Abstract
Background and aims
The pH of the growth medium influences Cu speciation in solution, the negativity of plasma membrane (PM) surface potential, and hence the rhizotoxicity of Cu.
Methods
Solution culture experiments were conducted with wheat (Triticum aestivum L.) seedlings to examine the toxicity of various Cu species at pH values ranging from 4.50 to 8.25. The toxic species of Cu was identified, giving particular consideration to the electrical properties at the plant cell membrane and ion activities at the PM surface.
Results
The solution culture studies showed that at pH < 6.60 (i.e., free Cu2+ >95 % of total Cu), the addition of cations (Ca2+ or H+) decreased the toxic effects of Cu by decreasing the negativity of the PM surface potential (and hence decreasing the activity of Cu2+ at the PM surface). For solutions with pH values from 7.50 to 8.25 (CuCO 03 >50 % of total Cu), an increase in pH significantly enhanced the toxicity of Cu, whilst the addition of Ca had negligible influence on toxicity.
Conclusions
Root growth in solution cultures was influenced primarily by the surface activities of free Cu2+ and CuCO 03 . Across all experiments, the data indicate that it was CuCO 03 , rather than CuOH+, that contributed Cu toxicity over pH > 7.00. Although our data do not explore the mechanism of toxicity, we propose that CuCO 03 has an important role in Cu rhizotoxicity in alkaline growth media.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Copper is an essential micronutrient required for normal plant growth and development. However, at elevated concentrations, it is also highly toxic to plant growth potentially resulting in complete inhibition of growth. Some contaminated soils have been reported to contain more than 1,000 mg Cu kg−1 (Kabata-Pendias and Pendias 2001; Luo et al. 2006). Most Cu in soil is present in strongly bound forms with the soil solids, with relatively low levels in the soil solution (and potentially bioavailable forms) (Luo et al. 2006). Of the Cu in the soil solution, Cu is present as the free Cu2+ ion and other species bound with inorganic (e.g., CO 2−3 , OH−) or organic ligands (Sauvé et al. 1997). Solution pH has an important influence on the distribution of Cu species. For example, in the Cu2+-H+-CO 2−3 system in equilibrium with atmospheric CO2, the free Cu2+ ion is the dominant species (>95 %) at pH < 6.60, whilst at pH > 7.50 CuCO 03 is the dominant Cu species (Powell et al. 2007; Millero et al. 2010).
It is well-established that the free Cu2+ ion (the activity of which is denoted as {Cu2+}) is the form available to organisms, as described by the free ion activity model (FIAM) (Campbell 1996). The effect of pH on the rhizotoxicity of Cu has been studied extensively in nutrient solutions at pH values <7.0. For these solutions (i.e. <7.0), an increase in pH (decrease in {H+}) has been reported to increase the rhizotoxicity of Cu to wheat (Triticum aestivum) (Parker et al. 1998; Luo et al. 2008; Wang et al. 2008), cowpea (Vigna unguiculata) (Kopittke et al. 2011a), and barley (Hordeum vulgare) (Lock et al. 2007). Recent analyses have demonstrated that this increased toxicity can be attributable to an increased activity of Cu2+ at the plasma membrane (PM) surface. Hereafter, the activity of Cu species M in the bulk solution is designated as {MZ}b while its activity at the outer PM surface is {MZ}0, where Z is the charge of the ion. Increasing pH can polarize the PM and increase the negativity of electrical potential at the outer surface of the PM (ψ 0), and thereby increase {Cu2+}0 and increase toxicity (Kinraide 2006; Wang et al. 2008; Kinraide and Wang 2010; Kopittke et al. 2011a).
Much less information is available regarding Cu toxicity in alkaline solutions (pH > 7.0) (which is important, for example, in the alkaline soils contaminated by Cu-containing fungicides (Poschenrieder et al. 2001; Komárek et al. 2010)). Interestingly, studying root elongation of barley (H. vulgare), Wang et al. (2009) reported that the EC50 for total Cu decreased three-fold as pH increased from 6.5 to 8.0. Similarly, Lock et al. (2007) reported that Cu-containing solutions were substantially more toxic to roots of barley at pH 7.7 than at pH 6.9 (and this increased toxicity was not associated with increased uptake of Cu). Over this pH range (ca. 7 to 8), changes in ψ 0 due to changes in H+ activity are too small (<1 mV) to account for increases in toxicity. However, it is noteworthy that the speciation of Cu changes substantially across these pH values and species other than Cu2+ dominate. This raises the possibility that Cu-species other than Cu2+ are toxic. Wang et al. (2009) attributed this enhanced toxicity to CuOH+. In another study with Daphnia, De Schamphelaere and Janssen (2002) also proposed intoxication from CuOH+. However, the study of the toxicity of pH-dependent, ligand-bound metals (e.g., CuOH+, CuHCO +3 , or CuCO 03 ) is difficult due to problems of inter-correlation among variables (including pH) and the added complication of changes in PM surface potentials (Kinraide 2006).
