Abstract
The effects of lead and copper on the arrangement of microtubule (MT) cytoskeleton in root tip cells of Allium sativum L. were investigated. Batch cultures of garlic were carried out under defined conditions in the presence 10−4 M Pb/Cu of various duration treatments. With tubulin immunolabelling and transmission electron microscopy (TEM), we found four different types of MT structures depending on the cell cycle stage: the interphase array, preprophase band, mitotic spindle and phragmoplast were typical for the control cells. Pb/Cu affected the mechanisms controlling the organization of MT cytoskeleton, and induces the following aberrations in interphase and mitotic cells. (1) Pb/Cu induced the formation of atypical MT arrays in the cortical cytoplasm of the interphase cells, consisting of skewed, wavy MT bundles, MT fragments and ring-like tubulin aggregations. (2) Pb/Cu disordered the chromosome movements carried out by the mitotic spindle. The outcome was chromosome aberrations, for example, chromosome bridges and chromosome stickiness, as well as inhibition of cells from entering mitosis. (3) Depending on the time of exposure, MTs disintegrated into shorter fragments or they completely disappeared, indicating MT depolymerization. (4) Different metals had different effects on MT organization. MTs were more sensitive to the pressure of Cu ions than Pb. Moreover, TEM observations showed that the MTs were relatively short and in some places wavy when exposed to 10−4 M Pb/Cu solutions for 1–2 h. In many sections MTs were no longer visible with increasing duration of treatment (>4 h). Based on these results, we suggested that MT cytoskeleton is primarily responsible for Pb/Cu-associated toxicity and tolerance in plants.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Both lead (Pb) and copper (Cu) are environmental contaminants. Human activity has led to high levels of Pb/Cu being accumulated from sewage sludge or urban composts, fertilizers, emissions from municipal waste incinerators, residues from metalliferous mining and the metal smelting industry (Alloway and Ayres 1994; Tuszynska et al. 2006). Pb is non-essential and potentially toxic for plants, animals and humans, whereas Cu is an essential micronutrient for all living organisms, critical to many biochemical processes, excessive accumulation is known to be toxic (Tuszynska et al. 2006). Among the negative effects, inhibition of root growth, disturbance of the antioxidant system and reduction in chlorophyll content and PSII efficiency are reported (Kastori et al. 1998; Dietz et al. 1999; Verma and Dubey 2003; Lombardi and Sebastiani 2005). Although evidence is increasing that the root tip plays a major role in heavy metal perception and response (Liu et al. 1994; Wierzbicka 1998; Liu and Kottke 2004), the mechanism of heavy metal-induced growth inhibition remains poorly understood and controversial. This phenomenon has been attributed to interactions of heavy metal with the cytoskeleton, supposedly interfering with its structure and function (Wasteneys and Galway 2003; Wasteneys 2004).
Microtubules (MTs), one of the cytoskeletal components, are composed primarily of monomeric globular polypeptides designated α- and β-tubulins. They function in essential roles ranging from cellular morphogenesis to cell movement, cell expansion, cell division, and even signal transduction (Hepler and Hush 1996; Barlow and Baluška 2000; Linda 2000; Alberts et al. 2002; Wasteneys and Galway 2003). In a living cell, MTs are dynamic and their polymerization and depolymerization is affected by many factors such as temperature, drugs or ions (Hepler and Hush 1996; Schroer 2001). Much evidence demonstrates that toxic metal interferes with cytoskeleton elements that could modify the cell architecture and cellular functions. Eun et al. (2000) examined that Pb treatment perturbed the alignment of MTs in a concentration-dependent manner beginning at 10 mM, and further suggested that MT organization in maize is a better bio-monitor for lead contamination than is micronuclei formation, since the concentration of lead destructive to MTs (10 mM) was much lower than that causing micronuclei formation (200 mM). Frantzios et al. (2000, 2001) reported that Al interfering with the MT organization disturbs the mitotic and cytokinetic process in root tip cells of Triticum turgidum. Dovgalyuk et al. (2003) found that heavy metals including Cd, Pb and Ni have a different action from Al in disrupting MTs in Allium cepa meristematic cells. Přibyl et al. (2005) investigated the interactions between cadmium ions and the cytoskeleton in interphase cells of the green alga Spirogyra decimina. Fusconi et al. (2007) elucidated that the MT cytoskeleton was highly sensitive to Cd, compared with the other different parameters used in short-term tests for environmental monitoring—apex size and viability, cell proliferation and so on. MT alterations appeared already after treatment with the lowest cadmium concentration (0.25 μM), pointing to MTs or MT-associated proteins among the main targets of this metal. These facts are suggested that MT cytoskeleton may play an important role in the mechanism of metal toxicity and tolerance in plants.
