Abstract
Aims
Whether Arbuscular mycorrhizal fungi (AMF) influence community composition by changing plant adaptation to resource limitation remains unclear. This study examined how AMF affect the biodiversity and functional traits of plant functional groups in a shrub-dominated community in Mu Us Desert.
Methods
In a field experiment, mycorrhizae were suppressed via benomyl to address how AMF alter the adaptive strategy of plants and community structure in a shrub-dominated community. The relationship between nutrient acquisition and proxies of growth and reproduction of dominant plant functional groups (shrubs and perennial grasses) were determined using a structure equation model.
Results
The diversity and aboveground biomass of plant functional groups were not affected by benomyl treatment. Shrubs’ vegetative biomass responded negatively to AMF owing to the nitrogen (N) limitation induced by phosphorous (P) increase, whereas a positive response in reproduction was related to foliar carbon (C) accumulation for drought tolerance. The abundance and height of perennial grasses varied with AMF, and it was correlated with foliar P and N contents.
Conclusions
Different local adaptive strategies of shrubs and perennial grasses were associated with AMF through the regulation of plant nutrient acquisition. The link between AMF and plant adaptation highlights a potential mechanism underlying plant community dynamics in the resource-limited desert ecosystem.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Arbuscular mycorrhizal fungi (AMF) form symbiotic associations with the roots of most plants in terrestrial ecosystems (Smith and Read 2008), and significantly contribute to soil carbon (C) cycling and nutrient retention (Phillips et al. 2013; Van der Heijden 2010; Wilson et al. 2009). This symbiosis influence plant growth and community processes by regulating plant nutrient acquisition, resistance to pathogens, and tolerance to water deficit (Bücking and Kafle 2015; Delavaux et al. 2017; Smith and Smith 2011). The nutrient uptake and stress tolerance via mycorrhiza are considered particularly crucial for plant survival in semiarid ecosystems, which are characterised by pronounced nutrient deficiency and water stress (Asmelash et al. 2016; Smith et al. 2010). However, it remains unclear how the plant−AMF symbiosis affects plant adaptation (growth, reproduction, and survival) and community structure, particularly in desert shrublands.
Mycorrhizal fungi can influence plant community composition (Klironomos et al. 2011; Van der Heijden et al. 1998). The effect of AMF on plant diversity can be negative (Hartnett and Wilson 1999; O’Connor et al. 2002), neutral (Yang et al. 2014), or positive (van der Heijden et al. 1998), depending mainly on the responsiveness of different species or functional groups within the plant community to mycorrhiza (Janos 2007; Lin et al. 2015; Urcelay and Díaz 2003).
The effect of AMF on plant species varies among plant functional groups (Hoeksema et al. 2010; Johnson and Graham 2013). There is evidence that dicotyledonous plants generally benefit more from mycorrhiza than grasses, C4 grasses more than C3 grasses, and leguminous plants more than non-leguminous plants (Hartnett and Wilson 1999; Hoeksema et al. 2010; Johnson and Graham 2013). The response of woody species to AMF is more favourable than that of grasses and forbs, probably because woody plants are relatively slow-growing and less dependent on mycorrhiza (Yang et al. 2016). However, although some studies have reported association of AMF with grass, herb, and shrub species in the semi-arid areas of northern China (Bai et al. 2009; Chen et al. 2012; He et al. 2010), how AMF influence plant functional groups and community structure remains unclear.
Because AMF significantly affect plant nutrient uptake, they influence plant growth and survival (Asmelash et al. 2016; Smith and Read 2008; Smith et al. 2010). It is usually considered that AMF can promote plant growth by increasing the uptake of nutrients, primarily nitrogen (N) and phosphorous (P) (Smith and Read 2008; Van der Heijden et al. 2015). However, numerous studies have reported that AMF inhibit or do not affect plant growth (Smith and Smith 2011; Smith et al. 2004). Some studies suggested that the effect of AMF depends on plant and soil stoichiometry (Johnson 2010; Van der Heijden et al. 2015). Other research indicated that AMF sometimes act as ‘cheaters’ (Kiers et al. 2011). For example, symbiotic AMF can obtain a large amount of photosynthetic C from the host plant while providing little benefits to the host (Smith and Smith 2011; Van der Heijden et al. 2015; Werner et al. 2018). Despite observations of AMF effects on plant growth and nutrient uptake (Smith and Smith 2011), whether AMF can affect adaptive strategies (trade−off among growth, reproduction, and survival) of plants by altering nutrient absorption in diverse plant functional groups has not yet been tested.
In semi-arid regions, in which nutrient and water availability are co-limiting, distinguishing the relationship between plant nutrition and AMF, as well as plant functional traits as a proxy of plant adaptation are essential to understand the plant−soil interaction. The southwestern edge of the Mu Us desert is a representative transition zone between arid and semi-arid climates and between desert shrubland and grassland ecosystems (Jia et al. 2014). In this region, nutrient availability is often limited, and AMF can play a central role in resource assimilation under stress conditions (Xu et al. 2014). In the present study, we aimed to evaluate the symbiotic relationships between plants and AMF in a shrub-dominated community, and to examine whether plant−AMF interaction is a critical driver in mediating plants’ adaptive strategies. We addressed here the following two questions: (1) how AMF affect plant diversity and adaptive strategies in a shrub-dominated community in a resource-limited desert; and (2) how AMF shape the growth-reproduction trade-off of plants by regulating plant nutrient acquisition. To answer these questions, we performed a field experiment in the shrub-dominated community in the Mu Us desert using benomyl to suppress AMF and evaluated the aboveground biomass and diversity of the plant community, as well as the correlations between plant nutrition and growth performance of dominant plant functional groups. We also assessed the potential role of AMF in the shrub-dominated community dynamics.
Materials and methods
Study site
The experiment was conducted at Yanchi Research Station, Ningxia, northern China, located on the southwestern edge of the Mu Us desert (37°42′ N, 107°14′ E, 1550 m above the sea level). The site is characterised by a mid-temperate semi-arid continental monsoon climate with mean annual temperature (1954–2004) of 8.1 °C and mean annual rainfall of approximately 284.8 mm (1955–2013; most of it falling during the period from July to September) (Wang et al. 2014). The growing season in the region lasts from May to September. The soil type is quartisamment, according to the US Soil Taxonomy (Gao et al. 2014). Historically, this region was covered in dry steppe, but since the 1980s it has experienced desertification and overgrazing (Bai et al. 2018). In recent years, some ecological restoration and rehabilitation programmes were introduced and the vegetation gradually recovered, being currently characterised by extensive xeric shrubs and scattered herbaceous plants. The dominant shrub is Artemisia ordosica with Hedysarum mongolicum, Salix psammophila and Caragana korshinskii maintaining cover ranges between 30 and 70%. The dominant herbaceous species are Leymus secalinus, Ixeris chinensis, Setaria viridis, and Cynanchum thesioides (Table S1, Supplementary Material).
