Abstract
Background and aims
Tundra soils, which usually contain low concentrations of soil nutrients and have a low pH, store a large proportion of the global soil carbon (C) pool. The importance of soil nitrogen (N) availability for microbial activity in the tundra has received a great deal of attention; however, although soil pH is known to exert a considerable impact on microbial activities across ecosystems, the importance of soil pH in the tundra has not been experimentally investigated.
Methods
We tested a hypothesis that low nutrient availability and pH may limit microbial biomass and microbial capacity for organic matter degradation in acidic tundra heaths by analyzing potential extracellular enzyme activities and microbial biomass after 6 years of factorial treatments of fertilization and liming.
Results
Increasing nutrients enhanced the potential activity of β-glucosidase (synthesized for cellulose degradation). Increasing soil pH, in contrast, reduced the potential activity of β-glucosidase. The soil phospholipid fatty acid concentrations (PLFAs; indicative of the amount of microbial biomass) increased in response to fertilization but were not influenced by liming.
Conclusions
Our results show that soil nutrient availability and pH together control extracellular enzyme activities but with largely differing or even opposing effects. When nutrient limitation was alleviated by fertilization, microbial biomass and enzymatic capacity for cellulose decomposition increased, which likely facilitates greater decomposition of soil organic matter. Increased soil pH, in contrast, reduced enzymatic capacity for cellulose decomposition, which could be related with the bioavailability of organic substrates.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The decomposition rates of plant litter and soil organic matter (SOM) in Arctic tundra ecosystems are slow; therefore, large quantities of terrestrial global carbon (C) are accumulated and stored in these ecosystems (Jonasson et al. 2001; Hobbie et al. 2002; De Deyn et al. 2008). Because of the predicted effects of climate change, it has become increasingly important to understand the factors that determine the decomposition rate of accumulated soil C. The quantity of SOM is regulated to a great extent by the capacity of soil microorganisms to degrade organic substrates via extracellular enzymes that hydrolyze these compounds into forms that can be assimilated as a C or nutrient source (Schimel and Weintraub 2003). As synthesizing extracellular enzymes requires a considerable investment of both energy and nutrients from soil microorganisms, resource availability to synthesize extracellular enzymes is fundamentally important for SOM degradation (Sinsabaugh et al. 2005, 2008; Weintraub et al. 2007; Grandy et al. 2008; Burke et al. 2011). Arctic tundra ecosystems are typically strongly N-limited (Jonasson et al. 2001; Schimel and Bennett 2004). As N constitutes an important component of microbial and enzymatic makeup, it has been suggested that SOM decomposition rates in tundra are limited by the N-limitation of soil microorganisms for growth and synthesizing extracellular enzymes (Schimel and Weintraub 2003; Wallenstein et al. 2009; Weedon et al. 2011; Sistla et al. 2012; Koyama et al. 2013; Sistla and Schimel 2013). Given that the ongoing climate change exerts significant effects on the tundra soil nutrient cycling (Weintraub and Schimel 2005) and experimental increase in soil nutrients has been shown to decrease the quantity of C stored in tundra soils (Nowinski et al. 2008), it is critically important to understand how soil nutrients regulate the SOM degradation potential of soil microbial communities.
To understand the control of soil nutrients over the SOM degradation, it is important to elucidate a multitude of different enzymatic processes. Extracellular enzymes (e.g., cellulases) degrading organic compounds that do not contain N (hereafter referred to as C-containing compounds) are synthesized for microbial C acquisition, whereas extracellular enzymes (e.g., proteases, chitinases) degrading organic compounds that contain both C and N (hereafter referred to as N-containing compounds) may primarily be synthesized for microbial N acquisition (Sinsabaugh et al. 2008). Although nutrient enrichment may enhance enzyme activities for microbial C acquisition (Sinsabaugh et al. 2005; Koyama et al. 2013), enzyme activities for nutrient acquisition are often reduced under conditions of high nutrient availability because the microbial need for nutrient acquisition via the decomposition of organic N- and P-containing compounds is reduced (Allison and Vitousek 2005; Moorhead and Sinsabaugh 2006; Sinsabaugh 2010). Thus, the degradation rates of C- and N-containing organic compounds respond differently to changes in soil nutrient availability.
Soil pH exerts a powerful control over extracellular enzyme activities across ecosystems (Sinsabaugh et al. 2008). Evidence from the experimental manipulations of the soil pH in the field suggests that low soil pH limits microbial growth and extracellular enzyme activities. For example, lime application (which increases the soil pH) in arable soils enhances the potential activities of many C- and N-acquiring extracellular enzymes, such as cellulase and protease (Haynes and Swift 1998; Acosta-Martínez and Tabatabai 2000; Ekenler and Tabatabai 2003) and increases the microbial biomass (Blagodatskaya and Anderson 1998; Aciego Pietri and Brookes 2008). The potential activities of P-acquiring enzymes (acid-phosphatase; AP), on the other hand, often decrease in response to liming (Haynes and Swift 1998; Acosta-Martínez and Tabatabai 2000). As tundra soils are drastically more nutrient-limited than arable soils (Schimel and Bennett 2004), the importance of soil pH on microbial activities and biomass in tundra could substantially differ from those found in other systems. The nutrient control over microbial activities could over-power the control by the soil pH; however, no experimental studies on the role of soil pH have been conducted in tundra. Given the high importance of soil pH for microbial activities across ecosystems and the global importance of tundra SOM stocks, mechanistic understanding on the role of pH in tundra soil microbial activities would be important. Tundra ecosystems, especially those dominated by evergreen dwarf shrubs, have a characteristically low soil pH (Hobbie and Gough 2002; Eskelinen et al. 2009). Soil pH in tundra is a powerful determinant of the microbial community composition (Männistö et al. 2007; Eskelinen et al. 2009) and the soil biochemistry (Whittinghill and Hobbie 2011, 2012). Earlier studies have found a negative correlation between soil pH and temperature in tundra (Sundqvist et al. 2011). A transition from tundra to a subarctic mountain birch forest due to climate warming could locally increase the soil pH by more than 1 unit (Sjögersten et al. 2003). Also changes in soil moisture and associated changes in plant species composition alter soil pH (Le Roux et al. 2013). If low soil pH limit the rates of soil microbial processes in tundra soils, increasing soil pH due to increasing temperatures or associated shifts in the vegetation or forest line could trigger enhanced microbial degradation of accumulated SOM.