In our previous investigations, Allium sativum L. represented a favorable experimental organism that can accumulate substantial amounts of heavy metal (Cd, Cu and Pb) from solutions deposited predominantly in the root (Jiang et al. 2001; Wang et al. 2001). Moreover, heavy metal was shown to disturb mitotic processes in A. sativum meristematic cells (Liu et al. 2003/2004). Recently it has been approved that Cd interfered with the MT organization in A. sativum root tip cells (data unpublished). Considering the above information, we described in this article direct evidence of changes in the morphology and function of MT cytoskeleton in interphase and mitotic root tip cells of A. sativum following cellular Pb/Cu uptake using indirect immunofluorescence microscopy and transmission electron microscopy (TEM). The study of the Pb/Cu effects on a cellular level will contribute not only in understanding the mechanisms of heavy metal-induced cell toxicity and tolerance, but also those of plant cell division and root growth.
Materials and methods
Plant materials and treatments
Healthy and equal-sized garlic cloves were chosen from bulbs that had not started the formation of green leaves or root growth. Before commencing the experiment, the dry scales of the bulbs were removed. The bases of bulbs remained submerged in tap water at a constant temperature of about 20°C to produce roots. When roots reached about 1 cm in length, they were treated with 10−4 M Pb(NO3)2 and 10−4 M CuSO4 solutions for 0.5, 1, 2, 4, 8 and 12 h. During the treatment the roots remained immersed in the Pb/Cu solution without altering the other environmental conditions. The Pb/Cu solutions were prepared in tap water.
Indirect immunofluorescence microscopy
For tubulin immunolocalization, root tips of control and Pb/Cu treated seedlings were fixed with 4% (w/v) paraformaldehyde in phosphate-buffered saline (PBS, pH 7.0) for 1–2 h at room temperature. Root tips were subsequently washed in the same buffer before being digested with a mixture of 2.5% cellulase and 2.5% pectolase. After washing in PBS the root tips were squashed on poly-l-lysine-coated slides, left to dry and extracted in freshly prepared 1% (v/v) Triton X-100 in PBS. After rinsing in PBS, the cells were incubated at 4°C overnight or for 45 min at 37°C with the mouse monoclonal anti-α-tubulin antibody (Sigma T-9026) diluted at 1/100 in a moist, sealed chamber to prevent evaporation. After three washes in PBS for 10 min each, cells were incubated in secondary antibodies. For detection of mouse primary antibodies a 1/50 dilution of secondary fluorescein isothiocyanate (FITC) sheep anti-mouse antibody (Sigma F-0257) was used. The cells were incubated in the dark for 45 min at 37°C. Following tubulin immunolabelling, nuclei were stained with 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI) at a final concentration of 1 μg per 1 ml for 15 min at room temperature. After a brief wash in PBS, the cells were mounted in antifade agent. The slides were stored at 4°C in the dark until viewed.
The immunofluorescent specimens were examined under a fluorescence microscope (Nikon, HB-10101AF) equipped with the digital camera Pixera Pro 600CL, using violet (355–425 nm) and blue (450–490 nm) specific filters. Images were processed with Adobe Photoshop 7.0.
