Abstract
Background and aims
While large differences in microbial biomass and extracellular enzyme activities (EEAs) between rhizosphere and bulk soils have been demonstrated, the potentially different response of microbes and their EEAs in rhizosphere and bulk soils to nitrogen (N) deposition is still not elucidated.
Methods
We analyzed the microbial biomass and EEAs in the rhizosphere and bulk soils of Sibiraea angustata in an alpine shrubland on the eastern Qinghai-Tibet Plateau after chronic N application. We also analyzed the stoichiometric linkages between plants, microbes, enzymes and soils to clarify the coupled responses of aboveground plants and belowground ecological processes.
Results
Microbial nutrient concentrations and activities of EAAs responded differently to N addition in the rhizosphere and bulk soils. In the rhizosphere, N addition caused a significant increase in microbial biomass carbon (C), N and phosphorus (P) concentrations and greater P-degrading enzyme activity (relative to the activities of C- and N-degrading enzymes), which induced a significant reduction in enzyme C:P and N:P ratios. The rhizosphere enzyme N:P ratio was negatively correlated with the N:P ratios of plant, soil and microbe, implying that increased plant and microbial P uptake under N addition may gradually aggravate rhizosphere P limitation. However, for the bulk soil, N addition did not affect microbial biomass but significantly enhanced C-degrading enzyme activity and decreased the enzyme C:N ratio. Meanwhile, the bulk-soil enzyme C:N ratio was negatively correlated with the soil C:N ratio but independent of the plant C:N ratio, implying that N addition may enhance bulk-soil microbial C limitation.
Conclusions
Our study suggests that elevated N deposition may induce differential microbial nutrient limitation between the rhizosphere and bulk soils due to the plant-microbe-soil interactions in the rhizosphere. This study highlights the importance of incorporating rhizosphere microbial processes into biogeochemical models describing environmental changes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Global atmospheric nitrogen (N) deposition has increased two-fold over the last century and has been predicted to increase further in most regions in the next decades (Galloway et al. 2008). Previous studies showed that N deposition could affect the cycles of carbon (C) and nutrients in terrestrial ecosystems by altering aboveground biomass and nutrient contents (Reay et al. 2008; Thomas et al. 2010), whereas considerable uncertainty is associated with the responses of belowground C and nutrient cycling to N enrichment. Specifically, N addition can increase soil C storage by suppressing heterotrophic respiration, decreasing microbial biomass and altering microbial community composition and C-degrading enzyme activity (Janssens et al. 2010 Liu and Greaver 2010), while others have reported that N deposition has neutral or negative effects on soil C storage (Lu et al. 2011; Mack et al. 2004). Moreover, exogenous N input was reported to decrease N mineralization as well as the activities of N-degrading enzymes (Midgley and Phillips 2016), while opposite findings were also observed in some studies (Corre et al. 2010; Dalmonech et al. 2010). Furthermore, N inputs can enhance phosphatase production and consequently cause some ecosystems to shift from relative N limitation to P limitation (Marklein and Houlton 2012). Given recent efforts to incorporate the interactions of aboveground and belowground processes into climate-C feedback models (Thomas et al. 2013), developing a unifying framework of how N deposition affects these processes is critical.
Soil microbes (bacteria and fungi) produce a series of extracellular enzymes to decay organic C, N and P (Allison et al. 2011). The relative activities of extracellular enzymes involved in C, N and P acquisition reflect the stoichiometric equilibrium between microbial biomass and resources (Sinsabaugh et al. 2009). Therefore, the stoichiometry of extracellular enzyme activities (EEAs) has been suggested to be a useful indicator of the relative resource limitations of microbial metabolism (Zechmeister-Boltenstern et al. 2015). By measuring EEAs such as β-1,4-glucosidase (BG), leucine aminopeptidase (LAP), β-1,4-N-acetyl-glucosaminidase (NAG), and acid phosphatase (AP) (Table 1), Sinsabaugh et al. (2009) observed a mean global ratio of C:N:P acquisition, ln(BG):ln(NAG+LAP):ln(AP), of approximately 1:1:1. Although a large amount of evidence suggests that N addition can affect microbial biomass and EEAs, most of these studies did not differentiate between bulk and rhizosphere soils (Treseder 2008; Jian et al. 2016; Yue et al. 2017). The interactions between plant, microbe and soil within the rhizosphere which is the soil zone directly impacted by root activity further increase the uncertainty and complexity of belowground C and nutrient cycling in response to N deposition (Hiltner 1904; Bennett and Klironmos 2018). Specifically, plants can regulate soil physicochemical and microbial properties not only through the uptake of water and nutrients (Moreau et al. 2019) but also by allocating 20~60% of net-fixed C into the rhizosphere soil to stimulate rhizosphere microbial biomass and EEAs and then accelerate soil organic matter (SOM) decomposition (Jones et al. 2009; Brzostek et al. 2013). When soil N availability is improved, plants likely reduce belowground C allocation to the rhizosphere microbial community and subsequently reduce their ability to break down SOM (Phillips et al. 2011; Carrara et al. 2018). In addition, N enrichment can affect rhizosphere microbial activity and composition by altering the physicochemical environment based on plant/root growth and turnover and the formation of mycorrhizae (Philippot et al. 2013; Freedman et al. 2015). All these changes may lead to different responses of rhizosphere microbial nutrient processes to N addition and changes in the underlying mechanisms compared with those of bulk soil. Although the responses of rhizosphere processes to N addition at ecosystem scales have received increasing attention recently (Yin et al. 2013; Carrara et al. 2018), how N addition affects the linkage of rhizosphere stoichiometry with aboveground stoichiometry has not been clearly examined.
Alpine ecosystems on the Qinghai-Tibet Plateau (QTP) are typical and important components of global alpine ecosystems and respond sensitively to environmental changes (Fu and Shen 2017). Alpine grasslands (including alpine steppes and shrub-meadows) are the most dominant ecosystems on this plateau, occupying more than 60% of its total area (~2.5 × 106 km2). The soil organic C and total N storages in the top 1 m of the mineral soil layer are estimated to be 7.36 Pg C and 0.96 Pg N (1 Pg = 1015 g), with an average density of 6.52 C kg m−2 and 0.86 N kg m−2, respectively (Yang et al. 2009). Previous studies have reported that N is a key limiting factor for plants in these alpine ecosystems on the QTP (Jiang et al. 2013), whereas microbes in the rhizosphere and bulk soils suffer from relative C and phosphorus (P) limitations compared to the N limitation, and these limitations are enhanced along an increasing altitudinal gradient (2800–3500 m a.s.l.) (Cui et al. 2019). Therefore, the responses of plant and microbe nutrient statuses in these alpine ecosystems to environmental changes remain unclear. Recently, background N deposition in this region was estimated to be 10–15 kg N ha−1 year−1 (Yu et al. 2019). Given that rhizosphere stoichiometry has been confirmed to be a useful tool for connecting the dynamics of aboveground and belowground processes (Bell et al. 2014), identifying the effect of N deposition on rhizosphere stoichiometry represents a key step in incorporating the coupled relationship of aboveground and belowground processes into ecosystem models and then accurately predicting biogeochemical consequences in these alpine ecosystems under future climatic conditions.