Here, we investigated the effects of nutrient availability and soil pH on extracellular enzyme activities and microbial biomass in acidic, nutrient-poor tundra heaths after 6 years of factorial treatments of fertilization and liming. Our previous investigation, using the same experiment, demonstrated that soil nutrient availability was a powerful determinant of the microbial community composition and decreased the fungal:bacterial ratio, whereas shifts in the soil pH had only minor effects (Stark et al. 2012). In the present study, we tested the effects of soil nutrient availability and pH on microbial activities and biomass. Based on the hypotheses that extracellular enzyme activities are determined by the stoichiometric requirement of C vs. nutrients for the soil microorganisms (Sinsabaugh et al. 2008) and that microbial biomass and enzymatic activity for C acquisition in tundra ecosystems are limited by nutrient availability (Schimel and Weintraub 2003; Sistla et al. 2012), we predicted that fertilization would increase microbial biomass and enzyme activities synthesized for C acquisition but decrease enzyme activities for microbial N and P acquisition. Given that liming in arable and forest soils has been detected to enhance microbial biomass and enzyme activities for C and N acquisition and reduce enzyme activities for P acquisition (Acosta-Martínez and Tabatabai 2000; Anderson et al. 2000; Ekenler and Tabatabai 2003), we predicted that liming would increase microbial biomass and cellulase and protease activities but decrease acid-phosphatase activities. Finally, we predicted that combined fertilization and liming would alleviate both the nutrient-limitation and the limitation of microbial activities by low soil pH, and to induce a particularly strong increase in microbial biomass and cellulase activities.
Materials and methods
Site description and experimental design
This study was conducted on acidic tundra heaths at Mt. Saana in Kilpisjärvi (69° 03′ N, 20° 50′ E), northwestern Finland. The long-term mean annual temperature of the study area from 1971 to 2000 was −2.3 ºC, and the mean annual precipitation was 420 mm (Drebs et al. 2002). The soil in the Kilpisjärvi area is classified as a Leptosol, which is shallow soil over hard rock or a deeper gravelly soil (Jones et al. 2010). Temperatures have clearly increased during the last 20 years (Virtanen et al. 2010). In August 2004, five acidic heath patches of ca. 30 m × 30 m (hereafter referred to as sites) were selected from the southwest-facing and northeast-facing slopes of Mt. Saana. The sites were located at altitudes ranging from 720 to 800 m a.s.l. and were separated by no more than 5 km of each other. Vegetation at the sites was dominated by dwarf shrubs (Empetrum nigrum ssp. hermaphroditum, Vaccinium vitis-idaea, V. uliginosum, Betula nana) and graminoids (e.g., Festuca ovina, Calamagrostis lapponica, Carex bigelowii). Eight 0.75 × 0.75 m plots were established at each of the five sites and assigned to the following treatments in a full factorial design: 1) fertilization (commercial rapidly dissolving NPK fertilizer [16 - 9 - 22] applied twice during each growing season, with a total addition of 9.6 g N m−2, 5.4 g P m−2 and 13.2 g K m−2 per year beginning in 2005); 2) liming (dolomite lime (CaMg(CO3)2) was applied twice per growing season in equal doses to give a total of 300 g/m2 per year in 2005 and a total of 600 g/m2 per year in 2006-2010. Same experimental site also contain experimental grazer exclosures (Eskelinen 2008), but plots with the grazer exclosure treatment were not used in the present study. All plots, including controls, were watered immediately following fertilization and liming with 500 ml of water from nearby brooks. Fertilization has increased the total plant biomass but has not affected the litter quantity (Eskelinen et al. 2012). Liming has had no effects on the plant biomass or the litter quantity (Eskelinen et al. 2012).
Sampling and soil analyses
We sampled the plots once during the peak growing season (August 2010), when we assumed that the nutrient limitation in the system was at its height (Weintraub and Schimel 2005; Wallenstein et al. 2009). Composite soil samples consisting of five soil cores (with a diameter of 3 cm) were collected from the humus layer of the border zone of each experimental plot approximately. 3 weeks after the second fertilization and liming event. The depth of the organic layer was recorded from each soil core at the time of the sampling. Soils were transported to the laboratory and kept in a cold room (+4 °C). Analyses were finished within 10 days of sample collection. The bulk density of the samples was analyzed by measuring the total fresh weight of the sample and dividing this value by the total sample volume. Bulk density values and the depth of the humus layer were used to calculate the parameters per soil area. All plant materials were removed from the soil samples by sieving (mesh of 3 mm), after which the soil was subsampled for different analyses. Soil moisture was determined by drying the samples (105 °C, 12 h), and the organic matter (SOM) content was analyzed based on the loss on ignition (475 °C, 4 h). Soil moisture and SOM content values were used to calculate the results per gram SOM. Soil pH was measured in 3:5 v/v soil:water suspensions (Denver Instrument Model 220). The total C concentration was analyzed from dry soil (60 °C, 48 h) using a CHN analyzer (EA 1110 CHN). We note that soil C in the limed plots may have contained an unknown quantity of carbonate-C (CO3; total added quantity approximately 6 g C/m−2 per year). We report the values of C% and soil C stock in the results to provide a rough comparison among the different treatments.
