Abstract
Litterfall and its subsequent decomposition in the soil are two essential ecosystem processes. In order to determine the biological potential of a species to aid soil restoration, it is necessary to evaluate litter production, its temporal variation, and rates of decomposition and nutrient cycling. In this study, we examined patterns of litterfall production, quality, and decomposition of two slow-growing (Crescentia alata and Eysenhardtia polystachya) and two fast-growing (Leucaena leucocephala and Pithecellobium dulce), multi-purpose indigenous species from seasonally dry tropical forest, in a 10-year-old plantation in Morelos, Mexico. Average litterfall was 7.82 ± 2.69 Mg ha−1 year−1 and varied significantly among species as follows: P. dulce > L. leucocephala > C. alata = E. polstachya. Leaf litter comprised the highest fraction in all species studied. In a litterbag experiment, all species had fast mass loss in the first 183 days of decomposition, coinciding with the rainy season. L. leucocephala had significantly higher decomposition than the other species. Nitrogen percentages increased significantly as decomposition progressed for all species except for E. polystachya while Carbon percentages during decomposition significantly decreased only in C. alata and L. leucocephala. C. alata had the highest average C/N ratio and L. leucocephala the lowest. We recommended for soil biological restoration, P. dulce because it is a fast-growing tree, with a rapid canopy closure and a high litter production and L. Leucocephala, which produces large amounts of rapidly decomposing mulch with high amount of nutrients, which can be rapidly released into the soil.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Seasonally dry tropical forest (SDTF) is regarded as the most altered and threatened tropical ecosystem in the world (Jaramillo and Sandford 1995). Because of its high diversity, SDTF should be a high priority for conservation and ecological or productive restoration (Miles et al. 2006; Ceccon 2011). In order to facilitate these activities, it is important to carry out studies to characterize the original species of this ecosystem, know their role in forest dynamics, and use this knowledge to inform sustainable management of these species or their potential use in agroforestry or restoration projects in degraded landscapes (Ceccon 2013).
Central issues in the study of ecosystem structure and function are understanding the dynamics of organic matter and how it contributes to energy flow and nutrient cycling (Bourlière and Hadley 1970), which in turn are essential for the continuity and stability of living systems (Pomeroy 1970). At the same time, restoration of degraded habitats requires the establishment of a self-sustaining soil–plant system. Vegetation management is often essential to combat soil erosion, restore soil fertility and accelerate natural recovery processes (Dobson et al. 1997; Holl 1999; Leitão et al. 2010; Ceccon et al. 2012).
The present study was conducted to understand the dynamics of litter production, leaf decomposition, and C and N concentration in four SDTF indigenous tree species growing in a 10 year experimental plantation. These species are commonly used by local people and are considered among the most important 20 multi-purpose tree species in the State of Morelos, Mexico (SAGARPA-INIFAP 2001). Two of these, are very abundant and fast -growing (Pithecellobium dulce and Leucaena leucocephala) and two less common and slow-growing (Eysenhardtia polystachya and Crescentia alata; SAGARPA-INIFAP 2001) within the State of Morelos. Our study is one of a handful of experiments to quantify the litter production, decomposition dynamics and its effects on the return of some nutrients in forest plantations, with the specific goal of producing useful information for the restoration of degraded soils and the potentially successful establishment of productive or agroforestry systems (Montagnini et al. 1993; Byard et al. 1996; Montagnini 2000).
Materials and methods
Study site
The study was carried out in the experimental field of the Center for Agricultural and Forestry Research (CIFAP in Spanish) in Zacatepec, Morelos, Mexico (18°92′N lat. and 99°12′W long., 920 m of altitude; Ornelas et al. 1997), belonging to National Institute for Agricultural and Forestry Research (INIFAP in Spanish).
The climate of the area is characterized as Awo (w) (i′) gw″ (Koppen, modified by García 1987), considered the driest of the warm sub-humid, with rain in summer and winter precipitation percentage of less than 5 % of the annual total rainfall. Total annual precipitation is ~919 mm. The driest period is from November to April (39 mm) and the wettest, from May to October (880 mm). The average annual temperature is 24.1 °C. The soil is a vertisol with clay texture (48.8 %), with high montmorillonite clay content expandable (2:1), and a neutral pH (7.3; FAO–UNESCO 1988). The vegetation is tropical deciduous forest and the majority of the trees lose their leaves during the dry season. The most abundant tree families are Fabaceae, Burseraceae and Euphorbiaceae (Trejo and Dirzo 2000).
Methods
Species distribution in the plantation
The trees were 10 years at the beginning of the study and were distributed in 8 blocks (replicates). Each block had 4 rows, with 10 trees of one species each. The spacing used among trees was 3 × 2 m corresponding to 1,666 trees per ha. In total, there were 80 individuals of each species in the plantation. Species characteristics are found in the Table 1.