Electron microscopy
The excised root tips of control and Pb/Cu treated seedlings were prefixed with 2.5% (v/v) glutaraldehyde and 2% (w/v) paraformaldehyde in 0.2 M PBS (pH 7.4) for 2 h at room temperature, post-fixed in 1% (w/v) OsO4 similarly buffered for 2 h at room temperature, dehydrated in acetone series, and finally embedded in Spurr’s resin. For ultrastructure observations, ultrathin sections of 75-nm thickness were cut on an ultramicrotome-LKB VI (Sweden) with a diamond knife. The sections were examined and photographed with TEM (Phillips EM 400 ST, Holland) after double staining with 2% uranyl acetate for 50 min and lead citrate for 15 min.
Results
Macroscopic effects of Cu and Pb on root tips
The evidence from this investigation indicates that effect of Pb and Cu (10−4 M) on root tips varied with different treatment time. The morphological changes of tips treated with Cu and Pb did not appear until 8 h for both when compared with the control tips (Fig. 1a–d). During 8–12 h treatment with Pb, the root tips showed slightly brownish (Fig. 1e), and dark brown (Fig. 1f) appearance. The morphology of the root was nearly normal during the whole Cu treatment, but after 8 h the root tips were soft.
The organization of MT cytoskeleton in control cells
Four typical arrays of MTs, interphase cortical arrays, preprophase band (PPB), mitotic spindle and phragmoplast, were clearly found in control root tip cells of A. sativum, indicating that the MTs were well labeled in these cells (Fig. 2).
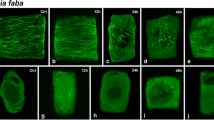
Showing the organization of microtubule cytoskeleton in control cells. DNA staining with DAPI (A1–E1, blue), tubulin immunolabelling (A2–E2, green) and merged images (A3–E3) of normal root tip cells. Bar 10 μm for all figures. A Interphase. The nucleus is surrounded by numerous cortical MTs that are orientated transversely to the long cell axis. B Early prophase. The PPB is forming around the nucleus. C Metaphase chromosome and spindles; D anaphase chromosome and spindles; E telophase chromosome and phragmoplast MTs
Cortical MTs of normal meristematic cells were very abundant during interphase. They were found roughly parallel to each other and were oriented perpendicular to the primary axis of cell expansion (Fig. 2a1–a3). In preprophase cells, the nuclear envelope remained intact. Simultaneously the cortical MTs gathered into a girdle-shaped preprophase band around the nucleus (Fig. 2b1–b3), which persisted up to the end of prophase. During mitosis, the preprophase band disappeared gradually from cortex, forming spindle and phragmoplast. In cells examined at metaphase and anaphase, specific anti-α-tubulin fluorescence was concentrated in the spindle array. To this end, spindle MTs became oriented into a bipolar array whose dyad axis divided the structure into two half spindles (Fig. 2c2, d2), and segregated sister chromatids by moving them to opposite poles (Fig. 2c1–c3, d2–d3). The phragmoplast was first seen as two opposing short bundles of MTs in the position of the equator at anaphase/telophase (Fig. 2e1–e3). At this stage MTs were oriented parallel to the longitudinal cell axis. Later, the phragmoplast expanded centrifugally until it contacted the parent cell walls, and finally formed regularly along both sides of the developing cell plate.
Pb effects on the organization of MT cytoskeleton in interphase cells
In our experiments, Pb caused change in the organization of microtubular cytoskeleton (Fig. 3a–d). Some cells displaying aberrant cortical MTs were found after 1 and 2 h treatment with Pb. In these cells microtubule organization was traversed by slightly skewed wavy (Fig. 3a2–a3). Exposure of A. sativum cells to 10−4 M Pb solution for 4 h, the cortical MTs of some cells lost their transverse organization. Instead, they were randomly oriented and often discontinuous, forming numerous short MT fragments of different size at the cell periphery. Moreover, the number of MTs decreased, resulted in their deficiency evidenced by some “large gaps” in some places (Fig. 3b–d). As shown in Fig. 3d1–d3, only small fragments of MTs were observed at the nuclear surface and cell periphery. However, most cells with a typical MT organization were present amongst cells with disarranged MTs. The proportion of abnormal MTs increased with prolonging Pb treatment time. In addition, after 12-h Pb treatment irregular nuclei in shape, nuclei condensed and fade nucleoli also occurred.
