Abstract
Background and aims
Nitrogen (N) deposition usually alters plant community structure and reduces plant biodiversity in grasslands. Seedling recruitment is essential for maintaining species richness and determines plant community composition. Arbuscular mycorrhizal fungi (AMF) are widespread symbiotic fungi and could facilitate seedling establishment. Here we conducted an experiment to address whether the influence of AMF on seedling recruitment depends on N addition and plant species.
Methods
Leymus chinensis were cultivated for 5 months in the microcosms that were inoculated with or without AMF at five N addition rates. Seeds of three main species (two C3 grasses and one non-N2-fixing forb) of the Eurasian steppe were sown to the 5-month-old microcosms. Seedling establishment was estimated by shoot biomass, N and P contents 7 weeks after seedling germination.
Results
AMF promoted seedlings recruitment of two C3 grasses at addition rates above 0.5 g N m−2. In contrast, seedling recruitment of the non-N2-fixing forb was increased by AMF at addition rates below 0.5 g N m−2 but was decreased above 2.5 g N m−2.
Conclusions
These results partly explain why N addition favored the dominance of grasses over forbs in perennial grassland communities. Our study indicates that AMF have the potential to influence plant community composition by mediating revegetation in the face of N deposition.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nitrogen (N) deposition resulting from intensive human activities has become a global concern owning to its negative effects on plant community composition and biodiversity in Europe (Bobbink et al. 1998; Stevens et al. 2004), North America (Suding et al. 2005; Clark and Tilman 2008) and the Eurasian steppe (Bai et al. 2010; Song et al. 2011). N addition generally alters the composition of plant communities by favoring fast-growing species such as grasses and reducing the abundance of forbs and legumes (Stevens et al. 2004; Bai et al. 2010). For instance, N addition increased aboveground production of grasses but decreased aboveground biomass and species richness of forbs (Song et al. 2011; Yang et al. 2012).
Although it has been widely recognized that N enrichment has negative impacts on plant communities by favoring the abundance of grasses over forbs, the interaction of N enrichment with other factors on seedling recruitment is still poorly understood. Successful recruitment into perennial grassland is difficult because of low levels of light and nutrients (Turnbull et al. 2000). Arbuscular mycorrhizal fungi (AMF) as support systems could enhance seedlings recruitment (van der Heijden 2004; Voets et al. 2009; Simard et al. 2012). However, few studies have so far investigated the effect of AMF on seedling recruitment along N deposition gradients. A better understanding of these would help us to predict the responses of plant communities to N deposition.
About 80 % of all terrestrial plant species can form symbiotic associations with AMF (Smith and Read 2008; Brundrett 2009). AMF supply nutrients to the plant and receive photosynthate in return (Smith and Read 2008; Johnson 2010). It has long been recognized that AMF can promote seedling recruitment by fast mycorrhizal colonization and by supplying soil nutrients and carbon allocation to the seedlings (Grime et al. 1987; van der Heijden 2004; Nakano-Hylander and Olsson 2007; Voets et al. 2009). Soil abiotic conditions can alter the effect of AMF on seedling recruitment. Janouskova et al. (2011) found that the extraradical hyphal network of AMF radiating from large plants depressed the growth of nearby seedlings in a nutrient-deficient substrate. However, other studies indicate that high soil nutrient availability can decrease the benefits that AMF provide to the host plant (Johnson 2010; Grman 2012). Seedlings benefitted greatly from the AMF in nutrient-poor conditions but that seedling growth was suppressed in fertile conditions. For instance, seedling growth of Plantago lanceolata was increased by AMF under nutrient-poor conditions (Grime et al. 1987; Francis and Read 1995) but was decreased when soil nutrient availability was higher (Nakano-Hylander and Olsson 2007). Whether N deposition can decrease the assistance of AMF on seedling recruitment remains unknown.