Experimental design
Twenty plots, 5 m × 5 m each, were established in 10 blocks (each block was approximately 12 m × 8 m and comprised two plots) at a lowland site before June 2017. Each plot was separated from the others by 1–2 m. Plots in each block were randomly assigned to one of two treatments (fungicide treatment with benomyl or control treatment without benomyl), each with 10 replicates. Benomyl (Lanfeng Biochemical Co., Ltd. in Jingsu, China), a general fungicide, is widely used to suppress AMF in field experiments (Helgason et al. 2007; Jiang et al. 2018; O’Connor et al. 2002; Yang et al. 2014; Zhang et al. 2016). The fungicide treated plots received benomyl as a soil drench (100 g of the active ingredient in 165 L of water per plot every 2 weeks). The control plots received 165 L of water per plot every 2weeks. Treatments were applied simultaneously from June to September (six times) 2017.
Plant sampling and analyses
The vegetative and reproductive twigs of A. ordosica developed during the growing season enabled testing the growth and reproduction of shrubs. A 1 m × 1 m quadrat was established in an A. ordosica patch within each plot to assess the aboveground biomass of this shrub. The dry mass of current-year twig (CYT) was used as the proxy for shrub aboveground net primary productivity (ANPP), which was estimated by using an improved non-destructive method (She et al. 2016). Briefly, current-year vegetative twig biomass (Biomassveg) and current-year reproductive twig biomass (Biomassrep) were determined based on the length and number of the vegetative and reproductive twigs [Biomassveg = Nveg × (0.029 × Lveg − 0.052); Biomassrep = Nrep × 0.0059 × Lrep1.493, where N represents the twig number and L denotes the average length of twigs in a patch]. The number of twigs and length of 10 vegetative and reproductive twigs were determined for random A. ordosica in the patch. Shrub coverage was determined along three parallel lines in each plot. The ANPP of the shrub was determined using shrub patch biomass and coverage in each plot.
The abundance and height of herbaceous plants were measured in each 1 m × 1 m quadrat established within each plot, and then averaged for each plant functional group (annual species, AS; perennial grasses, PG; perennial forbs, PF) for use in later analyses. The aboveground biomass of herbaceous plants was determined by harvesting the plants at the soil surface within the 1 m × 1 m quadrat in each plot, sorting them by functional groups, oven-drying at 80 °C for 48 h, and finally weighing each functional group. Perennial grasses, dominated by creeping grasses (L. secalinus), originated from a single original rhizome, and their height and abundance were expected to be proxies of growth and reproduction. Because AS and PF were poorly represented, these groups were excluded from foliar and root sampling as well as from further analysis.
Simultaneously, the healthy leaves of the shrub A. ordosica and perennial grass L. secalinus were sampled, oven-dried at 80 °C for 48 h, and ground to analyse total foliar C, N, and P concentrations. Carbon and N concentrations were measured using a C/N analyzer (vario EL cube CHNS Elemental Analyzer, Elementar Analysensysteme GmbH, Germany), and P was determined using inductively coupled plasma - optical emission spectrometry (iCAP 6300 spectrophotometer, Thermo Fisher Scientific, MA, USA). Three soil cores of 3.8 cm in diameter and 0–20 cm in depth were randomly collected under shrubs and PG within each plot. The roots were separated by plant functional groups (shrubs and PG) according to their colour and shape in the soil samples taken from each plot, split into two subsamples with sterile tweezers, and stored at −20 °C for the determination of AMF colonization and DNA extraction. All samples comprised 10 replicates, except for root samples. However, given the workload resulting from examining 10 replicates per sample and major concern on treatment effectiveness, we only examined five replicates per root sample per treatment, which were sufficient for statistical analyses.
Assessment of AMF root colonization
To assess the effect of benomyl on AMF colonization, root subsamples of shrubs and PG from each plot were pooled into a mixed root sample; roots within this mixed sample were cut into approximately 1-cm segments to determine AMF root colonization. Root segments were rinsed with tap water, cleared in 10% KOH (w/v) at 90 °C in a water bath for 60 min, and then washed and stained with 0.05% (w/v) trypan blue. Thirty root segments from each mixed root sample were examined under 200 × magnification to assess the percentage of the root length colonized by AMF (Trouvelot et al. 1986).
DNA extraction, polymerase chain reaction, and sequencing
Total genomic DNA was extracted from 0.25 g of each root sample using the Power Soil DNA Isolation Kit (MoBio Laboratories Inc., USA) following the manufacturer’s instructions. DNA concentration and purity were monitored on 1% agarose gels. According to the concentration, DNA was diluted to 1 ng/μL using sterile water.
Barcode primers ITS5-1737F (5′-GGAAGTAAAAGTCGTAACAAGG-3′) and ITS2-2043R (5′-GCTGCGTTCTTCATCGATGC-3′) targeting the ITS1 regions of fungal rRNA genes were used to analyse the fungal taxa (Schoch et al. 2012). All PCR reactions were performed in a total volume of 30 μL containing 15 μL Phusion® High-Fidelity PCR Master Mix (New England Biolabs, Inc., USA), 0.2 μM of each primer, and 10 ng template DNA. Thermal cycling involved an initial denaturation at 98 °C for 1 min; followed by 30 cycles of denaturation at 98 °C for 10 s, annealing at 50 °C for 30 s, and elongation at 72 °C for 60 s; with a final extension at 72 °C for 5 min. PCR products were detected by 2% agarose gel electrophoresis, and samples with bright main strip between 400 and 450 bp were chosen for further experiments. The mixed PCR products with equidensity ratios was purified with Qiagen Gel Extraction Kit (Qiagen, Inc., Germany). Sequencing libraries were generated using TruSeq® DNA PCR-Free Sample Preparation Kit (Illumina, Inc., USA) following manufacturer’s recommendations and index codes were added. The library quality was assessed on the Qubit@ 2.0 Fluorometer (Thermo Scientific, Inc., USA) and Agilent Bioanalyzer 2100 system. At last, the library was sequenced on an IlluminaHiSeq2500 platform (Illumina, Inc., USA) at Novogene (Beijing, China) and 250 bp paired-end reads were generated.
Paired-end reads were assigned to samples based on their unique barcode and truncated by cutting off the barcode and primer sequence. Paired-end reads were merged using FLASH (Magoč and Salzberg 2011), which was designed to merge paired-end reads when at least some of the reads overlap the read generated from the opposite end of the same DNA fragment, and the splicing sequences were called raw tags. Quality filtering on the raw tags were performed under specific filtering conditions to obtain the high-quality clean tags (Bokulich et al. 2013) according to the QIIME (V1.7.0, http://qiime.org/index.html) quality-controlled process. The tags were compared with the reference database (Unite Database, https://unite.ut.ee/) using UCHIME algorithm (Edgar et al. 2011) to detect chimera sequences, and then the chimera sequences were removed. Then the Effective Tags were finally obtained. Sequences with ≥ 97% similarity were assigned to the same OTUs by Uparse software (Uparse v7.0.1001, http://drive5.com/uparse/). Representative sequence for each OTU was screened for further annotation. For each representative sequence, the Unite Database (https://unite.ut.ee/) was used basing on Blast algorithm which was calculated by QIIME software (Version 1.7.0) to annotate taxonomic information. Abundance information of OTUs were normalized using a standard of sequence of sequence number corresponding to the sample with the least sequence for alpha diversity and beta diversity analysis.