Showing Pb/Cu effects on the organization of microtubule cytoskeleton. DNA staining with DAPI (A1–E1, blue), tubulin immunolabeling (A2–E2, green) and merged images (A3–E3) of Pb/Cu-affected root tip cells. Bar 20 μm for all figures. A Discontinuous wavy MTs traverse the cortical cytoplasm (10−4 M Pb, 1 h). B and C Showing numerous MT fragments traversing the cortical cytoplasm with MT deficiency in some region (10−4 M Pb, 4 h); D showing only small MT fragments that were randomly distributed at the nuclear surface and cell periphery (10−4 M Pb, 4 h). E Showing several intensely fluorescing MT fragments appeared among a few poorly organized discontinuous MTs (10−4 M Cu, 4 h)
Showing Pb/Cu effects on the organization of microtubule cytoskeleton. DNA staining with DAPI (A1–E1, blue), tubulin immunolabeling (A2–E2, green) and merged images (A3–E3) of Pb/Cu-affected root tip cells. Bar 20 μm for all figures. A Showing many short MT fragments appearing in the cell periphery and PPB MTs cracked (10−4 M Pb, 2 h); B showing variously orientated kinetochores-MT bundles (B2) with chromosomes condensed and dispersed all over the metaphase cell (B1) (10−4 M Pb, 4 h); C showing MT segments (C2) and anaphase bridges (C1; arrow) in the anaphase cell (10−4 M Pb, 4 h); D showing MT segments (D2) and chromosome stickiness (D1; arrow) (10−4 M Pb, 4 h). E and F Showing segments only of mitotic spindles (E2, F2) with chromosomes discernible (E1 and F1) in the anaphase cell (10−4 M Cu, 2 h)
Cu effects on the organization of MT cytoskeleton in interphase cells
Interestingly, a different type of microtubular perturbation was observed in the cells exposed to Cu, and a severer antimicrotubular activity of Cu than Pb was revealed. The first interphase cells in 10−4 M Cu solution displaying an aberrant cortical MT system were found after 30-min treatment. In these cells, the MTs formed an atypical network of slightly skewed, thick, wavy and randomly aligned MT bundles among which fine and well organized MTs could be distinguished. The treatment with Cu solutions at longer time induced a much smaller fragmentation of the MT cytoskeleton (4–12 h). A significant decrease in the number of MTs was shown in the interphase root tip cells exposed to 10−4 M Cu. This decrease could be attributable either to the direct interference of MT with the depolymerisation process of tubulin or to its effect on the mechanisms controlling MT dynamics. The treated cells displayed several intensely fluorescing MT fragments among a few poorly organized discontinuous MTs (Fig. 3e1–e3).
Pb effects on the organization of MT cytoskeleton in mitotic cells
After 2-h treatment with 10−4 M Pb all kinds of abnormal MTs can be seen clearly in mitotic cells. Pb inhibited PPB maturation and induced disorganization. For instance, the cell illustrated in Fig. 4a1–a3 exhibits that many short MT fragments appeared in the cell periphery and PPB MTs cracked. Pb affected the mechanisms controlling the organization of the MT cytoskeleton as well as tubule polymerization, which delayed MT disassembly during mitosis, resulting in the disorder of spindle MTs. In metaphase cells not all of the kinetochores were arranged on the equatorial plane with their chromosomes further condensed. Some of the chromosome arms displayed variously orientated kinetochores-MT bundles and incapacity to align the kinetochores on the spindle equator. Staining of nucleic acid showed that chromosomes in these cells were not aligned at the equatorial area of the cell. Instead, they were dispersed all over the cell (Fig. 4b1–b3). Anaphases were abnormal, consisting mainly of MT segments (Fig. 4c2–c3, d2–d3), which further induced anaphase bridges (Fig. 4c1) and chromosome stickiness (Fig. 4d1). After 12-h treatment various MT arrays were depolymerized in some cells.
