Abstract
Aims
We assessed the effects of native and exotic tree leaf litter on soil properties in two contrasting scenarios. The native Quercus robur and Pinus pinaster tree species coexist with the aliens Eucalyptus globulus and Acacia dealbata in acid soils of NW Spain. The native trees Fraxinus angustifolia and Ulmus minor coexist with the aliens Ailanthus altissima, Robinia pseudoacacia and Ulmus pumila in eutrophic basic riparian soils in Central Spain.
Methods
Four plastic trays per species were filled with homogenized top-soil of the site and covered with leaf litter. Before and after 9 months of incubation, litter mass, soil pH, organic matter, mineral and total N were measured. Available mineral N (NO −3 -N and NH +4 -N) was assessed every 2 months.
Results
Soil biological activity was higher in the basic than in the acid soil. Litter of the exotic trees tended to decompose less than litter of native species, probably due to the presence of secondary metabolites in the former. Soil pH, mineral and total N responded differently to different litter types, irrespective of their exotic or native origin (acid soil), or was similar across litter treatments (basic riparian soil). The similar response of the basic soil to the addition of different litter types may be due to the low contrast of litter quality between the species. E. globulus litter inhibitied soil microbial activity much more than the rest of the studied litter types, leading to a drastic impoverishment of N in soils.
Conclusion
Litter of exotic N-fixing trees (A. dealbata and R. pseudoacacia) did not increase soil N pools because of the inhibition of microbial activity by secondary compounds. Therefore, secondary metabolites of the litter played a major role explaining exotic litter impact on soil properties.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Changes in species composition or abundance driven by exotic plant introductions may alter many ecosystem functions, such as disturbance regime (Grigulis et al. 2005; Levine et al. 2003; Mack and D’Antonio 1999), water cycle (Ehrenfeld 2003; Le Maitre et al. 1996; Tennesen 2008; Ohte et al. 2003) or nutrient cycles (Vitousek and Walker 1989; Ehrenfeld 2003; Gomez-Aparicio and Canham 2008; Vanderhoeven et al. 2005). Nutrient availability, particularly nitrogen, limits primary production in many terrestrial ecosystems (Schlesinger 1991; Bowman et al. 1993; Soudzilovskaia and Onipchenko 2005) and affects the diversity of plant communities (Stevens et al. 2010; Gilliam and Dick 2010; Bai et al. 2007). Therefore, any alteration of the N cycle because of exotic plant invasion may also alter the ecosystem services provided by plant communities.
The introduction of exotic plants may alter the nutrient cycle of the system either directly, by modifying the quality and quantity of litter entering the soils beneath, or indirectly, by altering the physical-chemical site properties below their canopy (Tateno et al. 2007; Yelenik et al. 2007; Vitousek and Walker 1989; Follstad Shah et al. 2010). Both site and litter properties affect the structure and activity of macro-detritivores and microbial decomposers (Hawkes et al. 2005; Mack et al. 2001; Mayer et al. 2005), controlling litter decomposition and organic matter mineralization rates (Cornelissen and Thompson 1997; Gallardo and Merino 1993, 1992; Xiong et al. 2008), and finally affecting nutrient availability for plants.
Previous information suggests that exotic invasive plants tend to speed up litter decomposition and increase nutrient pools readily available for plant uptake (reviewed in Ehrenfeld 2003; Liao et al. 2008). This trend accords with the opportunistic resource-acquisition strategy frequently reported among exotic invasive plants (Grotkopp and Rejmánek 2007; Pattison et al. 1998; Baruch and Bilbao 1999), which involves leaf traits typically associated with high decomposition rate, i.e. low leaf mass per area, high N content, low investment in secondary metabolites, etc. (Lake and Leishman 2004; Cornelissen and Thompson 1997). However, it is not clear the extent to which this trend derives from a case-selection bias (i.e. many studies report the effects of N-fixing species invading N-poor soils (Vitousek and Walker 1989; Yelenik et al. 2007; Stock et al. 1995; Ley and D’Antonio 1998)) and/or publication bias over-representing the most significant results. The few studies performed on the Mediterranean Basin showed that exotic invasive plants have little effect on N-related soil properties (Vilà et al. 2006; Castro-Díez et al. 2009), or they even tend to slow down litter decomposition (Godoy et al. 2010). These contrasting results suggest that the effects of exotic invasive plants on the N cycle largely depend on the interaction between the functional properties of the exotic plant (N-fixing ability, lignin content, growth rate, etc.) and the properties of the invaded ecosystems (soil properties, climate, etc.). Most studies comparing exotic and native litter decomposition and mineralization have been performed in the field using reciprocal incubation designs (Castro-Díez et al. 2009; Ehrenfeld et al. 2001; Ashton et al. 2005; Rothstein et al. 2004). However, soil conditions are difficult to replicate due to the inherent high variability of soil properties at small scales (Castro-Díez et al. 2009; Gallardo and Parama 2007), blurring the effects of the exotic plants on N cycling processes.
