Abstract
Plant communities, soil organic matter and microbial communities are predicted to be interlinked and to exhibit concordant patterns along major environmental gradients. We investigated the relationships between plant functional type composition, soil organic matter quality and decomposer community composition, and how these are related to major environmental variation in non-acid and acid soils derived from calcareous versus siliceous bedrocks, respectively. We analysed vegetation, organic matter and microbial community compositions from five non-acidic and five acidic heath sites in alpine tundra in northern Europe. Sequential organic matter fractionation was used to characterize organic matter quality and phospholipid fatty acid analysis to detect major variation in decomposer communities. Non-acidic and acidic heaths differed substantially in vegetation composition, and these disparities were associated with congruent shifts in soil organic matter and microbial communities. A high proportion of forbs in the vegetation was positively associated with low C:N and high soluble N:phenolics ratios in soil organic matter, and a high proportion of bacteria in the microbial community. On the contrary, dwarf shrub-rich vegetation was associated with high C:N and low soluble N:phenolics ratios, and a high proportion of fungi in the microbial community. Our study demonstrates a strong link between the plant community composition, soil organic matter quality, and microbial community composition, and that differences in one compartment are paralleled by changes in others. Variation in the forb-shrub gradient of vegetation may largely dictate variations in the chemical quality of organic matter and decomposer communities in tundra ecosystems. Soil pH, through its direct and indirect effects on plant and microbial communities, seems to function as an ultimate environmental driver that gives rise to and amplifies the interactions between above- and belowground systems.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
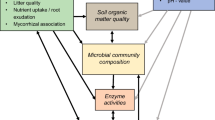
There is increasing recognition that plant community dynamics cannot be separated from the dynamics of belowground soil communities, but that these operate in concert with changes in plant community composition and diversity leading to concordant and predictable changes in soil nutrient cycling and microbial communities, and vice versa (Wardle 2002; Reynolds et al. 2003; Wardle et al. 2004; Bardgett 2005; Van der Heijden et al. 2008). Plant communities differing in species composition are likely to produce litter and organic matter that differ in their chemical composition, which may subsequently influence the composition of soil microbial communities, and the rates of decomposition and nutrient release (Hobbie 1996). These have further feedback effects on plant community composition, productivity and diversity (Reynolds et al. 2003; Ehrenfeld et al. 2005). Despite the acknowledgement of the importance of the interplay between plant and soil communities to ecosystem function, still surprisingly little is known about how the different components of aboveground and belowground systems, e.g., vegetation, soil organic matter and microbial communities, are associated with each other.
Plant species and growth forms may have substantial effects on decomposition and rates of nutrient cycling (Hobbie 1992) via the chemical quality of plant litter. In general, litter produced by herbaceous plants (forbs, graminoids) and/or deciduous shrubs have been shown to decompose faster than the litter of woody and evergreen plants (Hobbie 1996; Dorrepaal et al. 2005) and these differences in decomposition rates have been connected to C quality (Hobbie 1996), C:N ratios and concentrations of soluble phenolics and N (Pérez-Harguindeguy et al. 2000; Quested et al. 2003; Dorrepaal et al. 2005). Polyphenol-rich plant tissues may also impede decomposition because certain polyphenolic compounds (e.g. tannins) tend to form highly stable polyphenol-protein complexes that are unavailable to most decomposers (Northup et al. 1998; Hättenschwiler and Vitousek 2000). Eventually, differences in the plant group-specific litter quality should be reflected in the composition of soil organic matter, so that graminoid- and forb-rich vegetation produces high-quality organic matter that has low C:N ratios and a high proportion of labile compounds (e.g. soluble N, sugars), and woody plant-dominated vegetation produces organic matter that has high C:N ratios and high concentrations of polyphenolic compounds (e.g. lignin, condensed tannins). The link between aboveground vegetation and belowground organic matter quality in tundra ecosystems, however, is inadequately understood, and the few studies conducted so far have not found consistent patterns between the quality of vegetation and the chemical quality of accumulated soil organic matter (see Shaver et al. 2006).
Plant litter and soil organic matter, which act as substrates for soil decomposer organisms, are likely to support divergent microbial communities depending on the relative proportions of labile and recalcitrant substrates (Wardle 2002; Wardle et al. 2004; Bardgett 2005; Orwin et al. 2006). The dominance of bacterial-based soil communities has been suggested to result from the dominance of fast-growing plant species that produce easily decomposable N-rich organic matter, while fungal-based microbial communities would result from the dominance of slow-growing plant species that produce recalcitrant organic matter rich in phenolics and lignin (Wardle et al. 2004; see also Van der Heijden et al. 2008). Different plant species and plant community types have been found to promote different soil microbes and fungi:bacteria ratios (e.g. Kourtev et al. 2002; Zak and Kling 2006); however, there is only limited direct evidence concerning whether the prevalence of bacteria- or fungi-dominated decomposer systems can be predicted by the prevalence of certain plant functional groups (Van der Heijden et al. 2008). Furthermore, despite the close interdependence between the composition of vegetation, soil organic matter and decomposer organisms, we have not been able to find a study that would simultaneously relate patterns in vegetation to patterns in both soil organic matter and microbial communities.
Ecosystem- and habitat-specific discrepancies in abiotic environmental conditions may function as important ultimate drivers of plant-soil interactions (Reynolds et al. 2003; Wardle et al. 2004; Bezemer et al. 2006). One major abiotic factor, soil pH, has long been recognized as having a profound influence on plant community structure and diversity (Gough et al. 2000; Pärtel 2002; Virtanen et al. 2003; Crawley et al. 2005; Virtanen et al. 2006), and it has also been found to be a major determinant of soil microbial community composition (Bååth and Anderson 2003; Fierer and Jackson 2006; Högberg et al. 2007; Männistö et al. 2007). In Northern European arctic and alpine tundra, a major gradient in soil pH, paralleled with changes in soil nutrient availability (Eskelinen 2008), is formed by the underlying bedrock type. Siliceous bedrock types result in acidic heaths with low soil pH and dominance by a few species of dwarf shrubs (e.g. Empetrum nigrum and Vaccinium spp.), while calcareous bedrock types result in non-acidic heaths with high soil pH and species-rich forb- and graminoid-dominated plant communities. Given this drastic variation in the composition of plant communities and bedrock beneath them in alpine tundra, it is reasonable to expect that patterns in vegetation between acidic and non-acidic habitat types would be paralleled by concordant patterns in soil organic matter and microbial communities.