Cu effects on the organization of MT cytoskeleton in mitotic cells
After exposed of A. sativum cells to 10−4 M Cu solution for 30 min, the MT arrays of PPB in preprophase were distorted. This tendency became more apparent as PPB MTs split into many small fragments after 2 h of treatment. The two poles of the developing mitotic spindle were not distinct with the cell cycle advanced. It was also found that MTs of mitotic spindle were disturbed by Cu and fragments only of mitotic spindles were present; fluorescence labeling indicated that spindle individuals were no longer discernible (Fig. 4e1–e3, f1–f3).
Moreover, TEM observations showed that several rows of long and intact MTs were arranged round the cell wall and plasmalemma in control meristematic cells of A. sativum (Fig. 5a). When exposed to 10−4 M Pb/Cu solutions for 1–2 h, the MTs were relatively short, a phenomenon unusual in control cells (Fig. 5b–d). The MT arrays were more sensitive to Cu than Pb, since the number of cortical MTs in Cu solution was less and only MT fragments remained. In many sections MTs were not observed at the margins of the cell wall and plasmalemma with increasing duration of treatment (>4 h). The above fact suggested that Pb/Cu-affected interphase and mitotic cells, containing incomplete atypical MTs, which was consistent with the immunofluorescence observation. These disappeared later and the structure of MT was no longer visible, indicating MT depolymerization.
Electron micrographs of control (a) and Pb/Cu-treated (b, c, d) root tip cells of A. sativum as they appear in transverse sections. a Showing long and intact MTs arranged round the cell wall and plasmalemma in control meristematic cells of A. sativum. b, c, d Showing relatively short and wavy MTs near the cell walls. Microtubules (arrows) are located at these sites (b 10−4 M Pb, 1 h; c 10−4 M Pb, 2 h; d 10−4 M Cu, 2 h). Bar 0.25 μm for all figures. CW Cell wall, C cytoplasm, D dictyosome, ER endoplasmic reticulum, M mitochondria, MT microtubule, Ve vesicle
Discussion
As shown in Fig. 2a1–a3, MTs were localized throughout the cytoplasm and arranged perpendicular to the long axis of the interphase cell, which are commonly observed in many other plant species (Zhang et al. 1990; Hepler and Hush 1996; Inoué and Oldenbourg 1998; Vantard et al. 2000; Shamina 2005). The functions of cortical MTs associated with adaptive responses to environmental triggers, including exposure to heavy metals (Eun et al. 2000; Dhonukshe et al. 2003; Dovgalyuk et al. 2003; Pribyl et al. 2005; Fusconi et al. 2007) were discussed. After the root tip cells of A. sativum were treated with 10−4 M Pb/Cu, immunofluorescence labeling revealed obvious changes of microtubular distribution in cells (Figs. 3, 4). The effects of Pb/Cu on MTs of root tip cells varied with the different duration of treatment. With increased duration of treatment, the frequency of abnormal cells increased, and fluorescence were weaker indicating microtubule depolymerization, i.e., the appearance of many MT fragments randomly distributed in the cell after 10−4 M Pb/Cu for 4 h (Fig. 3). Similar effects were shown in A. cepa meristematic cells exposed to 50 μM Pb for 24 h (Dovgalyuk et al. 2003). However, unlike results obtained previously by fluorescence microscopy, Eun et al. (2000) found that Cu inhibited root growth of Zea mays significantly at a concentration (2 μM) that did not affect MTs, unlike Pb which disrupted MT arrays at a concentration (10 μM) lower than that causing root growth inhibition (20 μM). This experiment showed that the first visible MT changes (initial randomization) induced by 10−4 M Cu occurred after 30 min of treatment. The MT arrays in meristematic cells of A. sativum were more sensitive to Cu than Pb, since the first interphase cells in 10−4 M Cu solution displaying an aberrant cortical MT system were found after 30 min. The number of affected interphase and mitotic cells displaying atypical MT bundles was very small after 30-min Cu treatment, but dominated in root tips subjected to prolonged exposure (4–12 h). This could be explained by the different cell proportion, plant taxa and so on investigated. It seems that there is an inherent variability in the tubulin-based cytoskeletal architecture within the different taxonomic category. Thus, we might conclude that a fast response of MT cytoskeleton seems to be the general feature of heavy metal ion action.