Furthermore, species identity of AMF and plants has been shown to influence the effect of AMF on seedling recruitment (Grime et al. 1987; van der Heijden 2004; Moora and Zobel 2010). For instance, Trifolium pratense seedlings were largely promoted by grassland microcosms inoculated with Glomus geosporum Walker BEG 18 and Glomus sp. BEG 21, compared to Glomus sp. isolate Basle Pi and Glomus sp. BEG 19 (van der Heijden 2004). By contrast, seedlings of Bromus erectus, Brachypodium pinnatum and Prunella vulgaris did not respond significantly to mycorrhizal identity (van der Heijden 2004). Moora and Zobel (2010) reported that the AMF established by the adult plant (host) might be less beneficial for seedlings of the host species compared with seedlings of other plant species. Furthermore, Hoeksema et al. (2010) concluded that non-N2-fixing forbs responded more positively to mycorrhizal inoculation than C3 grasses. All these results show that seedling recruitment of non-N2-fixing forbs and C3 grasses species might show differential growth response to AMF. Thus, there is a need to include different functional plant groups in order to understand the general effect of AMF on seedling recruitment.
The Inner Mongolia grasslands are dominated by perennial C3 grasses and non-N2-fixing forbs are the subdominant species, and N enrichment enhances the dominance of grasses over forbs (Bai et al. 2010; Song et al. 2011). Leymus chinensis and Stipa krylovii Roshev (C3 grasses) and Artemisia frigida Willd (non-N2-fixing forb) are widely distributed in the Eurasian steppe (Bai et al. 2010) and were selected as model species for the two plant functional groups in our study. We hypothesized that (1) N addition could decrease the assistance of AMF on seedling recruitment and (2) the effect of AMF on seedling recruitment differs among plant species of different functional groups.
Materials and methods
Experimental design
The experiment was a factorial experiment laid out as complete randomized design with three factors: seedling species, AMF and N addition. There were in total 30 treatments consisting of the combinations of two levels of AMF (with/without AMF inoculation), three levels of seedling species (L. chinensis, S. krylovii and A. frigida) and five levels of N addition (0, 10, 50, 100 and 400 mg N kg−1 soil). Each treatment was replicated 7 times. Altogether, 210 plastic pots (16 cm in diameter, 17 cm in depth) were used and each was filled with 3.2 L (3.8 kg dry weight) of a mixture of 1 mm sieved autoclaved (121 °C; 120 min) soil obtained from grassland, autoclaved sand and grass peat (10:3:1 v/v/v). The total amounts of each N addition rate given to the pot equaled 0, 0.5, 2.5, 5.0 and 20.0 g N m−2 year−1, respectively, based on previous studies investigating the effects of N addition on plant communities in the Inner Mongolia grasslands (Bai et al. 2010; Song et al. 2011). The initial nutrient contents were 0.49 g total P and 2.24 g total N kg−1 soil. Total P content was determined by ammonium molybdate method after digestion of soil by perchloric and sulphuric acids and total N content was determined by Kjeldahl method (Carter and Gregorich 2008).
Plant and fungal material
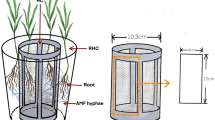
L. chinensis, S. krylovii and A. frigida were selected as model species and have been identified as arbuscular mycorrhizal plants (Bao and Yan 2004; Tian et al. 2009; Wu et al. 2009). Seeds were obtained from the native grasslands. The four AMF species used were Glomus intraradices, Glomus mosseae, Glomus versiforme and Glomus claroidum isolated from the field site, kindly provided by Professor Junling Zhang, mycorrhizal research team, College of resources and environment, China Agricultural University. The AMF isolates were propagated in a sandy soil (sieved autoclaved at 121 °C for 120 min before propagating) with red clover (Trifolium pratense L.) and maize (Zea mays L.) for 10 weeks in a glasshouse. The mycorrhizal inoculum comprised sandy soil, fungal spores (approximate 250 spores in 5 g soil), hyphae and root fragments. The AMF treatment (+AMF) was inoculated with 100 g mixture of all four AMF species (1:1:1:1). The non-AMF treatment (−AMF) was inoculated with the same amount of the autoclaved (121 °C; 120 min) mixture of these four AMF isolates. The inoculum was applied at the center of each pot (Fig. 1). Each pot received 150 mL filtered washing of grassland soil (without AMF propagules) in order to include microbial communities from natural grassland (Koide and Li 1989).