In this study, we conducted a field experiment of N addition (with levels of 0, 50, and 100 kg N ha−1 yr−1) in an alpine Sibiraea angustata (S. angustata) shrubland on the eastern QTP. S. angustata was chosen as a model plant for rhizosphere analysis in this study because shrubland is the second largest vegetation type on the eastern QTP and S. angustata is an endemic and representative shrub species (Wu 1998; Zhao et al. 2018). We examined microbial nutrient (C, N and P) concentrations and EEAs (involved in organic C, N and P degradation) in the rhizosphere and bulk soils of S. angustata to clarify the effects of N addition on the microbial nutrient status in the rhizosphere and bulk soils. We also examined the stoichiometric relationships between extracellular enzymes, microbial biomass, soil (rhizosphere and bulk soils) and plant (roots and leaves) to assess the coupled changes in aboveground responses and belowground processes under N addition. Our preliminary study revealed that N addition significantly reduced the C input by root exudation of S. angustata by nearly 45% compared with that in the controls (P < 0.001, He et al. 2017). We hypothesized that the difference in C source quality between the rhizosphere (labile easily degradable C as exudates) and bulk soils (more recalcitrant C as soil organic matter (SOM) and litter) (Land et al., 2006; Koranda et al., 2011; Bengtson et al., 2012) would result in different effects of N addition on microbial biomass and EEAs in the rhizosphere soil as compared to the bulk soil. In particular, we expected N addition to lower microbial biomass and EEAs in the rhizosphere soil through reduction of C-supply via root education; whereas in the bulk soil, N addition would have a weaker effect on microbial biomass and EEAs because of the relative low C availability which would constrain microbial growth and metabolism.
Materials and methods
Study site
This study was conducted at the Field Research Station of Alpine Shrubland Ecosystems located in Songpan County, Sichuan Province (32°59′N, 103°40′E, 3300 m a.s.l.). This region has a typical alpine climate, and the average annual temperature and precipitation are 4.8 °C and 693.2 mm, respectively (Wang et al. 2017). The plant community includes temperate and cold-temperate vegetation, and the dominant plant species was S. angustata in our study site (Table 1, Wang et al. 2017). The soil is classified as a Cambisol (IUSS Working Group, WRB).
Experimental treatments
The N addition experiment was initiated in May 2012. A randomized block design with three replicated blocks of three treatments was established prior to the experiment: control (no N fertilizer), low-N (50 kg N ha−1 year−1), and high-N (100 kg N ha−1 year−1). Each block included three 5 × 5 m plots, and each plot was surrounded by 10-m-wide buffer strips. An ammonium nitrate (NH4NO3) solution has been sprayed onto the forest floor in 6 equal doses in the first week of each month from May to October (the growing season) each year since 2012. For each N addition event, the fertilizer was weighed, dissolved in 20 L of water, and sprayed evenly using backpack sprayers. An equal volume of water was sprayed on the control plots.
Soil and plant sampling
Soil was collected from each plot three times during 2017 (May, July and September). Sampling was conducted one week before each N-treatment event. At each sampling point, we collected five cores from each plot with a 6-cm-diameter soil core (15-cm depth). The living roots of S. angustata in each core were empirically identified by features that included shape, color, and elasticity. The soil adhering to the roots was carefully separated from the roots using fine forceps. This compartment was operationally defined as rhizosphere soil, and nonadhering soil was considered bulk soil (Phillips et al. 2011). The mass ratios of rhizosphere soil to bulk soil were approximately 1:4, 1:7, and 1:9 in the control, low-N and high-N plots, respectively. All soil samples were passed through a 2-mm-mesh sieve on site and then transported to the laboratory on ice. Each soil sample was divided into three subsamples: one was stored at −20 °C for microbial biomass analysis, enzyme activity analysis and DNA extraction, one was stored at 4 °C for available nutrients analysis, and one was air-dried for chemical analysis. Mature leaves of S. angustata were randomly collected from 5 to 10 individuals in each plot in July 2017 and then mixed to form a sample for each plot. For elemental analysis, the sample from each plot was divided into 3 subsamples. However, the values from the three subsamples per plot were then pooled, and only the mean value per plot was used in statistical analysis. All plant material was oven-dried at 65 °C for 72 h and then milled to pass through a 0.15-mm-mesh sieve. The root biomass was weighed before milling.
The total C (TC) and N (TN) of the soil and plant tissue were analyzed using an elemental analyzer (Vario EL Cube, Elementar Corp., Germany). The plant tissue P concentration was determined by the molybdenum antimony colorimetric method after digestion with an H2SO4 + HClO4 solution (Liu 1996). Dissolved organic C (DOC) in soil was extracted with 0.5 M K2SO4 and then determined on a TOC analyzer (Vario TOC cube, Elementar Corp., Germany). Soil NH4+ and NO3− were extracted by 2 M KCl (soil:solution = 1:5) and then determined on a continuous flow injection analyzer (SEAL Analytical, Germany). The dissolved inorganic N (DIN) was the sum of NH4+ and NO3−. The soil available P was extracted with Bray-I solution (0.03 M NH4F - 0.025 M HCl) (Bray and Kurtz 1945) and then measured using the same colorimetric method used for total P determination.
Microbial nutrient concentrations
The concentrations of microbial biomass C, N and P (MBC, MBN and MBP) were determined by the chloroform fumigation method (Brookes et al. 1985). For chloroform fumigation, twenty grams of fresh rhizosphere and bulk soil from each treatment plot was fumigated with ethanol-free chloroform for 48 h in the dark before extraction. Subsamples of fumigated and nonfumigated soil were shaken with 0.5 M K2SO4 for 30 min (soil:solution = 1:5) (Vance et al. 1987). Extractable organic C and total N were determined simultaneously on a TOC analyzer (Vario TOC, Elementar Corp., UK) after tenfold dilution of the extracts. The remaining part of the fumigated and unfumigated soil was extracted by Bray-I solution (soil:solution = 1:4), and then the total P in the solution was determined by the colorimetric method after digestion with 0.2 mL of H2O2 (30%, v/v) and 0.5 mL of HClO4 (70%, v/v) (Wu et al. 2000). MBC, MBN and MBP were calculated based on the differences of extractable organic C, total N and TP between the fumigated and nonfumigated samples, respectively, and the values were corrected for unrecovered biomass using a factor of 0.45 for MBC and MBN and 0.40 for MBP (Jenkinson et al. 2004). The C:N, C:P and N:P ratios of soil microbial biomass were expressed on a molar basis.
Enzyme activity assays
The potential activities of one organic C-degrading enzyme (β-1,4-glucosidase, BG), two organic N-degrading enzymes (L-leucine aminopeptidase, LAP; β-N-acetylglucosaminidase, NAG) and one organic P-degrading enzyme (acid phosphatase, AP) were measured to evaluate the microbial nutrient status (Sinsabaugh et al. 2009). The specific functions of each enzyme are shown in Table 1. The EEAs were determined within 1 week after field sampling using a fluorometric microplate assay with 4-methyl-umbelliferyl-substrate conjugate (Saiya–Cork et al. 2002). The assay was conducted by homogenizing 2.00 g of soil in 125 mL of 50 mM sodium acetate buffer (pH = 5.0) in a homogenizer for 1 min to form a slurry. Black 96-well microplates were used for fluorometric analysis. Microplates were assigned to six parts, including the sample assay, sample control, quench control, reference standard, negative control, and blank wells. First, 200 μL of buffer was pipetted into the blank, reference standard and negative control wells. Second, 50 μL of buffer was pipetted into the blank and sample control wells. Third, 200 μL of the soil slurry was pipetted into the sample assay, sample control, and quench control wells, and then 50 μL of 10 μM 4-methylumbelliferyl (MUB, for BG, NAG and AP enzyme analysis) or 7-amino-4-methylcoumarin (AMC, for LAP enzyme analysis) was pipetted into the reference standard and quench standard wells. Finally, 50 μL of the corresponding substrate (200 μM) was pipetted into the negative control and sample assay wells. There were eight replicate wells for each enzyme per soil sample. After incubation for 4 h at 25 °C in the dark, 10 μL of 1 M NaOH was added to each well to terminate the reaction. The microplates were scanned on a Varioskan Flash multiplate reader (Thermo Scientific, USA) using a 365-nm excitation filter and 450-nm emission filter. EEAs are expressed in nmol MU g−1 soil (dry weight) h−1. The C:N, C:P and N:P stoichiometric ratios of extracellular enzymes are expressed as Ln(BG):Ln(LAP+NAG), Ln(BG):LN(AP) and Ln(LAP+NAG):Ln(AP) (Sinsabaugh et al. 2009).