Litterfall production
To measure litterfall production, 64 square traps 60 × 60 cm (0.36 m2) and 1 m high were used. The traps were placed randomly in the row corresponding to the species of study (16 traps per species). Litterfall was collected monthly during one year (December to November). Subsequently, the collected material was separated by species and components: leaves, reproductive structures (flowers, fruits and seeds) and twigs (fragments with a diameter >1 cm and bark). The material was dried and moved to a desiccator (Pyrex) with silica gel (dried) and weighed.
Organic matter decomposition
We used the litterbag method to determine the decomposition rate of leaves of each species (Wieder and Lang 1982). We focused on leaf litter rather than twigs because it represented a substantial portion (50–80 %) of total litter in terms of biomass produced (Sundarapandian and Swamy 1999), it has higher nutrient concentrations (Álvarez-Sánchez 2001) and decomposes faster than twigs and other wood materials (Anderson and Swift 1983), thus accelerating the cycling of nutrients in the soil. Freshly senesced leaves of each species were gathered and placed in decomposition bags (30 × 30 cm). Leaves of P. dulce and C. alata, with large leaflets, were collected directly from the plantation floor and were dried at room temperature. Leaves of E. polystachya and L. leucocephala were collected directly from trees of multiple individuals in the same period as the others, just before to abscission in order to avoid an overestimation in the nutrients content (Xuluc-Tolosa et al. 2003) because during senescence a decrease of nutrient content is observed in the leaves (Addicott 1978). Leaves of all species were dried at 60 °C for 72 h (Xuluc-Tolosa et al. 2003). The main reason for the different collections among species is that the latter two species have leaves with small leaflets, which are falling off, making it almost impossible to collect ground. Once dried, sub-samples of 15 g (dry weight) of L. leucocephala, 12 g of C. alata and P. dulce and 7 g of E. polystachya (Palm and Sanchez 1990) were placed in bags. The lower amount of E. polystachya in the bags, was due to the fact that this tree produced the smallest sized leaves and the lowest amount of leaves (Fig. 1) and there was few biomass of leaves just before the abscission in the collection periods. A total of 48 bags for 12 collections (one per month) with four replicates of each species were randomly placed and set in the ground by a metal stake under trees of each species. Four randomly selected mesh bags of each species were collected monthly for 12 months. After collection, the material was removed manually, including plant material (leaves, seeds, grasses, roots) and fauna, if present. Then they were sieved in mesh of 0.25 mm pore size to remove the smaller fragments and soil particles. The material was dried in an oven at 80 °C until reaching constant weight.
Dry weight of litterfall structures produced in one year by Pithecellobium dulce (Pd), Leucaena leucocephala (Ll), Eysenhardtia polystachya (Ep), Crescentia alata (Ca) tree species in a 10 year old plantation. Vertical bars represent standard errors. Different letters represent significant differences by the Duncan test (P < 0.05)
Remaining weight (RW) for each collection date was expressed as a percentage of initial weight as follows:
where RW = remaining weight; WT0 = weight at time 0; WT1 = weight after a given period.
The decomposition rate constant (K) was estimated for each collection date following the exponential model (Olson 1963) that characterizes the weight loss during the decomposition.
where X0 = weight (g) of litter at time 0; Xt = weight (g) of litter at time t (days); K = decomposition rate constant.
From K it was possible to obtain the average lifespan of the material sampled, in terms of time of decomposition, using the following equation (Olson 1963): t(0.5) = − ln(0.5)/kt(0.5) = 0.6931/k.
The time (t) necessary for 99 % organic matter loss was obtained by using the following equation (Olson 1963): t(0.99) = ln(1−0.99)/k.
According to Wieder and Lang (1982), time (t) and average lifespan of litterfall return is expressed as: t = 1/k.
Chemical quality of leaf litter
Approximately 30 g of leaf material per species was taken in a bulked sample of leaf litter to estimate initial litter quality. Three of the four bags of decomposed litter (29, 122, 212, 302 and 398 days after decomposition) of each species were selected and dried. The mixed samples of each species were analyzed by a NC 2500 model elemental auto analyzer to quantify carbon (C) and nitrogen (N).
The percentage of nutrients remaining in the decomposing material was determined by the following formula:
where C or Nt0 = initial concentration of C or N in the leaf litter; C or Nt1 = concentration after a t period; RW = remaining weight.
The C/N ratio was obtained by dividing the concentration of C by N (%) for each species at different times of decomposition of the material.
Statistical analysis
The data were transformed when was necessary and were subjected to a one-way ANOVA or to a t test. Differences in means were tested using a Duncan test. A Kruskal–Wallis test was made to compare N and C concentrations of species through the time of decomposition. A Pearson correlation was used to correlate the monthly remaining percentages of dry weight of each species with monthly precipitation.