Our objective was to determine whether the differences in plant communities imposed by local environmental conditions are paralleled by changes in soil organic matter and microbial communities, and how the different compartments are linked to each other and abiotic environmental conditions. To study this, we analysed vegetation and soil data from non-acidic and acidic mountain tundra in Northern Europe. We hypothesized that in dwarf shrub-dominated plant communities soil organic matter would be rich in C and recalcitrant compounds that would parallel a high proportion of fungi in soil microbial communities and low nutrient availability. In contrast, productive forb- and graminoid-dominated plant communities would produce N-rich and higher quality organic matter that would support a high proportion of bacteria in microbial communities and high nutrient availability.
Materials and methods
Study sites and data sampling
The study was carried out on Mt. Saana in Kilpisjärvi (69°03′N, 20°50′E), north-western Finland. In the area, the mean annual precipitation is 420 mm and the mean annual temperature is −2.6°C (Järvinen 1987). The tree line is formed by mountain birch (Betula pubescens ssp. czerepanowii) and lies at an altitude of 600–700 m a.s.l. The bedrock in the area consists of both siliceous rocks, resulting in acidic barren soils where dwarf shrub-dominated Empetrum heaths prevail, and of dolomitic rocks resulting in non-acidic and relatively fertile soils characterized by forb- and graminoid-rich Dryas heaths. These heath types are present on the mountain slopes at different exposures, and they often form vegetation mosaics where non-acidic and acidic heath patches alternate within short distances depending on the underlying bedrock material. We first chose five sampling areas where both non-acidic and acidic heath vegetation were present usually within a distance of no more than 100 m. At each sampling area, one non-acidic and one acidic heath vegetation patch (hereafter called “non-acidic” and “acidic” sites) representing as similar moisture conditions and topographical positions as possible were selected for sampling. The sampling areas were located on north-east and south-west slopes of Mt. Saana within a distance of 5 km and at altitudes ranging from 720 to 800 m a.s.l. Acidic heath sites were dominated by the evergreen dwarf shrub Empetrum nigrum ssp. hermaphroditum, and also other shrubs (e.g. Vaccinium vitis-idaea, Vaccinium uliginosum, Betula nana) and some graminoids (e.g. Festuca ovina, Calamagrostis lapponica, Carex bigelowii) were commonly encountered. Non-acidic heath sites were dominated by Dryas octopetala, and other abundant species included dwarf shrubs preferring high-pH sites (e.g. Cassiope tetragona, Rhododendron lapponicum, Salix reticulata), graminoids (e.g. Carex rupestris, Carex vaginata), and arctic-alpine forbs (e.g. Thalictrum alpinum, Saussurea alpina, Saxifraga oppositifolia, Silene acaulis, Astragalus frigidus). Nomenclature follows Hämet-Ahti et al. (1998).
At each site, eight plots of 0.25 × 0.25 m were randomly chosen (however, plots with stones and reindeer paths had to be discarded), all species in the plots were recorded, and the above-ground biomass of vegetation was harvested from the plots. The total number of sampled plots was 80. Plant material was later sorted into five groups of species [graminoids, dwarf shrubs (including families Ericaceae, Empetraceae, Salicaceae and Betulaceae), forbs, bryophytes, lichens], dried for 48 h at +60°C and weighed. Immediately after the collection of biomass samples, composite soil samples consisting of four soil cores (diameter 3 cm) were taken from the humus layer (7–15 cm deep) around each plot. All plant material was removed from the soil samples in the laboratory, after which the soil was homogenized and frozen at −25°C until analysed.
Soil chemical and microbial analysis
Total C and N of soil organic matter were analysed on a CHN element analyzer (Fisons Instruments, Milan). For the analysis of soil P, Ca, K and Mg concentrations, soils were extracted with acid (pH 4.65) 1 M ammonium acetate and determined colorimetrically with an atom absorption spectrophotometer (John 1970). The dry matter content of the soil was determined by drying the samples (105°C, 12 h) and organic matter content analysed by loss on ignition (475°C, 4 h). Soil pH was measured in 3:5 v:v soil:water suspensions (model 220, Denver Instruments). A sub-sample of ca. 3 g fresh soil was extracted with 50 ml of 0.5 M K2SO4, and the NH4 +–N concentration in the extracts was determined by flow injection analysis (FIA 5012; Perstorp). Total extractable N in the extracts was determined by oxidizing all the extractable NO3 − to N (Williams et al. 1995) and then analysing it as NO3 −–N by FIA. Extractable organic N was calculated as the difference between total extractable N and the extractable NH4 +–N concentrations. Microbial N was extracted from the samples using 0.5 M K2SO4 after chloroform fumigation (18 h) (Brookes et al. 1985), and analysed as total extractable N after oxidation as above. Microbial N was calculated by subtracting total extractable N in the unfumigated extracts from that in the fumigated ones. Soil extractable C and microbial C were determined on the unfumigated and fumigated extracts on a total organic C (TOC) analyzer (Shimadzu TOC-5000), and microbial C was calculated in the same way as for microbial N. The results were calculated per soil organic matter.