The aim of the present study was to identify the toxic ionic species of Cu, giving particular consideration to the electrical properties at the plant cell membrane and ion activities at the PM surface. Treatments were designed in order to minimise inter-correlation among the activities of the various species in the bulk culture media or at the PM surface. Furthermore, Ca was added to solutions to reduce the negativity of ψ 0 and thereby allow differentiation between Cu species with different valencies (through changing their activities at the PM surface).
Materials and methods
Solution culture design
Experiments were conducted in solution culture to study the effects of Cu speciation on toxicity over a wide range of pH values (4.50–8.25). Experiment 1 was designed to investigate the effect of pH on Cu toxicity when the total Cu concentrations were maintained constant. Solutions were arranged in a factorial design with eleven pH values (4.50, 5.00, 5.50, 6.00, 6.50, 7.00, 7.25, 7.50, 7.75, 8.00, and 8.25), three Ca(NO3)2 concentrations (0.25, 1.00, and 4.00 mM), and four Cu(NO3)2 concentrations (0–1.50 μM).
Experiment 2 was designed to investigate Cu toxicity in alkaline solutions by holding the activity of Cu2+ constant whilst varying the activity of other Cu-species. Toxicity was examined at four pH values (7.50, 7.75, 8.00, and 8.25), with {Cu2+}b being held constant at 0.064 μM by varying the total Cu(NO3)2 concentration at two Ca(NO3)2 concentrations (0.50 and 8.00 mM). With these treatments, the {Cu2+}0 was also constant (ca. 0.18 or 2.39 μM) given that the negativity of ψ 0 changed by <0.5 mV as pH increased from 7.5 to 8.25. Therefore, this experiment held {Cu2+}0 constant whilst activities of the other Cu species (i.e., CuCO 03 , CuOH+, CuHCO +3 ) varied in both the bulk solution and at the PM surface. For instance, {CuCO 03 }0 (which is equal to {CuCO 03 }b since it has a valency of zero) ranged from 0 to 2.53 μM.
Experiment 3 examined whether Ca alleviates Cu toxicity at pH 8.00 (as is observed in acidic solutions). Seven Ca concentrations (0.25 to 10.0 mM) were examined at pH 8.00 with the bulk Cu2+ activity being held constant at either 0.48 or 0.96 μM (with the corresponding CuCO 03 activity constant at 0.60 or 1.20 μM). An increase in Ca reduced the ψ 0 negativity, resulting in a decrease in the activities of Cu species with a positive charge (i.e., Cu2+, CuHCO +3 and CuOH+) at the PM surface, whilst changes in the activity of CuCO 03 were negligible as it is an uncharged ion-pair.
Solution pH values were maintained constant by the use of buffers. At pH ≤ 6.00, MES (2-2-[N-morpholino] ethane sulfonic acid, 2.0 mM) was used as a buffer, whilst at pH > 6.00, pH was maintained using MOPS (3-[N-morpholino] propane sulfonic acid, 3.6 mM) and addition of NaOH. For solutions with a pH < 5.50, HNO3 was used for pH adjustment. Both buffers do not form complexes with Cu (Kandegedara and Rorabacher 1999; Lock et al. 2007) and did not affect Cu toxicity to plants at the concentrations used in this study. Solution pH was measured both at the start and upon completion of each experiment, and the concentrations of Ca, Cu and Na were determined by inductively coupled plasma-atomic emission spectroscopy (ICP-AES; POEMS-II, TJA, USA).
For all three experiments, 2 d old wheat (T. aestivum L. cv. Yangmai 14) seedlings with uniform root length (1–2 cm) were cultured in darkness for 48 h at 25 °C in acid-washed polyethylene beakers containing 500 mL of continuously aerated test medium. For each treatment series (i.e. different pH or Ca concentration), a control without Cu was included. The treatments were replicated twice with seven seedlings per replicate. At the completion of the experiment, the two longest roots of each seedling were measured and the mean of the 14 measured values per replicate were recorded. Further details of the growth experiments and precautions for the preparation of the test solutions have been presented previously (Wang et al. 2008, 2011a).