In this study we aimed to isolate the effects of litter type on soil properties by means of a laboratory experiment, where different types of exotic and native litter were incubated in field-collected standard soils, under standard microclimatic conditions. We simulated two contrasting scenarios of invasion that occur in the Iberian Peninsula: 1) A temperate forest in NW Spain, developed on an acid soil and dominated by Quercus robur in the mature stage, and 2) a mixed riparian forest growing on eutrophic basic soils in central Spain, under continental Mediterranean climate. In the first scenario Q. robur has been largely displaced by forestry plantations of the native Pinus pinaster and the exotic Eucalyptus globulus. Both species are able to sustain stable populations in the region with no human assistance (Sanz Elorza et al. 2004). In addition, the N-fixing exotic tree Acacia dealbata, which was introduced for ornamental and agricultural purposes, has also naturalized in the region (Sanz Elorza et al. 2004). The four species can be found growing close to each other. The second scenario is dominated by native riparian trees, such as Populus nigra, P. alba, Fraxinus angustifolia and Ulmus minor. However, in disturbed places, near roads, crops or towns, these species coexist or have been displaced by exotic trees, such as Ailanthus altissima, Robinia pseudoacacia or Ulmus pumila (Sanz Elorza et al. 2004). All exotic trees of the study have been reported in Spain no longer than two centuries ago (Sanz Elorza et al. 2004), and the studied populations are not older than 30–50 years.
We address the following questions: Does the exotic litter tend to decompose faster and to increase soil N pools, as suggested by the literature, irrespective of the species and site? If not, is there any other consistent effect of all exotics in each site? Is there a correlation between litter properties and changes in soil and N cycle properties? Given the contrasting functional traits across exotic species we expect them to cause different effects on soil and N cycle properties (hypothesis 1). Given that the top soil largely derives from the decomposition of the incoming litter, and that native trees have been adding litter to the soil for a much longer time than exotic trees, we expect local soil properties to be closer to an equilibrium with the native litter, but not with the recently introduced exotic litter; therefore we expect exotic litter to alter the local soil properties more than native litter (hypothesis 2). Finally, litter decomposition is expected to be negatively related with leaf mass per area and positively with litter N content (hypothesis 3).
Materials and methods
Litter, leaf and soil collection
Between October and November 2007 we collected senescent or recently fallen leaf litter from 6–10 trees per species. Pinus pinaster, Quercus robur and Eucalyptus globulus, were collected in Poulo, Orense, NW Spain (latitude, 42° 12′ 05″N, longitude 8° 05′ 10″ W, altitude 489 m). In the case of E. globulus, we collected both true leaves of juveniles and phyllodes of adults. Although Acacia dealbata populations exist in the same site, leaves were not senescent at this time of the year, so its litter was collected in the colder place of Sierra de Gata, Cáceres, W-Spain (latitude 40° 14′ 33″, longitude 6°25′08″W, altitude 432 m). Although litter properties of A. dealbata may differ between populations, the magnitude of this variation is expected to be much lower than cross-species variation, which constitutes the focus of the study. Leaf litter of the native trees Fraxinus angustifolia and Ulmus minor, and the exotic trees Ailanthus altissima, Robinia pseudoacacia and Ulmus pumila was collected in Alcalá de Henares, Madrid, Central-Spain (latitude 40° 20–30′, longitude 3° 18–20′, altitude 600 m). Litter was taken to the lab, extended on paper sheets and left to dry at ambient temperature. All litter from the same species was pooled into a composite sample. Four replicates from this composite sample were oven-dried at 60°C for 48 h and grounded with a Culatti mill (<1 mm particle size) for analysis of N and ash content (see below). Additionally, five green leaves (both true leaves and phyllodes for E. globulus) or leaflets were collected from each of 7–9 trees per species to assess the leaf mass to leaf area ratio (see below). Soil was collected from the two main sites selected for the litter collection below the canopy of native forests (co-dominated by Q. robur and P. pinaster in Poulo and by F. angustifolia and U. minor in Alcalá de Henares), where no exotic trees were present. After removing the upper debris layer, the top soil was collected to a depth of 30 cm, dried at ambient temperature, pooled into a single composite sample, and passed through a 2 mm sieve to eliminate remaining debris and stones. A fraction of the pooled soil was kept for chemical analysis.
Experimental design
Thirty-six plastic trays (2 L, 6-cm depth, 22-cm long and 14-cm width), with holes drilled in the bottom to allow drainage, were filled with c.a. 900 g of air-dried soil (16 from Poulo and 20 from Alcalá) and covered with 10 g of air-dried litter of one species, matching the origin of litter and soil (four replicated trays per treatment). This amount of litter corresponds to the average litter layer thickness found below dense patches of the studied species during the peak of leaf abscission. Four additional samples of each soil type and litter type were kept to correct for the initial water content after >48 h at 60°C in the oven. The proportions of juvenile true leaves to adult phyllodes (E. globulus) and of leaflets to rachises (A. altissima, R. pseudoacacia, F. angustifolia and A. dealbata) was kept as found in the field, though A. altissima rachises had to be cut into smaller pieces to fit their size to the trays. On 2007 December 28th trays were introduced into a growth chamber (Walk-in Ibercex HR, A.S.L., S.A.) at dark and constant temperature (21°C) and air humidity (77%), until the end of the experiment on 2008 September 15th. During this time soil moisture was kept c.a. 70% of field capacity by weekly weighing every tray and adding the required amount of de-ionized water, after previous calibration. This moisture level was selected as the mean point between soil saturation and the point at which soil start to crack (see below). Litter moisture was kept high by weekly spraying the top of the trays with de-ionized water. After 9 months of incubation the litter was removed from the trays, gently brushed to remove the soil particles, and weighed after being oven-dried at 60°C for >48 h. A fraction of this litter (c.a. 1 g) was used to assess ash content after 2 h at 550°C in a muffle furnace. The percentage of mass loss by decomposition was calculated as the difference between the initial and final ash-free dry mass, divided by the initial mass.