The adult plants establishment
The L. chinensis seeds were sterilized with 10 % H2O2 for 10 min, washed with sterilized water, germinated and pre-grown in autoclaved sand. Seedlings were transplanted into the center of each pot, one plant per pot. Dead seedlings were replaced by new ones during the first 2 weeks. N was added as NH4NO3 one month later. The pots were cultivated for 5 months in a greenhouse with a 20–30/15–25 °C day/night temperature regime and natural lighting, watered daily and the location was randomized every 2 weeks. The 5-month-old L. chinensis plants were defined as adults because they flowered and set seed. The adult plants were clipped (3 cm above ground level) twice during the experiment before sowing the seeds in order to simulate grazing. The hyphal length in the AMF treatment quantified by a modified membrane filter technique (Jakobsen et al. 1992) reached average values 3.2 m g−1 soil before seedling recruitment.
Seedling recruitment
Seeds of L. chinensis, S. krylovii and A. frigida, originated from their native grassland, were sterilized as described above and were sown to the seedling species treatments, respectively. There were 25 seeds sown into each pot at 5 cm distance from the center of the pot (Fig. 1). Seeds germinated within one week for L. chinensis and S. krylovii and within 10 day for A. frigida. After 2 weeks, six seedlings of the same age were left and evenly spaced in each pot (Fig. 1). The other seedlings were removed. As the adult plants were growing at the center of the pot and were gently bound by elastic bands to avoid shading, there was no competition for light between the adult plants and seedlings.
Harvesting and element measurements
The adult plants and seedlings were harvested 7 weeks after sowing the seeds. The shoots of adult plants and seedlings were cut at the soil surface and oven dried at 65 °C for 72 h before weighing. The five seedlings in each pot were combined as one replicate in order to calculate the shoot biomass and determine element concentrations. Because there was insufficient shoot biomass of seedlings to measure both N and P concentrations in one replicate, three of the seven replicates were randomly selected to determine P concentration and the others for N concentration. Shoot N concentration was measured using a FOSS Kjeltec 2300 Analyzer Unit. Shoot P concentration was determined by spectrophotometer using ammonium molybdate and ascorbic acid as color reagents, following digestion of plant tissue by nitric and perchloric acids. Shoot N and P contents were determined by multiplying plant dry weight of one pot with the N and P concentration, respectively.
Roots directly beneath the adult plant and seedlings in each pot were separated from the soil by washing and were cut into approximately 1-cm lengths. The root samples were cleared in 10 % (w/v) KOH at 90 °C in a water bath for 30 min, and then washed and stained with 0.05 % (w/v) Trypan blue. Thirty root segments of each sample were examined microscopically to assess the percentage of root length colonized by AMF according to Trouvelot et al. (1986).
Mycorrhizal growth responses (MGR) were calculated for each replicate according to the equation MGR (%) = 100 × (M − NM mean )/NM mean , where M is the shoot dry weight in the given replicate of the AMF treatment and NM mean is the mean value of dry weight in the corresponding non-AMF treatment (Janouskova et al. 2011). MGR > 0 indicates seedling establishment is promoted by AMF; MGR < 0 indicates seedling establishment is suppressed by AMF.
Statistical analyses
Three-way factorial analysis of variance (ANOVA) was used to test the effects of seedling species, AMF inoculation, N addition and their interactions on N and P contents of the adult plants. Because the factor seedling species and its interactions with the other factors were not significant, the data for the adult plants were analyzed by two-way ANOVA with the factors AMF inoculation and N addition. One-way ANOVA was used to test the effect of N addition on MGRs of the adult plants.