In our experimental model Pb/Cu tested both had antimicrotubular activity of A. sativum though the toxic effect was different. Up to date, mechanisms of action of the heavy metals on the MTs in vitro and in vivo cells have not been sufficiently studied yet. In dividing root cells of maize, Pb may interact with tubulin and hinder the polymerization process at a high concentration, resulting in defective mitotic spindles, as in cells where the tubulin supply was limited (Olszewska et al. 1990; Rieder and Palazzo 1992). Here, the idea is that signaling molecules and the tubulin-associated proteins may be released to the cytoplasm and become active when MTs are depolymerized (Wasteneys 2004). This may involve MT disruption, which is likely to be associated with a sudden increase in cytosolic calcium which is essential for MT polymerization, or be stimulated by protein kinase activity (Foissner et al. 2002; Tian et al. 2004). In these situations, transmembrane protein receptors, such as integrin, binding to its ligand on cell’s surface is the first step in biological signal transduction (Reuzeau and Pont-Lezica 1995; Miller et al. 1997). Microtubular structure will be changed once the signal is received and physiological and biochemical changes are made by signal molecules, such as protein kinase C in the cell. These signal molecules will function by changing their status into active or inactive, or by phosphorylated substrate. Also, the movement of receptors on cell’s surface may be influenced in this process. MT, receptor and ion channel on cell’s surface, and tubulin related protein play very important role in cell signal transduction process. Depending on the intensity of the signal, the activation of signal transducers or repressors may be sufficient to elicit an appropriate metabolic response. The result is transient MT disassembly, which may integrate with the signaling cascade that eventually protects the plant by the production and secretion of organic acids. Finally, MTs may play a crucial role in remodeling the actin cytoskeleton (Wasteneys and Galway 2003), and this in turn, may explain how MTs regulate morphogenesis, possibly by controlling the length of cellulose microfibrils across the growth continuum. Progressive change in this microtubular alignment and related wall textures may account for the inhibition of root growth and deformation of cell shape which is a commonly observed effect in the cells after heavy metal treatment. These results provide evidence that the oriented cell growth in response to heavy metal stress requires the maintenance of intact microtubule–plasmalemma–cell wall adhesion. These findings contribute to the understanding of its toxicity in plants by illuminating one of the important cellular targets of heavy metal. In this paper we have provided evidence of a relationship between the MT cytoskeleton and heavy metal interactions. Although we do not know the nature of the signal molecules released by A. sativum prior to Pb/Cu ions, it seems that A. sativum can detect the presence of the incoming signals and change its accommodation program, particularly its MT cytoskeleton. Understanding how MT organization affects the activity and function of actin microfilaments will yield important knowledge about morphogenesis and the workings of plant cells. Future studies are in progress to define and measure the kinetics of heavy metal internalization and the role played by proteins in the medium under these controlled conditions. Whether heavy metal affects the organization of cytoskeletal elements by modifying protein phosphorylation and the cell wall structures connected to it and whether it causes disturbances of the Ca homeostasis and/or phytohormonal signaling needs to be clarified in future studies. However, Eun et al. (2000) hypothesized that the observed abnormal MTs could have resulted primarily from malfunction of organization rather than that of polymerization.