Statistical analysis
To estimate the effect of benomyl treatment on the root−associated fungal community of shrubs and perennial grasses, the dissimilarity of a fungal community under various treatments was computed by non-metric multidimensional scaling (NMDS) with Bray−Curtis distance using ‘metaMDS’ function in package ‘vegan’ (Oksanen 2015). The effect of treatment on fungal community composition was evaluated by permutational multivariate analysis of variance (PERMANOVA) using ‘adonis’ function in ‘vegan.’ To determine the fungal groups (Arbuscular mycorrhizal fungi, Plant endophyte, Plant pathogen, Animal Pathogen, Saprotroph, and Others) whether were affected by benomyl treatment, FUNGuild v1.0 (http://www.stbates.org/guilds/app.php) was used to identify the fungal functional guilds (Nguyen et al. 2016).
ANOVA was used to analyse the effect of the treatment on root colonization, fungal functional guilds richness, and plant performance, such as shrub’s twigs (twig biomass, density, and length), herbaceous aboveground biomass and plant diversity (Shannon-Wiener, Simpson, and Pielou index), and foliar nutritional status (C, N, and P concentration). Plant diversity was calculated with abundance of herbaceous species in “vegan” package. The block was analysed as a random factor. Before the analysis, all data were tested for homogeneity of variances using Levene’s test and the assumption of normality using Kolmogorov−Smirnov test, and data were log-transformed to meet the assumption. The non-parametric Kruskal−Wallis test were used for data as it did not fulfil the required conditions. To illustrate the mycorrhizal function that determine by nutrient, such as N and P, the mycorrhizal response (that is, the response of plant to AMF) was calculated by using a simple response ratio as loge (AM/NM) (Johnson 2010), where AM was the value of mycorrhizal plants and NM was mean value of a nonmycorrhizal treatment (Johnson 2010; Johnson et al. 2015; Yang et al. 2018). We examined the mycorrhizal response in terms of functional traits per plant functional groups (twig length, density and biomass for shrubs; height and abundance for herbaceous plants; as well as foliar nutrient concentration for shrubs and perennial grasses). Student’s t test was applied to determine whether the log response ratio was significantly different from zero. A mycorrhizal benefit existed when the mycorrhizal response was significantly higher than zero, negative when significantly less than zero, and neutral when no significantly different from zero. Modified Thompson Tau test (delta = 0.01) were used to reduce the influence of outliers.
Given the possible effect of AMF presence (control treatment) and suppression (benomyl treatment) on plant nutrient acquisition and plant growth performance, we used structural equation model (SEM) to evaluate the relationship between plant tissue nutrient concentration (foliar N, C, and P concentration) and performance of shrubs (vegetative and reproductive biomass) as well as PG (height, abundance, and aboveground biomass). Our a priori model is shown in Supplementary Material Fig. S1. The AMF manipulation (control and benomyl treatment) was set as categorical exogenous variables with two levels: 1 and 0 (Grace 2006). Model adequacy was determined through Chi-square test (χ2; the model has a good fit when 0 ≤ χ2/ df ≤ 2 and 0.05 < P ≤ 1.00) and the root square mean error of approximation (RMSEA; the model has a good fit when 0 ≤ RMSEA ≤0.05 and 0.10 ≤ P ≤ 1.00) (Schermelleh-Engel et al. 2003). To aid with the final interpretation of the SEM, standardized total effects of the AMF and plant nutrient on plant biomass were calculated. The SEM was conducted with ‘lavaan’ package. All analyses were performed in R software (R Core Team 2017).
Results
Root colonization, fungal community, and AMF richness
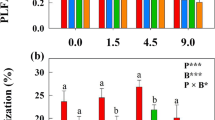
Field treatment of AMF with benomyl significantly decreased AMF root colonization (Fig. 1a; F = 38.66, P = 0.002) by approximately 40% relative to control treatment. The Illumina sequencing yielded 1,571,275 sequences for the 20 root samples, and 1,474,843 sequences (93.86% of all reads) were identified as fungal sequences (AMF, 1769 sequences). These fungal sequences were binned into 889 operational taxonomic units (OTUs) (AMF, 76 OTUs). The non-metric multidimensional scaling (NMDS) ordination of the fungal OTUs revealed that root-associated fungal community composition was significantly affected by the benomyl treatment (Fig. 1b, R2 = 0.184, P = 0.036 for treatment; R2 = 0.162, P = 0.061 for plant functional groups). Furthermore, the analysis of fungal ecological guilds using information retrieved from the FUNGuild database revealed that the Guild sequence richness of AMF in plant roots was reduced by the benomyl treatment (Fig. 2b, F = 6.57, P = 0.025 for treatment; F = 5.75, P = 0.034 for plant functional groups), but OTU richness did not change (Fig. 2a; F = 0.899, P = 0.363). Both sequence and OTU richness of other fungal groups (Plant Pathogen, Plant Endophyte, Saprotroph, Animal Pathogen and Others) were not significantly affected by the benomyl treatment (Fig. 2, all P > 0.05 for treatment).
AMF root colonization in different treatments (a), and NMDS patterns of community dissimilarities among treatments and plants determined using data on fungi OTU richness (b). The whiskers indicate the interquartile range. Solid and dashed ellipses with different colours are 95% confidence ellipses for centroids of each plant functional group under the two different treatments. Plant functional groups: shrubs and perennial grasses. Treatments: benomyl (Bnm) and control (Ctr). *, P < 0.05; **, P < 0.01; n = 5
Twig length and density and current-year biomass of shrubs
The benomyl treatment did not affect the total CYT biomass (Table 1, F = 0.657, P = 0.439). However, the twigs of shrubs were altered differently by the benomyl treatment when vegetative and reproductive twigs were evaluated separately. The fungicide treatment increased the proportion of reproductive biomass (Table 1, F = 8.958, P = 0.015), but significantly decreased the biomass and proportion of biomass of current-year vegetative twigs (Table 1, F = 5.218, P = 0.048; F = 8.958, P = 0.015). The length and density of vegetative and reproductive twigs were not significantly shifted by the benomyl treatment (Table 1, all P > 0.05). Although reproductive twig weakly responded to AMF (Fig. 3, t = 1.648, P = 0.134 for twig length; t = 0.862, P = 0.411 for twig density, and t = 1.416, P = 0.191 for CYT biomass), the response of both types of twigs was different. The vegetative twigs of the shrubs responded more negatively to AMF than the reproductive twigs (Fig. 3, t = − 2.253, P = 0.051 for twig length; t = − 2.097, P = 0.065 for twig density; and t = − 2.95, P = 0.016 for vegetative biomass). The proportion of vegetative biomass negatively responded to AMF (Fig. 3d, t = − 2.511, P = 0.033), while reproductive biomass positively responded to AMF (Fig. 3d, t = 2.402, P = 0.04).
Aboveground biomass, plant diversity, abundance, and height of herbaceous plants
The benomyl treatment did not affect the total plant biomass (Table 1, χ2 = 0.091, P = 0.762) and plant diversity index (Table 1, χ2 = 0.966, P = 0.326 for Shannon−Wiener; F = 1.463, P = 0.226 for Simpson; F = 1.178, P = 0.306 for Pielou evenness) of the herbaceous plants. The aboveground biomass of AS, PF, PG, and total herbaceous plants were not affected by the benomyl treatment (Table 1, all P > 0.05). However, responses to AMF in terms of height and abundance were different across plant functional groups. The heights of AS and PG positively responded to AMF (Fig. 4, t = 6.648, P < 0.001, and t = 9.512, P < 0.001, respectively), but PF height and PG abundance were marginally negatively affected by AMF (Fig. 4, t = − 2.055, P = 0.07, and t = − 2.124, P = 0.063, respectively); the abundance of AS and PF was not changed (Fig. 4, t = − 0.08, P = 0.94, and t = − 1.681, P = 0.127, respectively).