The N and P contents of each seedling species were analyzed separately by the two-way ANOVA with the factors AMF inoculation and N addition. The data for MGR of the seedlings were analyzed by two-way ANOVA with the factors seedling species and N addition. As no signs of AMF were detected in the non-AMF treatment, one-way ANOVA was used to test the effect of N addition on mycorrhizal root colonization of each seedling species and the adult plants in the AMF treatment. These ANOVAs were followed by a Tukey simultaneous test to detect differences between the AMF and non-AMF treatments and Duncan’s multiple-range test was used to compare the effects of seedling species or N addition for each rate. Biomass, and N and P contents data were log-transformed and the MGR data were arcsine-transformed before analyses to ensure normality (Janouskova et al. 2011). All statistical analyses were performed using SAS version 9.1 (SAS Institute, Cary, North Carolina, USA, 2002).
Results
Mycorrhizal growth responses
The MGRs of seedlings among all the N addition rates varied significantly between species (Fig. 2a; seedlings species × N addition: P < 0.01). Both the L. chinensis seedlings and adult plants showed positive MGRs across the N addition rates, with the highest at 0.5 g N m−2(Fig. 2a, b). MGR of the L. chinensis seedling was on average 47.63 ± 3.97 %, but was 24.72 ± 4.24 % for adult plants, indicating that seedlings and adult plants showed different growth responses to mycorrhizal inoculation. MGR of S. krylovii seedlings was increased by addition rates above 0.5 g N m−2 compared with zero-N addition (Fig. 2a). A. frigida showed positive MGR below 0.5 g N m−2 but N addition decreased it from positive to negative at addition rates above 2.5 g N m−2(Fig. 2a). These results suggest that establishment of A. frigida seedlings was suppressed by the mycorrhizal inoculation at addition rates above 2.5 g N m−2, but was increased for L. chinensis and S. krylovii at these N addition rates.
Mycorrhizal growth responses of the seedlings and the adult plants. a The effects of seedling species (S), N addition (N) and their interactions on the MGRs of seedlings. b The effect of N addition on the MGR of the adult plant (L. chinensis). Bar groups with different capital letters indicate a significant difference (P < 0.05) among five N addition treatments within each seedling species. Bars with different lowercase letters indicate a significant difference (P < 0.05) among three seedling species within each N addition rate. Data are means ± SE. * P < 0.05; ** P < 0.01; *** P < 0.001
N and P contents
The AMF treatment on average increased shoot N and P contents of L. chinensis seedlings compared with non-AMF treatments by 28 and 53 %, respectively (Fig. 3a, b). N addition significantly increased the shoot P and N content of L. chinensis seedlings (Fig. 3a, b; Table 1, P < 0.001). Shoot P content of S. krylovii seedlings was increased by both the mycorrhizal inoculation and N addition (Fig. 3c; Table 1, P < 0.001). Shoot N content of S. krylovii seedlings showed no significant relationship with mycorrhizal inoculation at an addition rate of zero-N and increased above 0.5 g N m−2 (Fig. 3d; Table 1, AMF × N addition: P < 0.001).
There was a significant interaction between N addition and the AMF treatment for shoot N or P content of A. frigida seedlings (Fig. 3e, f; Table 1, AMF × N addition: P < 0.001). Shoot P content was increased by inoculation with AMF at an addition rate of zero-N but was decreased above 2.5 g N m−2 (Fig. 3e). In addition, shoot N content was increased by AMF at addition rates below 0.5 g N m−2 but was decreased above 2.5 g N m−2 (Fig. 3f). The AMF treatment increased shoot N and P contents of the adult plants by 27 and 58 %, respectively (Fig. 3g, h), indicating that the seedlings and adult plants of L. chinensis obtained similar N and P benefits from mycorrhizal symbiosis and more in terms of P than N.
Mycorrhizal root colonization
Roots of the seedlings and adult plants in the non-AMF treatment were not colonized by AMF. Mycorrhizal root colonization of L. chinensis seedlings was slightly increased by N addition, and was highest at 2.5 and 20.0 g N m−2 (Fig. 4; P < 0.01). Mycorrhizal root colonization of S. krylovii seedlings was higher at addition rates of 0 and 20.0 g N m−2 than at 0.5, 2.5 and 5.0 g N m−2 (Fig. 4; P < 0.05). Mycorrhizal root colonization of A. frigida seedlings was decreased by 45 % at addition rate of 0.5 g N m−2 (Fig. 4; P < 0.05). The average mycorrhizal root colonization of adult plants was 40 ± 2 %, and was not significantly affected by N addition (Fig. 4).