Cu speciation
The accuracy of the equilibrium constants used to compute the activities of the Cu species may influence the conclusions regarding the identification of the toxic species. Table 1 presents ten sets of equilibrium constants compiled from various sources. It is noteworthy that there is no consensus regarding the stability constants for Cu complexes with OH−, which appear to depend largely upon the ionic strength (Paulson and Kester 1980; Santana-Casiano et al. 2008). For example, Paulson and Kester (1980) reported a value of −7.99 at an ionic strength of 0.05 M but −7.20 at 3.0 M. The modelling for the present study used the recommended and provisional stability constants from the International Union of Pure and Applied Chemistry (IUPAC) technical report (Powell et al. 2007), which are very close to two new measurements (Santana-Casiano et al. 2008; Millero et al. 2010). All thermodynamic modeling in the nutrient solutions was conducted using WHAM 6.5 with the stability constants of Cu complexes of IUPAC (Table 1). For the solution cultures, the equilibrium phases included atmospheric CO2 (PCO2 = 10−3.5 atm).
Ion surface activity at the PM surface
Values for ψ 0 were calculated using the Gouy-Chapman-Stern (GCS) model (computer program available from the authors) with the average of the measured pH values and other cation concentrations in each treatment. The GCS model combines electrostatic theory (Gouy-Chapman theory) with ion binding (Stern model) so that ψ 0 can be computed (Kinraide et al. 1998; Tatulian 1999; Yermiyahu and Kinraide 2005; Kinraide and Wang 2010). This model incorporates the intrinsic surface charge density (σ0) of a membrane, the ion composition of the bulk medium (BM), and ion binding to the membrane (see Yermiyahu and Kinraide (2005) and Kinraide and Wang (2010)). Knowledge of ψ 0 enables the calculation of ion activities at the PM surface. The activity of ion I Z at the PM surface ({I Z}0) is computed from the activity of I Z in the bulk-phase medium ({I Z}b) according to the Nernst equation:
where Z, F, R, and T are the charge on the ion, the Faraday constant, the gas constant, and temperature, respectively (F/(RT) = 1/25.7 at 25 °C when ψ 0 is expressed in mV).
Analysis of root elongation
Root elongation was evaluated as relative root elongation (RRE, %). The RRE was calculated using the formula:
where RL T represents the mean root length (RL) in the presence of Cu, RL C represents RL in the corresponding Cu-free control, and RL S represents RL in toxicant sufficient to saturate growth-inhibitory processes. The RL S is nearly equal to RL at the time of seedling transfer to the test media. When growth responds to measures of toxicant intensity (T, such as the activity (μM) of free Cu2+ activity or other species in the bulk or at the PM surface), the resulting curves can be expressed by the following equation:
where a 1 (μM−1) is a strength coefficient that increases with the strength of the metal toxicity, and β 1 (dimensionless) is a shape coefficient (Taylor et al. 1991; Wang et al. 2008, 2011a; Kopittke et al. 2011a). It is noteworthy that sometimes large differences in tolerance are observed among plant species. The differences in the a 1 and β 1 coefficients for Eq. 3 may denote differences in sensitivity (Wang et al. 2011b).
Statistics
All coefficients in equations were evaluated by multiple, non-linear regression analysis using SYSTAT 13 (Cranes Software International Ltd, India). Significance levels are P < 0.05 for all reported regression and coefficients. Root mean square error (RMSE) is also given to estimate how close the predictions are to the observations.
Results
Effect of pH on Cu speciation in the absence of DOM
Figure 1 shows a speciation diagram for the Cu2+-H+-CO 2−3 system from pH 4.00 to 8.50, based on the recommended stability constants of IUPAC (Martell et al. 1997) (Table 1). For solutions at pH < 6.60, the free Cu2+ ion accounts for >95 % of the total Cu. However, for solutions at pH > 6.60, the contribution of Cu2+ to the total Cu species decreases sharply. At pH > 7.50, CuCO 03 is the dominant species with CuOH+ also accounting for approximately 12.6 % of the total Cu at pH 7.50. CuHCO +3 formation constants are sufficiently small that CuHCO +3 is unlikely to be a significantly environmental species (CuHCO +3 for <0.37 % of the total Cu, Fig. 1) under normal environmental conditions. It can, however, become a significant species in condition with high levels of bicarbonate (Powell et al. 2007). It should be noted that CuHCO +3 maybe possibly contributes to Cu toxicity in some calcareous soils.