DNA extraction and quantitative PCR
In this study, we also measured the bacterial/fungal gene abundance and the ratios of fungi to bacteria (F:B ratio) in the rhizosphere and bulk soils to monitor the change of microbial community under N addition. DNA was extracted from 0.25 g of soil using MoBio Power Soil DNA isolation kits (MoBio Laboratories, CA, USA). DNA concentration was measured using a NanoDrop spectrophotometer (NanoDrop, DE, United States) and agarose gel electrophoresis (1% w/v in TAE), followed by storage at −20 °C prior to amplification. Quantitative PCR (qPCR) was utilized to quantify the gene copy numbers of bacterial 16S rRNA and fungal ITS rRNA using the primer pairs 515F/909R and ITS7F/ITS4R, respectively (Li et al., 2014; Schulz et al., 2018). Each 10-μL reaction contained 5 μL of SybrGreen (2×) PCR Master Mix (Bio-Rad, USA), 0.5 μL of each primer (10 pM), 2 μL of DNA template and 2.5 μL of sterilized water. Bacterial 16S rRNA amplification was performed at 95 °C for 5 min, followed by 40 cycles of 95 °C for 30 s, 55 °C for 30 s, and 72 °C for 30 s, with a final extension cycle for 8 min at 72 °C. The qPCR standards for quantification were prepared from PCR products of target genes from environmental DNA with each primer set using the method described by Hou et al. (2010). Each sample was tested with four replicates. The amplification efficiencies of the 16S rDNA gene and ITS gene were 90% and 92%, respectively, with R2 values higher than 0.99, and no signals were observed in the negative controls. The F:B ratio was calculated based on the gene copy numbers of fungi and bacteria.
Statistical analyses
Data were transformed to meet the assumptions of normality and variance homogeneity when necessary. One-way analysis of variance (ANOVA) with Tukey’s honestly significant difference (HSD) test was performed to test the effects of N addition on nutrient concentrations and their stoichiometric ratios in plants (roots and leaves) and in soils (rhizosphere and bulk soils). The effects of N addition on microbial and enzymatic parameters in the rhizosphere and bulk soil during the growing season were analyzed using repeated measures ANOVA with mixed-effect models. We included the dependent factors (season and soil compartment) and the independent factor (N doses) as fixed effects and the factor “block” as a random factor in the models (Fig. 1 and Fig. 2). Post-hoc comparisons among N-treatment plots were made using Tukey’s HSD test. Pearson correlations with P-values adjusted by Bonferroni correction were calculated to identify the stoichiometric relationships between plant nutrients, available nutrients, microbial biomass, and extracellular enzymes in the rhizosphere and bulk soils. All analyses were conducted using SAS 8.0 for Windows (SAS Institute Inc., Cary NC, USA). Graphic illustrations were generated using Origin 8.0 software (OriginLab Corporation, Northampton, MA, USA). Statistically significant differences were identified at P < 0.05 unless otherwise stated.
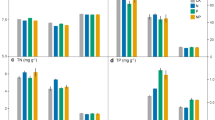
Effects of N addition on microbial biomass C, N and P (a-c) and their stoichiometric ratios (d-f) in the rhizosphere and bulk soils of an alpine shrubland during the sampling period (May, July and September 2017). Values are the means; bars are ±1 SE (n = 3). The concentrations and ratios of microbial elements (C, N and P) were expressed on a molar basis. The abbreviations “S”, “N”, “T”, “S × N”, “S × T”, “N × T” and “S × N × T” indicate the individual and interactive effects of soil compartment (“S”, rhizosphere vs. bulk soils), N addition (“N”, control, low-N and high-N: 0, 50, and 100 kg N ha−1 yr−1, respectively), and sampling time (“T”). P < 0.05: “*”; P < 0.01: “**”; P < 0.001: “***”
Effects of N addition on the activity of activity C-, N-, and P-degrading enzymes (a-d) and their stoichiometric ratios (e-f) in the rhizosphere and bulk soils during the sampling period (May, July and September 2017). Values are the means; bars are ±1 SE (n = 3). The abbreviations “S”, “N”, “T”, “S × N”, “S × T”, “N × T” and “S × N × T” indicate the individual and interactive effects of soil compartment (“S”, rhizosphere vs. bulk soils), N addition (“N”, control, low-N and high-N: 0, 50, and 100 kg N ha-1 yr-1, respectively), and sampling time (“T”). P < 0.05: “*”; P < 0.01: “**”; P < 0.001: “***”
Results
Nutrient concentration and stoichiometry in plants and in two soil compartments
A survey conducted in 2013 (Wang et al. 2017) showed that N addition had no significant effect on plant community structure, but N addition significantly increased the total biomass of S. angustata and grass (both aboveground and belowground; P = 0.04; Table 2). Nevertheless, the foliar biomass of S. angustata did not increase significantly (Table 2). N addition significantly increased the foliar N and P concentrations, leading to a significant decrease in the foliar C:N ratio but an increase in the foliar N:P ratio (all P < 0.05; Table 2). There was a significant increase in the root N concentration but a decrease in the root P concentration (both P < 0.05), which caused a significant increase in the root N:P ratio (P = 0.02) (Table 2).
The chemical and biological characteristics of the rhizosphere soil were significantly different from those of the bulk soil as the rhizosphere soil had significantly higher concentrations of TC, DOC, and available P and a higher C:N ratio than the bulk soil (Table 3). Additionally, rhizosphere and bulk soils differed in their responses to N addition. In the rhizosphere soil, the DIN concentration increased by 40% (P = 0.01) and the TC decreased by 16% (P = 0.03) in the high-N addition plots relative to the control plots, whereas N addition caused only a slight increase in DIN and a 30% increase in TC in bulk soil (Table 3). DOC was consistently high and was not affected by N application in the rhizosphere, whereas its concentration in bulk soil was significantly decreased by N addition (Table 3). In contrast, available P was not significantly affected by N addition in either rhizosphere o bulk soil (Table 3).
Microbial biomass and nutrient stoichiometry
N addition had a significant effect on microbial biomass in the rhizosphere soil but not in the bulk soil. For the rhizosphere soil, N addition significantly increased the microbial biomass C (P = 0.01) and P (P = 0.01), and the increases were most pronounced in the middle of the growing season and less pronounced in the early and late of the growing season. The same tendency, although not significant, was observed for microbial N (Fig. 1a-c). The mean values of microbial biomass C, N and P in the high-N plots increased by 39%, 34% and 23%, respectively, compared to the control plots. Due to the concomitant increases in microbial biomass nutrients, the rhizosphere microbial element ratios were not significantly affected by N addition (all P > 0.05; Fig. 1d-f), and the mean C:N:P ratio was approximately 45 (± 3):10 (± 0.3):1 (± 0.7) across treatment plots. In contrast, for the bulk soil, the microbial biomass C, N and P and their C:N:P stoichiometry were not significantly changed by N addition (all P > 0.05; Fig. 1). The mean C:N:P ratio in the bulk soil across treatment plots was approximately 47 (± 2):11 (± 0.3):1 (± 0.4).