Extracellular enzyme activities
The potential activities of five different enzymes involved in SOM decomposition were analyzed using the method described by Boerner et al. (2000). Although the liming treatment increased soil pH in the field, we analyzed the potential activities in a buffer (pH 5.0) that standardizes pH across samples. Our data thus do not reveal the direct effects of pH on enzymes but rather depict the effect of liming and fertilization on the potential production of extracellular enzymes. We focused on the treatment effects on the production of extracellular enzymes (i.e. indirect effects), because the current theoretical framework on SOM degradation in the tundra considers the production of extracellular enzymes to be the major factor limiting SOM degradation (Schimel and Weintraub 2003). Potential enzyme activity analyses thus do not necessarily reflect the actual degradation rates of the substrates in field conditions (Allison et al. 2007, 2011; German et al. 2011). We used the following substrates: paranitrophenyl(pNP)-β-glucopyranoside for β-glucosidase (BG), pNP-β-N-acetylglucosaminide for β-N-acetylglucosaminidase (NAG), leucine p-nitroanilide for leucine aminopeptidase (LAP), pNP-phosphate for acid-phosphatase (AP) and L-3,4-dihydroxyphenylalanine (L-DOPA) for phenol oxidase (PO). BG, NAG, LAP and AP catalyze reactions that hydrolyze the terminal linkages of oligomers released from larger polymers. Specifically, BG releases glucose from cellulose, NAG hydrolyzes N-acetyl glucosamide residues from chitin-derived oligomers, LAP catalyzes the hydrolytic release of leucine and other amino acids from peptides, AP catalyzes the release of phosphate by hydrolyzing the phosphoric ester bonds of phosphate groups in organic molecules, and PO catalyzes oxidative reactions in the decomposition of phenols (Weintraub et al. 2007; Sinsabaugh et al. 2008). According to a generalized model, soil microorganisms synthesize BG for C acquisition, NAG and LAP mainly for N acquisition and AP for P acquisition (Sinsabaugh et al. 2008), whereas PO can be synthesized for either C or N acquisition depending on which resource is required the most (Sinsabaugh 2010). We also calculated the sum of LAP and NAG activities, which is used to depict the total microbial N acquisition (Sinsabaugh et al. 2008). Potential enzyme activities were calculated both per gram SOM and per total PLFAs (used as an index of the amount of microbial biomass). Ratios of C-acquiring to N- and P-acquiring extracellular enzyme activities [resource acquisition activities; ln(BG) : ln(LAP + NAG), ln(BG): ln(AP)] were also calculated (Sistla and Schimel 2013).
Subsamples of 10 g of fresh soil were suspended in 100 ml of 50 mM sodium acetate buffer (at pH 5.0), after which 2 ml of the sample was mixed with 2 ml of the enzyme substrate (substrate concentration 5 mM). Blanks containing 2 ml of sodium acetate were also prepared. Enzyme activities were measured as the oxidation of chromogenic substrates. Following incubation at room temperature, samples were centrifuged; 100 μl of 1.0 M NaOH was added to 1 ml of supernatant; and after dilution to 5 ml with H2O, the absorbance at 410 nm (BG, NAG, LAP, AP) or 460 nm (PO) was measured using a Shimadzu UV-1700 spectrophotometer. The background absorbance of the soil (2 ml soil slurry + 2 ml buffer) and the enzyme substrate solution (2 ml buffer + 2 ml substrate solution) were subtracted from the sample absorbance. Extinction coefficients for calculating activities of BG, NAG, LAP and AP were obtained using paranitrophenol (BG, NAG, LAP, AP). The extinction coefficient for calculating the activity of PO was obtained by oxidizing L-DOPA with horseradish peroxidase.
Microbial biomass
Bacterial, fungal and total microbial biomass was estimated using phospholipid fatty acid (PLFA) analysis. Lipids were extracted overnight from 1 g (wet weight) of freeze-dried soil using 10 ml of a one-phase mixture (1 : 2 : 0.8 v/v/v) of chloroform, methanol and 0.05 M of sodium phosphate buffer (pH 7.4). The extraction was repeated with 5 ml of extraction solvent for 3 h, and 4 ml of chloroform and H2O were added to the solvent phase. After overnight separation, the lipids were separated into neutral lipids, glycolipids and phospholipids in silicic acid columns as described by Frostegård et al. (1991). Methyl nonadecanoate was added as an internal standard and the phospholipid fraction was subjected to mild alkaline methanolysis (White et al. 1979), after which the fatty acid methyl esters were analyzed by gas chromatography as described by Männistö and Häggblom (2006). Peaks were identified using a mixture of 26 bacterial fatty acid methyl esters (Supelco, Bellefonte, PA) and fatty acids extracted from reference strains for which the fatty acid composition is known (Männistö and Häggblom 2006). A total of 34 fatty acid peaks were identified and expressed as nmol g−1 to indicate total microbial biomass. Molar concentration of 18:2ω6,9c was used to indicate fungal biomass [including saprotrophic, ectomycorrhizal and ericoid mycorrhizal fungi (Olsson 1999; Ruess et al. 2002)], while the sum of PLFAs i15:0, a15:0, 15:0, i16:0, 16:1ω9c, i17:0, a17:0, 17:0, cyclo-17:0, 18:1ω7c and cyclo-19:0 was used to indicate the bacterial biomass (Frostegård and Bååth 1996). We also analyzed fungal:bacterial –ratio, some individual PLFAs (e.g. PLFA 10ME-18:0, PLFA 18:1ω9) and microbial community composition using multivariate analyses, but these results showed the same responses to the treatments as in our previous study (see Stark et al. 2012); therefore, these results are not presented in the current paper.
To analyze soil NH4-N concentrations and N and P immobilized in the microbial biomass, a sub-sample of ~3 g of fresh soil was extracted with 50 ml of 0.5 M K2SO4. Another subsample was extracted using the same method following chloroform fumigation (18 h) (Brookes et al. 1985). The NH4-N concentration in the unfumigated extracts was determined by flow injection analysis (FIA 5012, Perstorp). The total extractable N in both unfumigated and fumigated extracts was oxidized to NO3 (Williams et al. 1995) and then analyzed as NO3-N (FIA, Perstorp). Microbial N was calculated by subtracting the total extractable N in the unfumigated extracts from that in the fumigated extracts. Microbial P was analyzed from the same extracts using a Shimadzu UV-1700 spectrophotometer after the addition of a mixed color reagent (consisting of H2SO4, ascorbic acid, ammonium molybdate tetra hydrate and potassium antimonyl tartrate; Murphy and Riley 1962).