Soil moisture calibration
Before the beginning of the experiment, ten previously weighed trays were filled with 900 g of air-dry experimental soil (five with Poulo soil and five with Alcalá soil). Soils were saturated by submerging the bottom of the trays into water for several days and watering the top at intervals. Then trays were left to freely drain until they stopped dripping from the bottom. At that point trays were weighed, left to air-dry in the lab, and re-weighed 1–4 times per day until they reach c.a. 40% of the initial saturated weight. At this point the soil started to show cracks and to lose its structure. Finally, trays were oven-dried at 60°C for ≥3 day and weighed. Soil moisture was then calculated for every weighing time as the proportion of soil water mass (full tray weight minus empty tray weight minus dry soil weight) with respect to the saturated soil water content. The relation between tray weight and soil moisture (% of saturation water content) was used to calculate the amount of water required to keep trays at 70% during the experiment.
Leaf and litter properties
The four ground litter samples per species separated for analyses were divided into three aliquots. The first one was used to assess N concentration at Nutrilab (University of Rey Juan Carlos I, Móstoles, Madrid, Spain) with an Automated Wet Chemistry Analyzer (Skalar San ++, Breda, The Netherlands), after a digestion with H2SO4 and Cu-KSO4, which converts all organic nitrogen into ammonium (NH +4 -N). The second sample (c.a. 1 g) was used to assess water content, and the third one (1–1.5 g) to assess ash content after 2 h at 550°C in a muffle furnace. The two later values were used to express N content and litter mass per unit of ash-free dry mass. Values for two additional leaf litter properties (C/N and lignin content) were obtained from the literature. The leaf area of the fresh green leaves or leaflets (in the case of compound-leaf species) was assessed by means of a Delta-T Image Analysis System (Delta-T Devices LTD, Cambridge, England), and their dry mass was obtained after 2 days in the oven (60°C). The leaf mass per area (LMA) of each species was the average quotient between dry mass and leaf area of the eight sampled trees.
Soil properties
Soil pH, organic matter, mineral (NH +4 -N, NO −3 -N and the sum of both) and total N content was measured at the beginning (4–7 replicates per soil type) and end (4 replicates per species, i.e. one per tray) of the experiment. For soil pH assessment, 40 mL of deionised water was added to 20 g of soil, shaken and measured with a pH-meter (Allen et al. 1986). Organic matter was assessed by the Walkley-Black acid digestion method (Porta Casanellas et al. 1982). Total N content was assessed as explained before for leaf N content. Mineral N was extracted by adding 100 ml KCl 2 N to 5 g of soil and shaking the mix for 2 h. The solution was passed through 0.45 μm Millipore filters and preserved at −20°C until analysis of NO −3 -N and NH +4 -N at Nutrilab (University of Rey Juan Carlos I, Móstoles, Madrid, Spain) with an Automated Wet Chemistry Analyzer (Skalar San ++, Breda, The Netherlands). Available mineral N (NO −3 -N and NH +4 -N) in soils was monitored through the experiment by means of ion exchange membranes. Two 2.5 × 2.5 cm anion and two 2.5 × 2.5 cation exchange resin membranes (types I-100 and I-200, Electropure excellion-inc., Laguna Hills, California) fixed to two plastic labels were introduced in each tray and extracted 15 days later (Abrams and Jarrell 1992; Subler et al. 1995). This process was repeated every 2 months. Resin membranes were previously conditioned in the lab by immersing them in deionized water at 82–90°C for 48 h. Ions were extracted from the resins by shaking them for 2 h in 20–25 ml of 2 N KCl solution. Ammonium and nitrate content in the solution were assessed as before.
Statistical analysis
Analyses were separately performed for each location. LMA of green leaves, litter N concentration and proportion of litter mass loss were compared across species by means of a one-way ANOVA followed by a post-hoc Tukey test. The relation between litter mass loss and litter properties was assessed by Pearson correlation coefficient. Resin-assessed ammonium and nitrate soil availability were compared across species through the time by means of a repeated-measures ANOVA, using time as intra-subject factor and species as between-subject factor. The two values obtained per tray were averaged prior to analysis. When data did not fulfil sphericity, the Huynh-Feldt correction was applied (Field 2005). Changes in soil properties (pH, organic matter, NO −3 -N, NH +4 -N, total mineral N and total N content) were calculated by subtracting the average initial values from the final values in each treatment. The effects of litter origin (exotic or native) and litter species (nested in origin) on litter mass loss and on the change of soil properties between the beginning and the end of the experiment, were tested by means of a nested two-way ANOVA. Pair-wise comparisons of soil properties between trays covered by different litter types were performed by means of one-way ANOVA followed by a Tukey post-hoc test. In some cases data were Ln-transformed to achieve homogeneity of variances (checked by means of Levene test). All analyses were performed with SPSS 17.0.
Results
At the beginning of the experiment soils from Poulo exhibited lower pH, nitrate, mineral N and total N content, but higher ammonium content than Alcalá soils. Organic matter was similarly high in both sites (Table 1).