Results
Annual litterfall production
Average litterfall production for all species after 12 months was 7.82 ± 2.69 Mg ha−1 year−1. However, litterfall production differed significantly among species (F = 150.30, p < 0.0001). P. dulce had the highest litterfall production and C. alata had the lowest amount; Table 2).
Litterfall fractions
Leaf litter comprised the highest fraction in all species studied, being more than half of the total litterfall. The smallest fractions were twigs or reproductive structures. C. alata showed the highest percentage of leaf litter (98.41 %), while L. leucocephala the lowest percentage (57.43 %). L. leucocephala had the highest percentage of fallen twigs (20.90 %) while C. alata had the lowest percentage (0.52 %). The species with the highest percentage of reproductive structures was P. dulce (24.34 %) while C. alata had the lowest (1.06 %). The total annual amount of leaves in the litterfall was significantly different among species (F = 111.08, p = 0.000). P. dulce had significantly greater amount of leaf litter, followed by L. leucocephala compared to the two other species. The quantities of leaf litter for C. alata and E. polystachya did not differ from one another statistically (Fig. 1).
The amount of fallen twigs was significantly different among all species (F = 49.10, p < 0.0001, Fig. 1). L. leucocephala had the largest amount of twigs (56.36 % of total) and C. alata dropped the lowest amount of twigs (0.23 % of total). E. polystachya (17.95 % of total) and P. dulce (25.45 % of total) dropped similar amounts (Fig. 1).
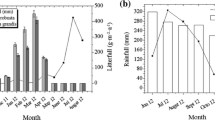
The species also differed significantly from each other in the total amount of reproductive structures fallen along the year (F = 60.47, p = 0.0001, Fig. 1). P. dulce stood out as having the significantly highest amount of reproductive structures than the other species, followed by L. leucocephala, which both differed from the other species. C. alata and E. polystachya dropped a small amount of reproductive structures and showed no significant differences between them (Fig. 1). Most of species increased the production of reproductive structures in the dry period (Fig. 2): L. leucocephala from February to April (59.73 %), P. dulce from March to May (79.60 %) and E. polystachya from December to March (76.02 %). By contrast, C. alata produced more reproductive structures at the onset of the rainy season (from May to June, 92.23 % of total).
Temporal dynamics of leaf litterfall production
Leaf litterfall production in C. alata followed a unimodal distribution, with increased production in the driest period (December to April, 80.72 % of total) and the lowest production from June to November (rainy season; 6.81 % of the total; Fig. 3). E. polystachya showed fluctuations, with a low production from December to August (19.54 % of total) and high production only in September and October (rainy season, 80.47 % of the total, Fig. 3). L. leucocephala leaf litterfall production was relatively constant through the year, with two small peaks of production in April (13.03 % of total) and October (11.86 %) and two lowest productions in January (4.99 %) and July (4.50 %; Fig. 3). P. dulce showed a maximum peak in May (the month in which the rain season begins, 16.89 %), a minimum in July (wettest month of the year; 2.53 %) and maintained a constant litterfall production along the year.
Organic matter decomposition
After 398 days in the plantation ground, the percentage of average dry weight remaining differed significantly among species (F = 19.90, p = 0.000). In all species mass loss in the first 183 days was rapid, coinciding with the rainy season (May to November). After this period, the mass loss stabilized showing small losses that were similar and continuous (Fig. 4). In fact, remaining percentages of dry weight of C. alata (R = 0.87, p = 0.000), L. leucocephala (R = 0.75, p = 0.005) and P. dulce (R = 0.66, p = 0.020) were significantly and positively correlated with precipitation, but E. polystachya did not show a significant correlation (R = 0.48, p = 0.110). L. leucocephala had the significantly lowest percentage of material remaining after 398 days (16.75 %) and consequently had the fastest decomposition rate (F = 9.73, p < 0.0001), losing approximately half of litter mass after 59 days (Fig. 4).
The decomposition rate constant K differed significantly among species (F = 21.90, P = 0.000). L. leucocephala had the highest value of k(3.06) and was significantly different from the other species (C. alata, P. dulce y E. polystachya), which did not differ from one another. The average lifespan and the time to lose 99 % of leaf fall suggest the same pattern. C. alata was the species with the lowest rate of decomposition (Table 3).