Speciation of Cu as function of pH in solution containing a total of 1.0 μM Cu(NO3)2 and 1.0 mM Ca(NO3)2. The speciation was modelled using the equilibrium constants of IUPAC (Powell et al. 2007, Table 1) in equilibrium with atmospheric CO2 (pCO2 = 10−3.5 atm). Only the five most abundant species are presented
Effects of pH and Ca on Cu toxicity
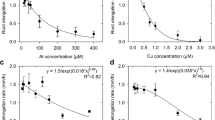
In the range of pH from 4.50 to 6.50, increases in Ca2+ or H+ (i.e. decreases in pH) alleviated Cu toxicity, but there was no significant relationship between RRE and {Cu2+}b (P > 0.05) (Fig. 2). The Ca- or H-induced alleviation of Cu toxicity resulted not from specific competition (also see Wang et al. 2012 for detail), but from a non-specific reduction in the negativity of ψ 0 which decreased {Cu2+}0 (and hence decreased inhibition of root growth). Indeed, RRE was significantly related to {Cu2+}0 (R 2 = 0.823; P < 0.001, Fig. 2) irrespective of solution pH and Ca concentration, indicating that the effects of Ca2+ and H+ in this pH range on Cu toxicity were attributed to the electrostatic effect of ψ 0. Using the fitted curves, an EA50{Cu2+}0 value (i.e., the effective PM activity of Cu2+ causing a 50 % inhibition in growth) can be derived as 15.6 μM. This value is similar to the value of 14.1 μM for wheat (Wang et al. 2008), and higher than 4.4 μM {Cu2+}0 for cowpea (Kopittke et al. 2011a). Thus, ion activities at the PM surface are more appropriate for examining plant-ion interactions than are bulk activities.
The data indicate that in the pH range of 6.50 to 8.25 (where species such as CuCO 03 become increasingly important, see Fig. 1 and discussion above), species other than Cu2+ probably also contribute to toxicity. For example, for solutions in which the activity of Cu2+ was held constant (but total Cu was increased due to the formation of other species), toxicity increased as pH increased; an increase in pH from 7.50 to 8.25 decreased RRE by 60 %, despite a constant {Cu2+}b of 0.064 μM (Fig. 3).
So which of the other Cu-species formed in alkaline solutions is toxic? Interestingly, in Experiment 2 when solution pH was varied (and free Cu2+ ion was maintained constant by varying the total Cu), root elongation correlated with {CuCO 03 }0 (R 2 = 0.980, P < 0.001) more closely than to {CuOH+}0 (R 2 = 0.884, P < 0.001), or {Cu2+}0 (P > 0.05) (Fig. 4). These results regarding the toxicity of CuCO 03 were extended by Experiment 3, which investigated the effect of Ca on Cu toxicity in solutions at pH 8.00. In this experiment, the addition of up to 10 mM Ca (which decreased ψ 0 negativity from −55.2 to −10.9 mV) had no significant impact on root growth in Cu-containing solutions at pH 8.00 (P > 0.05) (Fig. 5), which was in contrast to the alleviatory-effect of Ca observed in acid solutions where Cu2+ dominates (Fig. 2). This observation supports the proposed toxicity of the CuCO 03 species as being uncharged its activity at the PM surface is not influenced by the addition of depolarizing cations and the associated changes in the negativity of ψ 0. Furthermore, it is noteworthy that RREs were related to {CuCO 03 }0 (R 2 = 0.986, P < 0.001), but not to {Cu2+}0 or {CuOH+}0 (P > 0.05) in these solutions at pH 8.00 (Fig. 6). These results suggest that the increased Cu rhizotoxicity caused by an increase in pH of that range resulted from increases in {CuCO 03 }0, rather than {CuOH+}0.