The chromosome aberrations could be the effect of Pb/Cu on DNA and RNA synthesis (Christie and Costa 1984). This explains the strong inhibition of mitosis in root tips after prolonged Pb/Cu treatments (Fig. 4b1–f1). It is well known that MTs play key roles in both nuclear division and mitosis in plants. As described above, Pb/Cu directly or indirectly affected the dynamic condition of MTs mainly by disturbing MT assembly/disassembly in dividing root tip cells of A. sativum. So it is reasonable to speculate that Pb/Cu may interact with tubulin and hinder the polymerization process, resulting in defective mitotic spindles, as in cells where the organization and function of the MTs was disturbed.
References
Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P (2002) Molecular biology of the cell. Garland Science, New York, pp 1463
Alloway BJ, Ayres DG (1994) Transport and behavior of pollutants in the environment. In: Alloway BJ, Ayres DC (eds) Chemical principles of environmental pollution. Blackie Academic & Professional, New York, pp 16–42
Barlow PW, Baluška F (2000) Cytoskeletal perspectives on root growth and morphogenesis. Annu Rev Plant Phys 51:289–322
Christie NT, Costa M (1984) In vitro assessment of the toxicity of metal compounds IV. Disposition of metals in cell: interactions with membranes, glutathione, metallothionein and DNA. Biol Trace Elem Res 6:139–158
Dhonukshe P, Laxalt AM, Goedhart J, Gadella TW, Munnik T (2003) Phospholipase D activation correlates with microtubule reorganization in living plant cells. Plant Cell 15:2666–2679
Dietz KJ, Baier M, Kramer U (1999) Free radicals and reactive oxygen species as mediators of heavy metal toxicity in plants. In: Prasad MNV, Hageneyer J (eds) Heavy metal stress in plant from molecular to ecosystems. Springer, Berlin, pp 73–98
Dovgalyuk AI, Kalynyak TB, Blumea YB (2003) Heavy metals have a different action from aluminium in disrupting microtubules in Allium cepa meristematic cells. Cell Biol Int 27:193–195
Eun SO, Youn HS, Lee Y (2000) Lead disturbs microtubule organization in the root meristem of Zea mays. Physiol Plant 110:357–365
Foissner I, Grolig F, Obermeyer G (2002) Reversible protein phosphorylation regulates the dynamic organization of the pollen tube cytoskeleton: effects of calyculin A and okadaic acid. Protoplasma 220:1–15
Frantzios G, Galatis B, Apostolakos P (2000) Aluminium effects on microtubule organization in dividing root-tip cells of Triticum turgidum I. Mitotic cells. New Phytol 145:211–224
Frantzios G, Galatis B, Apostolakos P (2001) Aluminium effects on microtubule organization in dividing root-tip cells of Triticum turgidum II. Cytokinetic cells. J Plant Res 114:157–170
Fusconi A, Gallo C, Camusso W (2007) Effects of cadmium on root apical meristems of Pisum sativum L.: cell viability, cell proliferation and microtubule pattern as suitable markers for assessment of stress pollution. Mutat Res 632:9–19
Hepler PK, Hush JM (1996) Behavior of microtubules in living plant cell. Plant Physiol 112:455–461
Inoué S, Oldenbourg R (1998) Microtubule dynamics in mitotic spindle displayed by polarized light microscopy. Mol Biol Cell 9:1603–1607
Jiang WS, Liu DH, Hou WQ (2001) Hyperaccumulation of cadmium by roots, bulbs and shoots of garlic (Allium sativum L.). Bioresource Technol 71:9–13
Kastori R, Plesnicar M, Sakac Z, Pankovic D, Arsenijevic-Maksimovic I (1998) Effect of excess lead on sunflower growth and photosynthesis. J Plant Nutr 21:75–85
Linda AA (2000) Focusing-in on microtubules. Curr Opin Struct Biol 10:241–266