Quality of leaf litter after decomposition
The C and N concentrations in the leaf litter
The average of N and C concentrations along the time of decomposition was significantly different among species according to Kruskal–Wallis test (HN38.52, p = 0.0001; HC = 11.09, p = 0.011). C. alata increased significantly N % through time (H = 13.05, p = 0.023). N percentage was 1.72 % ± 0.11 after 29 days exposure and 2.84 % ± 0.1 after 302 days of exposure. On the other hand, C percentages significantly decreased (H = 13.47, p = 0.019) through decomposition time (43.36 % ± 0.22 in the first day of exposure and 34.55 % ± 2.86 after 122 days of exposure). L. leucocephala showed the same pattern, increasing significantly in N % through time (H = 11.92, p = 0.036), had the lowest N percentage (3.19 % ± 0.07) at the start of the decomposition process (0 days exposure) and the highest (3.78 % ± 0.2) after 212 days of exposure. C percentage significantly decreased in relation to time (H = 12.74, p = 0.026), had the lowest percentage (38.89 % ± 1.11) after 398 days of exposure and the highest (46.87 % ± 0.4) at 29 days of exposure. Also P. dulce increased significantly in N percentages over time of exposure (H) = 11.93, p = 0.0357), showed the lowest N percentage (2.46 % ± 0.12) at the start of the decomposition process and the highest (3.5 % ± 0.05) at 302 days after exposure. However, in terms of C percentage there was no significant differences through the time of decomposition (H = 9.25, p = 0.0856). Meanwhile, E. polystachya showed no significant differences in N and C percentages (H = 4.77, p = 0.445; H = 8.48, p = 0.131 respectively) among days of exposure (Fig. 5).
The C/N ratio of the leaf litter along the decomposition time
The C/N ratio differed significantly among species (F = 10.87, p = 0.000) and time (F = 10.50, p = 0.000). The average ratio of C. alata was significantly higher from other species, the ratio of E. Polystachya and P. dulce followed in magnitude and were significantly similar, L. leucocephala was the species with the lowest C/N ratio and similar to P. dulce (and was significantly different from E. polystachya, that was similar to P. dulce (Table 4). In average, species showed a significantly higher C/N ratio in the beginning of decomposition (18.06 and 17.51 for 0 and 29 days, respectively) than in subsequent times, that were significantly similar to each other (12.74, 13.55, 12.81 and 13.26 for 122, 212, 302 and 398 days respectively).
Discussion and conclusion
The quantity of biomass per unit area over time constitutes the primary data needed to understand the flow of nutrients and water through terrestrial ecosystems. The average of productivity in terms of litterfall (7.82 ± 2.69) of the 10 year-old plantations of four species from SDTF in Morelos, Mexico was not too much lower than the productivity of a secondary SDTF in Yucatán, also in Mexico (9.2 ± 0.6 Mg ha−1 year−1, Campo and Vázquez-Yanes 2004).
P. dulce and L. leucocephala produced the highest annual litterfall, possibly because both are fast-growing species, and after 10 years they had the highest basal area, cover and crown volume; which correlate with litterfall production (Álvarez-Sánchez 2001; Descheemaeker et al. 2006). The annual litterfall production of L. leucocephala in this study was similar to reported by Parrotta (1999) for this species in a mixed plantation in Puerto Rico (9.7 Mg ha−1 year−1).
In this study, leaves constituted the largest proportion of total litterfall (57.4–98.4 %) similar to other studies in SDTF: in upland forests of Ethiopia (57–77 %; Lisanework and Michelsen 1994), in Chamela (70 %; Martínez-Yrízar 1995) and in Tigray uplands in Ethiopia (68–86 %; Descheemaeker et al. 2006). Regarding the small proportion of reproductive structures found in this study, according to Vitousek and Hooper (1998), in SDTF, plants generally produce more structures responsible for photosynthesis than reproductive structures. The zoochorous species P. dulce and L. leucocephala showed the highest production of reproductive structures because they are fast growing species and at 10 years, they already reached the reproductive age. The early reproduction and zoochory are two characteristic potentially important for restoration ecology projects, since these species shortly after implantation will be attracting the fauna to eat their fruits and increasing the seed dispersion by defecation, catalyzing the regeneration of other species in the restoration area (Holl 1999; Leitão et al. 2010, among others).
Most of reproductive structures of the species was produced in the dry season, in all SDTF, seed dispersal occurs mainly in this season (Bullock and Solis-Magallanes 1990). Seeds remain on the forest floor until the next rainfall season when they encounter more favorable conditions for germination and growth (Ceccon et al. 2006).
There was a marked effect of precipitation seasonality in the leaf litterfall of P. dulce and C. alata, while E. polystachya and L. leucocephala did not show this effect, because they are evergreen species. P. dulce produced the highest amount of leaf litterfall followed by L. leucocephala. Both species have a great potential to recuperate the organic matter of degraded soils.
All species of the study had significantly faster mass loss in the first 183 days of exposure, which coincided with the rainy season (May to November), then stabilizing with smaller but continuous mass loss. This behavior was similar to what happened in others SDTF (Nuñez-Quevedo 1998, Sundarapandian and Swamy 1999; Goma-Tchimbakala and Bernhard-Reversat 2006). At same time, decomposition rates of this study were similar to those of other species in plantations with similar climatic conditions (Lisanework and Michelsen 1994; Attignon et al. 2004; Goma-Tchimbakala and Bernhard-Reversat 2006).