Modelling RRE in solution culture
Across all three experiments, no relationships were found between RRE and any single Cu species using Eq. 3 (P > 0.05 for regression or coefficients). Equation 3, therefore, was modified to incorporate the toxicity of multiple Cu species (e.g., Cu2+ and CuCO 03 ) (see Kinraide 1999):
In this equation, the value of the coefficient a 12 represents the relative rhizotoxicity of the Cu species {CuCO 03 }0 relative to the rhizotoxicity of the Cu species {Cu2+}0. For example in Eq. 4, an a 12 value of 10 means that {CuCO 03 }0 is 10-times more toxic than {Cu2+}0. Fitting all data (Experiments 1–3) to this equation yielded an R 2 value 0.912 (cf. R 2 of 0.858 when considering {Cu2+}b and {CuCO 03 }b), with a 1 = 0.021, a 12 = 56.1, and β 1 = 0.419 (Table 2, Fig. 7). The value of a 12 indicates that the same level of growth inhibition as defined by {CuCO 03 }0 required 56.1 times of {Cu2+}0. Interestingly, when species {CuCO 03 }0 was replaced with {CuOH+}0, no significant coefficient (P > 0.05) was obtained for a 12 .
The relative root elongation (RRE) as functions of the activity of CuCO 03 and Cu2+ at the plasma membrane surface ({CuCO 03 }0, {Cu2+}0). Regression coefficients are presented in Table 2
Discussion
At pH values <6.50, the addition of cations (Ca2+ or H+) to the bathing solution tended to alleviate the rhizotoxicity of Cu2+. Similar results have been reported previously (Parker et al. 1998; Kinraide 2006; Lock et al. 2007; Wang et al. 2008, 2012; Kopittke et al. 2011a). This alleviatory effect can be attributed to non-specific changes in {Cu2+}0 (Kinraide 2006; Wang et al. 2008; Kopittke et al. 2011b). The addition of these cations reduces the negativity of ψ 0 and thereby decreases {Cu2+}0.
In the pH range from 6.50 to 8.25, an increase in pH significantly enhanced Cu toxicity (due to changes in Cu species), whilst the addition of Ca, (contributing substantially to changes in the negativity of ψ 0) did not influence root growth in these Cu-containing solutions (Figs. 3 and 5). Based on the following evidence, we propose that it is CuCO 03 , rather than CuOH+, that contributes substantially to Cu toxicity at pH > 6.50:
-
(i)
RRE in Experiment 2 decreased sharply across the entire pH range from 7.50 to 8.25 (Fig. 3), in accordance with the changes in speciation for CuCO 03 , (rather than for CuOH+, for which there is a maximum in formation at pH 7.5, see Fig. 1).
-
(ii)
RRE in Experiment 3 was dependent only on CuCO 03 at pH 8.00, not on {Cu2+} or {CuOH+} (Fig. 6).
-
(iii)
High Ca (up to 10.0 mM) alleviated the toxicity of Cu2+, but had no influence on toxicity at pH 8.00 (therefore suggesting that the toxic ion was uncharged, CuCO 03 ).
-
(iv)
RRE in Experiment 2 correlated better with {CuCO 03 }0 (R 2 = 0.980) than with {CuOH+}0 (R 2 = 0.884) or {Cu2+}0 (P > 0.05) (Fig. 4).
-
(v)
Across all three solution culture experiments, Eq. 4 yielded a significant coefficient (P < 0.05) for {CuCO 03 }0 but not for {CuOH+}0 (P > 0.05, Table 2). An equation incorporating both {Cu2+}0 and {CuCO 03 }0 explains 92.7 % of variation of RRE in these experiments.
-
(vi)
Analysis of RRE vs. ion species with different equilibrium constants (Table 1) also confirmed that RRE depends upon the formation of both {Cu2+}0 and {CuCO 03 }0 (Table 2).