Discussion
The present study showed that mycorrhizal inoculation promoted seedling recruitment of the C3 grasses, L. chinensis and S. krylovii, but reduced that of the non-N2-fixing forb A. frigida at high N addition rates. Because plant diversity of a community depends on the dynamic equilibrium between the number of lost species and the new arrivals which establish successfully (MacArthur and Wilson 1967; Tilman 1997), the suppressed establishment of non-N2-fixing forb seedlings might result in reduced plant diversity in response to N addition. Earlier studies have shown that N enrichment favored the growth of grasses over forbs and legumes in terrestrial ecosystems worldwide, often leading to biodiversity loss (Stevens et al. 2004; Suding et al. 2005; Clark and Tilman 2008; Bai et al. 2010; Song et al. 2011). The results presented here suggest that biodiversity loss might be partly explained by the different seedling establishment of C3 grasses and forbs at high N addition rates. The threshold of N addition for seedling establishment suppression of the non-N2-fixing forb was 2.5 g N m−2 year−1, consistent with the N-induced biodiversity loss at N addition rate in the range of 1.75–3.00 g N m−2 year−1 in the Eurasian grasslands (Bai et al. 2010; Song et al. 2011).
Seedling recruitment has important implications for predicting the effects of AMF on plant diversity (van der Heijden et al. 1998; van der Heijden 2004). Previous studies showed that the influence of AMF on diversity depends on the mycorrhizal responsiveness of the dominant and subordinate species (Hartnett and Wilson 1999; Collins and Foster 2009). van der Heijden et al. (2008) suggested that AMF can reduce the negative effects of N enrichment on plant diversity by enhancing the growth of the legumes and reducing the relative abundance of grasses. However, our results showed that seedling recruitment of non-N2-fixing forb (subordinate species) was suppressed by AMF, indicating that AMF may decrease the plant diversity by reducing the abundance of the subordinate species at elevated N addition rate. Consequently, AMF may influence seedling recruitment at high N addition rates, resulting in negative effects of N addition on the plant communities.
As the AMF inoculum was applied at the centre of each pot, the colonization of the seedlings germinating 5 months later in the AMF treatment was likely to result from contact with extraradical mycelium networks radiating from the roots of the adult plant, suggested by Pietikainen and Kytoviita (2007). Mycorrhizal inoculation enhanced the growth and N and P uptake of L. chinensis seedlings but reduced that of A. frigida at high N addition rates. The results suggest that the extraradical mycelium networks might favor the growth and nutrient uptake of the AMF host species seedling (L. chinensis) while it will reduce the growth of the non-host species (A. frigida). In addition, seedlings of another C3 grass, S. krylovii, were also enhanced by the extraradical mycelium network radiating from C3 grass, indicating that plant functional group might be more important than species in predicting seedling growth responses to AMF, consistent with the previous result (Hoeksema et al. 2010). The significant interaction between seedling species and N addition shows that the growth responses of seedlings to mycorrhizal inoculation are nutrient dependent. Janouskova et al. (2011) reported that the extraradical mycelium of AMF radiating from large plants depressed the growth of nearby seedlings in a nutrient-deficient substrate because of N depletion by the adult plant. However, our results suggest that mycorrhizal inoculation facilitate the establishment of A. frigida seedlings at low N addition rate and had an opposite effect at high N addition rates (Fig. 2a). van der Heijden and Horton (2009) suggested that these contrasting results might be explained by the strongest effect of AMF on plant growth at low soil fertility. Nutrient addition can induce parasitic effects of AMF on plants when soil nutrient are abundant (Johnson 2010) and the present study showed that N addition might induce parasitism of AMF (negative MGRs) on the A. frigida seedling (Fig. 2a).