Plant nutrient status
Foliar P concentration of both shrubs and PG was significantly reduced by the benomyl treatment (Table 1, F = 21.076, P < 0.001; and F = 76.22, P < 0.001, respectively) while their foliar C and N concentrations showed a very different response to the treatment. The benomyl treatment increased foliar N concentration of PG (Table 1, χ2 = 12.728, P < 0.001) and marginally reduced the foliar C concentration of the shrub (Table 1, χ2 = 3.158, P = 0.076), but no significant difference was detected in the foliar N of shrubs and foliar C of PG (Table 1, F = 0.012, P = 0.914, and F = 0.0, P = 0.995, respectively). The foliar C and P of shrubs and foliar P of PG positively responded to AMF (Fig. S2, t = 4.591, P = 0.001; t = 8.018, P < 0.001; t = 5.506, P < 0.001, respectively), whereas the foliar N of PG negatively responded to AMF (Fig. S2, t = − 22.642, P < 0.001). The responses of the foliar N of shrubs and foliar C of PG to AMF were not detected (Fig. S2, t = − 0.275, P = 0.789; t = − 0.019, P = 0.985).
Correlations among plant nutrient status and plant biomass, growth, and reproduction
The SEM explained 87% and 65% of the variance in aboveground biomass of shrubs and PG, respectively, and provided a good fit based on the χ2 test and Root Mean Square Error of Approximation (RMSEA) (Fig. 5a, χ2 = 4.415, P = 0.882, RMSEA = 0.0, P = 0.898 for shrubs; Fig. 5b, χ2 = 8.525, P = 0.384, RMSEA = 0.057, P = 0.422 for PG). In shrubs, the biomass of vegetative and reproductive twig showed significant correlations with the foliar P and C contents, which were affected by AMF (Fig. 5a). Remarkably, the increased foliar P content of shrubs due to AMF reduced the positive effect of the biomass of vegetative twigs on ANPP, whereas an increase in the foliar C content promoted the effect of the biomass of reproductive twigs on ANPP. The standardized total effects derived from the SEM revealed that the ANPP of shrubs was mainly driven by vegetative biomass and foliar P, followed by reproductive biomass and foliar C (Fig. 5c). The effect of foliar P and C on the ANPP of shrubs tended to counterbalance each other (Fig. 5a). The abundance of PG showed a significant and positive correlation with aboveground biomass, whereas foliar P and N concentrations were positively and negatively associated with AMF, respectively (Fig. 5b). Particularly, increases in the foliar P concentrations of PG enhanced the positive effect of the abundance of PG and the negative effect of the height of PG on aboveground biomass, whereas reduced foliar N concentrations decreased the positive effect of PG abundance on aboveground biomass. The standardized total effects derived from the SEM revealed that the aboveground biomass of PG was mainly driven by the abundance and height of PG, followed by foliar P and foliar N concentrations (Fig. 5d). The effects of foliar P and N concentrations on the aboveground biomass of PG tended to counterbalance each other (Fig. 5b).
Structural equation model (SEM) identifying the direct and indirect effects of AMF on the ANPP of shrubs (a) and aboveground biomass of perennial grasses (b). Standardized total effects derived from the SEMs of shrubs (c) and perennial grasses (d). For simplicity, only effects with a P < 0.05 are reported. Arrow width indicates the strength of the causal effect. Numbers adjacent to arrows indicate the effect size. R2 denotes the proportion of variance explained. Colour arrows indicate positive or negative relationships. Biomassveg, biomass of vegetative twigs of shrubs; Biomassrep, biomass of reproduction twigs of shrubs. *, P < 0.05; **, P < 0.01; ***, P < 0.001; n = 10
Discussion
AMF affect the growth performance of shrubs and herbaceous plants
The benomyl treatment did not affect the ANPP of shrubs but differently affected the proportions of vegetative and reproductive biomasses (Table 1, Fig. 3), suggesting that AMF contributes to regulate the vegetative-reproductive trade-off of shrubs by restraining vegetative growth and promoting reproduction. In a desert ecosystem, characterized by water and nutrient deficiencies, AMF might perform other functions in shrubs, such as resource capture and drought resistance (Aguilar-Trigueros et al. 2017; Augé 2004) by allocating host photosynthates to mycorrhiza for nutrient and water uptake (Smith and Read 2008; Smith et al. 2010), which results in the depression of vegetative growth. Although AMF not always benefit plant vegetative growth, they contribute to stress resistance or root exudates in adverse conditions by trading off plant growth (Smith and Read 2008; Smith et al. 2009; Vannette and Hunter 2011). Recent studies have shown that plant reproductive and vegetative traits are correlated to mycorrhizal colonization (Grilli et al. 2013; Koide 2010). The uptake of limiting resources via mycorrhiza might promote vegetative and reproductive structures (Koide 2010; Smith and Read 2008). However, a study along a gradient of forest fragmentation demonstrated the negative effect of AMF on vegetative and reproductive outputs, probably because ruderal plants (Euphorbia acerensis and Euphorbia dentata) with a short life history and high efficiency in acquiring available nutrients rarely depend on AMF for plant adaptation (Grilli et al. 2013). Our results suggest that, this might be the case in our ecosystem, where growth and reproduction trade-off in the plants associated with AMF. The relationship between the allocation of resource to growth and reproduction in plants reflects adaptive strategies based on drought avoidance and competition, respectively (Ravenscroft et al. 2014). The results of foliar nutrient analyses in the shrub A. ordosica also support this finding, as AMF promoted P uptake inducing lower N/P ratios and reduced resource allocation to vegetative growth, and increased C accumulation and the allocation of resource to reproduction; both strategies are expected to contribute to plant drought tolerance (Table 1; Fig. 5a; Fig. S2a,b).
Recent studies have suggested that the effect of AMF on plant growth and reproduction is linked to successional traits of the plants (Koziol and Bever 2015, 2017). The early successional species are less responsive to mycorrhizal fungi and there is massive resource allocation to reproduction to rapidly colonise disturbed lands (Koziol and Bever 2015). In the Mu Us desert, A. ordosica is pioneer for ecological rehabilitation on bare sand, and it actively facilitates the establishment of the herbaceous community in the early stages of succession (Bai et al. 2018). In the present study, we hypothesized that AMF regulated the allocation of resources to vegetative growth and reproduction in A. ordosica for stress resistance in the early stages of succession. This hypothesis was supported by a meta-analysis study, which suggested that early successional plants associated with AMF could rapidly colonise of disturbed lands with substantial resource allocation to reproduction (Van Kleunen et al. 2010). Our results indicate that AMF might facilitate the shrub establishment in the desert environment, leading to a successful ecological rehabilitation due to cooperation of the aboveground and belowground biotic interactions in a resource-limited environment. However, we do not have a direct test of whether AMF influence species colonisation in the early successional stages. Further work is needed to assess the relationship between AMF and plant species along successional stages.