Leaucaena leucocephala was the species that decomposed faster (lost 83.25 % of its leaf litter in 398 days) and the lifespan average of leaf litter was only 4.3 months, because this species contains much water that is lost at the beginning, and makes its weight greatly reduced, water-soluble compounds are released as carbohydrates and proteins, which creates a moist and nutrient rich environment for microbial activity and decomposition (Parrotta 1999). Furthermore, its N fixing status, its smooth, small and thin leaflets with cell walls that have high concentrations of N, Ca, Mg and K in the tissues, a low C/N ratio and a high initial N contents, as in this study, that make its leaf litter much more palatable (Heal et al. 1997; Parrotta 1999). Due to these characteristics, L. leucocephala is commonly used in alley cropping systems in tropical zones (Shelton and Brewbaker 1994) and it is recognized as a very efficient species in reclamation of physical properties of the soil, particularly porosity and bulk density, due to the rapid incorporation of humus in mineral soil.
P. dulce had less rapid decomposition rates than L. leucocephala presumably due to the morphology of their more rigid and thick leaves, but its decomposition rate depended on the quality of the resource expressed as a similar to L. leucocephala C/N ratio. Therefore, this species presents high biological potential for soil restoration.
The low rate of decomposition in E. polystachya certainly does not derive from its leaf litter quality (its initial C/N ratio was similar to L. leucocephala). This low rate probably resulted from the existence of a sunny, hot and dry microclimate under its open tree canopy (Byard et al. 1996; Barlow et al. 2007) that affected dynamic population of the microorganisms responsible for the decomposition (Valle-Arango 2003). Moreover, some authors (Singh 1969; Ewel 1976; Palm and Sanchez 1990; Byard et al. 1996) found that species with high nitrogen content in the leaves not always decompose faster than those with low concentrations, lignin and polyphenols can be important factors in determining the rate of decomposition and release retard decomposition of N, joining N compounds of the plant tissues.
C. alata was the species presenting the highest initial ratio C/N and lowest initial N concentration in the leaves, but its decomposition rate was not significantly different from that of P. dulce and E. polystachya, possibly due to decomposition depended not only on the quality of litter, but also of the microclimate condition of the soil microorganisms. It is likely that it decomposed faster by the shade provided by neighboring species (P. dulce and L. leucocephala) that afford an optimal microclimate for decomposition and mineralization of humus, as also reported by Attignon et al. (2004) for a species of lower quality leaves.
Remaining mass correlated significantly with precipitation in all species except for E. polystachya, probably because its decomposition was more influenced by microenvironmental conditions under its canopy, an open canopy allows greater exposure to solar radiation and therefore there is less moisture in the soil. While in other species, rainfall keeps the leaf litter in wet conditions to their decay (Aerts 1997).
The release of deposited nutrients depends on the rate of decomposition. In this study, the only species that showed a rate similar to a natural seasonally dry tropical forest in India [among 4–4.5 months for 50 % decomposition; Roy and Singh 1994)] was L. Leucocephala. In this case, mixing leaves from species with differing resource quality and leaf structure may change the chemical environment and physically alter the total litter surface where decomposition is occurring (Kaneko and Salamanca 1999; Hector et al. 2000) and these alterations can also affect decomposer abundance and activity (Wardle 2002). Thus, chemical and physical changes in leaf mixes can influence decomposition rates. At same time, the relatively slow leaf litter decomposition of other tree species in this study should lead to soil organic matter build up in the long run and is expected to provide benefits of mulching such as preventing soil erosion and conserving soil moisture (Yadav 1963).
The analysis of quality of leaf litter coupled with mulch decomposition is of great importance for the understanding of energy flow, primary production and nutrient cycling in forest ecosystems (Vitousek 1984; Sundarapandian and Swamy 1999). Mulch with a high concentration of N tends to decompose faster and much of the N released is taken by microorganisms for the synthesis of cells and only a small part of it is removed from the soil by denitrification and uptake by plants (Singh 1969). Nitrogen is one of the most limiting factors in litter decomposition because it determines the growth and transfer of microbial biomass to organic C mineralized; therefore, mulch with a high concentration of N tends to decompose faster (Heal et al. 1997). In this study N percentage increased significantly with the time of decomposition for C. alata, L. leucocephala and P. dulce except for E. polystachya. An increasing of N along the decomposition also happens for the most species in other studies (Montagnini et al. 1993; Xuluc-Tolosa et al. 2003; Berg and Mc Claugherty 2008). Some possible reasons for this accumulation of nitrogen were due nitrogen fixation, atmospheric ammonia absorption, caulinar flow, dust, fungal translocation and/or immobilization (Melillo et al. 1989; Xuluc-Tolosa et al. 2003) and microbial growth (Berg and Mc Claugherty 2008). Concentration of C during the decomposition process decreased in C. alata and L. leucocephala leaf litter, because during the decomposition, possibly C was the source of energy for decomposers, N fixation and denitrification (Berg and Mc Claugherty 2008).