Johnson (2010) reported that plants benefit more from mycorrhizal symbiosis in terms of P than N because of the high N demand of both the plant and fungal tissues. In line with this, both the L. chinensis seedlings and adults obtained more nutrient benefits in terms of P than N in the present study. In addition, the seedlings and adult plants of L. chinensis obtained similar N and P benefits from mycorrhizal symbiosis. Nevertheless, the seedlings of L. chinensis showed more positive growth response to mycorrhizal inoculations than the adult plants, indicating that N and P benefits were not consistent with MGRs. These results illustrate that mycorrhizal inoculation (extraradical mycelium network from the adult plant) may promote seedling growth by other assistances, such as supplying soil nutrients other than N and P or transferring carbon from the adult plants to the seedlings. Previous studies have proved that AMF facilitate plant growth by providing them with nutrients including N, P, zinc, copper and potassium (Smith and Read 2008), and the extraradical mycelium network can transfer nutrient and carbon from one plant to another (Walder et al. 2012). Hence, future studies should investigate the diverse assistance of the extraradical mycelium network for seedling recruitment.
The seedlings and adult plants of L. chinensis showed different growth responses to mycorrhizal inoculation, and high N addition reduced MGR, indicating that the response of plants to AMF might be age dependent (the adults and seedlings were 5 months and 7 weeks old, respectively) and depending on N addition. These were consistent with a previous study (van der Heijden 2004). Mycorrhizal root colonization of the L. chinensis seedlings and adult plants were differently affected by N addition, indicating that mycorrhizal root colonization may also be age dependent. Besides, mycorrhizal root colonization was not consistently linked with MGRs and N and P benefits from AMF in the present study, consistent with earlier studies (McGonigle 1988; Collins and Foster 2009).
Conclusions
Our results confirmed the hypothesis that N addition decreased the assistance of AMF on seedling recruitment and the effect of AMF on seedling recruitment differs in plant species. The contrasting effects of AMF on seedling establishment of C3 grasses and forbs at high N addition rates partly explained why N addition favors the dominance of grasses over forbs. These results reveal the effect of AMF on mediating revegetation in the face of N deposition and help us to predict the responses of plant communities to N deposition.
References
Bai YF, Wu JG, Clark CM, Naeem S, Pan QM, Huang JH, Zhang LX, Han XG (2010) Tradeoffs and thresholds in the effects of nitrogen addition on biodiversity and ecosystem functioning: evidence from inner Mongolia Grasslands. Global Change Biol 16(1):358–372. doi:10.1111/j.1365-2486.2009.01950.x
Bao YY, Yan W (2004) Arbuscular mycorrhizae and their structural types on common plants in grasslands of mid-western Inner Mongolia. Biodivers Sci 12(5):501–518
Bobbink R, Hornung M, Roelofs JGM (1998) The effects of air-borne nitrogen pollutants on species diversity in natural and semi-natural European vegetation. J Ecol 86(5):717–738. doi:10.1046/j.1365-2745.1998.8650717.x
Brundrett M (2009) Mycorrhizal associations and other means of nutrition of vascular plants: understanding the global diversity of host plants by resolving conflicting information and developing reliable means of diagnosis. Plant Soil 320(1–2):37–77. doi:10.1007/s11104-008-9877-9
Carter MR, Gregorich EG (2008) Soil sampling and methods of analysis, 2nd edn. CRC Press, Boca Raton