As noted in the Introduction, the toxicity of ionic species other Cu2+ has been reported previously. Interestingly, CuCO 03 has been reported previously to be toxic in aquatic species, including in rainbow trout (Salmo gairdneri Richardson) (Shaw and Brown 1974). Similarly, De Schamphelaere et al. (2002) included CuCO 03 (and CuOH+) in a revised biotic ligand model for Daphnia magna. Furthermore, in studying Cu toxicity to plants, both Wang et al. (2009) and Lock et al. (2007) reported that the values of EC50{Cu2+}b for barley root were increased significantly when the pH increased in high-pH range from about 7.0 to 8.0, and indicated that ionic species other than Cu2+ were toxic to roots of barley. Wang et al. (2009) attributed this toxicity to CuOH+, and stated that CuOH+ was more toxic than Cu2+ due to the stronger binding of CuOH+ at the biotic ligand. The authors used the Cu stability constants from NIST (Table 1). By using these constants, it is estimated that concentrations of CuOH+ are higher than those of CuCO 03 for solutions at pH < 7.50, and that CuOH+ accounts for approximately 28.5 % of the total Cu at pH 7.50 (Fig. S1). They included CuOH+ in the BLM construct (see Eq. 7 of Wang et al. 2009) and found the linear relationship between CuOH+/Cu2+ and 1/EC50{Cu2+}b (Fig. 5 in that article). Actually, if CuOH+ is replaced with CuCO 03 in Eq. 7 of that study, a linear relationship is also derived between CuCO 03 /Cu2+ and 1/EC50{Cu2+}b (R 2 = 0.98). Similarly, a value of 0.336 is obtained for KCuCO3BL/KCuBL. The values for (Cu2+ + 2.92 × CuOH+) are significantly linear with the values for (Cu2+ + 0.336 × CuCO 03 ) (R 2 = 0.83). Given this high correlation, RRE should be also highly correlated with the values for (Cu2+ + 0.336 × CuCO 03 ), indicating that CuCO 03 could also interpret the enhanced toxicity observed in Wang et al. (2009). However, the attribution to CuOH+ toxicity cannot explain the fact that RRE almost did not change with {CuOH+}0 varying from 0.09 to 0.89 μM in the present study, provided that {CuCO 03 } is held constant (Fig. 6; Experiment 3).
So how are these various Cu-species toxic? The free Cu2+ ion binds very strongly to soft ligands (Soft Ligand Scale of 0.88) but comparatively weakly to hard ligand (High Ligand Scale of −0.99) (Kinraide 2009). Recent in situ synchrotron-based experiments using hydrated root tissues of cowpea have demonstrated that the intoxication of Cu2+ results in accumulation of the Cu in the rhizodermis and outer cortex (Kopittke et al. 2011b), possibly inhibiting loosening of the cell wall as part of the elongation process (Kopittke et al. 2008, 2009). Additionally, a study examining Cu transport showed that more than 99.7 % of total Cu in tomato and chicory xylem sap was in a bound form (Liao 2000). However, the observation that CuCO 03 is also toxic (and, in fact, more toxic than Cu2+) was unexpected given that CuCO 03 is an uncharged ion-pair which is considered to bind only weakly to physiologically active sites. According to the biotic ligand model, CuCO 03 is toxic by binding to the biotic ligand, although the binding constant of CuCO 03 for the biotic ligand was defined as being 10-fold lower than for Cu2+ (De Schamphelaere et al. 2002). We suggest, however, that CuCO 03 may be transported easily through the apoplast (in contrast to Cu2+) and possibly be taken up as the uncharged ion-pair (as observed for the uptake of undissociated H3BO3 by plant roots by mass flow). An alternative explanation would be that CuCO 03 complex is not strong, therefore may dissociate at the membrane surface where Cu2+ transporter proteins are located. Because of neutrality, CuCO 03 is much more easily transported through the apoplast to the membrane surface (followed by dissociation and uptake of Cu2+), therefore appear to be more toxic. Exploring the mechanisms by which CuCO 03 is toxic requires further investigation.
Some of the foregoing discussions are based on the assumption that the equilibrium constants (from IUPAC, Table 1) used in this study are valid. The equilibrium constants used to compute the activities of the Cu species may influence the conclusions regarding the identification of the toxic species. Actually, the Cu species calculated using the three sets of constants are highly correlated (R 2 > 0.98). A sensitivity analysis was conducted which demonstrated that using the constants from the default database of WHAM (same as Visual MINTEQ 3.0) and NIST produced similar results (see Table 2), indicating CuCO 03 toxicity in alkaline solutions (i.e., significant values were obtained for coefficient ‘a 12’), with the values for ‘a 12’ as large as those obtained based on the constants of IUPAC. This study focused on the root PM. A question of the study’s validates may be raised if the sites of Cu intoxication lie within the cell wall rather than the PM? It should be noted, however, that the electrostatic characteristics of the cell wall are similar to the PM (Shomer et al. 2003; Kinraide 2004).