Native species from Poulo exhibited the highest (P. pinaster) and the lowest (Q. robur) LMA values, while exotic species (E. globulus and A. dealbata) showed intermediate and similar values. No significant difference of LMA was found between true leaves and phyllodes of E. globulus. Initial leaf litter N concentration was the lowest in E. globulus phyllodes and P. pinaster leaves, followed by E. globulus leaves and Q. robur leaves. The highest N concentration was found in A. dealbata leaf litter. Among Alcalá species, the two exotics, A. altissima and R. pseudoacacia, showed the lowest LMA values, followed by the native F. angustifolia and the two Ulmus species exhibited the highest values. Leaf litter N content was similar across species, being only differences between U. minor (the highest) and U. pumila (the lowest) (Table 2).
The proportion of litter mass loss at the end of the incubation period was much lower in Poulo (0–15%) than in Alcalá species (25–48%, Fig. 1). Among Poulo species, the exotic litter tended to decomposed less than the native litter (p = 0.099, Table 3), due to the negligible decomposition found in E. globulus (Fig. 1). The same trend was found among Alcalá species (Table 3), due to the low decomposition of the exotic litters of R. pseudoacacia and U. pumila. However, the litter of the remaining exotic, A. altissima, decomposed as fast as the litter of U. minor, which exhibited the highest decomposition (Fig. 1). Litter with higher N concentration tended to lose more mass than N-poor litter (Pearson correlation coefficients between litter N and proportion of litter mass loss for Poulo species, Alcalá species and all species were r = 0.74 p = 0.26, r = 0.74 p = 0.16 and for r = 0.70 p = 0.049, respectively); however, no significant correlation was found between litter mass loss and LMA (r = −0.37 p = 0.63, r = 0.51 p = 0.38 and r = −0.26 p = 0.51 for Poulo species, Alcalá species and all species, respectively).
Percentage of leaf litter mass loss after 9 months of incubation in a growth chamber. a) Species from Poulo (mesic forest of NW Spain) and b) species from Alcalá (riparian forest of central Spain). Grey columns represent exotic species and dashed columns native species. Different letters between columns in each graph means significant differences on the basis of an ANOVA test followed by a post-hoc Tukey test (Significant values P ≤ 0.05)
Poulo soil properties showed contrasting responses to the addition of different litter types, while Alcalá soil showed virtually the same trends irrespective of the type of added litter. During the experiment, soil pH of Poulo tended to increase when covered with P. pinaster or E. globulus litter, but decreased below Q. robur litter, and remained similar below A. dealbata litter. However, the only significant pH difference was found between E. globulus and Q. robur treatments (Table 3, Fig. 2a). We found no support to hypothesis 2, which suggested that exotic litter changed soil properties more than native litter. The percentage of organic matter in Poulo soil increased below the native litter (P. pinaster and Q. robur), but decreased below the exotic litter of E. globulus. Below A. dealbata litter, soil organic matter slightly decreased, but its value neither differed from that of E. globulus nor the native litter treatments (Fig. 2b). On average, NO −3 -N increased more below the native than the exotic litter (Table 3). However, pair-wise comparisons revealed that this effect was driven by the negligible increase of nitrate below E. globulus litter, as the other exotic (A. dealbata) produced a similar effect as the native P. pinaster, and the highest increase of NO −3 -N was found below the native Q. robur litter (Fig. 2c). Accordingly, resin-assessed nitrate availability increased through time below all litter types, except E. globulus (Table 4, Fig. 3). NH +4 -N increased signifincantly more below the exotic than the native litter (p = 0.025, Table 3), although pair-wise differences across species were not significant (Fig. 2d). Resin-assessed ammonium availability in P. pinaster, Q. robur and E. globulus trays decreased the first 4 months, peaked in the sixth month and declined again in the last month of incubation. By contrast, below A. dealbata litter, ammonium constantly increased since the second month (Table 4, Fig. 3). The highest increase of total soil mineral N was found below the litter of the native Q. robur and the exotic A. dealbata, closely followed by P. pinaster. Below E. globulus litter we found the least increase of mineral N, which significantly differed from the values found in the A. dealbata and Q. robur treatments, but not from that of P. pinaster (Fig. 2e). Total soil N (including organic and inorganic N) increased in all cases, but slightly more below P. pinaster litter (the significance of the species factor in the ANOVA analysis was marginally significant (p = 0.063) Table 3, Fig. 2f).
Changes of soil properties between the beginning and the end of the experiment in trays filled with soil from Poulo (left, A–F) and Alcalá (right, G–L), and covered with leaf litter of different native (dashed columns) and exotic (grey columns) tree species. Different letters across columns mean significant differences after an ANOVA followed by a post-hoc Tukey test (Significant values P ≤ 0.05)
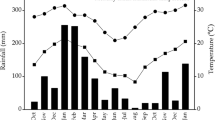
Nitrate (NO −3 -N) and ammonium (NH +4 -N) availability over time assessed by means of adsorption resin membranes in soils of Poulo (above) and Alcalá (below) covered by different species litter (exotic litter represented by open symbols and native litter by filled symbols). Note the different scales between graphs
Among Alcalá species, litter origin only affected the organic matter content of the soil, decreasing more below the native than below the exotic litter (Table 3, Fig. 2h). Soil pH decreased, and NO −3 -N and mineral N increased below all litter types (Fig. 2g, i, k). Resin-assessed nitrate availability speeded up after 4–6 months of incubation (Table 4, Fig. 3). NH +4 -N was marginally affected by origin and species (p = 0.081 and p = 0.086, respectively), decreasing slightly more below F. angustifolia, U. minor and R. pseudoacacia litter than below the rest of the species (Table 3, Fig. 2j). Resin-assessed ammonium availability decreased during the first 2 months, recovered until the sixth month and declined again in the last month of incubation (Fig. 3). Total N tended to increase only below A. altissima, and to remain constant below the rest of the species, but the species effect was not significant (Table 3, Fig. 2l).