Clark CM, Tilman D (2008) Loss of plant species after chronic low-level nitrogen deposition to prairie grasslands. Nature 451(7179):712–715. doi:10.1038/nature06503
Collins CD, Foster BL (2009) Community-level consequences of mycorrhizae depend on phosphorus availability. Ecology 90(9):2567–2576. doi:10.1890/08-1560.1
Francis R, Read DJ (1995) Mutualism and antagonism in the mycorrhizal symbiosis, with special reference to impacts on plant community structure. Can J Bot 73(S1):1301–1309. doi:10.1139/b95-391
Grime JP, Mackey JML, Hillier SH, Read DJ (1987) Floristic diversity in a model system using experimental microcosms. Nature 328(6129):420–422. doi:10.1038/328420a0
Grman E (2012) Plant species differ in their ability to reduce allocation to non-beneficial arbuscular mycorrhizal fungi. Ecology 93(4):711–718. doi:10.1890/11-1358.1
Hartnett DC, Wilson GWT (1999) Mycorrhizae influence plant community structure and diversity in tallgrass prairie. Ecology 80(4):1187–1195. doi:10.1890/0012-9658(1999)080[1187:MIPCSA]2.0.CO;2
Hoeksema JD, Chaudhary VB, Gehring CA, Johnson NC, Karst J, Koide RT, Pringle A, Zabinski C, Bever JD, Moore JC, Wilson GWT, Klironomos JN, Umbanhowar J (2010) A meta-analysis of context-dependency in plant response to inoculation with mycorrhizal fungi. Ecol Lett 13(3):394–407. doi:10.1111/j.1461-0248.2009.01430.x
Jakobsen I, Abbott LK, Robson AD (1992) External hyphae of vesicular-arbuscular mycorrhizal fungi associated with trifolium-subterraneum L.1. Spread of hyphae and phosphorus inflow into roots. New Phytol 120(3):371–380. doi:10.1111/j.1469-8137.1992.tb01077.x
Janouskova M, Rydlova J, Puschel D, Szakova J, Vosatka M (2011) Extraradical mycelium of arbuscular mycorrhizal fungi radiating from large plants depresses the growth of nearby seedlings in a nutrient deficient substrate. Mycorrhiza 21(7):641–650. doi:10.1007/s00572-011-0372-4
Johnson NC (2010) Resource stoichiometry elucidates the structure and function of arbuscular mycorrhizas across scales. New Phytol 185(3):631–647. doi:10.1111/j.1469-8137.2009.03110.x
Koide RT, Li M (1989) Appropriate controls for vesicular-arbuscular mycorrhiza research. New Phytol 111(1):35–44. doi:10.1111/j.1469-8137.1989.tb04215.x
MacArthur RH, Wilson EO (1967) The theory of island biogeography. Princeton University Press, Princeton
McGonigle TP (1988) A numerical analysis of published field trials with vesicular-arbuscular mycorrhizal fungi. Funct Ecol 2(4):473–478. doi:10.2307/2389390
Moora M, Zobel M (2010) Arbuscular mycorrhiza and plant-plant interactions. Impact of invisible world on visible patterns. In: Pugnaire FI (ed) Positive plant interactions and community dynamics. CRC Press, Boca Raton
Nakano-Hylander A, Olsson P (2007) Carbon allocation in mycelia of arbuscular mycorrhizal fungi during colonisation of plant seedlings. Soil Biol Biochem 39(7):1450–1458. doi:10.1016/j.soilbio.2006.12.031
Pietikainen A, Kytoviita MM (2007) Defoliation changes mycorrhizal benefit and competitive interactions between seedlings and adult plants. J Ecol 95(4):639–647. doi:10.1111/j.1365-2745.2007.01257.x
Simard SW, Beiler KJ, Bingham MA, Deslippe JR, Philip LJ, Teste FP (2012) Mycorrhizal networks: mechanisms, ecology and modelling. Fungal Biol Rev 26(1):39–60. doi:10.1016/j.fbr.2012.01.001
Smith SE, Read DJ (2008) Mycorrhizal symbiosis, 3rd edn. Elsevier, New York
Song L, Bao X, Liu X, Zhang Y, Christie P, Fangmeier A, Zhang F (2011) Nitrogen enrichment enhances the dominance of grasses over forbs in a temperate steppe ecosystem. Biogeosciences 8(8):2341–2350. doi:10.5194/bg-8-2341-2011
Stevens CJ, Dise NB, Mountford JO, Gowing DJ (2004) Impact of nitrogen deposition on the species richness of grasslands. Science 303(5665):1876–1879. doi:10.1126/science.1094678