In the present study, the benomyl treatment did not significantly altered the aboveground biomass and diversity of herbaceous plants (Table 1, Fig. 4). However, our results indicated different responses to AMF in terms of the height and abundance of the plant functional groups (AS, PG, and PF). Although AMF increased the height of PG, it slightly reduced the abundance of this functional group resulting in non-significant changes in its aboveground biomass (Table 1, Fig. 4). Given the intrinsic low height of AS and PF, change in their heights did not induce differences in the aboveground biomass (Table 1, Fig. 4). In addition, the increased height of AS and slightly reduced height of PF probably led to non-significant changes in the aboveground biomass of herbaceous plants (Table 1, Fig. 4). Several studies have indicated that the effect of AMF on plant community diversity and production depends on the particular response of a specific species or functional group to AMF (Hartnett and Wilson 1999; Urcelay and Díaz 2003; Van der Heijden 2004). For example, mycorrhizal responsiveness of target and neighbouring plant species can determine the effect of AMF on the competitive outcome of plant species; this, in turn, affects plant species diversity and community composition (Klabi et al. 2014). When there are compensatory effects of AMF on plant functional groups, plant community diversity and productivity are likely maintained. For example, a recent field study in a grassland ecosystem of the Inner Mongolian steppe, suggested that suppressing AMF does not affect plant productivity and diversity due to compensatory effects between C3 grasses and non-N2-fixing forbs (Yang et al. 2014). Our results partly support this relationship in terms of the height of plant functional groups.
Inconsistently with previous studies where the relationship between AMF and AS was negative or neutral (Hoeksema et al. 2010; Lin et al. 2015), our results suggest that the relationship between AMF and AS is positive, and that the positive response observed in the height of AS to AMF was due to the strong photosynthetic capacity of this functional group dominated by S. viridis (C4 grass, Jiang et al. 2013) when sufficient quantities of C were provided to the AMF to maintain a mutualistic relationship (Lin et al. 2015; Yang et al. 2016). The marginally negative response of PF height to AMF is probably related to this species having a rhizome or alternative symbioses, such as legumes with rhizobia, which probably inhibit AMF development (Werner et al. 2018). In addition, except for the different responses of plant functional groups to AMF in terms of growth, we also found opposite effects of AMF on different functional traits, such as height and abundance of PG. Future studies should explore the relationship between functional traits of different plants incorporating AMF and its determinants.
Benomyl may present limitations because it suppresses non-target organisms (Fitter and Nichols 1988), such as pathogenic and saprotrophic fungi, but these were not affected by the benomyl treatment in the present study. Thus, we can exclude the potential influence of the pathogens and saprophytes on plant growth and reproduction. Previous field studies have shown that manipulation of AMF by benomyl could effectively suppress AMF, despite having little influence on other fungi (Helgason et al. 2007; Yang et al. 2014, 2018; Zhang et al. 2016).
Relationships between nutrient uptake and growth performance of shrubs and perennial grasses
The results of the current study show that AMF significantly promoted foliar P acquisition in shrubs, which was correlated with their reduced vegetative biomass (Fig. 5). These results imply that AMF contributes to the excess uptake of P by plants (Yang et al. 2014). Previous studies also reported that AMF could promote plant P uptake via mycorrhizal hyphae by enhancing nutrient foraging ability, regardless of the soil conditions and mycorrhizal responses (Helgason et al. 2007; Smith et al. 2011; Smith and Smith 2011). Because AMF contributes disproportionally more to P nutrition than to N nutrition leading to lower N/P ratios (Fig. S2), we observed the suppression of resource allocation to vegetative growth in shrubs. The tissue N/P ratios of plants may be a useful indicator of plant responsiveness to AMF (Johnson 2010; Johnson et al. 2015; Hoeksema et al. 2010). Interestingly, we found that AMF tended to increase the foliar C assimilation of shrubs, thereby contributing to the allocation of resource to reproduction. AMF probably increased plant water use efficiency or high-carbon content organic matter in foliar tissues for stress tolerance (Smith and Smith 2011; Zhu et al. 2012). It is possible that AMF can eliminate one resource limitation, while inducing another limitation, which results in plant stress leading to the allocation of resource to reproduction (Johnson 2010; Koide 2010). Further work is required to test whether AMF convert one resource stress to another, influencing the trade-off between plant traits in different functional groups under various environments.
In PG, foliar P concentration was also increased by AMF, while N concentration was reduced (Fig. 5, Fig. S2). Phosphate ions are often tightly bound to soil particles and have low mobility (Schachtman et al. 1998). The increased surface area provided by a symbiotic mycelium facilitates P uptake (Smith and Read 2008; Smith et al. 2011). The contribution of AMF to plant N nutrition is often negligible (Hodge and Storer 2015) or even negative (George et al. 1995) and depends on soil conditions (Giovannetti et al. 2017). In the present study, the reduced N absorption of PG due to AMF was likely related to the competition for soil-dissoluble N between AMF and grass roots. For example, the concentration of N in the external hyphae of AMF is 4–7 times greater than that of plant shoots and at least 10 times greater than that of roots, resulting in significant amounts of N immobilised in mycelia (Hodge et al. 2010). The SEM showed that increased foliar P concentrations presented positive correlations with the abundance and height of PG, while reduced foliar N concentrations were negatively associated with the abundance of PG, indicating that nutrient uptake through AMF influenced plant functional traits. The effect of increased foliar P concentration due to AMF on the aboveground biomass tended to counteract to the effect of reduced foliar N concentration due to AMF through abundance. The PG associated with AMF tended to adopt a competitive strategy by increasing individual growth rather than population expansion.
Although we observed that AMF affected the plant’s adaptive strategy by mediating nutrient uptake via the mycorrhizal pathway in the Mu Us desert, other researchers reported that AMF play a crucial role in regulating water use strategy in such dry and nutrient-poor environments (Augé 2004; Zhang et al. 2018). Further attention should be dedicated to exploring the role of AMF in altering the water use strategy of plant functional groups and in elucidating potential trade-offs between nutrient and water use strategies mediated by AMF.
Possible effects of AMF on plant community dynamics
In the present study, the shrubs associated with AMF were prone to an adaptive strategy with higher resource allocation to reproduction for stress tolerance, whereas PG preferred a competitive strategy based on individual growth. We hypothesised that the strategies of plant functional groups with different local adaptations via AMF probably drives this shrub-dominated community dynamics (Fig. 6). As a pioneer shrub in the early successional stage of bare sand land, A. ordosica associated with AMF was characterised by stress avoidance via nutrient uptake and growth trade−off, which led to increased tolerance and rapid colonisation in the early successional stage. This strategy enables the shrub to provide shelter for herbaceous plants, facilitating their subsequent growth (Bai et al. 2018). However, while PG tended to show more competitive traits than tolerance syndromes, the positive effects of the benefactor on its beneficiary can result in negative feedback effects of the beneficiary on the benefactor and reduced fitness of the benefactor (Bai et al. 2018; Schöb et al. 2014). In such a habitat, the competitiveness of PG is probably increased by AMF, and this may induce a negative feedback effect on the shrubs (Table S2). Additionally, the regeneration of A. ordosica in later succession is limited by a thick litter layer in later succession, especially from herbaceous and biological soil crusts, separating the shrub seeds from the soil (Bai et al. 2018). Our results indicate that AMF potentially help the shrubs colonise the bare land during the early successional stage rapidly, but PG associated with AMF probably caused the decline of shrubs in a later successional community (Fig. 6). Although we did not detect any changes in plant community composition and thus could not address succession, the relationships between AMF and plant functional groups, especially between shrubs and PG, added to our knowledge for predicting plant community dynamics. Long-term field experiments should test the interaction between AMF and plant community from early to late successional stages.