Extracellular enzyme activities (EEAs) and enzyme stoichiometry
The effects of N addition on EEAs and enzymatic C:N:P stoichiometric ratios were different in the rhizosphere and bulk soils. Compared to enzymatic stoichiometric ratios (C:N, C:P and N:P), the N-effect on the absolute values of enzyme activity was almost greater and the significance level was greater in the rhizosphere soil than in the bulk soil. Specifically, in the rhizosphere soil, N addition significantly increased the activities of C-acquiring enzymes (BG; P = 0.02; Fig. 2a) and P-acquiring enzymes (AP; P < 0.001; Fig. 2d) during the growing season. While the effect of N addition on the activity of N-acquiring enzymes (NAG and LAP) was not significant (P = 0.183 and P = 0.097 for the NAG and LAP, respectively; Fig. 2b, c), LAP activities also had a tendency to increase with N dose (Fig. 2b). Corresponding to the changes in MBC and MBP in the rhizosphere, the increase in BG and AP activities was most pronounced in the middle of the growing season and less pronounced in the early and late of the growing season (Fig. 2a, d). LAP activity also showed a similar tendency during the sampled seasons (P = 0.34), although the change without statistical significance. The mean BG activity was 28% higher in the high-N plots than in the control plots, while the mean AP activity increased by 48% and 69% in the low-N plots and high-N plots, respectively, compared to the control plots. The enzyme C:P (P < 0.001) and N:P (P = 0.004) ratios significantly decreased with increasing N input over time (Fig. 2f, g). The mean enzyme C:P ratio decreased from 1.20 ± 0.02 in the control plots to 1.09 ± 0.01 in the high N plots. The mean enzyme N:P ratio decreased from 0.99 ± 0.01 in the control plots to 0.90 ± 0.01 in the high N plots.
In the bulk soil, N addition had no significant effect on the activities of N- and P-degrading enzymes (P > 0.05; Fig. 2b-d) but significantly increased the BG enzyme activity (P = 0.02) during all seasons (Fig. 2a). The BG activity showed an increase of 35% in the high-N plots compared to the control plots. N addition significantly increased the enzyme C:N ratio (P = 0.014) and C:P ratio during all seasons (P = 0.025; Fig. 2e, f). The mean enzyme C:N ratio increased from 1.20 ± 0.02 in the control plots to 1.27 ± 0.01 in the high N plots. The mean enzyme C:P ratio increased from 1.26 ± 0.02 in the control plots to 1.31 ± 0.02 in the high N plots.
Microbial gene abundance and F:B ratio
Analysis of bacterial/fungal gene abundance was performed the soil collected in the middle of the growing season (July) because soil microbes are most active (highest microbial biomass and enzyme activities, Figs.1 and 2) at this time due to optimal environmental conditions. The response patterns of bacterial gene abundance to N addition were consistent with the response pattern of microbial biomass, showing a positive effect of N addition in the rhizosphere but no effect in bulk soil (Fig. 3a). In the rhizosphere, bacterial gene abundance was greatest in the high-N addition plots (P = 0.01), but fungal gene abundance was not affected by N addition (P > 0.05; Fig. 3b). The F:B ratio decreased after the addition of N (P = 0.02; Fig. 3c). In contrast, N addition had no effect on either bacterial or fungal gene abundance or the F:B ratio in the bulk soil.
The abundance (106 copies g−1 dry soil) of total bacterial genes (16S) and fungal genes (ITS) and the ratio of fungi to bacteria (F:B) in the rhizosphere and bulk soils of an alpine shrubland site after five years of experimental N addition. For analysis of bacterial/fungal gene abundance, soil samples collected in the middle of the growing season (July) in 2017 were used. Different uppercase letters denote a significant difference (P < 0.05) between the rhizosphere and bulk soils, and different lowercase letters denote a significant difference (P < 0.05) between treatments in the rhizosphere or bulk soils
Relationships between aboveground and belowground stoichiometry
The aboveground stoichiometric ratios, to some extent, were closely correlated with rhizosphere stoichiometric ratios (Table 4). Following the increase of N addition, the N:P ratios of the leaves and roots had positive relationships with the rhizosphere soil N:P ratio (the correlation coefficients (r) were 0.883 and 0.828, respectively, with P = 0.02 and P = 0.04). The enzyme N:P ratio was negatively correlated with the microbial N:P ratio (r = −0.859; P = 0.03) and soil N:P ratio in the rhizosphere soil (r = −0.880, P = 0.01). In the bulk soil, however, the soil C:N ratio was positively correlated with the foliar C:N ratio (r = 0.896; P = 0.01) and negatively correlated with the enzyme C:N ratio (r = −0.879; P = 0.02).
Discussion
The lack of data on rhizosphere stoichiometry in response to N addition limits our further understanding of plant–microbe interactions associated with ecosystem-level processes under N deposition (Bell et al. 2014). Here, we started to close this gap and provided direct evidence that N addition has differentiated effects on the stoichiometric ratios of microbes and extracellular enzymes between rhizosphere and bulk soils and depends on their relationships with aboveground stoichiometry in an alpine shrubland ecosystem dominated by S. angustata (Fig. 4). In the rhizosphere soil, N addition increased the microbial biomass C, N and P concentrations and induced greater AP enzyme activity (relative to C- and N-degrading enzyme activities), resulting in a lower enzyme N:P ratio. Meanwhile, the significant negative relationships between the rhizosphere enzyme N:P ratio and the N:P ratios of plant tissues, rhizosphere soil and microbes under N addition suggest that the mitigation of N limitation of plant and microbes may enhance the P demand of S. angustata and the rhizosphere microbial community and then aggravates P limitation in the rhizosphere soil. On the contrary, in the bulk soil, N addition had no significant effect on the microbial biomass C, N and P or their stoichiometry but significantly enhanced C-degrading enzyme activity and the enzyme C:N ratio. The enzyme C:N ratio was negatively correlated with the soil C:N ratio following N addition, suggesting that N addition may aggravate microbial C limitation in bulk soil. Previous studies have revealed the close relationship between enzyme stoichiometry and microbial nutrient limitation. For example, the enzyme N:P ratio decreased from 1.73 to 1.49 following an increase in DIN concentration, suggesting that microbial nutrient limitation shifted from N to P limitation (Fanin et al. 2016). Waring et al. (2014) found that in tropical soils, which are characterized by relatively low P bioavailability, the enzyme C:P and N:P ratios (0.21 ± 0.05 and 0.13 ± 0.05 for the C:P and N:P ratios, respectively) were lower than those in the temperate soils (Sinsabaugh et al. 2008) and the soils in our current study. Similarly, in neighboring alpine ecosystems, the enzyme N:P ratios in rhizosphere soils decreased from 0.63 ± 0.98 to 0.15 ± 0.15 as the elevating of altitudes, suggesting that microbial P limitation was gradually enhanced and thus soil microbes in high-altitude ecosystems will be more vulnerable to nutrient imbalance under excess N addition (such as N deposition or fertilization) (Cui et al. 2019).
