J Exp Bot 53:825–833
Piccini D, Azcon AR (1987) Effect of phosphate-solubilizing bacteria and vesicular-arbuscular mycorrhzial fungi on the utilization of Bayovar rock phosphate by alfalfa plants using a sand-vermiculite medium. Plant Soil 101:45–50
Pickart AJ, Miller LM, Duebendorfer TE (1998) Yellow bush lupine invasion in Northern California coastal dunes I. Ecological impacts and manual restoration techniques. Restor Ecol 6:59–68
Rickey MA, Anderson RC (2004) Effects of nitrogen addition on the invasive grass Phragmites australis and a native competitor Spartina pectinata. J Appl Ecol 41:888–896
Roberts H, Boddrell J (1983) Seed survival and periodicity of seedling emergence in eight species of Cruciferae. Ann Appl Biol 103:301–309
Shimoji H, Tokuda G, Tanaka Y, Moshiri B, Yamasaki H (2006) A simple method for two-dimensional color analyses of plant leaves. Russ J Plant Physiol 53:126–133
Spencer DF, Sher A, Thornby D, Ksander GG (2007) Non-destructive assessment of Arundo donax poaceae leaf quality. J Freshw Ecol 22:277–285
Stinson KA, Campbell SA, Powell JR, Wolfe BE, Callaway RM, Thelen GC, Hallett SG, Prati D, Klironomos JN (2006) Invasive plant suppresses the growth of native tree seedlings by disrupting belowground mutualisms. PLoS Biol 4:e140
Tilman DA (1982) Resource competition and community structure. Princeton University Press, Princeton, 296 pp
von Wirén N, Gazzarrini S, Gojon A, Frommer WB (2000) The molecular physiology of ammonim uptake and retrieval. Curr Opin Plant Biol 3:254–261
Zhang F-C, Kang S-Z, Li F-S, Zhang J-H (2007) Growth and major nutrient concentrations in Brassica campestris supplied with different NH4 +/NO3 − ratios. J Integr Plant Biol 49:455–462
Acknowledgments
The authors thank Heather Throop for advice on our drip system and nutrient solutions. Further thanks to Sheena Gayomba, Crystal Thompson, Josh Crossley and Danielle Cheong for helping with midterm measurements and the final harvest. This work was supported by the Merck-American Association for the Advancement of Science Undergraduate Science Research Program at Rider University and National Science Foundation Research at Undergraduate Institutions grant #DEB 0344218 to LAH.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hewins, D.B., Hyatt, L.A. Flexible N uptake and assimilation mechanisms may assist biological invasion by Alliaria petiolata . Biol Invasions 12, 2639–2647 (2010). https://doi.org/10.1007/s10530-009-9671-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-009-9671-5