Conclusion
Overall, AMF manipulation did not change the aboveground biomass and plant diversity of the shrub-dominated community; however, the abundance and height of plant functional groups (AS, PG, and PF) responded differently to AMF. In particular, the growth and reproduction of shrubs and PG were regulated by AMF via altering nutrient uptake. Thus, the present study revealed the link between AMF, nutrient uptake, and plant adaptation. Further, AMF might play an essential role in driving the plant community dynamics via mediating the adaptive strategies of shrubs and PG. More importantly, the plant−AMF symbiosis is an important mechanism underlying the plant−soil interaction in a resource-limited desert ecosystem. Future studies should evaluate the effects of AMF on various plant functional traits and plant−plant interactions along environmental gradients.
Abbreviations
- AMF:
-
Arbuscular mycorrhizal fungi
- ANPP:
-
Aboveground net primary productivity
- AS:
-
Annual species
- C :
-
Carbon
- CYT:
-
Current-year twig
- N:
-
Nitrogen
- NMDS:
-
Non-metric multidimensional scaling
- OTU:
-
Operational taxonomic unit
- P:
-
Phosphorus
- PF:
-
Perennial forbs
- PG:
-
Perennial grasses
- SEM:
-
Structure equation model
References
Aguilar-Trigueros CA, Rillig MC, Crowther TW (2017) Applying allometric theory to fungi. ISME J 11:2175–2180. https://doi.org/10.1038/ismej.2017.86
Asmelash F, Bekele T, Birhane E (2016) The potential role of arbuscular mycorrhizal Fungi in the restoration of degraded lands. Front Microbiol 7:1095. https://doi.org/10.3389/fmicb.2016.01095
Augé RM (2004) Arbuscular mycorrhizae and soil/plant water relations. Can J Soil Sci 84:373–381. https://doi.org/10.4141/S04-002
Bai C, He X, Tang H, Shan B, Zhao L (2009) Spatial distribution of arbuscular mycorrhizal fungi, glomalin and soil enzymes under the canopy of Astragalus adsurgens Pall. in the Mu Us sandland, China. Soil Biol Biochem 41:941–947. https://doi.org/10.1016/j.soilbio.2009.02.010
Bai Y, She W, Michalet R, Zheng J, Qin S, Zhang Y (2018) Benefactor facilitation and beneficiary feedback effects drive shrub-dominated community succession in a semi-arid dune ecosystem. Appl Veg Sci 21:595–606. https://doi.org/10.1111/avsc.12388
Bokulich NA, Subramanian S, Faith JJ, Gevers D, Gordon JI, Knight R, Mills DA, Caporaso JG (2013) Quality-filtering vastly improves diversity estimates from Illumina amplicon sequencing. Nat Methods 10:57–59. https://doi.org/10.1038/nmeth.2276
Bücking H, Kafle A (2015) Role of arbuscular mycorrhizal Fungi in the nitrogen uptake of plants: current knowledge and research gaps. Agronomy 5:587–612. https://doi.org/10.3390/agronomy5040587
Chen Z, He X, Guo H, Yao X, Chen C (2012) Diversity of arbuscular mycorrhizal fungi in the rhizosphere of three host plants in the farming–pastoral zone, North China. Symbiosis 57:149–160. https://doi.org/10.1007/s13199-012-0186-y
Delavaux CS, Smith-Ramesh LM, Kuebbing SE (2017) Beyond nutrients: a meta-analysis of the diverse effects of arbuscular mycorrhizal fungi on plants and soils. Ecology 98:2111–2119. https://doi.org/10.1002/ecy.1892
Edgar RC, Haas BJ, Clemente JC, Quince C, Knight R (2011) UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 27:2194–2200. https://doi.org/10.1093/bioinformatics/btr381
Fitter AH, Nichols R (1988) The use of benomyl to control infection by vesicular–arbuscular mycorrhizal fungi. New Phytol 110:201–206. https://doi.org/10.1111/j.1469-8137.1988.tb00253.x
Gao G-L, Ding G-D, Zhao Y-Y, Wu B, Zhang Y-Q, Qin S-G, Bao Y-F, Yu M-H, Liu Y-D (2014) Fractal approach to estimating changes in soil properties following the establishment of Caragana korshinskii shelterbelts in Ningxia, NW China. Ecol Indic 43:236–243. https://doi.org/10.1016/j.ecolind.2014.03.001
George E, Marschner H, Jakobsen I (1995) Role of arbuscular mycorrhizal Fungi in uptake of phosphorus and nitrogen from soil. Crit Rev Biotechnol 15:257–270. https://doi.org/10.3109/07388559509147412
Giovannetti M, Volpe V, Salvioli A, Bonfante P (2017) Fungal and plant tools for the uptake of nutrients in arbuscular mycorrhizas: a molecular view. In: Mycorrhizal mediation of soil. Elsevier, Amsterdam pp 107–128
Grace JB (2006) Structural equation modeling and natural systems. Cambridge University Press, Cambridge
Grilli G, Urcelay C, Galetto L (2013) Linking mycorrhizal fungi and soil nutrients to vegetative and reproductive ruderal plant development in a fragmented forest at Central Argentina. Forest Ecol Manag 310:442–449. https://doi.org/10.1016/j.foreco.2013.08.052
Hartnett DC, Wilson GWT (1999) Mycorrhizae influence plant community structure and diversity in tallgrass prairie. Ecology 80:1187–1195. https://doi.org/10.1890/0012-9658(1999)080[1187:MIPCSA]2.0.CO;2
He X, Li Y, Zhao L (2010) Dynamics of arbuscular mycorrhizal fungi and glomalin in the rhizosphere of Artemisia ordosica Krasch. in Mu Us sandland, China. Soil Biol Biochem 42:1313–1319. https://doi.org/10.1016/j.soilbio.2010.03.022
Helgason T, Merryweather J, Young JPW, Fitter AH (2007) Specificity and resilience in the arbuscular mycorrhizal fungi of a natural woodland community. J Ecol 95:623–630. https://doi.org/10.1111/j.1365-2745.2007.01239.x
Hodge A, Storer K (2015) Arbuscular mycorrhiza and nitrogen: implications for individual plants through to ecosystems. Plant Soil 386:1–19. https://doi.org/10.1007/s11104-014-2162-1
Hodge A, Helgason T, Fitter AH (2010) Nutritional ecology of arbuscular mycorrhizal fungi. Fungal Ecol 3:267–273. https://doi.org/10.1016/j.funeco.2010.02.002
Hoeksema JD, Chaudhary VB, Gehring CA, Johnson NC, Karst J, Koide RT, Pringle A, Zabinski C, Bever JD, Moore JC, Wilson GWT, Klironomos JN, Umbanhowar J (2010) A meta-analysis of context-dependency in plant response to inoculation with mycorrhizal fungi. Ecol Lett 13:394–407. https://doi.org/10.1111/j.1461-0248.2009.01430.x
Janos DP (2007) Plant responsiveness to mycorrhizas differs from dependence upon mycorrhizas. Mycorrhiza 17:75–91. https://doi.org/10.1007/s00572-006-0094-1