Conceptual framework of the effects of nitrogen input on microbial biomass and enzymatic activities and the relationship between aboveground and belowground stoichiometry in the rhizosphere (a) and bulk soils (b) of an alpine shrubland. The red numbers with plus signs indicate the increases in parameters (expressed in percentages) in the high-N addition plots relative to the control plots, while the green numbers (with minus signs) indicate the decrease in parameters. “NS” indicates no significant difference between the high-N addition and control plots
It should be noted that the level of N addition in the current study was 3- and 5- fold higher than the maximum of background N deposition in this region. The N-treatment level was chosen for the following three reasons: (1) the low-N treatment was chosen to study the ecological consequences of alpine shrubland ecosystems beyond the maximum “critical load threshold” (50 kg N ha−1 yr−1, Zhu et al., 2015); (2) since a cumulative effect of N addition (or low-level N deposition) can drive N-limited ecosystems to gradually become N-saturation ecosystems (e.g., Emmett et al., 1998; Templer et al., 2007; Blanes et al., 2013), high-N addition can mitigate the N-limitation of alpine shrubland ecosystems in a shorter time; and (3) the two levels of N addition were a useful comparison with those in many previous N fertilization experiments conducted in various ecosystems (Xiao et al., 2018; Zhou et al. 2017).
Effects of N addition on microbial biomass and stoichiometry in the rhizosphere and bulk soils
The increase in microbial biomass in the rhizosphere was unexpected since it was contrary to our hypothesis that it would decrease due to decreased root-C exudation under an elevated N supply. Previous studies showed that N addition has positive, neutral, and negative effects on the rhizosphere microbial biomass in temperature forests (Drake et al. 2013; Phillips et al. 2011; Carrara et al. 2018). The differing results suggest that various factors, e.g., soil C availability, the imbalanced C:N stoichiometry between microbes and resources (root exudates), the different response of microbial communities (bacteria vs. fungi), mycorrhizal infection, and root exudation rate, may control rhizosphere microbes in response to N addition. One possible reason for the increase in rhizosphere microbial biomass with N addition observed in the current study is that the rhizosphere environment is characterized by a surplus of easily available C, which results in strong nutrient limitation rather than C limitation for rhizosphere microbes (Kuzyakov 2002). This finding was supported by our observation that the DOC concentration and soil C:N ratio (DOC:DIN) were higher in the rhizosphere soil than in the bulk soil (Table 3). The literature clearly indicates that rhizosphere microbes are able to acquire enough N to support the synthesis of additional biomass and exoenzymes, which have a low C:N ratio and thus a high N requirement (Elser et al. 2000; Sterner and Elser 2002; Cleveland and Liptzin 2007). In this case, N addition can relieve the imbalanced C:N stoichiometry between microbes and root exudates and then stimulate microbial activity, which is similar to the findings of other studies (Waldrop et al. 2004; Zhou et al. 2017). Another possible reason is that the additional N alleviated N limitation of microbial growth in the rhizosphere, which is rich in bioavailable labile C but not N, which is taken up by plants as a nutrient. It has been widely confirmed that different microbial groups have different nutrient preferences, with bacteria preferring to utilize labile C and N pools and their growth and activity reaching maximum values in soils with high nutrient content and fungi showing greater competition in decomposing substrates with low N availability (Fierer et al. 2007; Ramirez et al. 2012). Our results were similar to those of previous studies that showing a significant positive effect of N addition on bacterial biomass (most likely copiotrophic bacteria; Cusack et al. 2011; Ramirez et al. 2010). Although the labile C pool size and net C mineralization were not measured in this study, we found that N addition significantly decreased the total C concentration in the rhizosphere soil (Table 3), which means that microbes (mainly bacteria) may consume other labile C sources in the rhizosphere soil. Further research is needed to elucidate the linkages among SOC pools (e.g., light vs. heavy fraction or labile vs. recalcitrant fraction), C fluxes and microbial community structure in the rhizosphere and bulk soils as well as their responses to N addition.
However, in the bulk soil, N addition did not significantly affect the microbial biomass, which was mirrored by the pattern of microbial gene abundance in the fertilized and unfertilized bulk soils (Fig. 3). The most likely reason for the absence of a microbial response to N addition in the bulk soil was the enhanced C limitation in the microbial community induced by increased N availability (Rappe–George et al. 2017; Chen et al. 2018). This was supported by our finding that the decrease in DOC and the soil C:N ratio due to increased N input was more pronounced in the bulk soil than in the rhizosphere soil under N addition (Table 3). Berg and Matzner (1997) has indicated that when labile C pools are depleted or the soil C:N ratio is close to the microbial C:N ratio, the response of microbial biomass to long-term N addition is neutral or even negative. The molar ratios of C:N:P in the microbial biomass remained constant (homeostatic) despite large changes in the rhizosphere and bulk-soil nutrient ratios due to N addition (Fig. 1). These results suggest that soil microbial communities, to some extent, can maintain stoichiometric homeostasis under changes in resource nutrient status. To maintain elemental homeostasis, microbes can alternatively adjust their extracellular enzyme production to maximize the mobilization of substrates rich in the limiting elements (Mooshammer et al. 2014b). This was also observed in this study, where enhanced AP activity resulted in a decrease in the enzymatic N:P ratio in rhizosphere soil and enhanced BG activity resulted in an increased in enzyme C:P ratio in bulk soil because the corresponding enzymes that hydrolyzed nonlimiting N and P, respectively, were not concomitantly increased (Fig. 4). In accordance with other studies (Allison and Vitousek 2005; Marklein and Houlton 2012), the observed negative correlations of soil and microbial biomass stoichiometry (N:P in rhizosphere, C:N in bulk soil) can be explained by a selective increase in the enzymatic activities hydrolyzing the rarer, limiting element.
Effects of N addition on extracellular enzyme activity and stoichiometry in the rhizosphere and bulk soils
As mentioned above, the divergent effects of N addition on extracellular enzyme activity between the rhizosphere and bulk soils were strongly dependent on the changes in microbial nutrient demand. In the rhizosphere soil, AP enzyme activity was more significantly stimulated by N addition relative to the activities of other classes of enzymes, leading to lower enzyme C:P and N:P ratios (Fig. 4a). This result was consistent with the results of previous studies (Dalmonech et al. 2010; Turner and Wright 2013) and large-scale meta-analyses (Deng et al. 2017; Marklein and Houlton 2012). Meanwhile, we found that the microbial biomass P concentration and bacterial gene abundance were correspondingly increased by N addition. This finding supports the view that the fast growth of bacteria requires large amounts of P because P is one of the essential elements for the synthesis of the bacterial cell wall (i.e., phospholipid fatty acids) and RNA nucleotides (Elser et al. 2000). Moreover, we found that significantly negative relationships between the enzyme N:P ratio and the N:P ratio of plant tissues, soil and microbes following N addition, which further suggests that N addition can stimulate the growth of plants and microbes and consequently aggravate competition between plants and microbes for available P (Kuzyakov and Xu 2013). As a consequence, microbes need to produce more phosphatase to stimulate P mobilization in the context of N enrichment (Li et al. 2016; Deng et al. 2017). There were two pieces of evidence to support this inference in this study: (1) the total plant biomass of S. angustata increased gradually with the increasing N level; and (2) The plant N pool (foliar N content + root N content) was increased from 19.92 ± 0.90 kg N m−2 to 24.69 ± 1.90 kg N m−2 with the increasing N level. However, regardless of the difference in P allocation between leaves and fine roots, the plant P pool (foliar P content + root P content) was not significantly affected by N addition, ranging from 1.24 ± 0.11 kg P m−2 to 1.51 ± 0.18 kg P m−2 among the treatment plots. As a consequence, the fine root and foliar N:P ratios were increased by N addition. The imbalance in soil availability of N and P under greater N input stimulates plants to take up more N for biomass production but limits their P uptake. This increases their demand for phosphorus and further induces P limitation, resulting in negative feedback on plant growth in response to the increase in foliar N concentration under N addition (Li et al. 2016; Deng et al. 2017). Although the fine root biomass and P concentration did not significantly increase with N addition, the coarse root biomass significantly increased from 3.18 ± 0.01 g m−2 to 4.98 ± 0.02 g m−2 at our study site (P = 0.02; Wang et al. 2017), suggesting that the function of roots shifts from nutrient absorption to nutrient transport under N addition (Zadworny et al. 2017; Ostonen et al. 2017). This may explain the significant shift in P allocation from fine roots to leaves under elevated N input (Table 2). We recognize that using plant biomass data from an earlier study may underestimate the changes in plant nutrient pools due to the lagging response of plants as compared to a relative faster response of microbes. Additionally, the cumulative effect of consecutive N application every year exacerbates differences since plots had received less N over the years at the time of plant harvest than at the time of soil and enzyme measurements. Thus, longer-term monitoring of plant and microbial responses at the sample site is needed in future studies.