Organic matter fractionation and protein precipitation capacity
We used the sequential fractionation method, also known as proximate analysis, for investigating the chemical composition of soil organic matter (Ryan et al. 1990). For a detailed description of the procedure see Hilli et al. (2008). The fractionation method separates the soil organic matter into four fractions: non-polar extractives (NPE; e.g. fatty acids and lipids), water-soluble extractives (WSE; e.g. sugars and soluble phenolics), the acid-soluble fraction (AS; e.g. cellulose and hemicellulose), and the acid-insoluble fraction (acid-insoluble residue; AIR) which constitutes the most recalcitrant fraction of organic matter. Although previously regarded solely as comprising lignin or lignin-like compounds, the AIR fraction is a mixture derived from insoluble alkyl-C (mainly cutin, surface waxes, or suberin), tannins and lignin (Lützow et al. 2006). All the results were calculated on an organic matter basis, and the organic matter content was determined separately after each extraction and from acid-insoluble residue (Wieder and Starr 1998). The WSE fraction was further analysed for soluble N concentrations (FIA Tecator) and soluble phenolic concentrations. Phenolics were determined by the Folin-Ciocalteau method (Suominen et al. 2003), using commercial tannic acid (Ph Eur.; VWR BDH Prolabo) as a standard. Although this method does not produce an accurate quantification of phenolics, it is a useful indicator of the relative amount of phenolics in ecological studies.
The protein precipitation capacity (PPC) was analysed using the method by Hagerman and Butler (1978). A solution containing 1 mg ml−1 bovine serum albumin (BSA) was added to ca. 50 mg of dry soil and insoluble tannin-protein complexes were isolated by centrifugation. Protein concentration was analysed from the supernatant using the method by Bradford (1976). PPC was calculated by subtracting the precipitated proteins from the total added BSA (μg g−1 dry soil).
Phospholipid fatty acid analysis
In order to study soil microbial community composition, we analysed soil phospholipid fatty acid (PLFA) patterns from the soil samples. Fatty acids were extracted from 5 g (wet weight) of soil with 15 ml of a one-phase mixture (1:2:0.8 v/v/v) of chloroform, methanol and 0.05 M sodium phosphate buffer (pH 7.4) overnight as described by Ruess et al. (2005). The extraction was repeated with 7.5 ml extraction solvent for 3 h, and 5.9 ml of methanol and H2O was added to the solvent phase. After overnight separation, the lipids were separated to neutral lipids, glycolipids and phospholipids in silicic acid columns. The phospholipid fraction was saponified and methylated as described by Ruess et al. (2005) and fatty acid methyl esters were analysed as described earlier (Männistö and Häggblom 2006). PLFA 18:2ω6c was used to indicate fungal biomass [including saprotrophic, ectomycorrhizal and ericoid mycorrhizal fungi (Olsson 1999; Ruess et al. 2002)] while the sum of PLFAs i15:0, a15:0, 15:0, i16:0, 16:1ω9c, i17:0, a17:0, 17:0, cyclo-17:0, 18:1ω7c and cyclo-19:0 was used to indicate bacterial biomass (Frostegård and Bååth 1996). The fungi:bacteria ratios were estimated as fungal PLFAs divided by bacterial PLFAs. PLFA 18:10ME, which indicates actinobacteria (Kroppenstedt 1985), was tested separately (as mol % from the total PLFAs).
Statistical analyses
First, to assess whether and how the two habitat types, non-acidic and acidic heaths, differed from each other with respect to the abundance of plant functional groups, soil chemical and microbial variables, abundance of soil organic matter fractions and microbial community composition (measured as fungi:bacteria ratios, actinobacteria), we performed linear mixed effects models (Pinheiro and Bates 2000; Crawley 2007) with the habitat type (non-acidic versus acidic) as a fixed factor. Since the study plots were nested within sites, we used site (ten levels) as a random variable in the analyses, thereby defining a hierarchical model that takes into account the spatial correlation in the data (Crawley 2007).
Second, to detect and identify major patterns in plant community data, organic matter fraction data and PLFA data we applied principal component analysis (PCA) to each data set separately. The first PCA axis accounted for 91.7% of the variation in the plant data [Appendix 1 in the Electronic supplementary material (ESM)] and correlated strongly with the forb:shrub ratio (Pearson correlation, r = −0.909, P < 0.0001; plant data were log-transformed to linearize the relationship). The first PCA axis for organic matter fraction data accounted for 65.2% of the variation in the data (Appendix 1 in the ESM) and showed a strong correlation with soil C:N ratio (r = −0.808, P < 0.0001) and soluble N:soluble phenols ratio (r = 0.815, P < 0.0001, log-transformed to linearize the relationship). Similarly, the first PCA axis for the PLFA data accounted for 60.3% of the variation in the data (Appendix 1 in the ESM) and was strongly related to the fungi:bacteria ratio (r = 0.973, P < 0.0001). Since the resulting first axes from PCAs explained most of the variation in all three data sets (i.e. the data sets were one-dimensional) and correlated well with real measurements from each system, we chose to use the forb:shrub ratio, soil C:N ratio, soluble N:soluble phenols ratio and fungi:bacteria ratio rather than the axis scores from PCA (which are difficult to interpret) in the subsequent analyses. The PCA results are not discussed any further.
Third, to examine the links between plant community composition (i.e. forb:shrub ratio), organic matter composition (i.e. C:N ratio, soluble N:soluble phenols ratio, individual fractions), microbial community composition (fungi:bacteria ratio) and bedrock-driven variation in soil pH, we performed linear mixed effects models where each variable was explained by those factors that could act as potential predictors for the given variable on a priori grounds. However, potentially reciprocal relationships were tested only to one direction. In these analyses, the forb:shrub ratio was fitted in relation to soil pH and the fungi:bacteria ratio, the C:N ratio was fitted in relation to the forb:shrub ratio, and the fungi:bacteria ratio was fitted in relation to soil pH and the soil C:N ratio. For the soluble N:soluble phenols ratio and other organic matter fractions (NPE, WSE, AS, AIR), graminoid, bryophyte and lichen biomasses, the forb:shrub ratio and fungi:bacteria ratio were used as explanatory variables. We also included the habitat type (non-acidic versus acidic) as a fixed variable in these analyses, thereby attempting to test whether the variation in each response variable is best explained by a categorical explanatory variable (i.e. habitat type) or continuous ecological variables derived from the variation in bedrock material. In all analyses, the site (ten levels) was used as a random variable. Since some predictor variables were likely to be correlated, we tested the significance of each predictor variable by single term deletion F-tests from the full model (also known as type III analysis) which should reduce the significance of all correlated terms. For the figures, we illustrated significant relationships by performing several separate simple regressions.