Liu D, Kottke I (2004) Subcellular localization of copper in the root cells of Allium sativum by electron energy loss spectroscopy (EELS). Bioresource Technol 94:153–158
Liu DH, Jiang WS, Lu C, Zhao FM, Hao YQ, Guo L (1994) Effects of copper sulfate on the nucleolus of Allium cepa root tip cells. Hereditas 120:87–90
Liu D, Jiang W, Gao X (2003/2004) Effects of cadmium on root growth, cell division and nucleoli in root tip cells of garlic. Biol Plantarum 47:79–83
Lombardi L, Sebastiani L (2005) Copper toxicity in Prunus cerasifera: growth and antioxidant enzymes responses of in vitro grown plants. Plant Sci 168:797–802
Miller D, Hable W, Gottwald J, Ellard-Ivey M, Demura T, Lomax T, Carpita N (1997) Connections: the hard wiring of the plant cell for perception, signaling, and response. Plant Cell 9:2105–2117
Olszewska MJ, Marciniak K, Kuran H (1990) The timing of synthesis of proteins required for mitotic spindle and phragmoplast in partially synchronized root meristems of Vicia faba L. Eur J Cell Biol 53:89–92
Přibyl P, Cepák V, Zachleder V (2005) Cytoskeletal alterations in interphase cells of the green alga Spirogyra decimina in response to heavy metals exposure: I. The effect of cadmium. Protoplasma 226:231–240
Reuzeau C, Pont-Lezica RF (1995) Comparing plant and animal extracellular matrix-cytoskeleton connections—are they alike? Protoplasma 186:113–121
Rieder CL, Palazzo RE (1992) Colcemid and the mitotic cycle. J Cell Sci 102:387–392
Schroer TA (2001) Microtubules don and doff their caps: dynamic attachments at plus and minus ends. Curr Opin Struct Biol 13:92–96
Shamina NV (2005) Formation of division spindles in higher plant meiosis. Cell Biol Int 29:307–318
Tian GW, Smith D, Gluck S, Baskin TI (2004) Higher plant cortical microtubule array analyzed in vitro in the presence of the cell wall. Cell Motil Cytoskelet 57:26–36
Tuszynska S, Davies D, Turnau K, Ashford AE (2006) Changes in vacuolar and mitochondrial motility and tubularity in response to zinc in a Paxillus involutus isolate from a zinc-rich soil. Fungal Genet Biol 43:155–163
Vantard M, Cowling R, Delichère C (2000) Cell cycle regulation of the microtubular cytoskeleton. Plant Mol Biol 43:691–703
Verma S, Dubey RS (2003) Lead toxicity induces lipid peroxidation and alters the activities of antioxidant enzymes in growing rice plants. Plant Sci 164:645–655
Wang W, Liu Z, Jiang W, Liu D, Hou W (2001) Effects of copper on Allium sativum and accumulation of Cu2+ by its roots, bulbs and shoots. Acta Bot Boreal Occident Sin 21:306–312
Wasteneys GO (2004) Progress in understanding the role of microtubules in plant cells. Curr Opin Struct Biol 7:651–660
Wasteneys GO, Galway ME (2003) Remodelling the cytoskeleton for growth and form: an overview with some new views. Annu Rev Plant Phys 54:691–722
Wierzbicka M (1998) Lead in the apoplast of Allium cepa L. root tips—ultrastructural studies. Plant Sci 133:105–119
Zhang DH, Wadsworth P, Hepler PK (1990) Microtubule dynamics in living dividing plant cells: confocal imaging of fluorescent brain tubulin. Proc Natl Acad Sci USA 87:8820–8824
Acknowledgments
This project was supported by the National Natural Science Foundation of China. The authors wish to express their appreciation to the reviewers of this paper.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by E. Prinsen.
Rights and permissions
About this article
Cite this article
Liu, D., Xue, P., Meng, Q. et al. Pb/Cu effects on the organization of microtubule cytoskeleton in interphase and mitotic cells of Allium sativum L.. Plant Cell Rep 28, 695–702 (2009). https://doi.org/10.1007/s00299-009-0669-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-009-0669-3