In the bulk soil, N addition selectively enhanced C-degrading activity (BG enzyme), while significant changes were not observed in the N- and P-degrading enzyme activities. As a result, the enzyme C:N ratio was significantly increased by N addition (Fig. 4b). The positive effects of N addition on C-degrading enzymes have been confirmed in many studies (Keeler et al. 2008; Cusack et al. 2011; Midgley and Phillips 2016). Furthermore, the enzyme C:N ratio was negatively correlated with the soil C:N ratio following N addition but independent of the plant C:N ratios. Previous studies showed that plant materials are the major N resource for microbial decomposers in litter and soil, thus plant C:N ratios generally control microbial activity and enzymatic production (Aneja et al. 2006; Moorhead et al. 2012). Our finding suggests that the microbial nutrient demand in the bulk soil switches from organic N to mineral N and that the growth and activity of microbes is controlled by soil C availability under N addition (Ågren et al. 2001; Mooshammer et al. 2014a, 2014b), which reveals that increased N availability aggravated microbial C limitation in the bulk soil of this alpine shrubland. In this case, the microbes need to increase their investment in the production of C-degrading enzymes relative to N- and P-degrading enzymes.
Conclusions and implications
Our results show contrasting effects of long-term N addition on microbial biomass and EEAs between the rhizosphere and bulk soils in an alpine shrubland. Here, we propose a novel framework to identify the microbial responses in the rhizosphere and bulk soils in this alpine shrubland ecosystem. The complex plant-microbe-soil interactions in the rhizosphere soil drive the responses of rhizosphere enzyme C:N:P stoichiometry to N addition. Due to the mitigation of plant and rhizosphere N limitation after N input, plant and microbial P demands were enhanced, which consequently caused more pronounced enhancement of AP enzyme activity and a decrease in enzyme C:P and N:P ratios in the rhizosphere soil (Fig. 4a). In contrast, the aboveground stoichiometry (leaf and root) had no significant correlation with the stoichiometry of the microbes and enzymes in the bulk soil. The improved N availability significantly enhanced the C-degrading enzyme activity and induced a higher enzyme C:N ratio (Fig. 4b). Specifically, our findings suggest that elevated N deposition may aggravate P limitation in the rhizosphere soil while aggravating microbial C limitation in the bulk soil, which consequently leads to different feedbacks in C-N-P cycles between the rhizosphere and bulk soils. Previous studies conducted in different ecosystems (subalpine, temperature and tropical forests) showed that the response of microbial metabolism and activity to N-addition is highly dependent on inherent ecosystem parameters that differ between the contrasting ecosystems (such as temperature, humidity, soil initial N status, soil C availability, soil nutrient ratios and colonization of mycorrhizal fungi) (Boot et al. 2016; Carrara et al. 2018; Wang et al. 2018; Corrales et al. 2017). However, it must be noted that most of the above-mentioned studies took the mineral soil in its entirety and did not differentiate between rhizosphere and bulk soils; thus, potential differences between rhizosphere and bulk soil (as discovered in this study) remained undetected. Therefore, further elucidation of the differential responses of biogeochemical processes between the rhizosphere and bulk soils and their underlying mechanisms in various ecosystems under long-term high N deposition should be considered a priority in future studies to deepen our mechanistic understanding of plant-soil interactions and associated ecological consequences. Given that rhizosphere processes are quantitatively important components of terrestrial carbon and nutrient cycles, our findings also highlight the need to incorporate rhizosphere processes into biogeochemical models to better predict ecosystem feedbacks under global changes (Finzi et al. 2015; Goll et al. 2012).
References
Ågren GI, Bosatta E, Magill AH (2001) Combining theory and experiment to understand effects of inorganic nitrogen on litter decomposition. Oecologia 128(3):94–08
Allison SD, Vitousek PM (2005) Responses of extracellular enzymes to simple and complex nutrient inputs. Soil Biol Biochem 37(5):937–944
Allison SD, Weintraub MN, Gartner TB, Waldrop MP (2011) Evolutionary ecosystem principles as regulators of soil enzyme production and ecosystem function. In: Shukla G, Varma A (Eds.), Soil Enzymology. Springer-Verlag, Berlin, Germany, pp. 229–243
Aneja MK, Sharma S, Fleischmann F, Stich S, Heller W, Bahnweg G et al (2006) Microbial colonization of beech and spruce litter – influence of decomposition site and plant litter species on the diversity of microbial community. Microb Ecol 52:127–135
Boot CM, Hall EK, Denef K, Baron JS (2016) Long-term reactive nitrogen loading alters soil carbon and microbial community properties in a subalpine forest ecosystem. Soil Biol Biochem 92:211–220
Bell C, Carrillo Y, Boot CM, Rocca JD, Pendall E, Wallenstein M (2014) Rhizosphere stoichiometry: are C : N : P ratios of plants, soils, and enzymes conserved at the plant species–level? New Phytol 201(2):505–517
Bennett JA, Klironmos J (2018) Mechanisms of plant–soil feedback: interaction among biotic and abiotic drivers. New Phytol 222(1):91–96
Berg B, Matzner E (1997) Effect of N deposition on decomposition of plant litter and soil organic matter in forest systems. Environ Rev 5:1–25
Bray RH, Kurtz LT (1945) Determination of total, organic, and available forms of phosphorus in soils. Soil Sci 59:39–45
Brookes PC, Landman A, Pruden G, Jenkinson DS (1985) Chloroform fumigation and the release of soil nitrogen: a rapid direct extraction method to measure microbial biomass nitrogen in soil. Soil Biol Biochem 17:837–842
Brzostek ER, Greco A, Drake JE, Finzi AC (2013) Root carbon inputs to the rhizosphere stimulate extracellular enzyme activity and increase nitrogen availability in temperate forest soils. Biogeochemistry 115(1):65–76
Carrara JE, Walter CA, Hawkins JS, Peterjohn WT, Averill C, Brzostek ER (2018) Interactions among plants, bacteria, and fungi reduce extracellular enzyme activities under long–term N fertilization. Glob Chang Biol 24(6):2721–2734
Chen H, Li D, Zhao J, Zhang W, Xiao K, Wang K (2018) Nitrogen addition aggravates microbial carbon limitation: evidence from ecoenzymatic stoichiometry. Geoderma 329:61–64
Cleveland CC, Liptzin D (2007). C:N:P stoichiometry in soil: is there a “Redfield ratio” for the microbial biomass? Biogeochemistry 85(3):235–252