Discussion
Temporal changes in soil properties were different between the two studied soils, which may be due to differences in their initial properties, differences between the litter added to each soil, or both. Growth and activity of decomposing soil microorganisms may have been more limited in Poulo than in Alcalá soil because of a lower litter quality among Poulo species (e.g. higher lignin content, see Table 2), because of the lower soil pH (Whalen and Sampedro 2009; Chamier 1987; Kittle et al. 1995; Motavalli et al. 1995) and/or because of the lower soil N content, which is a requirement for the growth of microbes (Whalen and Sampedro 2009; Yoshitake et al. 2007; Eiland et al. 2001). These arguments may explain the overall higher litter decomposition (Fig. 1) and the larger decline of soil organic matter (Fig. 2b, i) found in Alcalá treatments, as compared to Poulo treatments. Mineralization in Poulo soil was co-dominated by ammonification and nitrification processes, while in Alcalá it was dominated by nitrification processes (Fig. 2c, d, i, j). This suggests that there are differences in composition or activity rate of microbial communities between both sites. Nitrification in the Poulo soil may be inhibited by the low pH, while favoured in the Alcala soil by the lower C availability. This is suggested by the low organic matter to N ratio of the Alcalá soil, as compared to the Poulo soil, (Table 1) which may promote autotrophic nitrification (Aerts and Chapin 2000). In spite of the higher litter mass loss of Alcalá trays, total soil N tended to increase more in Poulo treatments (Fig. 2f, l), maybe because denitrification processes may compensate for N gains in the former.
The results of this study did not agree with the general trends of exotic invasive plants increasing soil N pools and speeding up the N cycle, shown by previous reviews (Ehrenfeld 2003; Liao et al. 2008). Conversely, the least decomposed litter in both soils belonged to exotic species, including the N-fixing R. pseudoacacia (Fig. 3). The higher decomposition of Q. robur and A. dealbata litter among Poulo species and of U. minor and A. altissima litter among Alcalá species may be attributed to their relatively low LMA and C/N, to a relatively high N content, or a combination of both (Cornelissen and Thompson 1997; Gallardo and Merino 1993). However, contrary to hypothesis 3, these litter properties failed to explain the low decomposition of some exotic litters. For instance, R. pseudoacacia showed the lowest LMA and C/N among Alcalá species. Instead, the presence of unassessed recalcitrant compounds, such as lignin in R. pseudoacacia litter (Castro-Díez et al. 2009; Alonso et al. 2010), or soluble polyphenols, as reported in E. globulus litter (Corbeels et al. 2003), may explain this result.
Organic matter content in the acid soil of Poulo increased below the native litter (P. pinaster and Q. robur) and decreased below the exotic litter (E. globulus, A. dealbata, Fig. 2b). Therefore, the release of organic matter from the native litter seemed to over-compensate the soil losses by mineralization. By contrast, organic matter declined below A. dealbata litter, in spite of the relatively high litter decomposition, suggesting a higher soil microbial activity. The same occurred in the basic Alcalá soil, whose organic matter declined in all cases. This decline was lower below the exotic litter (Table 3), suggesting that compounds released by this litter may partially inhibit soil mineralization activity. In the case of R. pseudoacacia the inhibitory compounds may be polyphenols, which are known to form complexes with proteins that are highly resistant to decomposition (Taylor et al. 1989; Hattenschwiler and Vitousek 2000). In the case of A. altissima, it is known to release allelopathic compounds, such as ailanthone, that are toxic to many plant species (Heisey and Heisey 2003; Kowarik and Saumel 2007), although their effects on soil microbes are not clear (Heisey and Heisey 2003; Castro-Díez et al. 2009).
In Alcalá soil no differences were found in pH, mineral N or total N across litter type treatments (Table 3). This can be attributed to the fact that the variation range of litter properties was lower across Alcalá species than across Poulo species (Table 2). In the case of Poulo, litter impacts on soil properties depended more on the species which produced the litter than on the origin of the species (native or exotic). Soil pH changes may reflect a shift in the balance between incomes (through litter decomposition) and losses (through mineralization) of organic acids (Finzi et al. 1998). In the case of the E. globulus treatment, where soil organic matter declined and litter mass remained unchanged at the end of the incubation (Figs. 2a, b and 1a), this balance was negative, leading to a pH increase. In the case of Q. robur treatment, the fast litter decomposition may lead to a positive balance and to a pH decrease. The relatively high increase of ammonium and nitrate at the end of the incubation period below A. dealbata litter (Fig. 1c, d, e) indicates a notable N mineralization activity, which accords with the decline of soil organic matter. By contrast, the small increase of mineral N in soils beneath E. globulus litter (Fig. 1f), fails to explain the relatively large decline of soil organic matter and suggests that the litter of this species inhibits more the mineralization of organic N than the mineralization of organic C, as suggested by previous studies (Mendham et al. 2004; Rovira and Vallejo 1997). Accordingly, the increase of mineral N, particularly nitrate, below E. globulus litter, was the lowest among all the studied litter types.
The relatively low nitrate availability shown by adsorption resins in all treatments during the first months of incubation (Fig. 3) can be attributed to N-poor litter organic compounds (simple sugars, starch, etc.) decomposing earlier than N-rich compounds (Whalen and Sampedro 2009). The subsequent nitrate increase suggests that the peak of protein breakdown occurred after 4–6 months of incubation. Ammoniun availability showed declines and increases during the incubation period, consistent across most treatments, indicating that the balance between ammonification activity and microbial uptake and/or conversion to nitrate was reversed several times during the 9-months incubation period.