References
Baes CF, Mesmer RE (1981) The thermodybamics of cation hydrolysis. Am J Sci 281:935–962
Campbell PGC (1996) Metal speciation and bioavailability in aquatic systems. Wiley, New York
De Schamphelaere KAC, Janssen CR (2002) A biotic ligand model predicting acute copper toxicity for Daphnia magna: the effects of calcium, magnesium, sodium, potassium, and pH. Environ Sci Technol 36:48–54
De Schamphelaere KAC, Heijerick DG, Janssen CR (2002) Refinement and field validation of a biotic ligand model predicting acute copper toxicity to Daphnia magna. Comp Biochem Phys C 133:243–258
Kabata-Pendias A, Pendias H (2001) Trace elements in soils and plants. CRC Press, Boca Raton
Kandegedara A, Rorabacher DB (1999) Noncomplexing tertiary amines as “better” buffers covering the range of pH 3–11. Temperature dependence of their acid dissociation constants. Anal Chem 71:3140–3144
Kinraide TB (1999) Interactions among Ca2+, Na+ and K+ in salinity toxicity: quantitative resolution of multiple toxic and ameliorative effects. J Exp Bot 50:1495–1505
Kinraide TB (2004) Possible influence of cell walls upon ion concentrations at plasma membrane surfaces. Toward a comprehensive view of cell-surface electrical effects upon ion uptake, intoxication, and amelioration. Plant Physiol 136:804–3813
Kinraide TB (2006) Plasma membrane surface potential (ψ PM) as a determinant of ion bioavailability: a critical analysis of new and published toxicological studies and a simplified method for the computation of plant ψ PM. Environ Toxicol Chem 25:3188–3198
Kinraide TB (2009) Improved scales for metal ion softness and toxicity. Environ Toxicol Chem 28:525–533
Kinraide TB, Wang P (2010) The surface charge density of plant cell membranes (σ): an attempt to resolve conflicting values for intrinsic σ. J Exp Bot 61:2507–2518
Kinraide TB, Yermiyahu U, Rytwo G (1998) Computation of surface electrical potentials of plant cell membranes. Correspondence to published Zeta potentials from diverse plant sources. Plant Physiol 118:505–512
Komárek M, Čadková E, Chrastný V, Bordas F, Bollinger JC (2010) Contamination of vineyard soils with fungicides: a review of environmental and toxicological aspects. Environ Int 36:138–151
Kopittke PM, Blamey FPC, Menzies NW (2008) Toxicities of soluble Al, Cu, and La include ruptures to rhizodermal and root cortical cells of cowpea. Plant Soil 303:217–227
Kopittke PM, McKenna BA, Blamey FPC, Wehr JB, Menzies NW (2009) Metal-induced cell rupture in elongating roots is associated with metal ion binding strengths. Plant Soil 322:303–315
Kopittke PM, Kinraide TB, Wang P, Blarney FPC, Reichman SM, Menzies NW (2011a) Alleviation of Cu and Pb rhizotoxicities in cowpea (Vigna unguiculata) as related to ion activities at root-cell plasma membrane surface. Environ Sci Technol 45:4966–4973
Kopittke PM, Menzies NW, de Jonge MD, McKenna BA, Donner E, Webb RI, Paterson DJ, Howard DL, Ryan CG, Glover CJ, Scheckel KG, Lombi E (2011b) In situ distribution and speciation of toxic Copper, Nickel, and Zinc in hydrated roots of cowpea. Plant Physiol 156:663–673
Liao M (2000) Mechanisms of copper uptake and transport in plants. Massey University, New Zealand
Lindsay W (1979) Chemical equilibrium in soils. Wiley, New York
Lock K, Criel P, De Schamphelaere KAC, Van Eeckhout H, Janssen CR (2007) Influence of calcium, magnesium, sodium, potassium and pH on copper toxicity to barley (Hordeum vulgare). Ecotox Environ Safe 68:299–304
Luo XS, Zhou DM, Wang YJ (2006) Free cupric ions in contaminated agricultural soils around a copper mine in eastern Nanjing City, China. J Environ Sci-China 18:927–931
Luo XS, Li LZ, Zhou DM (2008) Effect of cations on copper toxicity to wheat root: implications for the biotic ligand model. Chemosphere 73:401–406
Martell AE, Smith RM, Motekaitis RJ (1997) Critical stability constants of metal complexes database, Version 4.0. NIST Standard Reference Database 46; National Institute of Standards and Technology, Gaithersburg, MD