The homogeneity of variances was checked using diagnostic residual plots, and when necessary, the data were log-transformed (if the data contained zeros, the smallest observed value of the response variable was added to every value). All analyses were performed using R statistical environment (R Development Core Team 2007).
Results
Differences between non-acidic and acidic habitats
Soils from non-acidic and acidic habitats varied clearly with respect to soil chemical properties. Non-acidic heaths had significantly higher soil pH, higher concentration of Ca and lower concentrations of K and P than acidic heaths (Table 1). Non-acidic and acidic heaths also showed contrasting patterns of soil properties related to nutrient cycling: concentrations of inorganic and extractable organic N and extractable organic C (nearly significant) were higher in non-acidic than in acidic heaths (Table 1). However, there were no significant differences in microbial biomass C and N between the habitats (Table 1). Contrary to our expectations and despite the significant differences in the composition of vegetation (see below), the PPC of the soil samples was unaffected by the habitat type (Table 1).
As expected, there was a clear contrast in the composition of vegetation between non-acidic and acidic heaths. Forbs and shrubs showed distinct abundances between the habitats; forbs were significantly more abundant in non-acidic than in acidic habitats while shrubs were significantly more abundant at acidic sites (F 1,8 = 191.7, P < 0.0001, log-transformed, and F 1,8 = 130.8, P < 0.0001, respectively; Fig. 1a). Overall, the biomass of other plant groups formed only a minority of the total biomass of vegetation (Fig. 1a), and there were no significant differences in these (statistics not shown). Of the soil organic matter fractions, the concentration of the AS fraction consisting mainly of cellulose and hemicellulose was significantly higher in non-acidic than in acidic heaths (F 1,8 = 6.5, P = 0.0342, log-transformed; Fig. 1b) while concentrations of WSE (F 1,8 = 2.2, P = 0.1795) and NPE (F 1,8 = 3.0, P = 0.1224, log-transformed; Fig. 1b) did not differ between the habitat types. However, there were clear differences in the quality of WSE: the concentration of soluble N was significantly higher in the non-acidic heaths than acidic heaths (F 1,8 = 12.3, P = 0.0081), whereas the concentration of soluble phenols was significantly higher in the acidic compared to non-acidic heaths (F 1,8 = 15.2, P = 0.0046; Fig. 1c). Regardless of the striking disparity between the vegetation in non-acidic and acidic habitats, there was no difference in the concentration of AIR (a fraction including the most recalcitrant substances) between the habitats (F 1,8 = 0.6, P = 0.4668; Fig. 1b).
The total soil C:N ratio was significantly higher in shrub-dominated acidic heaths compared to forb-rich non-acidic heaths (F 1,8 = 15.8, P = 0.0041; Fig. 2a). This increase in the soil C:N ratio was paralleled by the increase in the soil fungi:bacteria ratio (F 1,8 = 21.6, P = 0.0017, log-transformed; Fig. 2b). Of the tested individual PLFAs, the proportion of actinobacterial fatty acid 18:10ME was significantly higher in non-acidic than in acidic heaths (1.80 ± 0.07 mol% and 1.13 ± 0.07 mol%, mean ± SE, respectively, F 1,8 = 11.1, P = 0.0103), thereby following the difference in the proportion of total bacteria.
Relationships between plant community composition, soil organic matter and microbial communities
In the mixed effects model with the forb:shrub ratio as a dependent variable and soil pH, the fungi:bacteria ratio and the habitat type as explanatory variables, the forb:shrub ratio significantly negatively correlated with the fungi:bacteria ratio (F 1,58 = 4.1, P = 0.0485; Fig. 3a) but was independent of soil pH (F 1,58 = 0.7, P = 0.3988) when the habitat was included in the model. However, if the habitat was not included in the model, soil pH had a nearly significant positive relationship with the forb:shrub ratio (F 1,58 = 3.8, P = 0.0559), indicating that the relationship with soil pH is important but that the categorical “habitat” variable (non-acidic versus acidic) better explains the variation in pH values (for the habitat, F 1,8 = 49.3, P = 0.0001). In a model with the soil C:N ratio as dependent variable and the forb:shrub ratio and the habitat as explanatory variables, soil C:N ratio significantly correlated with the forb:shrub ratio (F 1,59 = 8.8, P = 0.0044) with high relative abundance of shrubs in above-ground plant communities relating to a high proportion of total C in soil organic matter (Fig. 3b). However, soil C:N ratio was independent of the habitat (F 1,8 = 4.1, P = 0.0779), implying that bedrock-driven variation in forb:shrub ratio better explains variation in soil C:N ratio than habitat type as such. In the mixed effect model with the fungi:bacteria ratio as the dependent variable and soil pH, the soil C:N ratio and the habitat as explanatory variables, we found that fungi:bacteria ratio was significantly correlated with the soil C:N ratio (F 1,58 = 51.6, P < 0.0001) and with soil pH (F 1,58 = 7.7, P = 0.0073) with a high proportion of C in soil organic matter and low soil pH relating to a high proportion of fungi in soil microbial communities (Fig. 3c, d). In this model, the fungi:bacteria ratio was independent of the habitat type (F 1,8 = 1.6, P = 0.2477), suggesting a greater explanatory power of the continuous pH variable than the categorical habitat type.