The above results mostly support hypothesis 1, suggesting that the effects of plant litter on soil properties were species-specific, rather than origin-specific (i.e. native or exotic). Although some soil properties were significantly affected by the origin of species, the found trends differed between the two scenarios (i.e. soil organic matter and ammonium), or were clearly driven by one species (i.e. soil pH and nitrate trends found below exotic litter in Poulo were driven by E. globulus).
We found no support to hypothesis 2, suggesting that the addition of native litter would alter soil properties less than the addition of exotic litter, in either scenario, as native plant litter was expected to be in equilibrium with the local soil. However, the properties of other plant parts shed by the same species, such as fine roots or stems, may largely differ from those of the leaf litter (Hobbie et al. 2010). In the case of Alcalá floodplain soil, deposition of sediments from upstream represents an additional and relevant source of nutrients and organic matter to the soils (Noe and Hupp 2005; Olde Venterink et al. 2006). Therefore, soil properties are only partially determined by the input of native leaf litter. Our experiment also lacks detritivorous meso- and macrofauna, which fragment and make organic compounds available for microbial attack (Whalen and Sampedro 2009; Gallardo and Merino 1993). Under field conditions these fauna might be negatively affected by the addition of exotic litter, amplifying the effect of this litter on soil processes and properties. Further studies are needed to explore these possibilities.
We conclude that the litter produced by the main exotic trees living in two scenarios of the Iberian Peninsula did not decompose faster than that of the native trees. Moreover, litter of three out of the five exotic trees decomposed less rapidly than the coexisting native litter, contrary to the general trends reported in the literature. However, this trend was not consistently translated into changes of soil pH, total and mineral N pools, which varied irrespective of the added litter (Alcalá soil), or responded to each litter type, irrespective of their origin (Poulo soil). E. globulus litter caused the most drastic impact on the soil, strongly inhibiting nitrification and reducing soil N content. Litter of the N-fixing exotic trees did not increase soil N pools more than the rest of the species, probably because the transfer of litter N to the soil was slowed down by the presence of recalcitrant secondary compounds in the litter.
References
Abrams MM, Jarrell WM (1992) Bioavailability index for phosphorus using ion exchange resin impregnated membranes. Soil Sci Am J 56:1532–1537
Aerts R, Chapin FS (2000) The mineral nutrition of wild plants revisited: a re-evalutation of processes and patterns. Adv Ecol Res 30:1–67
Allen SE, Grimshaw HM, Rowland AP (1986) Chemical analysis. In: Moore PD, Chapman SB (eds) Methods in plant ecology, 2nd edn. Blackwell Sci. Pub, Oxford, pp 285–344
Alonso A, González-Muñoz N, Castro-Díez P (2010) Comparison of leaf decomposition and macroinvertebrate colonization between exotic and native trees in a freshwater ecosystem. Ecol Res 25:647–653
Ashton IW, Hyatt LA, Howe KM, Gurevitch J, Lerdau MT (2005) Invasive species accelerate decomposition and litter nitrogen loss in a mixed deciduous forest. Ecol Appl 15(4):1263–1272
Bai YF, Wu JG, Pan QM, Huang JH, Wang QB, Li FS, Buyantuyev A, Han XG (2007) Positive linear relationship between productivity and diversity: evidence from the Eurasian Steppe. J Appl Ecol 44(5):1023–1034
Baruch Z, Bilbao B (1999) Effects of fire and defoliation on the life history of native and invader C-4 grasses in a Neotropical savanna. Oecologia 119(4):510–520
Bowman WD, Theodose TA, Schardt JC, Conant RT (1993) Constraints of nutrient availability on primary production in two alpine tundra communities. Ecology 74(7):2085–2097
Castro-Díez P, González-Muñoz N, Alonso A, Gallardo A, Poorter L (2009) Effects of exotic invasive trees on nitrogen cycling: a case study in Central Spain. Biol Invasion 11:1973–1986
Chamier AC (1987) Effect of pH on microbial-degradation of leaf litter in seven streams of the English-Lake-District. Oecologia 71(4):491–500
Corbeels M, O’Connell AM, Grove TS, Mendham DS, Rance SJ (2003) Nitrogen release from eucalypt leaves and legume residues as influenced by their biochemical quality and degree of contact with soil. Plant Soil 250(1):15–28
Cornelissen JHC, Thompson K (1997) Functional leaf attributes predict litter decomposition rate in herbaceous plants. New Phytol 135:109–114
Ehrenfeld JG (2003) Effects of exotic plant invasions on soil nutrient cyling processes. Ecosystems 6:503–523
Ehrenfeld JG, Kourtev P, Huang W (2001) Changes in soil functions following invasions of exotic understory plants in deciduous forests. Ecol Appl 11:1287–1300