Data analysis and presentation
We used linear mixed-effects models (LME, Pinheiro and Bates 2000; Crawley 2007) to examine the effects of the experimental treatments (fertilization and liming) on the analyzed variables. In all analyses, the treatments were nested within sites (a random factor) to account for the hierarchical structure of the experiment. The heteroscedasticity of variances and the normality of errors in each model were checked using model diagnostic plots (Crawley 2007). When necessary, the response variables were log- or square-root transformed prior to analyses to meet the model assumptions of variance homogeneity and normality of errors. The transformed response variables are indicated in Table 1. All analyses were conducted using the R statistical environment (R Development Core Team 2011).
Results
Extracellular enzyme activities
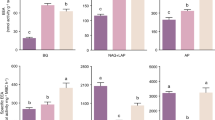
Fertilization and liming caused opposite effects on potential BG activity: while fertilization significantly increased BG activity per gram SOM, liming significantly decreased BG activity per gram SOM (Fig. 1, Table 1; significant main effects of fertilization and liming). When calculated per microbial biomass, there were no effects of fertilization on BG activity, but liming still significantly decreased BG activity (Tables 1 and 2). There were no effects of fertilization or liming on LAP, NAG, PO and AP activity per gram SOM, but fertilization decreased AP activity and tended to decrease LAP and NAG activities (P < 0.10) when calculated per microbial biomass (Fig. 1; Tables 1 and 2). Liming in unfertilized plots tended to reduce C:N acquisition activity [ln(BG):ln(NAG + LAP)]; 1.36 ± 0.10 in unfertilized and unlimed, and 1.20 ± 0.11 in unfertilized and limed plots; 1.47 ± 0.14 in fertilized and unlimed, and 1.39 ± 0.09 in fertilized and limed plots, respectively; P < 0.10, Table 1). C:P acquisition activity [ln(BG): ln(AP)] was not influenced by the treatments (values not shown).
Activity of β-glucosidase (BG), phenol oxidase (PO), leucine-aminopeptidase (LAP, N-acetylglucosidase (NAG) and acid-phosphatase (AP) in nutrient-poor tundra heaths after 6 years of fertilization and liming factorial treatments. N = 5. Note that the figures also include variation from the random variable (site) that was taken into account in the mixed-effects models. Significance levels are * P < 0.05, ** P < 0.01
Soil PLFA concentrations and microbial N and P
Fertilization significantly increased the total PLFAs, bacterial PLFAs and microbial N and P, but there were no effects of liming (Fig. 2; Table 1). The effect of fertilization on microbial P was much stronger than that on microbial N, as fertilization almost doubled the amount of P immobilized in the microbial biomass. Fertilization in unlimed plots tended to increase fungal PLFAs (fertilization × liming –interaction; P < 0.10; Tables 1 and 2).
Concentration of total and bacterial PLFAs, and microbial N and P after 6 years of factorial fertilization and liming treatments. N = 5. Note that the figures also include variation from the random variable (site) that was taken into account in the mixed-effects models. Significance levels are * P < 0.05, ** P < 0.01
Abiotic soil properties
Although soil NH4-N concentrations were substantially higher in fertilized than unfertilized plots, the effect was not statistically significant due to high variation among plots (Tables 1 and 2). Fertilization or liming did not influence soil NO3-N concentrations (Tables 1 and 2). Liming significantly increased the soil pH (Tables 1 and 2). Soil moisture was 64.5 ± 1.4 % across all samples (mean + S.E.) and was not affected by fertilization or liming (statistics not shown). Liming significantly decreased bulk density (Tables 1 and 2).
Fertilization did not significantly influence the soil C stock per soil area (Table 1; 6.7 ± 1.4 kg C m−2 in unfertilized and 5.6 ± 0.7 kg C m−2 in fertilized plots). Similarly, there were no effects of liming on the soil C stock per soil area (Table 1; 4.7 ± 0.8 kg C m−2 in limed and 5.0 ± 0.8 kg C m−2 in limed and fertilized plots; note that these values may contain an unknown quantity of CO3 - C from lime). The same was true for soil C% (Table 1; 37.7 ± 3.4 % in unfertilized, 38.7 ± 2.4 % in fertilized plots, 34.0 ± 2.7 % in limed plots and 39.2 ± 2.8 % in limed and fertilized plots).
Discussion
Our long-term field manipulations showed that increased nutrients enhanced microbial biomass and enzymatic activity for cellulose degradation, indicating increased potential of the soil microbial community to decompose C-containing compounds. Increased soil pH, on the other hand, exerted no effects on microbial biomass while decreasing enzymatic activity for cellulose degradation. These findings provide the first experimental evidence to demonstrate the importance of soil pH for enzymatic activities in Arctic tundra soils.