Suding KN, Collins SL, Gough L, Clark C, Cleland EE, Gross KL, Milchunas DG, Pennings S (2005) Functional- and abundance-based mechanisms explain diversity loss due to N fertilization. Proc Natl Acad Sci U S A 102(12):4387–4392. doi:10.1073/pnas.0408648102
Tian H, Gai J, Zhang J, Christie P, Li X (2009) Arbuscular mycorrhizal fungi associated with wild forage plants in typical steppe of eastern Inner Mongolia. Eur J Soil Biol 45:321–327. doi:10.1016/j.ejsobi.2009.05.003
Tilman D (1997) Community invasibility, recruitment limitation, and grassland biodiversity. Ecology 78(1):81–92. doi:10.1890/0012-9658(1997)078[0081:cirlag]2.0.co;2
Trouvelot A, Kough JL, Gianinazzi-Pearson V (1986) Mesure dutaux de mycorrhization VA dun systeme radiculaire. Recherche de methodes destimation ayant une signification functionnelle. In: Gianinazzi-Pearson V, Gianinazzi S (eds) Physiological and Genetic Aspects of Mycorrhizae. Paris, INRA, p 217–221
Turnbull LA, Crawley MJ, Rees M (2000) Are plant populations seed-limited? A review of seed sowing experiments. Oikos 88(2):225–238. doi:10.1034/j.1600-0706.2000.880201.x
van der Heijden MGA (2004) Arbuscular mycorrhizal fungi as support systems for seedling establishment in grassland. Ecol Lett 7(4):293–303. doi:10.1111/j.1461-0248.2004.00577.x
van der Heijden MGA, Verkade S, de Bruin SJ (2008) Mycorrhizal fungi reduce the negative effects of nitrogen enrichment on plant community structure in dune grassland. Global Change Biol 14(11):2626-2635. doi:10.1111/j.1365-2486.2008.01691.x
van der Heijden MGA, Horton TR (2009) Socialism in soil? The importance of mycorrhizal fungal networks for facilitation in natural ecosystems. J Ecol 97(6):1139–1150. doi:10.1111/j.1365-2745.2009.01570.x
van der Heijden MGA, Klironomos JN, Ursic M, Moutoglis P, Streitwolf-Engel R, Boller T, Wiemken A, Sanders IR (1998) Mycorrhizal fungal diversity determines plant biodiversity, ecosystem variability and productivity. Nature 396(6706):69–72
Voets L, de la Providencia I, Fernandez K, Ijdo M, Cranenbrouck S, Declerck S (2009) Extraradical mycelium network of arbuscular mycorrhizal fungi allows fast colonization of seedlings under in vitro conditions. Mycorrhiza 19(5):347–356. doi:10.1007/s00572-009-0233-6
Walder F, Niemann H, Natarajan M, Lehmann MF, Boller T, Wiemken A (2012) Mycorrhizal networks: common goods of plants shared under unequal terms of trade. Plant Physiol 159(2):789–797. doi:10.1104/pp. 112.195727
Wu E, Li CY, Zhang Y, Wang CM, Yun JF (2009) Effect of grassland degradation on arbuscular mycorrhizal symbiosis of Leymus chinensis(Trin.) Tzvel. in typical steppe. Acta Agrestia Sinica 17(6):731–734
Yang H, Jiang L, Li L, Li A, Wu M, Wan S (2012) Diversity-dependent stability under mowing and nutrient addition: evidence from a 7-year grassland experiment. Ecol Lett 15(6):619–626. doi:10.1111/j.1461-0248.2012.01778.x
Acknowledgments
We are grateful to two anonymous reviewers for their valuable comments on the manuscript. This project was supported by the earmarked fund for Modern Agro-industry Technology Research System (CARS-35) and National Natural Science Foundation of China (31270375).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Timothy Cavagnaro.
Lina Zhen and Gaowen Yang contributed equally to this work.
Rights and permissions
About this article
Cite this article
Zhen, L., Yang, G., Yang, H. et al. Arbuscular mycorrhizal fungi affect seedling recruitment: a potential mechanism by which N deposition favors the dominance of grasses over forbs. Plant Soil 375, 127–136 (2014). https://doi.org/10.1007/s11104-013-1950-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-013-1950-3