Eiland F, Klamer M, Lind AM, Leth M, Baath E (2001) Influence of initial C/N ratio on chemical and microbial composition during long term composting of straw. Microb Ecol 41(3):272–280
Field A (2005) Discovering statistics using SPSS. Sage Publications, London
Finzi AC, Canham CD, Van Breemen N (1998) Canopy tree-soil interactions within temperate forests: species effects on pH and cations. Ecol Appl 8(2):447–454
Follstad Shah JJ, Harner MJ, Tibbets TM (2010) Elaeagnus angustifolia elevates soil inorganic nitrogen pools in riparian ecosystems. Ecosystems 13:46–61
Gallardo A, Merino J (1992) Nitrogen inmobilization in leaf litter at two Mediterranean ecosystems of SW Spain. Biogeochem 15:213–228
Gallardo A, Merino J (1993) Leaf decomposition in two Mediterranean ecosystems of southwest Spain: influence of substrate quality. Ecology 74(1):152–161
Gallardo A, Parama R (2007) Spatial variability of soil elements in two plant communities of NW Spain. Geoderma 139:199–208
Gilliam FS, Dick DA (2010) Spatial heterogeneity of soil nutrients and plant species in herb-dominated communities of contrasting land use. Plant Ecol 209(1):83–94
Godoy O, Castro-Díez P, Van Logtestijn RSP, Cornelissen JHC, Valladares F (2010) Leaf litter traits of invasive species slow down decomposition compared to Spanish natives: a broad phylogenetic comparison. Oecologia 162:781–790
Gomez-Aparicio L, Canham CD (2008) Neighborhood models of the effects of invasive tree species on ecosystem processes. Ecol Monogr 78(1):69–86
Grigulis K, Lavorel S, Davies ID, Dossantos A, Lloret F, Vila M (2005) Landscape-scale positive feedbacks between fire and expansion of the large tussock grass, Ampelodesmos mauritanica in Catalan shrublands. Glob Chang Biol 11(7):1042–1053
Grotkopp E, Rejmánek M (2007) High seedling relative growth rate and specific leaf area are traits of invasive species: Phylogenetically independent contrasts of woody angiosperms. Am J Bot 94(4):526–532
Hattenschwiler S, Vitousek PM (2000) The role of polyphenols in terrestrial ecosystem nutrient cycling. Trends Ecol Evol 15(6):238–243
Hawkes CV, Wren IF, Herman DJ, Firestone MK (2005) Plant invasion alters nitrogen cycling by modifying the soil nitrifying community. Ecol Lett 8(9):976–985
Heisey RM, Heisey TK (2003) Herbicidal effects under field conditions of Ailanthus altissima bark extract, which contains ailanthone. Plant Soil 256(1):85–99
Hobbie SE, Reich PB, Oleksyn J, Ogdahl M, Zytkowiak R, Hale C, Karolewski P (2006) Tree species effects on decomposition and forest floor dynamics in a common garden. Ecology 87(9):2288–2297
Hobbie SE, Oleksyn J, Eissenstat DM, Reich PB (2010) Fine root decomposition rates do not mirror those of leaf litter among temperate tree species. Oecologia 162(2):505–513
Kittle DL, McGraw JB, Garbutt K (1995) Plant litter decomposition in wetlands receiving acid-mine drainage. J Environ Qual 24(2):301–306
Kowarik I, Saumel I (2007) Biological flora of Central Europe: Ailanthus altissima (Mill.) Swingle. Perspect Plant Ecol 8(4):207–237
Lake JC, Leishman MR (2004) Invasion success of exotic plants in natural ecosystems: the role of disturbance, plant attributes and freedom from herbivores. Biol Conserv 117:215–226
Le Maitre DC, van Wilgen BW, Chapman RA, McKelly DH (1996) Invasive plants and water resources in the Western Cape Province, South Africa: modelling the consequences of a lack of management. J Appl Ecol 33:161–172
Levine JM, Vila M, D’Antonio CM, Dukes JS, Grigulis K, Lavorel S (2003) Mechanisms underlying the impacts of exotic plant invasions. Proc R Soc Lond [Biol] 270(1517):775–781
Ley RE, D’Antonio CM (1998) Exotic grass invasion alters potential rates of N fixation in Hawaiian woodlands. Oecologia 113:179–187
Liao CZ, Peng RH, Luo YQ, Zhou XH, Wu XW, Fang CM, Chen JK, Li B (2008) Altered ecosystem carbon and nitrogen cycles by plant invasion: a meta-analysis. New Phytol 177(3):706–714
Mack MC, D’Antonio CM (1999) Impacts of biological invasions on disturbance regimes. Trends Ecol Evol 13:195–198
Mack MC, D’Antonio CM, Ley RE (2001) Alteration of ecosystem nitrogen dynamics by exotic plants: a case study of C4 grasses in Hawaii. Ecol Appl 11:1323–1335
Mayer PM, Tunnell SJ, Engle DM, Jorgensen EE, Nunn P (2005) Invasive grass alters litter decomposition by influencing macrodetritivores. Ecosystems 8(2):200–209
Mendham DS, Heagney EC, Corbeels M, O’Connell AM, Grove TS, McMurtrie RE (2004) Soil particulate organic matter effects on nitrogen availability after afforestation with Eucalyptus globulus. Soil Biol Biochem 36(7):1067–1074
Motavalli PP, Palm CA, Parton WJ, Elliott ET, Frey SD (1995) Soil pH and organic C dynamics in tropical forest soils: Evidence from laboratory and simulation studies. Soil Biol Biochem 27(12):1589–1599
Noe GB, Hupp CR (2005) Carbon, nitrogen, and phosphorus accumulation in floodplains of Atlantic coastal plain rivers, USA. Ecol Appl 15(4):1178–1190