Millero FJ, Santana-Casiano JM, Gonzalez-Davila M (2010) The formation of Cu(II) complexes with carbonate and bicarbonate ions in NaClO4 solutions. J Solution Chem 39:543–558
Parker DR, Pedler JF, Thomason DN, Li HY (1998) Alleviation of copper rhizotoxicity by calcium and magnesium at defined free metal-ion activities. Soil Sci Soc Am J 62:965–972
Paulson AJ, Kester DR (1980) Copper(II) ion hydrolysis in aqueous solution. J Solution Chem 9:269–277
Poschenrieder C, Bech J, Llugany M, Pace A, Fenés E, Barceló J (2001) Copper in plant species in a copper gradient in Catalonia (North East Spain) and their potential for phytoremediation. Plant Soil 230:247–256
Powell KJ, Brown PL, Byrne RH, Gajda T, Hefter G, Sjoberg S, Wanner H (2007) Chemical speciation of environmentally significant metals with inorganic ligands—Part 2: the Cu2+-OH−, Cl−, CO 2−3 , SO 2−4 , and PO 3−4 systems—(IUPAC technical report). Pure Appl Chem 79:895–950
Santana-Casiano JM, Gonzalez-Davila M, Millero FJ (2008) The examination of the activity coefficients of Cu(II) complexes with OH− and Cl− in NaClO4 using pitzer equations: application to other divalent cations. J Solution Chem 37:749–762
Sauvé S, McBride MB, Norvell WA, Hendershot WH (1997) Copper solubility and speciation of in situ contaminated soils: effects of copper level, pH and organic matter. Water Air Soil Pollut 100:133–149
Shaw TL, Brown VM (1974) The toxicity of some forms of copper to rainbow trout. Water Res 8:377–382
Shomer I, Novacky AJ, Pike SM, Yermiyahu U, Kinraide TB (2003) Electrical potentials of plant cell walls in response to the ionic environment. Plant Physiol 133:411–422
Tatulian SA (1999) Surface electrostatics of biological membranes and ion binding. Marcel Dekker, New York, pp 871–922
Taylor GJ, Stadt KJ, Dale MRT (1991) Modelling the phytotoxicity of aluminum, cadmium, copper, manganese, nickel, and zinc using the Weibull frequency distribution. Can J Bot 69:359–367
Tipping E, Rieuwerts J, Pan G, Ashmore MR, Lofts S, Hill MTR, Farago ME, Thornton I (2003) The solid-solution partitioning of heavy metals (Cu, Zn, Cd, Pb) in upland soils of England and Wales. Environ Poll 125:213–225
Wang P, Zhou DM, Kinraide TB, Luo XS, Li LZ, Li DD, Zhang HL (2008) Cell membrane surface potential (ψ 0) plays a dominant role in the phytotoxicity of copper and arsenate. Plant Physiol 148:2134–2143
Wang XD, Ma YB, Hua L, McLaughlin MJ (2009) Identification of hydroxyl copper toxicity to barley (Hordeum vulgare) root elongation in solution culture. Environ Toxicol Chem 28:662–667
Wang P, Kinraide TB, Zhou DM, Kopittke PM, Peijnenburg WJGM (2011a) Plasma membrane surface potential: dual effects upon ion uptake and toxicity. Plant Physiol 155:808–820
Wang P, Kopittke PM, De Schamphelaere KAC, Zhao FJ, Zhou DM, Lock K, Ma YB, Peijnenburg WJGM, McGrath SP (2011b) Evaluation of an electrostatic toxicity model for predicting Ni2+ toxicity to barley root elongation in hydroponic cultures and in soils. New Phytol 192:414–427
Wang P, De Schamphelaere KAC, Kopittke PM, Zhou DM, Peijnenburg WJGM, Lock K (2012) Development of an electrostatic model predicting copper toxicity to plants. J Exp Bot 63:659–668
Yermiyahu U, Kinraide TB (2005) Binding and electrostatic attraction of trace elements to plant root surfaces. In: Huang PM, Gobran GR (eds) Biogeochemistry of trace elements in the rhizosphere. Elsevier, Amsterdam, pp 365–389
Acknowledgments
This work was supported financially by the National Natural Science Foundation (grant no. 31172034).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Henk Schat.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 62 kb)
Rights and permissions
About this article
Cite this article
Wang, P., Menzies, N.W., Wang, YM. et al. Identifying the species of copper that are toxic to plant roots in alkaline nutrient solutions. Plant Soil 361, 317–327 (2012). https://doi.org/10.1007/s11104-012-1260-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-012-1260-1