Links between plant community composition, soil organic matter quality and microbial community composition: the relationship between fungi:bacteria ratio and forb:shrub ratio (a) the relationship between forb:shrub ratio and soil C:N ratio (b), the relationship between soil C:N ratio and fungi:bacteria ratio (c), the relationship between soil pH and fungi:bacteria ratio (d) and the correlations of soluble N:soluble phenols ratio to forb:shub ratio (e) and to fungi:bacteria ratio (f). R 2-, F-, and P-values refer to simple regressions that were performed to illustrate significant relationships in the linear mixed effects models with the site as a random variable, and the F- and P-values therefore deviate from the original results. In all regressions n = 70
The soluble N:soluble phenols ratio was significantly positively correlated with the forb:shrub ratio (F 1,55 = 4.9, P = 0.0308; Fig. 3e) and significantly negatively correlated with the fungi:bacteria ratio (F 1,55 = 11.4, P = 0.0013; Fig. 3f) but independent of the habitat type (F 1,8 = 0.8, P = 0.4077). In the analysis of other individual organic matter fractions, there was a significant positive correlation between AIR and the abundance of graminoids (F 1,55 = 5.7, P = 0.0208). The habitat did not have a significant effect on AIR concentrations (F 1,8 = 0.02, P = 0.8802). There were no other significant correlations between organic matter fractions, plant functional groups and microbial communities (statistics not shown).
Discussion
We expected that the substantial difference between non-acidic and acidic habitats in plant community composition, non-acidic heaths having significantly more forbs and less ericoid shrubs than acidic heaths (Fig. 1a), would result in a divergent composition of soil organic matter between these habitats. Indeed, there was a strong relationship between vegetation composition and quality of organic matter: an increasing proportion of soluble N, and decreasing proportions of soluble phenolics and C:N ratios clearly correlated with an increasing proportion of forbs. These findings concur with several previous studies showing that forbs produce N-rich litter with a low concentration of phenolics and a low C:N ratio, whereas shrubs produce N-poor litter with a high concentration of phenolics and high C:N ratio (Hobbie 1996; Pérez-Harguindeguy et al. 2000; Shaw and Harte 2001; Dorrepaal et al. 2005). Furthermore, forbs in non-acidic heaths encompass legumes (Astragalus spp.), other N-fixers (Dryas octopetala) and hemiparasites (Bartsia alpina), which are rare or absent from acidic sites, and may through the production and release of N-rich litters constitute an important compartment of labile N pools in these habitats (Quested et al. 2003). Taken together, our findings suggest that strong compositional linkages prevail between plant functional types, litter and soil organic matter. These linkages imply that in tundra ecosystems major patterns in the quality of soil organic matter are linked with the forb-shrub gradient of vegetation, a gradient that has not been considered in previous studies of tundra areas (see e.g. Shaver et al. 2006).
The N-poor and phenol-rich organic matter produced by shrub-dominated vegetation was connected to a high proportion of fungi in microbial communities whereas organic matter rich in soluble and total N produced by forb-rich vegetation positively correlated with the proportion of bacteria. These findings are in accordance with those by, e.g., Högberg et al. (2007) and indicate that high proportions of phenolics and total C combined with low N concentrations in substrates favour fungi in decomposer communities. Concomitant findings were also reported by Kourtev et al. (2002) who found a higher amount of fungal PLFA in soils below Vaccinium-dominated vegetation and suggested this resulted from the ericoid mycorrhizal symbionts of the Vaccinium species. In our study, most of the vegetation in acidic habitats comprised shrubs possessing ericoid mycorrhiza (Empetrum nigrum ssp. hermaphroditum and Vaccinium spp.), which may contribute to the high proportion of fungi beneath shrubs. Nevertheless, to our knowledge our investigation is the first that shows in replicated field sites that the fungi:bacteria ratio is connected to the prevalence of both certain plant functional groups and organic matter fractions.
The concentration of the AS organic matter fraction, consisting mainly of cellulose and hemicellulose, was significantly higher in the non-acidic than in acidic heaths, thus coinciding with the higher abundance of cellulose-rich graminoids and forbs in the vegetation (Hobbie 1996). None of the plant groups had a direct correlation with AS, and the difference between the two habitat types therefore likely resulted from a combined effect of both forbs and graminoids. In contrast, although we observed a negative correlation between the graminoids and AIR, there were neither differences between the habitat types in AIR concentrations nor positive relationship between the proportion of dwarf shrubs and AIR, which forms the most recalcitrant fraction. This result is in contrast with the high concentration of AIR, including lignin and other polyphenolic compounds, in the litter of woody dwarf shrubs (Hobbie 1996). In a recent study, Shaver et al. (2006) found that organic matter beneath graminoid-dominated vegetation possessed higher concentrations of AIR than that beneath heath vegetation rich in woody plants. The discrepancy between AIR concentrations in the litter and soil organic matter could be explained by the chemical composition of this recalcitrant fraction. Although traditionally regarded as lignin, AIR in the soil organic matter is chemically versatile and consists of both aromatic and aliphatic (non-aromatic) substances that predominantly are not derived from lignin (Lützow et al. 2006). It is possible that the AIR beneath forb- and graminoid-dominated vegetation was composed of non-aromatic substances to a greater extent than beneath shrubs, thereby resulting in convergence in AIR concentrations despite divergence in vegetation. However, further chemical characterization of the AIR is needed to determine its origin and content in soil organic matter under differing vegetation. Furthermore, given the capability of saprotrophic and ericoid mycorrhizal fungi to metabolize polyphenols (Paul and Clark 1996; Bending and Read 1997; Read and Perez-Moreno 2003; Mutabaruka et al. 2007) and the strong positive relationship between fungal proportion and shrub prevalence in our data, the microbial community beneath woody dwarf shrubs could be better adapted to degrading recalcitrant polyphenols. The high accumulation of recalcitrant AIR in the non-acidic heaths, despite its low concentrations in the plant litter, could therefore partly result from differences in the degradation potential of the microbial community.
To our surprise, we did not find difference between the non-acidic and acidic heaths in PPC, usually considered to correlate with the occurrence of polyphenols (Hättenschwiler and Vitousek 2000). Empetrum, the dominant plant in acidic heaths, produces large quantities of recalcitrant and highly persistent phenolic compounds (e.g. batatasin III) (Gallet et al. 1999) that seem to have strong capability to bind with proteins to form recalcitrant N-containing compounds and, therefore, reduce plant nutrient availability (Nilsson et al. 2002). However, it has been suggested that in nutrient-poor ecosystems specific plant-mycorrhizal interactions have evolved to derive nutrients from these complexes (Northup et al. 1995, 1998), and recent studies also suggest that phenolics may not exert negative effects on micro-organisms if the microbial community is able to degrade polyphenols (Mutabaruka et al. 2007).