Ohte N, Koba K, Yoshikawa K, Sugimoto A, Matsuo N, Kabeya N, Wang LH (2003) Water utilization of natural and planted trees in the semiarid desert of Inner Mongolia, China. Ecol Appl 13(2):337–351
Olde Venterink H, Vermaat JE, Pronk M, Wiegman F, van der Lee GEM, van den Hoorn MW, Higler L, Verhoeven JTA (2006) Importance of sediment deposition and denitrification for nutrient retention in floodplain wetlands. Appl Veg Sci 9(2):163–174
Pattison RR, Goldstein G, Ares A (1998) Growth, biomass allocation and photosynthesis of invasive and native Hawaiian rainforest species. Oecologia 117:449–459
Porta Casanellas J, López-Acevedo Reguerín M, Rodríguez Ochoa R (1982) Técnicas y Experimentos en Edafología. Universitat de Lleida
Rothstein DE, Vitousek PM, Simmons BL (2004) An exotic tree alters decomposition and nutrient cycling in a Hawaiian montane forest. Ecosystems 7:805–814
Rovira P, Vallejo VR (1997) Organic carbon and nitrogen mineralization under Mediterranean climatic conditions: the effects of incubation depth. Soil Biol Biochem 29(9–10):1509–1520
Sanz Elorza M, Dana Sánchez ED, Sobrino Vesperinas E (2004) Atlas de las plantas alóctonas invasoras en España. Dirección General para la Biodiversidad. Ministerio de Medio Ambiente, Madrid
Schlesinger WC (1991) Biogeochemistry. Academic, New York
Soudzilovskaia NA, Onipchenko VG (2005) Experimental investigation of fertilization and irrigation effects on an alpine heath, northwestern Caucasus, Russia. Arct Antarct Alp Res 37(4):602–610
Stevens CJ, Dupre C, Dorland E, Gaudnik C, Gowing DJG, Bleeker A, Diekmann M, Alard D, Bobbink R, Fowler D, Corcket E, Mountford JO, Vandvik V, Aarrestad PA, Muller S, Dise NB (2010) Nitrogen deposition threatens species richness of grasslands across Europe. Environ Pollut 158(9):2940–2945
Stock WD, Wienand KT, Baker AC (1995) Impacts of Invading N2-Fixing Acacia species on patterns of nutrient cycling in two Cape ecosystems - Evidence from soil incubation studies and N-15 natural-abundance values. Oecologia 101(3):375–382
Subler S, Blair JM, Edwards CA (1995) Using anion-exchange membranes to measure soil nitrate availability and net nitrification. Soil Biol Biochem 27 (7):911–917
Tateno R, Tokuchi N, Yamanaka N, Du S, Otsuki K, Shimamura T, Xue ZD, Wang SQ, Hou QC (2007) Comparison of litterfall production and leaf litter decomposition between an exotic black locust plantation and an indigenous oak forest near Yan’an on the Loess Plateau, China. For Ecol Manag 241(1–3):84–90
Taylor BR, Parkinson D, Parsons WFJ (1989) Nitrogen and lignin content as predictors of litter decay-rates - a microcosm test. Ecology 70(1):97–104
Tennesen M (2008) When Juniper and woody plants invade, water may retreat. Science 322:1630–1361
Vanderhoeven S, Dassonville N, Meerts P (2005) Increased topsoil mineral nutrient concentrations under exotic invasive plants. Plant Soil 275:169–179
Vilà M, Tessier M, Suehs CM, Brundu G, Carta L, Galanidis A, Lambdon P, Manca M, Médails F, Moragues E, Traveset A, Roumbis AY, Hulme PE (2006) Local and regional assessment of the impacts of plant invaders on vegetation structure and soil properties of Mediterranean islands. J Biogeogr 33(5):853–861
Vitousek PM, Walker LR (1989) Biological invasion by Myrica faya in Hawai’i: plant demography, nutrient fixation, ecosystem effects. Ecol Monogr 59:247–265
Whalen JK, Sampedro L (2009) Soil ecology and management. CAB International,
Xiong YM, Xia HX, Li ZA, Cai XA, Fu SL (2008) Impacts of litter and understory removal on soil properties in a subtropical Acacia mangium plantation in China. Plant Soil 304(1–2):179–188
Yelenik SG, Stock WD, Richardson DM (2007) Functional group identity does not predict invader impacts: differential effects of nitrogen-fixing exotic plants on ecosystem function. Biol Invasion 9:117–125
Yoshitake S, Sasaki A, Uchida M, Funatsu Y, Nakatsubo T (2007) Carbon and nitrogen limitation to microbial respiration and biomass in an acidic solfatara field. Eur J Soil Biol 43(1):1–13
Acknowledgements
We acknowledge Dr. Esther Pérez-Corona for her revision of this manuscript. This study was supported by the grants CGL2007-61873/BOS, CGL2010-16388/BOS of the Spanish Ministry of Science and Innovation, POII10-0179-4700 of Junta de Comunidades de Castilla-La Mancha and the REMEDINAL network S2009/AMB-1783 (Comunidad de Madrid). NGM was supported by a grant of the Spanish Ministry of Science and Innovation (FPI fellowship. BES-2008-002457).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Alfonso Escudero.
Rights and permissions
About this article
Cite this article
Castro-Díez, P., Fierro-Brunnenmeister, N., González-Muñoz, N. et al. Effects of exotic and native tree leaf litter on soil properties of two contrasting sites in the Iberian Peninsula. Plant Soil 350, 179–191 (2012). https://doi.org/10.1007/s11104-011-0893-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-011-0893-9