Corre MD, Veldkamp E, Arnold J, Wright SJ (2010) Impact of elevated N input on soil N cycling and losses in old–growth lowland and montane forests in Panama. Ecology 91:1715–1729
Corrales A, Turner BL, Tedersoo L, Anslan S, Dalling JW (2017) Nitrogen addition alters ectomycorrhizal fungal communities and soil enzyme activities in a tropical montane forest. Fungal Ecol 27:14–23
Cui Y, Bing H, Fang L, Jiang M, Shen G, Yu J, Wang X, Zhu H, Wu Y, Zhang X (2019) Extracellular enzyme stoichiometry reveals the carbon phosphorus limitations of microbial metabolisms in the rhizosphere and bulk soils in alpine ecosystems. Plant Soil:1–14. https://doi.org/10.1007/s11104-019-04159-x
Cusack D, Silver W, Torn M, Burton S, Firestone M (2011) Changes in microbial community characteristics with nitrogen addition and effects on soil organic matter in two tropical forests. Ecology 92:1–33
Dalmonech D, Lagomarsino A, Moscatelli MC, Chiti T, Valentini R (2010) Microbial performance under increasing nitrogen availability in a Mediterranean forest soil. Soil Biol Biochem 42:1596–1606
Deng Q, Hui D, Dennis S, Reddy KC (2017) Responses of terrestrial ecosystem phosphorus cycling to nitrogen addition : a meta–analysis. Global Ecology and Biogerography 26:713–728
Drake JE, Darby BA, Giasson MA, Kramer MA, Phillips RP, Finzi AC (2013) Stoichiometry constrains microbial response to root exudation—insights from a model and a field experiment in a temperate forest. Biogeosciences 10(2):821–838
Elser JJ, Sterner RW, Gorokhova E, Fagan WF, Markow TA, Cotner JB, Harrison JF, Hobbie SE, Odell GM, Weider LW (2000) Biological stoichiometry from genes to ecosystems. Ecol Lett 3:540–550
Fierer N, Bradford MA, Jackson RB (2007) Toward an ecological classification of soil bacteria. Ecology 88:1354–1364
Fanin N, Moorhead D, Bertrand I (2016) Eco-enzymatic stoichiometry and enzymatic vectors reveal differential C, N, P dynamics in decaying litter along a land-use gradient. Biogeochemistry 129:21–36
Finzi AC, Abramoff RZ, Spiller KS, Brzostek ER, Darby BA, Kramer MA, Phillips RP (2015) Rhizosphere processes are quantitatively important components of terrestrial carbon and nutrient cycles. Glob Chang Biol 21(5):2082–2094
Freedman ZB, Romanowicz KJ, Upchurch RA, Zak D (2015) Differential responses of total and active soil microbial communities to long–term experimental N deposition. Soil Biol Biochem 90:275–282
Fu G, Shen Z-X (2017) Response of alpine soils to nitrogen addition on the Tibetan plateau: a meta-analysis. Appl Soil Ecol 114:99–104
Galloway JN, Townsend AR, Erisman JW et al (2008) Transformation of the nitrogen cycle: recent trends, questions, and potential solutions. Science (New York, N.Y.) 320(5878):889–892
Goll DS, Brovkin V, Parida BR et al (2012) Nutrient limitation reduces land carbon uptake in simulations with a model of combined carbon, nitrogen and phosphorus cycling. Biogeosciences 9:3547–3569
He W, Yang X, Xiao J, Zhang Z, Jiang Z, Yuan Y et al (2017) Effects of nitrogen enrichment on root exudative carbon inputs in Sibiraea angustata shrubbery at the eastern fringe of the Qinghai–Xizang plateau. Chin J Plant Ecol 41(6):610–621 (in Chinese with English abstract)
Hiltner L (1904) Über neuere Erfahrungen und Probleme auf dem Gebiete der Bodenbakteriologie unter besonderer Berücksichtigung der Gründüngung und Brache. Arbeiten der Deutschen Landwirtschaftlichen Gesellschaft 98:59–78
Janssens IA, Dieleman W, Luyssaert S et al (2010) Reduction of forest soil respiration in response to nitrogen deposition. Nat Geosci 3:315–322
Jenkinson DS, Brookes PC, Powlson DS (2004) Measuring soil microbial biomass. Soil Biol Biochem 36:5–7
Jian S, Li J, Chen J et al (2016) Soil extracellular enzyme activities, soil carbon and nitrogen storage under nitrogen fertilization : a meta–analysis. Soil Biol Biochem 101:32–43
Jiang J, Zong N, Song M, Shi P, Ma WL, Fu G, Shen Z, Zhang X, Ouyang H (2013) Responses of ecosystem respiration and its components to fertilization in an alpine meadow on the Tibetan plateau. Eur J Soil Biol 56:101–106
Jones DL, Nguyen C, Finlay RD (2009) Carbon flow in the rhizosphere: carbon trading at the soil–root interface. Plant Soil 321:5–33
Keeler BL, Hobbie SE, Kellogg LE (2008) Effects of long–term nitrogen addition on microbial enzyme activity in eight forested and grassland sites: implications for litter and soil organic matter decomposition. Ecosystems 12:1–15
Kuzyakov Y (2002) Review: factors affecting rhizosphere priming effects. J Plant Nutr Soil Sci 165(4):382–396
Kuzyakov Y, Xu X (2013) Competition between roots and microorganisms for nitrogen: mechanisms and ecological relevance. New Phytol 198(3):656–669
Li Y, Niu S, Yu G (2016) Aggravated phosphorus limitation on biomass production under increasing nitrogen loading: a meta–analysis. Glob Chang Biol 22(2):934–943
Liu G (1996) Standard methods for the observation and analysis of Chinese ecosystem research network: soil analysis and profile description. Standards Press of China, Beijing (in Chinese)
Liu LL, Greaver TL (2010) A global perspective on belowground carbon dynamics under nitrogen enrichment. Ecol Lett 13:819–828
Yu G, Jia Y, He N, Zhu J, Chen Z, Wang Q et al (2019) Stabilization of atmospheric nitrogen deposition in China over the past decade. Nat Geosci 12(6):424–429
Lu M, Zhou X, Luo Y, Yang Y, Fang C, Chen J, Li B (2011) Minor stimulation of soil carbon storage by nitrogen addition: a meta–analysis. Agric Ecosyst Environ 140(1–2):234–244
Mack MC, Schuur EAG, Bret–Harte MS, Shaver GR, Chapin FS (2004) Ecosystem carbon storage in arctic tundra reduced by long–term nutrient fertilization. Nature 431:440–443
Marklein A, Houlton B (2012) Nitrogen inputs accelerate phosphorus cycling rates across a wide variety of terrestrial ecosystems. New Phytol 193(3):696–704
Moreau D, Barggett R, Finlay RD, Jones DL, Phillippot L (2019) A plant perspective on nitrogen cycling in the rhizosphere. Funct Ecol 33:540–552
Midgley MG, Phillips RP (2016) Resource stoichiometry and the biogeochemical consequences of nitrogen deposition in a mixed deciduous forest. Ecology 97(12):3369–3378
Mooshammer M, Wanek W, Hämmerle L et al (2014a) Adjustment of microbial nitrogen use efficiency to carbon:nitrogen imbalances regulates soil nitrogen cycling. Nat Commun 5:3694
Mooshammer M, Wanek W, Zechmeister–Boltenstern S, Richter A (2014b) Stoichiometric imbalances between terrestrial decomposer communities and their resources: mechanisms and implications of microbial adaptations to their resources. Front Microbiol 5:22