Jia X, Zha TS, Wu B, Zhang YQ, Gong JN, Qin SG, Chen GP, Qian D, Kellomäki S, Peltola H (2014) Biophysical controls on net ecosystem CO2 exchange over a semiarid shrubland in Northwest China. Biogeosciences 11:4679–4693. https://doi.org/10.5194/bg-11-4679-2014
Jiang H, Barbier H, Brutnell T (2013) Methods for performing crosses in Setaria viridis, a new model system for the grasses. J Vis Exp 80:e50527. https://doi.org/10.3791/50527
Jiang S, Liu Y, Luo J, Qin M, Johnson NC, Öpik M, Vasar M, Chai Y, Zhou X, Mao L, Du G, An L, Feng H (2018) Dynamics of arbuscular mycorrhizal fungal community structure and functioning along a nitrogen enrichment gradient in an alpine meadow ecosystem. New Phytol 220:1222–1235. https://doi.org/10.1111/nph.15112
Johnson NC (2010) Resource stoichiometry elucidates the structure and function of arbuscular mycorrhizas across scales. New Phytol 185:631–647. https://doi.org/10.1111/j.1469-8137.2009.03110.x
Johnson NC, Graham JH (2013) The continuum concept remains a useful framework for studying mycorrhizal functioning. Plant Soil 363:411–419. https://doi.org/10.1007/s11104-012-1406-1
Johnson NC, Wilson GWT, Wilson JA, Miller RM, Bowker MA (2015) Mycorrhizal phenotypes and the law of the minimum. New Phytol 205:1473–1484. https://doi.org/10.1111/nph.13172
Kiers ET, Duhamel M, Beesetty Y, Mensah JA, Franken O, Verbruggen E, Fellbaum CR, Kowalchuk GA, Hart MM, Bago A, Palmer TM, West SA, Vandenkoornhuyse P, Jansa J, Bücking H (2011) Reciprocal rewards stabilize cooperation in the mycorrhizal symbiosis. Science 333:880–882. https://doi.org/10.1126/science.1208473
Klabi R, Hamel C, Raies A, Schellenberg MP, Iwaasa A, St-Arnaud M (2014) Interaction between legume and arbuscular mycorrhizal fungi identity alters the competitive ability of warm-season grass species in a grassland community. Soil Biol Biochem 70:176–182. https://doi.org/10.1016/j.soilbio.2013.12.019
Klironomos J, Zobel M, Tibbett M, Stock WD, Rillig MC, Parrent JL, Moora M, Koch AM, Facelli JM, Facelli E, Dickie IA, Bever JD (2011) Forces that structure plant communities: quantifying the importance of the mycorrhizal symbiosis. New Phytol 189:366–370. https://doi.org/10.1111/j.1469-8137.2010.03550.x
Koide RT (2010) Mycorrhizal symbiosis and plant reproduction. In: Arbuscular mycorrhizas: physiology and function. Springer, Dordrecht, pp 297–320
Koziol L, Bever JD (2015) Mycorrhizal response trades off with plant growth rate and increases with plant successional status. Ecology 96:1768–1774. https://doi.org/10.1890/14-2208.1
Koziol L, Bever JD (2017) The missing link in grassland restoration: arbuscular mycorrhizal fungi inoculation increases plant diversity and accelerates succession. J Appl Ecol 54:1301–1309. https://doi.org/10.1111/1365-2664.12843
Lin G, McCormack ML, Guo D (2015) Arbuscular mycorrhizal fungal effects on plant competition and community structure. J Ecol 103:1224–1232. https://doi.org/10.1111/1365-2745.12429
Magoč T, Salzberg SL (2011) FLASH: fast length adjustment of short reads to improve genome assemblies. Bioinformatics 27:2957–2963. https://doi.org/10.1093/bioinformatics/btr507
Nguyen NH, Song Z, Bates S, Branco S, Tedersoo L, Menke J, Schiling JS, Kennedy PG (2016) FUNGuild: An open annotation tool for parsing fungal community datasets by ecological guild. Fungal Ecol 20:241–248. https://doi.org/10.1016/j.funeco.2015.06.006
O’Connor PJ, Smith SE, Smith FA (2002) Arbuscular mycorrhizas influence plant diversity and community structure in a semiarid herbland. New Phytol 154:209–218. https://doi.org/10.1046/j.1469-8137.2002.00364.x
Oksanen J (2015) Vegan: an introduction to ordination. URL http://cran.r-project.org/web/packages/vegan/vignettes/introvegan.pdf 8:19. Accessed 16 May 2018
Phillips RP, Brzostek E, Midgley MG (2013) The mycorrhizal-associated nutrient economy: a new framework for predicting carbon-nutrient couplings in temperate forests. New Phytol 199:41–51. https://doi.org/10.1111/nph.12221
R Core Team (2017) R: a language and environment for statistical computing. https://www.r-project.org/. Accessed 30 Jun 2017
Ravenscroft C, Fridley JD, Grime JP (2014) Intraspecific functional differentiation suggests local adaptation to long-term climate change in a calcareous grassland. J Ecol 102:65–73. https://doi.org/10.1111/1365-2745.12168
Schachtman DP, Reid RJ, Ayling SM (1998) Phosphorus uptake by plants: from soil to cell. Plant Physiol 116:447–453. https://doi.org/10.1104/pp.116.2.447
Schermelleh-Engel K, Moosbrugger H, Müller H (2003) Evaluating the fit of structural equation models: tests of significance and descriptive goodness-of-fit measures. Methods Psychol Res Online 8:23–74
Schöb C, Callaway RM, Anthelme F, Brooker RW, Cavieres LA, Kikvidze Z, Lortie CJ, Michalet R, Pugnaire FI, Xiao S, Cranston BH, García M, Hupp NR, Llambí LD, Lingua E, Reid AM, Zhao L, Butterfield BJ (2014) The context dependence of beneficiary feedback effects on benefactors in plant facilitation. New Phytol 204:386–396. https://doi.org/10.1111/nph.12908
Schoch CL, Seifert KA, Huhndorf S, Robert V, Spouge JL, Levesque CA, Chen W, Fungal Barcoding Consortium (2012) Nuclear ribosomal Internal Transcribed Spacer (ITS) region as a universal DNA barcode marker for fungi. Proc Natl Acad Sci U S A 109:6241–6246. https://doi.org/10.1073/pnas.1117018109
She W, Zhang Y, Qin S, Wu B, Bai Y (2016) Increased precipitation and nitrogen Alter shrub architecture in a desert Shrubland: implications for primary production. Front Plant Sci 7:1908. https://doi.org/10.3389/fpls.2016.01908
Smith SE, Read DJ (2008) Mycorrhizal symbiosis. Academic Press, Cambridge
Smith SE, Smith FA (2011) Roles of arbuscular mycorrhizas in plant nutrition and growth: new paradigms from cellular to ecosystem scales. Annu Rev Plant Biol 62:227–250. https://doi.org/10.1146/annurev-arplant-042110-103846
Smith SE, Smith FA, Jakobsen I (2004) Functional diversity in arbuscular mycorrhizal (AM) symbioses: the contribution of the mycorrhizal P uptake pathway is not correlated with mycorrhizal responses in growth or total P uptake. New Phytol 162:511–524. https://doi.org/10.1111/j.1469-8137.2004.01039.x