The proportion of forbs in plant communities was considerably higher in the non-acidic than acidic heaths, implying that high pH habitats favour forbs in relation to shrubs. Besides the distinct physiological tolerances of plant species to soil pH (Kinzel 1983), the strong interdependence between pH and plant community composition (Gough et al. 2000; Pärtel 2002; Virtanen et al. 2003; Crawley et al. 2005; Virtanen et al. 2006) has been attributed to higher nutrient availability in high pH soils (Peet et al. 2003; Nordin et al. 2004). Indeed, the higher N concentrations in the non-acidic than acidic heaths in our study are likely to favour forbs having higher nutrient demands, intrinsic growth rates, and foliar nutrient concentrations than shrubs (Chapin 1980; Hobbie and Gough 2002). Furthermore, in these systems the chemical quality of forb-produced plant litter and organic matter probably acts as a factor that reinforces the existing patterns of plant species composition and productivity (Berendse 1994). Therefore, although the distinct vegetation patterns may have originally evolved from differences in the soil pH and nutrient availability, the indirect effect of the plant species on soil nutrient cycling feeds back on the vegetation further favouring plant species adapted to N-rich conditions in non-acidic heaths and plant species adapted to N-poor conditions in acidic heaths.
The strong correlation between the soil pH and microbial community is in line with several recent studies emphasizing the role of soil pH in controlling the composition (Bååth and Anderson 2003; Högberg et al. 2007; Fierer et al. 2007; Männistö et al. 2007) and diversity (Fierer and Jackson 2006) of microbial communities. In our data, the relationship between soil pH and the fungi:bacteria ratio remained significant even though the organic matter quality was included in the same model indicating that soil pH acted as an independent driver of microbial community composition. In general, optimal conditions for the growth of fungi as a group occur at lower pH compared to that of most bacteria (Madigan et al. 2003), which may explain why the proportion of bacteria increased with pH. High pH values might thereby promote the evolution of bacteria-based microbial communities which coincide with forb- and graminoid-rich plant communities, and the properties of forb-produced organic matter, i.e. high concentrations of labile N, low concentrations of phenolics and low C:N ratios, may further benefit the prevalence of soil bacteria. We suggest that soil pH may foster important ecosystem processes in tundra through its direct and indirect effects on both plant and microbial communities, and function as an ultimate environmental controller for the development of vegetation-soil-microbe interactions.
The environmental control of the pH also influences higher trophic levels, as Eskelinen (2008) showed in a previous study that plants in the forb-dominated and productive non-acidic heaths were more heavily consumed by mammal grazers than those in the unproductive acidic heaths. Together these investigations support the theory put forward by Wardle et al. (2004) that fertile habitats with higher net primary productivity, higher quality plant tissues and organic matter and bacteria-based decomposer systems support higher herbivory than infertile habitats with low net primary production, low-quality plant tissues and fungi-based decomposer systems.
In conclusion, our correlative investigation found support for the general framework (Wardle et al. 2004; Bardgett 2005; Van der Heijden et al. 2008) that above- and below-ground systems are strongly interconnected with productive, fast-growing plant species (forbs) producing high-quality, N-rich organic matter that supports bacteria-based microbial communities and high nutrient availability and less productive, slow-growing plant species (ericoid dwarf shrubs) producing phenol-rich and N-poor organic matter that favours fungi-based microbial communities and slow nutrient cycling. Given the direct effects that soil pH may have on both microbes and plants and its relationship with plant and microbial communities globally (e.g. Pärtel 2002; Fierer and Jackson 2006), soil pH may be the ultimate factor driving the vegetation and microbial community patterns in tundra. However, our study suggests that these patterns may be further reinforced by the influence of plant species on the quality of soil organic matter and consequently, microbial communities. Experimental investigations are still required to test the causal relationships between plants, soil organic matter, microbes and pH, and the feedback mechanisms operating behind these relationships. Nevertheless, it is conceivable that plant species in acidic heaths (i.e. Empetrum nigrum ssp. hermaphroditum, Vaccinium spp.) may positively feed back on their own performance through producing acidic, low-quality organic matter that further decreases soil pH (Cornelissen et al. 2006), favours fungi in microbial communities and decreases nutrient availability. All these are likely to reinforce the dominance of ericoid dwarf shrubs (Reynolds et al. 2003; Ehrenfeld et al. 2005; Van der Heijden et al. 2008) that possess several favourable traits connected to an adaptation to low nutrient concentrations (e.g. slow growth rate, long-lived tissues, high tissue C:N ratios) (Chapin 1980; Cornelissen et al. 2001) and are capable of accessing stable, complex organic N sources via their ericoid mycorrhizal symbionts (Bending and Read 1997; Read and Perez-Moreno 2003).