C. alata had the highest annual average C/N ratio and L. leucocephala the lowest and similar to reported by Tian et al. (1992). L. leucocephala by its low C/N ratio can be considered the species with the best quality leaf litter, additionally it is a legume that fixes N. Meanwhile, C. alata is a slow-growing species that has thick leaves, leathery texture and low concentration of nutrients per unit of dry weight, by the accumulation of carbon compounds, resulting in a high C/N ratio in their leaves, which are long-lived and are decomposed very slowly (Chapin 1980). The C/N ratios of all studied species were higher at the beginning of the decomposition process (at 0 and 29 days) and lowest in the following days as happened with the species studied by Singh (1969). The C/N ratio decreases along the degradation process, because the concentration of N in the residual matter incrementing along the process and is immobilized in the microbial structure while organic C is respired as CO2, in this way towards the end of the process, much of this tissue is the microbial residue that has replaced the original substrate (Waring and Schleslnger 1985 in Sachman 2004).
When the goal of restoration is the soil protection and nutrients recuperation, P. dulce is recommended, because it is a fast-growing tree, with a rapid canopy closure and a high litter production, which contributes to the establishment of mulch that protects against erosion and reduces the soil compaction, while slowly releasing nutrients, mainly N. This mulch could retain moisture preventing water loss and low soil temperature; it also prevents (due the mulch thick) the growth of weeds, promoting the regeneration of tree shade species.
When the objective is to increase soil fertility as soon as possible, the appropriate species according to the results of the study is L. leucocephala, which produces large amounts of rapidly decomposing mulch with high amount of nutrients, which can be rapidly released into the soil. This mulch can be used as fertilizer in N deficient soils due to the rapid biological fixation of its N content, favoring the growth of crops and plants associated with this species. Therefore, for fast STDF biological soil restoration, the combination of both species is recommended.
Although the study was conducted on a plantation and may have somewhat limited correspondence with the natural dynamics of the species used, it can provide support for studies in natural ecosystems and also provide basic information necessary for the selection of species in restoration projects of degraded areas and agroforestry systems.
References
Addicott T (1978) Abscisión strategies in the behavior of tropical trees. In: Tomson PB, Zimmermann MH (eds) Tropical Trees as living systems. Cambridge University Press, Cambridge, pp 381–400
Aerts R (1997) Climate, leaf litter chemistry and leaf litter decomposition in terrestrial ecosystems: a triangular relationship. Oikos 79:439–449
Álvarez-Sánchez J (2001) Descomposición y ciclo de nutrientes en ecosistemas terrestres de México. Acta Zoo Mex 1:11–27
Anderson JM, Swift MJ (1983) Decomposition in tropical forest. In: Sutton SL, Whitmore TC, Chadwick AC (eds) Tropical rain forest: ecology and management. Blackwell, Oxford, pp 287–309
Attignon SE, Weibel D, Lachat T, Sinsin B, Nagel P, Peveling R (2004) Leaf litter breakdown in natural and plantation forests of the Lama forest reserve in Benin. Appl Soil Ecol 27:109–124
Barlow J, Gardner TA, Ferreira LV, Peres CA (2007) Litter fall and decomposition in primary, secondary and plantation forests in the Brazilian Amazon. Forest Ecol Manag 247:91–97
Batis MAI, Alcocer MI, Gual M, Sánchez C, Vazquez-Yanez C (1999) Árboles y arbustos nativos potencialmente valiosos para la restauración ecológica y la reforestación. CONABIO, México
Berg B, Mc Claugherty C (2008) Plant litter decomposition, humus formation carbon secuestration. Springer, Berlin
Bourlière F, Hadley M (1970) The ecology of tropical savannas. Ann Rev Ecol Evol Syst 1:125–152
Bullock SH, Solis-Magallanes JA (1990) Phenology of canopy trees of a tropical deciduous forest in Mexico. Biotropica 22:22–35
Byard R, Lewis KC, Montagnini F (1996) Leaf litter decomposition and mulch performance from mixed and monospecific plantations of native tree species in Costa Rica. Agric Ecosyst Environ 58:145–155
Campo J, Vázquez-Yanes C (2004) Effects of nutrient limitation on aboveground carbon dynamics during tropical dry forest regeneration in Yucatan, Mexico. Ecosystems 7(3):311–319