Nutrients increased microbial biomass and degradation potential of C-containing compounds
We hypothesized that increased nutrient availability would increase the microbial biomass and the potential enzyme activity for C acquisition but reduce the potential enzyme activity for nutrient acquisition. Consistent with our hypothesis, potential BG activity was higher in fertilized than unfertilized plots, confirming the earlier findings that soil N availability limits the synthesis of microbial extracellular enzymes in tundra (Schimel and Weintraub 2003; Wallenstein et al. 2009; Weedon et al. 2011; Sistla et al. 2012; Koyama et al. 2013). The theory of N-limitation of microbial activity opposes the traditional view that soil microorganisms in tundra ecosystems are limited by C availability (Michelsen et al. 1995; Schmidt et al. 2000; Hobbie et al. 2002). As indicated by the increased concentration of total PLFAs in response to nutrient addition, the amount of soil microbial biomass was limited by soil nutrient availability. We detected a significant fertilization effect on BG activity, but not when activities were calculated per total PLFAs, indicating that the increased BG activity in response to fertilization was mediated by a shift in microbial abundance rather than activity. These findings confirm our hypothesis and support a recent study showing that, through limiting the amount of soil microbial biomass, nutrients regulate the microbial enzymatic capacity for C decomposition in nutrient-poor tundra soils (Sistla et al. 2012). Soil carbohydrates constitute a major proportion of accumulated SOM in tundra ecosystems (Sjögersten et al. 2003). Higher microbial potential for carbohydrate degradation might thus reduce the total C stock in the long-term and explain reductions in soil C stock following nutrient additions in field experiments that extend to several decades (Nowinski et al. 2008; Koyama et al. 2013).
The potential activities of enzymes for nutrient acquisition (LAP, NAG, AP; Sinsabaugh et al. 2008, PO; Sinsabaugh 2010) were not affected by nutrient addition when calculated per SOM; however, nutrient addition decreased potential AP activity and tended to decrease potential LAP and NAG activities (P < 0.10) when calculated per total PLFAs. Contrasting with previous studies (Waldrop and Zak 2006; Grandy et al. 2008; Sinsabaugh 2010), we did not find negative effects of nutrient addition on PO activity. These findings indicate that increased nutrient availability did not down-regulate the total microbial potential for deriving N or P via SOM degradation, but – in line with our hypothesis – nutrient addition did reduce microbial nutrient acquisition per unit microbial biomass. Several previous studies in boreal and temperate forests (DeForest et al. 2004; Allison and Vitousek 2005) and alpine tundra (Nemergut et al. 2008) have shown decreased enzyme activities for N and P acquisition. In a recent study in Arctic tundra, Koyama et al. (2013) found negative effects of nutrient addition on AP activity (synthesized for P acquisition), but no effects on LAP and NAG activities (synthesized for N acquisition). They suggested that soil microorganisms may still keep synthesizing LAP and NAG in N-enriched conditions for deriving C from proteins and chitin. In our experiment, we detected reduced nutrient acquisition per microbial biomass in response to nutrient addition, but the increased microbial biomass counteracted this decrease, resulting in no effects when activities were expressed per SOM. Thus, the degradation potential of N-containing compounds stayed at the same level in N-rich conditions even when the activity for N acquisition per microbial biomass decreased. Put together, the enhanced degradation potential for C-compounds and the lack of down-regulation of degradation potential for N-compounds highlights that increased soil nutrients indeed have a powerful potential to increase SOM degradation in nutrient-poor tundra.
Increased soil pH did not influence microbial biomass but reduced BG and PO activities
We hypothesized that increased soil pH would enhance microbial biomass and potential BG and LAP activities but reduce potential AP activity and that fertilization and liming in combination would exert a particularly strong positive effect on microbial biomass and BG activity. Contrary to these predictions, liming had no effect on microbial biomass, LAP, PO or AP activity and exerted a negative effect on BG activity. Also contrasting with predictions, there were no interactive effects of combined fertilization and liming. To our knowledge, our results provide the first experimental evidence on the effects of increasing soil pH on microbial biomass and extracellular enzyme activities in tundra. These effects were contrasting with the findings in arable soils, which show positive effects on microbial biomass (Blagodatskaya and Anderson 1998; Aciego Pietri and Brookes 2008; Rousk et al. 2010) or BG activity (Acosta-Martínez and Tabatabai 2000; Ekenler and Tabatabai 2003) in response to increasing soil pH, in conditions where the soil microorganisms are adapted to more nutrient-rich conditions and a higher pH range than the soil microorganisms in tundra soils.
We suggest that weak effects of increased soil pH on microbial biomass and community composition results from the strong nutrient-limitation of soil microbial growth in tundra ecosystems (Sistla et al. 2012; Stark et al. 2012). The importance of soil pH in controlling soil microbial abundance or community composition may thus depend on the strength of soil nutrient control over microbial communities. The lack of experimental liming effects on fungal:bacterial –ratio contrasts with the observation that microbial community composition varies greatly among tundra ecosystems with varying soil pH (Männistö et al. 2007; Eskelinen et al. 2009); however, the commonly observed important links between the microbial community composition and the soil pH at the ecosystem level (Chu et al. 2010) might result from a combination of environmental factors, such as plant species composition, soil nutrient availability and chemical composition of SOM (Eskelinen et al. 2009; Sundqvist et al. 2011) rather than solely from soil pH.
At this point, the underlying mechanisms and ecological consequences for the decreased potential BG activity per both SOM and microbial biomass in response to increasing soil pH remain unknown. The degradation rates of cellulose and lignin in soils are considered to be tightly interconnected because cellulose in the SOM is often physically protected by lignin (Sinsabaugh and Follstad Shah 2011). We therefore suggest that the negative effects of increasing soil pH on potential BG activity could reflect a change in the potential of the soil microbial community for the degradation of ligno-cellulose in the SOM. It is unclear, however, whether this decreased enzymatic potential results from increased or decreased bioavailability of organic substrates in the soil matrix. Increased soil pH may often enhance the solubility and degradability of SOM by increasing the number of negative charges in the soil components and the repulsion among molecules (Anderson et al. 2000; Kalbitz et al. 2000; Oste et al. 2002; Kleber 2010; Whittinghill and Hobbie 2012). Enhanced SOM solubility might improve the accessibility of the ligno-cellulose in the soil matrix for the extracellular enzymes because substrate diffusion – an important factor regulating the reaction rates between extracellular enzymes and organic substrates – would have a less limiting effect on the substrate availability for the extracellular enzymes. Consequently, a smaller investment of resources from soil microorganisms to extracellular enzymes would yield similar levels of C acquisition via ligno-cellulose degradation. In support for this conclusion, Leifeld et al. (2013) recently found in alpine soils that the SOM turnover time decreased with increasing soil pH, suggesting higher SOM degradation rates. Alternatively, decreased potential BG activity in response to increasing soil pH could also indicate that the microbial potential for SOM degradation was reduced by lime addition. This response might be caused by the reduced availability of substrates for the extracellular enzymes, as increased concentrations of Ca2+ ions in the soil have been shown to enhance SOM stability by creating bridges among the soil particles and, in this way, to decelerate the SOM degradation rates (Whittinghill and Hobbie 2011, 2012).