Smith FA, Grace EJ, Smith SE (2009) More than a carbon economy: nutrient trade and ecological sustainability in facultative arbuscular mycorrhizal symbioses. New Phytol 182:347–358. https://doi.org/10.1111/j.1469-8137.2008.02753.x
Smith SE, Facelli E, Pope S, Smith FA (2010) Plant performance in stressful environments: interpreting new and established knowledge of the roles of arbuscular mycorrhizas. Plant Soil 326:3–20. https://doi.org/10.1007/s11104-009-9981-5
Smith SE, Jakobsen I, Gronlund M, Smith FA (2011) Roles of arbuscular mycorrhizas in plant phosphorus nutrition: interactions between pathways of phosphorus uptake in arbuscular mycorrhizal roots have important implications for understanding and manipulating plant phosphorus acquisition. Plant Physiol 156:1050–1057. https://doi.org/10.1104/pp.111.174581
Trouvelot A, Kough JL, Gianinazzi-Pearson V (1986) Mesure du taux de mycorhizationVA dun systeme radiculaire. Recherche de methodes destimation ayant une signification fonctionnelle. In: GianinazziPearson V, Gianinazzi S (eds) Physiological and genetical aspects of mycorrhizae. INRA Press, Paris, pp 217–221
Urcelay C, Díaz S (2003) The mycorrhizal dependence of subordinates determines the effect of arbuscular mycorrhizal fungi on plant diversity. Ecol Lett 6:388–391. https://doi.org/10.1046/j.1461-0248.2003.00444.x
Van der Heijden MGA (2004) Arbuscular mycorrhizal fungi as support systems for seedling establishment in grassland. Ecol Lett 7:293–303. https://doi.org/10.1111/j.1461-0248.2004.00577.x
Van der Heijden MGA (2010) Mycorrhizal fungi reduce nutrient loss from model grassland ecosystems. Ecology 91:1163–1171. https://doi.org/10.1890/09-0336.1
Van der Heijden MGA, Klironomos JN, Ursic M, Moutoglis P, Streitwolf-Engel R, Boller T, Wiemken A, Sanders IR (1998) Mycorrhizal fungal diversity determines plant biodiversity, ecosystem variability and productivity. Nature 396:69–72. https://doi.org/10.1038/23932
Van der Heijden MGA, Martin FM, Selosse MA, Sanders IR (2015) Mycorrhizal ecology and evolution: the past, the present, and the future. New Phytol 205:1406–1423. https://doi.org/10.1111/nph.13288
Van Kleunen M, Weber E, Fischer M (2010) A meta-analysis of trait differences between invasive and non-invasive plant species. Ecol Lett 13:235–245. https://doi.org/10.1111/j.1461-0248.2009.01418.x
Vannette RL, Hunter MD (2011) Plant defence theory re-examined: nonlinear expectations based on the costs and benefits of resource mutualisms. J Ecol 99:66–76. https://doi.org/10.1111/j.1365-2745.2010.01755.x
Wang B, Zha TS, Jia X, Wu B, Zhang YQ, Qin SG (2014) Soil moisture modifies the response of soil respiration to temperature in a desert shrub ecosystem. Biogeosciences 11:259–268. https://doi.org/10.5194/bg-11-259-2014
Werner GDA, Cornelissen JHC, Cornwell WK, Soudzilovskaia NA, Kattge J, West SA, Kiers ET (2018) Symbiont switching and alternative resource acquisition strategies drive mutualism breakdown. Proc Natl Acad Sci U S A 115:5229–5234. https://doi.org/10.1073/pnas.1721629115
Wilson GWT, Rice CW, Rillig MC, Springer A, Hartnett DC (2009) Soil aggregation and carbon sequestration are tightly correlated with the abundance of arbuscular mycorrhizal fungi: results from long-term field experiments. Ecol Lett 12:452–461. https://doi.org/10.1111/j.1461-0248.2009.01303.x
Xu P, Liang L-Z, Dong X-Y, Xu J, Jiang P-K, Shen R-F (2014) Response of soil phosphorus required for maximum growth of Asparagus officinalis L. to inoculation of arbuscular mycorrhizal fungi. Pedosphere 24:776–782. https://doi.org/10.1016/S1002-0160(14)60064-3
Yang G, Liu N, Lu W, Wang S, Kan H, Zhang Y, Xu L, Chen Y (2014) The interaction between arbuscular mycorrhizal fungi and soil phosphorus availability influences plant community productivity and ecosystem stability. J Ecol 102:1072–1082. https://doi.org/10.1111/1365-2745.12249
Yang H, Xu J, Guo Y, Koide RT, Dai Y, Xu M, Bian L, Bian X, Zhang Q (2016) Predicting plant response to arbuscular mycorrhizas: the role of host functional traits. Fungal Ecol 20:79–83. https://doi.org/10.1016/j.funeco.2015.12.001
Yang R, Li S, Qin Z, Cai X, Li X, Christie P, Zhang J, Feng G, Gai J (2018) Importance of AM fungi and local adaptation in plant response to environmental change: field evidence at contrasting elevations. Fungal Ecol 34:59–66. https://doi.org/10.1016/j.funeco.2018.04.006
Zhang B, Li S, Chen S, Ren T, Yang X, Zhao H, Liang Y, Han X (2016) Arbuscular mycorrhizal fungi regulate soil respiration and its response to precipitation change in a semiarid steppe. Sci Rep 6:19990. https://doi.org/10.1038/srep19990
Zhang F, Zou Y-N, Wu Q-S (2018) Quantitative estimation of water uptake by mycorrhizal extraradical hyphae in citrus under drought stress. Sci Hortic 229:132–136. https://doi.org/10.1016/j.scienta.2017.10.038
Zhu XC, Song FB, Liu SQ, Liu TD, Zhou X (2012) Arbuscular mycorrhizae improves photosynthesisand water status of Zea mays L. under drought stress. Plant Soil Environ 58:186–191. https://doi.org/10.17221/23/2011-PSE
Acknowledgements
This study was funded by the National Key Research and Development Program of China (Grant no. 2016YFC0500905), the National Natural Science Foundation of China (Grant no. 31800611), and the Fundamental Research Funds for the Central Universities (Grant no. 2015ZCQ-SB-02). We would like to thank Shijun Liu, Jie Fu, Liang Liu, and Yongqi Sun for their help with field work.
Data availability
The raw sequences datasets generated during the current study were submitted to the NCBI repository (www.ncbi.nlm.nih.gov/bioproject/PRJNA540163) and are accessible in the BioProject PRJNA540163.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible Editor: Tatsuhiro Ezawa.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 34069 kb)
Rights and permissions
About this article
Cite this article
Qiao, Y., Bai, Y., Zhang, Y. et al. Arbuscular mycorrhizal fungi shape the adaptive strategy of plants by mediating nutrient acquisition in a shrub-dominated community in the Mu Us Desert. Plant Soil 443, 549–564 (2019). https://doi.org/10.1007/s11104-019-04253-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-019-04253-0