Ceccon E (2011) Los bosques tropicales estacionalmente secos: ¿una prueba ácida para la restauración? In: Vargas-Ríos O, Reyes S PB (Eds.). La Restauración Ecológica en la Práctica. Memorias en extenso del I Congreso Colombiano de Restauración Ecológica y II Simposio Nacional de Experiencias en Restauración. Universidad Nacional de Colombia, pp 119–130
Ceccon E (2013) Restauración en bosques tropicales: fundamentos ecológicos, prácticos y sociales. Ediciones Díaz de Santos/UNAM, Mexico. ISBN 978-84-9969-615-7
Ceccon E, Huante P, Rincón E (2006) Abiotic factors influencing tropical dry forest regeneration. Braz Arch Biol Techn BABT 49(2):305–312
Ceccon E, Almazo-Rogel A, Martínez-Romero E, Toledo I (2012) The effect of inoculation of indigenous bacteria on the early growth of Acacia farnesiana in a degraded area. Rev CERNE 18(1):49–57
Cervantes-Sánchez MA (1994) Calendario fenológico de 4 especies de latifoliadas de Selva Baja Caducifolia del Estado de Morelos En: Informe Anual del Centro de Investigaciones Regionales del Centro Campo Experimental de Zacatepec Morelos SAGAR. INIFAP, Mexico, pp 17–26
Chapin FS (1980) The mineral nutrition of wild plants. Annu Rev Ecol Evol Syst 11:233–260
Descheemaeker K, Muys B, Nyssen J, Poesen J, Raes D, Haile M, Deckers J (2006) Litter production and organic matter accumulation in exclosures of the Tigray highlands, Ethiopia. Forest Ecol Manag 233:21–35
Dobson AP, Bradshaw AD, Baker AA (1997) Hopes for the future: restoration ecology and conservation biology. Science 277(5325):515–522
Ewel J (1976) Litter fall and leaf decomposition in a tropical forest succession in eastern Guatemala. J Ecol 64(1):293–308
FAO–UNESCO (1988) FAO–UNESCO Soil Map of the World—Revised Legend (Reprint of FAO World Soil Resources Report 60). Technical Paper 20, International Soil Reference and Information Centre, Wageningen
García E (1987) Modificaciones al sistema de clasificación climática de Köppen. Universidad Nacional Autonoma de Mexico, Mexico, p 220
Goma-Tchimbakala J, Bernhard-Reversat F (2006) Comparison of litter dynamics in three plantations of an indigenous timber-tree species (Terminalia superba) and a natural tropical forest in Mayombe, Congo. Forest Ecol Manag 229:304–313
Heal OW, Anderson JM, Swift MJ (1997) Plant litter quality and decomposition: An historical overview. In: Cadish G, Giller KE (eds) Driven by nature: plant litter quality and decomposition. Cab Publishing, New York, pp 3–30
Hector A, Beale AJ, Minns A, Otway SJ, Lawton JH (2000) Consequences of the reduction of plant diversity for litter decomposition: effects through litter quality and microenvironment. Oikos 90:357–371
Holl KD (1999) Factors limiting tropical rain forest regeneration in abandoned pasture: seed rain, seed germination, microclimate, and soil. Biotropica 31:229–242
Janzen DH (1983) Costa Rican Natural History. The university of Chicago Press, Chicago
Jaramillo V, Sandford RL (1995) Nutrient cycling in tropical deciduous forests. In: Bullock SH, Mooney HA, Medina F (eds) Seasonality dry tropical forests. Cambridge University Press, Cambridge, pp 346–361
Kaneko N, Salamanca EF (1999) Mixed leaf litter effects on decomposition rates and soil microarthropod communities in an oak-pine stand in Japan. Ecol Res 14:131–138
Leitão FHM, Marques MCM, Ceccon E (2010) Young restored forests increase seedling recruitment in abandoned pastures in the Atlantic rainforest. Rev Biol Trop 58(4):1271–1282
Lisanework N, Michelsen A (1994) Litterfall and nutrient release by decomposition in three plantations compared with a natural forest in the Ethiopian highland. Forest Ecol Manag 65:149–164
Martínez-Yrízar A (1995) Biomass distribution and primary productivity of tropical dry forest. In: Bullock SH, Mooney HA, Medina F (eds) Seasonality dry tropical forests. Cambridge University Press, Cambridge, pp 326–345
Melillo JM, Aber JD, Linkins AE, Ricca A, Fry B, Nadelhoffer J (1989) Carbon and nitrogen dynamics along the decay continuum: plant litter to soil organic matter. Plant Soil 115:189–198
Miles L, Newton AC, DeFries RS, Ravilious C, May I, Blyth S, Kapos V, Gordon JE (2006) A global overview of the conservation status of tropical dry forests. J Biogeogr 33:491–505
Montagnini F (2000) Accumulation in above-ground biomass and soil storage of mineral nutrients in pure and mixed plantations in humid tropical lowland. Forest Ecol Manag 134:257–270
Montagnini F, Sancho F, Ramstad K (1993) Litter fall, litter decomposition and the use of mulch of four indigenous tree species in the Atlantic lowlands of Costa Rica. Agroforest Syst 23:39–61