Implications and future directions for study
According to the theory of eco-enzymatic stoichiometry, soil nutrient availability directs the activities of extracellular enzymes towards a balance between C and nutrient acquisition depending on the microbial requirement and the relative availability of C and nutrients in the soil environment (Sinsabaugh et al. 2008; Sinsabaugh and Follstad Shah 2012). Then, for understanding the regulation of tundra SOM decomposition it is important to ask which resources soil microorganisms are deriving from SOM in each particular set of environmental conditions. We found that nutrient addition through fertilization enhanced microbial biomass and potential to decompose C-rich soil carbohydrates. It has remained an open question to which extent soil microbial activity is limited by resource availability (i.e., supply driven) or induced by resource deficiency (i.e., demand driven; Weedon et al. 2011); our findings point towards a conclusion that microbial activity was limited by nutrients and, in this way, supply driven. In line with expectations, nutrient addition down-regulated enzyme activities for nutrient acquisition when expressed per soil microbial biomass. However, nutrient addition also increased the microbial biomass, which counteracted the reduction in microbial nutrient acquisition, resulting in no effects on potential enzyme activities for degrading N- and P-containing compounds per SOM. Although N-containing organic compounds can be important sources of C as well as N (Sinsabaugh and Follstad Shah 2012; Koyama et al. 2013), our results support the idea that increased nutrients may decrease microbial nutrient acquisition based on C:nutrient stoichiometry, but that changes in the amount of soil microorganisms in response to nutrient addition constitute another important mechanisms by which increasing nutrients influence microbial potential for SOM degradation.
Contrasting with the effects of increased nutrient availability, increased soil pH decreased BG activity, while it had no effects on the total, bacterial or fungal biomass. Thus, increasing soil pH reduced extracellular enzyme activity for C acquisition despite the fact that the microbial requirement of C and nutrients remained unaffected. The cause of this phenomenon is uncertain, but may reflect altered substrate diffusion due to changing soil pH (Kleber 2010) or direct effects of pH on substrate degradation (Sinsabaugh et al. 2008). The mechanisms underlying how soil pH regulates microbial activity and the accessibility of soil C substrates for soil microorganisms in tundra ecosystems would thus be an important subject for further research. Put together, our results highlight that in nutrient-poor and acidic tundra, soil nutrient availability and soil pH jointly control microbial activity but with opposing consequences: increased nutrient availability alleviates the nutrient-limitation of microbial growth and enzyme synthesis for degrading carbohydrates, while increased pH decreases the microbial potential for degrading carbohydrates. Given the important control of soil pH over microbial activities, more information is needed on the effects of land-use and climate warming on the soil pH across tundra systems, as shifts in the soil pH are likely to function as an important mechanism influencing SOM degradation in these systems.
References
Aciego Pietri JC, Brookes PC (2008) Relationship between soil pH and microbial properties in a UK arable soil. Soil Biol Biochem 40:1856–1861
Acosta-Martínez V, Tabatabai MA (2000) Enzyme activities in a limed agricultural soil. Biol Fertil Soils 31:85–91
Allison SD, Vitousek PM (2005) Responses of extracellular enzymes to simple and complex nutrient inputs. Soil Biol Biochem 37:937–944
Allison SD, Gartner TB, Holland K, Weintraub MN, Sinsabaugh RL (2007) Soil enzymes: linking proteomics and ecological processes. Manual of Environmental Microbiology, 3rd edn. p 704–711. ASM Press
Allison SD, Weintraub MN, Gartner TB, Waldrop MP (2011) Evolutionary-economic principles as regulators of soil enzyme production and ecosystem function. In: Shukla D and Varma A (ed) Soil Enzymology, Soil Biology 22. Springer-Verlag Berlin Heidelberg, p 229–243
Anderson S, Nilsson IS, Saetre P (2000) Leaching of dissolved organic carbon (DOC) and dissolved organic nitrogen (DON) in mor humus as affected by temperature and pH. Soil Biol Biochem 32:1–10
Blagodatskaya EV, Anderson T-H (1998) Interactive effects of pH and substrate quality on the fungal-to-bacterial ratio and qCO2 of microbial communities in forest soils. Soil Biol Biochem 30:1269–1274
Boerner REJ, Decker KLM, Sutherland E (2000) Prescribed burning effects on soil enzyme activity in a southern Ohio hardwood forest: a landscape-scale analysis. Soil Biol Biochem 32:899–908
Brookes PC, Kragt JF, Powlson DS, Jenkinson DS (1985) Chloroform fumigation and the release of soil nitrogen: the effects of fumigation time and temperature. Soil Biol Biochem 17:831–835
Burke DJ, Weintraub MN, Hewins CR, Kalisz S (2011) Relationship between soil enzyme activities, nutrient cycling and soil fungal communities in a northern hardwood forest. Soil Biol Biochem 43:795–803
Chu H, Fierer N, Lauber CL, Caporaso JG, Knight R, Grogan P (2010) Soil bacterial diversity in the Arctic is not fundamentally different from that found in other biomes. Environ Microbiol 12:2998–3006