Moorhead DL, Lashermes G, Sinsabaugh RL (2012) A theoretical model of C- and N-acquiring exoenzyme activities, which balances microbial demands during decomposition. Soil Biol Biochem 53:133–141
Ostonen I, Truu M, Helmisaari H-S, Lukac M, Borken W, Vanguelova E, Godbold DL, Lõhmus K, Zang U, Tedersoo L, Preem JK, Rosenvald K, Aosaar J, Armolaitis K, Frey J, Kabral N, Kukumägi M, Leppälammi-Kujansuu J, Lindroos AJ, Merilä P, Napa Ü, Nöjd P, Parts K, Uri V, Varik M, Truu J (2017) Adaptive root foraging strategies along a boreal-temperate forest gradient. New Phytol 215:977–991
Philippot L, Raaijmakers JM, Lemanceau P, van der Putten WH (2013) Going back to the roots: the microbial ecology of the rhizosphere. Nat Rev Microbiol 11:789–799
Phillips RP, Finzi AC, Bernhardt ES (2011) Enhanced root exudation induces microbial feedbacks to N cycling in a pine forest under long–term CO2 fumigation. Ecol Lett 14:187–194
Ramirez KS, Craine JM, Fierer N (2012) Consistent effects of nitrogen amendments on soil microbial communities and processes across biomes. Glob Chang Biol 18(6):1918–1927
Ramirez KS, Lauber CL, Knight R, Bradford MA, Fierer N (2010) Consistent effects of nitrogen fertilization on soil bacterial communities in contrasting systems. Ecology 91:3463–3470
Rappe–George MO, Choma M, Čapek P, Börjesson G, Kaštovská E, Šantrůčková H, Gärdenäs AI (2017) Indications that long–term nitrogen loading limits carbon resources for soil microbes. Soil Biol Biochem 115:310–321
Reay DS, Dentener F, Smith P, Grace J, Feely RA (2008) Global nitrogen deposition and carbon sinks. Nat Geosci 1(7):430–437
Saiya–Cork KR, Sinsabaugh RL, Zak DR (2002) The effects of long term nitrogen deposition on extracellular enzyme activity in an Acer saccharum forest soil. Soil Biol Biochem 34:1309–1315
Sinsabaugh RL, Hill BH, Follstad Shah JJ (2009) Ecoenzymatic stoichiometry of microbial organic nutrient acquisition in soil and sediment. Nature 462(7274):795–798
Sinsabaugh RL, Lauber CL, Weintraub MN, Ahmed B, Allison SD, Crenshaw C, Contosta AR, Cusack D, Frey S, Gallo ME, Gartner TB, Hobbie SE, Holland K, Keeler BL, Powers JS, Stursova M, Takacs-Vesbach C, Waldrop MP, Wallenstein MD, Zak DR, Zeglin LH (2008) Stoichiometry of soil enzyme activity at global scale. Ecol Lett 11:1252–1264
Sterner RW, Elser JJ (2002) Ecological Stoichiometry: The Biology of Elements from Molecules to the Biosphere. Princeton University Press, Princeton, NJ.
Thomas RQ, Canham CD, Weathers KC, Goodale CL (2010) Increased tree carbon storage in response to nitrogen deposition in the US. Nat Geosci 3:13–17
Thomas RQ, Bonan GB, Goodale CL (2013) Insights into mechanisms governing forest carbon response to nitro– gen deposition: a model–data comparison using observed responses to nitrogen addition. Biogeosciences 10:3869–3887
Treseder KK (2008) Nitrogen addition and microbial biomass: a meta–analysis of ecosystem studies. Ecol Lett 1:1111–1120
Turner BL, Wright JS (2013) The response of microbial biomass and hydrolytic enzymes to a decade of nitrogen, phosphorus, and potassium addition in a lowland tropical rain forest. Biogeochemistry 117(1):115–130
Vance ED, Brookes PC, Jenkinson DS (1987) An extraction method for measuring soil microbial biomass C. Soil Biol Biochem 19:703–707
Waldrop MP, Zak DR, Sinsabaugh RL, Gallo M, Lauber C (2004) Nitrogen deposition modifies soil carbon storage through changes in microbial enzymatic activity. Ecol Appl 14(4):1172–1177
Wang D, He H, Gao Q, He W, Zhao C, Yin H, Liu Q (2017) Effects of short–term N addition on plant biomass allocation and C and N pools of the Sibiraea angustata scrub ecosystem. Eur J Soil Sci 68(2):212–220
Wang C, Lu X, Mori T, Mao Q, Zhou K, Zhou G et al (2018) Responses of soil microbial community to continuous experimental nitrogen additions for 13 years in a nitrogen-rich tropical forest. Soil Biol Biochem 121:103–112
Waring BG, Weintraub SR, Sinsabaugh RL (2014) Ecoenzymatic stoichiometry of microbial nutrient acquisition in tropical soils. Biogeochemsitry 117:101–113
Wu J, He Z, Wei W, O’Donnell AG, Syers JK (2000) Quantifying microbial biomass phosphorus in acid soils. Biol Fertil Soils 32(6):500–507
Wu N (1998) The community types and biomass of Sibiraea angustata scrub and their relationship with environment factors in northwestern Sichuan. Acta Bot Sin 40:860–870
Yang Y, Fang J, Smith P, Tang Y, Chen A, Ji C et al (2009) Changes in topsoil carbon stock in the Tibetan grasslands between the 1980s and 2004. Glob Chang Biol 15(11):2723–2729
Yin H, Li Y, Xiao J, Xu Z, Cheng X, Liu Q (2013) Enhanced root exudation stimulates soil nitrogen transformations in a subalpine coniferous forest under experimental warming. Glob Chang Biol 19(7):2158–2167
Yue K, Fornara DA, Yang W, Peng Y, Li Z, Wu F, Peng C (2017) Effects of three global change drivers on terrestrial C:N:P stoichiometry: a global synthesis. Glob Chang Biol 23(6):2450–2463
Zadworny M, McCormack ML, Z ˇytkowiak R, Karolewski P, Mucha J, Oleksyn J (2017) Patterns of structural and defense investments in fine roots of Scots pine (Pinus sylvestris L.) across a strong temperature and latitudinal gradient in Europe. Glob Change Biol 23:1218–1231
Zechmeister-Boltenstern S, Keiblinger KM, Mooshammer M, Peñuelas J, Richter A, Sardans J, Wanek W (2015) The application of ecological stoichiometry to plant-microbial-soil organic matter transformations. Ecol Monogr 85:133–155
Zhao W, Reich PB, Yu Q, Zhao N, Yin C, Zhao C et al (2018) Shrub type dominates the vertical distribution of leaf C : N : P stoichiometry across an extensive altitudinal gradient. Biogeosciences 15(7):2033–2053
Zhou Z, Wang C, Zheng M, Jiang L, Luo Y (2017) Patterns and mechanisms of responses by soil microbial communities to nitrogen addition. Soil Biol Biochem 115:433–441
Acknowledgments
This study was supported jointly by the Priority Research Program of Frontier Science, Chinese Academy of Science (QYZDB-SSW-SMC023), the National Natural Science Foundation of China (No. 31670449, 31872700), and the Sichuan Key R & D Program (21018SZ0336). We are also grateful for the funding provided by the Ecological Security and Protection Key Laboratory of Sichuan Province, Mianyang Normal University (ESP1702).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Elizabeth M Baggs.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 45 kb)
Rights and permissions
About this article
Cite this article
Zhu, X., Liu, M., Kou, Y. et al. Differential effects of N addition on the stoichiometry of microbes and extracellular enzymes in the rhizosphere and bulk soils of an alpine shrubland. Plant Soil 449, 285–301 (2020). https://doi.org/10.1007/s11104-020-04468-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-020-04468-6