References
Bååth E, Anderson T-H (2003) Comparison of soil fungal/bacterial ratios in a pH gradient using physiological and PLFA-based techniques. Soil Biol Biochem 35:955–963
Bardgett R (2005) The biology of soil: a community and ecosystem approach. Oxford University Press, Oxford
Bending GD, Read DJ (1997) Lignin and soluble phenolic degradation by ectomycorrhizal and ericoid mycorrhizal fungi. Mycol Res 101:1348–1354
Berendse F (1994) Litter decomposability—a neglected component of plant fitness. J Ecol 82:187–190
Bezemer TM, Lawson CS, Hedlund K, Edwards AR, Brook AJ, Igual JM, Mortimer SR, van der Putten WH (2006) Plant species and functional group effects on abiotic and microbial soil properties and plant–soil feedback responses in two grasslands. J Ecol 94:893–904
Bradford M (1976) A rapid and sensitive method for quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal Biochem 72:248–254
Brookes P, Kragt JF, Powlson DS, Jenkinson DS (1985) Chloroform fumigation and the release of soil nitrogen: the effects of fumigation time and temperature. Soil Biol Biochem 17:831–835
Chapin FS III (1980) The mineral nutrition of wild plants. Annu Rev Ecol Syst 11:233–260
Cornelissen JHC, Aerts R, Cerabolini B, Werger MJA, van der Heijden MGA (2001) Carbon cycling traits of plant species are linked with mycorrhizal strategy. Oecologia 129:611–619
Cornelissen JHC, Quested HM, van Logtestijn RSP, Pérez-Harguindeguy N, Gwynn-Jones D, Díaz S, Callaghan TV, Press MC, Aerts R (2006) Foliar pH as a new plant trait: can it explain variation in foliar chemistry and carbon cycling processes among subarctic plant species and types? Oecologia 147:315–326
Crawley MJ (2007) The R book. Wiley, Chichester
Crawley MJ, Johnston AE, Silvertown J, Dodd M, de Mazancourt C, Heard MS, Henman DF, Edwards GR (2005) Determinants of species richness in the Park Grass Experiment. Am Nat 165:192–197
Dorrepaal E, Cornelissen JHC, Aerts R, Wallén RB, van Logtestijn RSP (2005) Are growth forms consistent predictors of leaf litter quality and decomposability across peatlands along latitudinal gradient? J Ecol 93:817–828
Ehrenfeld JG, Ravit B, Elgersma K (2005) Feedback in the plant–soil system. Annu Rev Environ Resour 30:75–115
Eskelinen A (2008) Herbivore and neighbour effects on tundra plants depend on species identity, nutrient availability and local environmental conditions. J Ecol 96:155–165
Fierer N, Jackson RB (2006) The diversity and biogeography of soil bacterial communities. Proc Natl Acad Sci 17:626–631
Fierer N, Morse JL, Berthrong ST, Bernhardt ES, Jackson RB (2007) Environmental controls on the landscape-scale biogeography of stream bacterial communities. Ecology 88:2162–2173
Frostegård Å, Bååth E (1996) The use of phospholipid fatty acid analysis to estimate bacterial and fungal biomass in soil. Biol Fert Soils 22:59–65
Gallet C, Nilsson M-C, Zackrisson O (1999) Phenolic metabolites of ecological significance in Empetrum hermaphroditum leaves and associated humus. Plant Soil 210:1–9
Gough L, Shaver GR, Carroll J, Royer DL, Laundre JA (2000) Vascular plant species richness in Alaskan arctic tundra: the importance of soil pH. J Ecol 88:54–66
Hagerman AE, Butler LG (1978) Protein precipitation method for the quantitative determination of tannins. J Agric Food Chem 26:809–812
Hämet-Ahti L, Suominen J, Ulvinen T, Uotila P (eds) (1998) Retkeilykasvio (Field flora of Finland), 4th edn. Finnish Museum of Natural History, Botanical Museum, Helsinki
Hättenschwiler S, Vitousek PM (2000) The role of polyphenols in terrestrial ecosystem nutrient cycling. Trends Ecol Evol 15:238–243
Hilli S, Stark S, Derome J (2008) Carbon quality and stocks in organic horizons in boreal forest soils. Ecosystems 11:270–282
Hobbie S (1992) Effects of plant species on nutrient cycling. Trends Ecol Evol 7:336–339
Hobbie S (1996) Temperature and plant species control over litter decomposition in Alaskan tundra. Ecol Monogr 66:503–522
Hobbie SE, Gough L (2002) Foliar and soil nutrients in tundra on glacial landscapes of contrasting ages in northern Alaska. Oecologia 131:453–462
Högberg MN, Högberg PP, Myrold DD (2007) Is microbial community composition in boreal forest soils determined by pH, C-to-N ratio, the trees, or all three? Oecologia 150:590–601
Järvinen A (1987) Basic climatological data on the Kilpisjärvi area, NW Finnish Lapland. Kilpisjärvi Notes 10:1–16
John MK (1970) Colorimetric determination of phosphorous in soil and plant materials with ascorbic acid. Soil Sci 100:214–220
Kinzel H (1983) Influence of limestone, silicates and soil pH on vegetation. In: Lange OL, Nobel PS, Osmond CB, Ziegler H (eds) Physiological plant ecology. III. Responses to the chemical and biological environment. Springer, Berlin, pp 201–244
Kourtev PS, Ehrenfeld JG, Häggblom M (2002) Exotic plant species alter the microbial community structure and function in the soil. Ecology 83:3152–3166
Kroppenstedt RM (1985) Fatty acid and menaquinone analysis of Actinomycetes and related organisms. In: Goodfellow M, Minnikin DE (eds) Chemical methods in bacterial systematics. Academic Press, London, pp 173–199
Lützow M, Kögel-knabner I, Ekschmitt K, Matzer E, Guggenberger G, Marschner B, Flessa H (2006) Stabilization of organic matter in temperate soils: mechanisms and their relevance under different soil conditions—a review. Eur J Soil Sci 57:426–445
Madigan M, Martinko J, Parker J (2003) Brock biology of microorganisms. Prentice Hall, Upper Saddle River
Männistö MK, Häggblom MM (2006) Characterization of psychrotolerant heterotrophic bacteria from Finnish Lapland. Syst Appl Microbiol 29:229–243
Männistö MK, Tiirola M, Häggblom MM (2007) Bacterial communities in Arctic fjelds of Finnish Lapland are stable but highly pH-dependent. FEMS Microbiol Ecol 59:452–465
Mutabaruka R, Hairiah K, Cadisch G (2007) Microbial degradation of hydrolysable and condensed tannin polyphenol-protein complexes in soils from different land-use histories. Soil Biol Biochem 39:1479–1492