Crawley MJ (2007) The R book. Wiley, Chichester
De Deyn GB, Cornelissen JHC, Bardgett RD (2008) Plant functional traits and soil carbon sequestration in contrasting biomes. Ecol Lett 11:516–531
DeForest JL, Zak DR, Pregitzer KS, Burton AJ (2004) Atmospheric nitrate deposition, microbial community composition, and enzyme activity in northern hardwood forests. Soil Sci Soc Am J 68:132–138
R Development Core Team (2011) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria, http://www.R-project.org
Drebs A, Nordlund A, Karlsson P, Helminen J, Rissanen P (2002) Climatological statistics of Finland 1971–2000. Clim Stat Finland 2002:1–99
Ekenler M, Tabatabai MA (2003) Effects of liming and tillage systems on microbial biomass and glycosidases in soils. Biol Fertil Soils 39:51–61
Eskelinen A (2008) Herbivore and neighbour effects on tundra plants depend on species identity, nutrient availability and local environmental conditions. J Ecol 96:155–165
Eskelinen A, Stark S, Männistö M (2009) Links between plant community composition, soil organic matter quality and microbial communities in contrasting tundra habitats. Oecologia 161:113–123
Eskelinen A, Harrison S, Tuomi M (2012) Plant traits mediate consumer and nutrient control on plant community productivity and diversity. Ecology 93:2705–2718
Frostegård Å, Bååth E (1996) The use of phospholipid fatty acid analysis to estimate bacterial and fungal biomass in soil. Biol Fert Soils 22:59–65
Frostegård A, Tunlid A, Bååth E (1991) Microbial biomass measured as total lipid phosphate in soils of different organic content. J Microbiol Methods 14:151–163
German DP, Weintraub MN, Grandy AS, Lauber CL, Rinkes ZL, Allison SD (2011) Optimization of hydrolytic and oxidative enzyme methods for ecosystem studies. Soil Biol Biochem 43:1387–1397
Grandy AS, Sinsabaugh RL, Neff JC, Stursova M, Zak DR (2008) Nitrogen deposition effects on soil organic matter chemistry are linked to variation in enzymes, ecosystems and size fractions. Biogeochemistry 91:37–49
Haynes RJ, Swift RS (1998) Effects of lime and phosphate additions on changes in enzyme activities, microbial biomass and levels of extractable nitrogen, sulphur and phosphorus in an acid soil. Biol Fertil Soils 6:153–158
Hobbie EA, Gough L (2002) Foliar and soil nutrients in tundra on glacial landscapes of contrasting ages in Northern Alaska. Oecologia 131:453–462
Hobbie SE, Nadelhoffer K, Högberg P (2002) A synthesis: the role of nutrients as constraints on carbon balances in boreal and arctic regions. Plant Soil 242:163–170
Jonasson S, Chapin FS III, Shaver GR (2001) Biogeochemistry in the Arctic: patterns, processes and controls. In: Global Biogeochemical cycles in the climate system. Academic, San Diego, pp 139–150
Jones A, Stolbovay V, Tarnocai C, Broll G, Spaargaren O, Montanarella L (2010) Soil atlas of the Northern Circumpolar Region. European Commission
Kalbitz K, Solinger S, Park JH, Michalzik B, Matzner E (2000) Controls of the dynamics of dissolved organic matter in soils: a review. Soil Sci 165:277–304
Kleber M (2010) What is recalcitrant soil organic matter? Environ Chem 7:320–332
Koyama A, Wallenstein MW, Simpson RT, Moore JC (2013) Carbon-degrading enzyme activities stimulated by increased nutrient availability in arctic tundra soils. PLoS One 8:e77212
Le Roux PC, Aalto J, Luoto M (2013) Soil moisture’s underestimated role in climate change impact modelling in low-energy systems. Glob Chang Biol 19:2965–2975
Leifeld J, Bassin S, Conen F, Hajdas I, Elgi M, Fuhrer J (2013) Control of soil pH on turnover of belowground organic matter in subalpine grassland. Biogeochemistry 112:59–69
Männistö MK, Häggblom MM (2006) Characterization of psychrotolerant heterotrophic bacteria from Finnish Lapland. Syst Appl Microbiol 29:229–243
Männistö MK, Tiirola M, Häggblom MM (2007) Bacterial communities in Arctic fields of Finnish Lapland are stable but highly pH-dependent. FEMS Microbiol Ecol 59:452–465
Michelsen A, Schmidt IK, Jonasson S, Dighton J, Jones HE, Callaghan TV (1995) Inhibition of growth, and effects on nutrient uptake of arctic graminoids by leaf extracts - allelopathy or resource competition between plants and microbes. Oecologia 103:407–418
Moorhead DL, Sinsabaugh RL (2006) A theoretical model of litter decay and microbial interactions. Ecol Monogr 76:151–174
Murphy J, Riley JP (1962) A modified single solution method for the determination of phosphate in natural waters. Anal Chim Acta 27:31–36
Nemergut DR, Townsend AR, Sattin SR, Freeman KR, Nierer N, Neff JC, Bowman WD, Schadt CW, Weintraub MN, Schmidt SK (2008) The effects of chronic nitrogen fertilization on alpine tundra soil microbial communities: implications for carbon and nitrogen cycling. Environ Microbiol 10:3093–3105
Nowinski NS, Trumbore SE, Schuur EAG, Mack MC, Shaver GR (2008) Nutrient addition prompts rapid detabilization of organic matter in an arctic tundra ecosystem. Ecosystems 11:16–25
Olsson PA (1999) Signature fatty acids provide tools for determination of the distribution and interactions of mycorrhizal fungi in soil. FEMS Microbiol Ecol 29:303–310
Oste LA, Temminghoff EJM, van Riemsdijk WH (2002) Solid-solution partitioning of organic matter in soils as influenced by an increase in pH or Ca concentration. Environ Sci Technol 36:208–214
Pinheiro JC, Bates DM (2000) Mixed-effects models in S and S-PLUS. Springer, New York