Nuñez-Quevedo S (1998) Producción de hojarasca, dinámica de mantillo, descomposición foliar y potencial microbiano del suelo en tres comunidades contrastantes del desierto de Sonora. Dissertation, Facultad de Ciencias. UNAM, Mexico
Olson JS (1963) Energy storage and the balance of producers and decomposers in ecological systems. Ecology 44:322–331
Ornelas RF, Ambriz R, Bustamante JD (1997) Delimitación y definición de agrohábitats del estado de morelos. Publicación Especial, 11. INIFAP, New York
Palm CA, Sanchez PA (1990) Decomposition and nutrient release patterns of the leaves of three tropical legumes. Biotropica 22(4):330–338
Parrotta JA (1991) Pithecellobium dulce (Roxb.) Benth. Guamùchil, Madras thorn. SO-ITF-SM-40. Department of Agriculture, Forest Service, Southern Forest Experiment Station. New Orleans, p 5
Parrotta JA (1992) Leucaena leucocpehala (Lam.) de Wit. Leucaena, tantan. SO-ITF-SM-52. Department of Agriculture, Forest Service, Southern Forest Experiment Station, New Orleans. 8 p
Parrotta JA (1999) Productivity, nutrient cycling, and succession in sigle- and mixed-species plantations of Casuarina equisetifolia, Eucalyptus robusta and Leucaena leucocephala in Puerto Rico. Forest Ecol Manag 124:45–77
Pérez-Negron E, Casas A (2007) Use, extraction rates and spatial availability of plant resources in the Tehuacán-Cuicatlán Valley, México: the case of Santiago Quiotepec, Oaxaca. J Arid Environ 70:356–379
Pomeroy LR (1970) The strategy of mineral cycling. Annu Rev Ecol Syst 1:17–190
Roy S, Singh JS (1994) Consequences of habitat heterogeneity for availability of nutrients in a dry tropical forest. J Ecol 82:503–509
Sachman RB (2004) Efecto de la introducción de especies en la recuperación de la dinámica de C y N de un sitio perturbado de SBC en Chamela, Jal. Dissertation. Instituto de Ecología. UNAM, México
SAGARPA-INIFAP (2001) Diagnostico Forestal del Estado de Morelos. Publicación especial, 7, SAGARPA-INIFAP, New York, pp 169
Shelton H, Brewbaker JL (1994) Leucaena leucocephala: the most widely used forage tree legume. In: Gutteridge HM, Shelton RC (eds) Forage tree legumes in tropical agriculture. CAB International, Wallingford, pp 15–29
Singh KP (1969) Studies in decomposition of leaf litter of important trees of tropical deciduous forest at Varanasi. Trop Ecol 10:292–311
Sundarapandian SM, Swamy PS (1999) Litter production and leaf-litter decomposition of selected tree species in tropical forests at Kodayar in Western Ghats, India. Forest Ecol Manag 123:231–244
Tian G, Kang BT (1992) Brussaard L (1992) Biological effects of plant residues with contrasting chemical compositions under humid tropical conditions—Decomposition and nutrient release. Soil Biol Biochem 24:1051–1060
Trejo I, Dirzo R (2000) Deforestation of seasonally dry tropical forest: a national and local analysis in México. Biol Cons 94:133–142
Valle-Arango JI (2003) Descomposición de la hojarasca fina en bosques pantanosos del pacífico colombiano. Interciencia 28(3):148–153
Vitousek PM (1984) Litterfall, nutrient cycling, and nutrient limitation in tropical forests. Ecology 65:285–298
Vitousek PM, Hooper DU (1998) Effects of plant composition and diversity on nutrient cycling. Ecol Monog. 68(1):121–149
Wardle DA (2002) Communities and ecosystems: linking the aboveground and belowground components. Princeton University Press, Osaka
Waring RH, Schleslnger WH (1985) Forest ecosystems: concepts and management. Academic Press, Orlando
Wieder RK, Lang GE (1982) A critique of the analytical methods used in examining decomposition data obtained from litter bags. Ecology 63(3):1636–1642
Xuluc-Tolosa FJ, Vester HFM, Ramírez-Marcial N, Castellanos-Albores J, Lawrence D (2003) Leaf litter decomposition of tree species in three successional phases of tropical dry secondary forest in Campeche, México. Forest Ecol Manag 174:401–412
Yadav JSP (1963) Site and soil characteristics of bamboo forests. Indian For 89(3):177–193
Acknowledgments
We very much appreciate PAPIIT-UNAM IN-300112 and IN-304409, a CONACYT-CNPq Bi-national Joint Project on the Dynamics of Mexico-Brazil Tropical Forests grants. Special thanks for INIFAP-Morelos for the field support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ceccon, E., Sánchez, I. & Powers, J.S. Biological potential of four indigenous tree species from seasonally dry tropical forest for soil restoration. Agroforest Syst 89, 455–467 (2015). https://doi.org/10.1007/s10457-014-9782-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10457-014-9782-6