Nilsson M-C, Wardle DA, Zackrisson O, Jäderlund A (2002) Effects of alleviation of ecological stresses on an alpine tundra community over an eight-year period. Oikos 97:3–17
Nordin A, Schmidt IK, Shaver GR (2004) Nitrogen uptake by arctic soil microbes and plants in relation to soil nitrogen supply. Ecology 85:955–962
Northup RR, Yu Z, Dahlgren RA, Vogt KA (1995) Polyphenol control of nitrogen release from pine litter. Nature 377:227–229
Northup RR, Dahlgren RA, McColl JG (1998) Polyphenols as regulators of plant-litter-soil interactions in northern California’s pygmy forest: a positive feedback? Biogeochemistry 42:189–220
Olsson PA (1999) Signature fatty acids provide tools for determination of the distribution and interactions of mycorrhizal fungi in soil. FEMS Microbiol Ecol 29:303–310
Orwin KH, Wardle DA, Greenfield LG (2006) Ecological consequences of carbon substrate identity and diversity in a laboratory study. Ecology 87:580–593
Pärtel M (2002) Local plant diversity patterns and evolutionary history at the regional scale. Ecology 83:2361–2366
Paul EA, Clark FE (1996) Soil microbiol biochemistry, 2nd edn. Academic Press, San Diego
Peet RK, Fridley JD, Gramling JM (2003) Variation in species richness and species pool size across a pH gradient in forests of the Southern Blue Ridge Mountains. Folia Geobot 38:391–401
Pérez-Harguindeguy N, Díaz S, Cornelissen JCH, Verdramini F, Cabido M, Castellanos A (2000) Chemistry and toughness predict leaf litter decomposition rates over a wide spectrum of functional types and taxa in central Argentina. Plant Soil 218:21–30
Pinheiro JC, Bates DM (2000) Mixed-effects models in S and S-PLUS. Springer, New York
Quested HM, Cornelissen JHC, Press MC, Callaghan TV, Aerts R, Trosien F, Riemann P, Gwynn-Jones D, Kondratchuk A, Jonasson SE (2003) Decomposition of sub-arctic plants with differing nitrogen economies: a functional role for hemiparasites. Ecology 84:3209–3221
R Development Core Team (2007) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. ISBN 3-900051-07-0, URL http://www.R-project.org
Read DJ, Perez-Moreno J (2003) Mycorrhizas and nutrient cycling in ecosystems–a journey towards relevance? New Phytol 157:475–492
Reynolds HL, Packer A, Bever JD, Clay K (2003) Grassroots ecology: plant–microbe–soil interactions as drivers of plant community structure and dynamics. Ecology 84:2281–2291
Ruess L, Häggblom MM, García Zapata EJ, Dighton J (2002) Fatty acids of fungi and nematodes–possible biomarkers in the soil food chain? Soil Biol Biochem 34:745–756
Ruess L, Schütz K, Haubert D, Häggblom MM, Kandeler E, Scheu S (2005) Application of lipid analysis to understand trophic interactions in soil. Ecology 86:2075–2082
Ryan MG, Melillo JM, Ricca A (1990) A comparison of methods for determining proximate carbon fractions of forest litter. Can J For Res 20:166–171
Shaver GR, Giblin AE, Nadelhoffer KJ, Thieler KK, Downs MR, Laundre JA, Rastetter EB (2006) Carbon turnover in Alaskan tundra soils: effects of organic matter quality, temperature, moisture and fertilizer. J Ecol 94:740–753
Shaw MR, Harte J (2001) Control of litter decomposition in a subalpine meadow-sagebrush steppe ecotone under climate change. Ecol Appl 11:1206–1223
Suominen K, Kitunen V, Smolander A (2003) Characteristics of dissolved organic matter and phenolic compounds in forest soils under silver birch (Betula pendula), Norway spruce (Picea abies) and Scots pine (Pinus sylvestris). Eur J Soil Sci 54:287–293
Van der Heijden MGA, Bardgett RD, van Straalen NM (2008) The unseen majority: soil microbes as drivers of plant diversity and productivity in terrestrial ecosystems. Ecol Lett 11:296–310
Virtanen R, Dirnböck T, Dullinger S, Grabherr G, Pauli H, Staudinger M, Villar L (2003) Patterns in the plant species richness of European high mountain vegetation. In: Nagy L, Grabherr G, Körner Ch, Thompson DBA (eds) Alpine biodiversity in Europe, Ecological Studies 167. Springer, Berlin, pp 149–172
Virtanen R, Oksanen J, Oksanen L, Razzhivin VY (2006) Broad-scale vegetation-environment relationships in Eurasian high-latitude areas. J Veg Sci 17:519–528
Wardle DA (2002) Communities and ecosystems: linking the aboveground and belowground components. Monographs in population biology 34. Princeton University Press, NJ
Wardle DA, Bardgett RD, Klironomos JN, Setälä H, van der Putten WH, Wall DH (2004) Ecological linkages between aboveground and belowground biota. Science 304:1629–1633
Wieder RK, Starr ST (1998) Quantitative determination of organic fractions in highly organic Sphagnum peat soils. Commun Soil Sci Plant Anal 29:847–857
Williams BL, Shand CA, Hill M, O’Hara C, Smith S, Young ME (1995) A procedure for the simultaneous oxidation of total soluble nitrogen and phosphorus in extracts of fresh and fumigated soils and litters. Commun Soil Sci Plant Anal 26:91–106
Zak DR, Kling GW (2006) Microbial community composition and function across an arctic tundra landscape. Ecology 87:1659–1670
Acknowledgments
We are grateful to Risto Virtanen and Jari Oksanen for discussions and comments on the earlier versions of the manuscript. Jari Oksanen is also appreciated for statistical advice. We thank Riitta Nielsen and Tuulikki Pakonen for helping with the soil analyses and organic matter fractionation. Kilpisjärvi Biological Station is thanked for providing laboratory facilities, assistance and lodging during the fieldwork. This study was financially supported by grants from the Societas pro Fauna et Flora Fennica, the Oskar Öflund Foundation and the Oulu University Scholarship Foundation (to A. Eskelinen). All experiments complied with the laws of Finland at the time the experiments were performed.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Stephan Hättenschwiler.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Eskelinen, A., Stark, S. & Männistö, M. Links between plant community composition, soil organic matter quality and microbial communities in contrasting tundra habitats. Oecologia 161, 113–123 (2009). https://doi.org/10.1007/s00442-009-1362-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-009-1362-5