Rousk J, Brookes PC, Bååth E (2010) Investigating the mechanisms for the opposing pH relationships of fungal and bacterial growth in soil. Soil Biol Biochem 42:926–934
Ruess L, Häggblom MM, García Zapata EJ, Dighton J (2002) Fatty acids of fungi and nematodes - possible biomarkers in the soil food chain. Soil Biol Biochem 34:745–756
Schimel JP, Bennett J (2004) Nitrogen mineralization: challenges of a changing paradigm. Ecology 85:591–602
Schimel JP, Weintraub MN (2003) The implications of exoenzyme activity on microbial carbon and nitrogen limitation in soil: a theoretical model. Soil Biol Biochem 35:549–563
Schmidt IK, Ruess L, Bååth E, Michelsen A, Ekelund F, Jonasson S (2000) Long-term manipulation of the microbes and microfauna of two subarctic heaths by addition of fungicide, bactericide, carbon and fertilizer. Soil Biol Biochem 32:707–720
Sinsabaugh RL (2010) Phenol oxidase, peroxidase and organic matter dynamics of soil. Soil Biol Biochem 42:391–404
Sinsabaugh RL, Follstad Shah JJ (2011) Ecoenzymatic stoichiometry of recalcitrant organic matter decomposition: the growth rate hypothesis in reverse. Biogeochemistry 102:31–43
Sinsabaugh RL, Follstad Shah JJ (2012) Ecoenzymatic stoichiometry and ecological theory. Annu Rev Ecol Evol Syst 43:313–343
Sinsabaugh RL, Gallo ME, Lauber C, Waldrop MP, Zak DR (2005) Extracellular enzyme activities and soil organic matter dynamics for northern hardwood forests receiving simulated nitrogen deposition. Biogeochemistry 75:201–215
Sinsabaugh RL, Lauber CL, Weintraub MN, Bony A, Allison SD, Crenshaw C, Contosta AR, Cusack D, Frey S, Gallo ME, Gartner TB, Hobbie SE, Holland K, Keeler BL, Powers JS, Stursova M, Takacs-Vesbach C, Waldrop MP, Wallenstein MD, Zak DR, Zeglin LH (2008) Stoichiometry of soil enzyme activity at global scale. Ecol Lett 11:1252–1264
Sistla SA, Schimel JP (2013) Seasonal patterns of microbial extracellular enzyme activities in an arctic tundra soil: Identifying direct and indirect effects of long-term summer warming. Soil Biol Biochem 66:119–129
Sistla SA, Asao S, Schimel JS (2012) Detecting microbial N-limitation in tussock tundra soil: implications for Arctic soil organic carbon cycling. Soil Biol Biochem 55:78–84
Sjögersten S, Turner BL, Mahieu N, Condron LM, Wookey PA (2003) Soil organic matter biochemistry and potential susceptibility to climatic change across the forest-tundra ecotone in the Fennoscandian mountains. Glob Chang Biol 9:759–772
Stark S, Eskelinen A, Männistö MK (2012) Regulation of microbial community composition and activity by soil nutrient availability, soil pH, and herbivory in the tundra. Ecosystems 15:18–33
Sundqvist MK, Giesler R, Graae BJ, Wallander H, Fogelberg E, Wardle DA (2011) Interactive effects of vegetation type and elevation on aboveground and belowground properties in a subarctic tundra. Oikos 120:128–142
Virtanen R, Luoto M, Rämä T, Mikkola K, Hjort J, Grytnes JA, Birks HJB (2010) Recent vegetation changes at the high-latitude tree line ecotone are controlled by geomorphological disturbance, productivity and diversity. Glob Ecol Biogeogr 19:810–821
Waldrop MP, Zak DR (2006) Response of oxidative enzyme activities to nitrogen deposition affects soil concentrations of dissolved organic carbon. Ecosystems 9:921–933
Wallenstein MD, McMahon SK, Schimel JP (2009) Seasonal variation in enzyme activities and temperature sensitivities in Arctic tundra soils. Glob Chang Biol 15:1631–1639
Weedon JT, Aerts R, Kowalchuk GA, Bodegom BM (2011) Enzymology under global change: organic nitrogen turnover in alpine and sub-Arctic soils. Biochem Soc T 39:309–314
Weintraub MN, Schimel JP (2005) Nitrogen cycling and the spread of shrubs control changes in the carbon balance of arctic tundra ecosystems. Bioscience 55:408–415
Weintraub MN, Scott-Denton LE, Schmidt SK, Monson RK (2007) The effects of tree rhizodeposition on soil exoenzyme activity, dissolved organic carbon, and nutrient availability in a subalpine forest ecosystem. Oecologia 154:327–338
White DC, Davis WM, Nickels JS, King JD, Bobbie RJ (1979) Determination of the sedimentary microbiall biomass by extractible lipid phosphate. Oecologia 40:51–62
Whittinghill KA, Hobbie SA (2011) Effects of landscape age on soil organic matter processing in Northern Alaksa. Soil Sci Soc Am J 75:907–917
Whittinghill KA, Hobbie SA (2012) Effects of pH and calcium on soil organic matter dynamics in Alaskan tundra. Biogeochem 111:569–581
Williams BL, Shand CA, Hill M, O’Hara C, Smith S, Young ME (1995) A procedure for the simultaneous oxidation of total soluble nitrogen and phosphorus in extracts of fresh and fumigated soils and litters. Commun Soil Sci Plant Anal 26:91–106
Acknowledgments
The authors sincerely thank Risto Virtanen for help in developing the ideas for this experiment, Kuisma Ranta for help during the establishment of the field experiment and Sirkka Aakkonen for help in the laboratory. This study was funded by the Academy of Finland (projects 218121, 130507 and 252323).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Kees Jan van Groenigen..
Rights and permissions
About this article
Cite this article
Stark, S., Männistö, M.K. & Eskelinen, A. Nutrient availability and pH jointly constrain microbial extracellular enzyme activities in nutrient-poor tundra soils. Plant Soil 383, 373–385 (2014). https://doi.org/10.1007/s11104-014-2181-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-014-2181-y