Abstract
Purpose
Land reclamation, as a measure of increasing cultivated land area, is being popularized in China in the past decades. However, the impact of land reclamation on soil carbon (C), nitrogen (N), and phosphorus (P) stoichiometry, microbial quantity, and enzyme activities has been rarely studied. The objective of this study was to know how land reclamation affected soil properties in agricultural soils.
Materials and methods
Soil samples were collected at 0–20, 20–40, and 40–60 cm depths before and after land reclamation of degraded soils in a village of southwest China. The samples were used to determine soil bulk density, porosity, moisture content, texture, pH, soil C, N, and P nutrient properties, microbial quantity, and enzyme activities in agricultural soils.
Results and discussion
The soil clay content was increased by 59.7%, while the soil organic matter (SOM), available phosphorus (AP), total potassium, microbial biomass carbon (MBC), microbial biomass nitrogen (MBN), and activities of catalase and urease enzymes were decreased by 49.9, 83.4, 32.8, 20.3, and 47.9%, respectively, at 0–20 cm of the soil. The decrease of SOM contributed to the decrease of MBC and MBN at 0–20 cm after the land reclamation. The soil texture had better capacity of water and fertilizer retention. Medium sand (> 0.25 mm) content, AP, the ratio of C to N, and activities of catalase were decreased, but pH and the ratio of N to P were increased at 20–40 and 40–60 cm. The clay content, medium silt (0.006–0.01 mm), total N, and quantity of fungi and actinomycetes were increased at 40–60 cm after the land reclamation.
Conclusions
Soil nutrient and enzyme activities at 0–20 cm were decreased but soil total microbial biomass was not disturbed at 0–20, 20–40, and 40–60 cm depths, after the land reclamation. The decrease of soil organic matter at 0–20 cm contributed to the decrease of MBC and MBN affected by land reclamation activities. Therefore, topsoil requires better management to preserve soil nutrients and enzyme activities after land reclamation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
In China, anthropogenic factors (i.e., deforestation and rapid urbanization) caused soil degradation and loss of large quantities of farmland (Zheng et al. 2005; Long 2014). Land reclamation of degraded agricultural soil is the comprehensive measure to restore farmland, water, roads, and forests (Chatzistathis and Koutsos 2017; Howell and Mackenzie 2017). It can help to increase the cultivated land area (Wang et al. 2014), improve soil quality of the cultivated land, increase productivity of arable land (Jürgenson 2016), increase vegetation cover, and reduce erosion events (Mihara 1996). However, there are some negative impacts, including decreasing landscape diversity and ecological service value (Yu et al. 2010). Thus, effective soil reclamation activities require a healthy biodiversity combining physical, chemical, and biological components of the ecosystem (Shrestha and Lal 2011).
Soil fertility is one of the key points of agriculture, and land reclamation is considered to be an important strategy in improving soil fertility (Mihara 1996; Sağlam 2015). Therefore, how we could increase the nutrient properties properly is of importance when conducting land reclamation. During land reclamation, carbon (C), nitrogen (N), and phosphorus (P) in the soil are intertwined together (Lal 2004). Ecological stoichiometry, the relation between elemental composition mostly for C, N, and P and the ecosystem energy balance (Elser et al. 2010), provides a way of investigating the ecological interactions among the organisms, plants, and the surrounding environment (Elser et al. 1996, 2000; Hu et al. 2016). The study on soil stoichiometric characteristics can be used for the regulation of soil fertility (Heuck and Spohn 2016) and soil C storage (Gao et al. 2014) in reclamation activities. The change of environmental conditions also affects diversity of microbial communities in soil (Manickam et al. 2010; Zhang et al. 2013a). Soil microorganisms play decisive roles in the soil nutrient transformation cycle, system stability, and anti-jamming capability (Peng et al. 2017). Soil microbial biomass carbon (MBC) and microbial biomass nitrogen (MBN) have positive effects on the cycling of soil organic C and N (Iqbal et al. 2010). Soil enzyme activities indicate the potential of soil to support biochemical processes that are vital for the maintenance of soil quality (Moscatelli et al. 2001). However, the effect of land reclamation on soil C, N, and P stoichiometry and biological characteristics in terms of soil fertility was not well studied.
Currently, some studies were conducted on the changes of soil physical and chemical properties after land reclamation (Juwarkar et al. 2010; 2008; Shrestha and Lal 2011; Wang et al. 2014), but the results are varied. Shrestha and Lal (2011) reported that the soil organic matter (SOM) was decreased but Nyamadzawo et al. (2008) reported that the soil C concentration and stocks were increased over years after land reclamation. In addition, the soil ecological stoichiometric characteristics, during land reclamation, soil microorganisms, and activity of enzymes in degraded agricultural soils of different soil depths were still unknown. Thus, the primary goals of this study are to (1) compare the changes of physical and chemical properties in different depths of soils, (2) investigate changes in C, N, and P ecological stoichiometric characteristics at 0–20 cm soil depth before and after land reclamation, and (3) try to answer if land reclamation could affect soil microorganisms and activity of enzymes.
2 Materials and methods
2.1 Study area
The study area is located at the Sanba, Fengdu County (Fig. 1), (107°42′52.8″–107°45′30″E, 29°35′32.7″–29°39′4.6″N), which is part of the Three Gorges Reservoir Area. The research region is 8.9 km2 and featured a subtropical humid monsoon climate. The elevation ranges from 1100 to 1690 m. The annual precipitation is 1320 mm (from 1980 to 2013). In addition, the average annual temperature is 14 °C at night and 19 °C in the day. From east to west, the elevation becomes higher with stable geological structure. The main soil type belongs to Alisols (FAO soil classification system) (FAO 2006), which is developed from the parent material of Jialing River limestone. Before the land reclamation, the study area included a majority of farmland, a small fraction of agroforestry, and a large part of the wasteland in karst areas (Fig. 2). The main problems included soils with a large number of bare rocks, fragmented plots, shallow and uneven soil layers in some fields, and soil acidification.
Distribution of sampling sites during and before the land reclamation, in which, (a) 6 collection samples belong to degraded soils in garden areas, 39 collection samples belong to wasteland which belonged to non-irrigated farmland, 6 collection samples belong to degraded soils in forest land, 1 collection sample belongs to agricultural facility land; n = 52. After the land reclamation (b), 3 collection samples belong to arable soils in garden areas, 20 collection samples belong to non-irrigated farmland, 4 collection samples belong to agroforestry soils, and 1 collection sample belongs to agricultural facility land; n = 28
2.2 Experimental design
The land reclamation project for Sanba, Fengdu County, was planned in May 2013 and completed in March 2014 (Fig. S1, Electronic Supplementary Material (ESM)). The project included arable land formation, irrigation and drainage construction tasks, and arable land formation task, which was the core project. At the beginning of the arable land formation tasks, plots of topsoil were stripped and rocks were excavated mechanically. Next, fragmentary plots (irregular plots and corner plots) were merged to larger plots. Then, stone sills were built on the plots with higher elevation difference to reduce the slope of the plots and soil erosion. Finally, new soil was added if the surface soil thickness was insufficient. After the reclamation, the average soil thickness was 60 cm. The new soil added is light clay soil which also belongs to Alisols (FAO soil classification system) with clay content at 28.9%, fine silt at 22.3%, medium silt at 12.8%, coarse silt at 26.2%, fine sand at 5.1%, medium sand at 4.7%, pH at 6.5, SOM at 15.2 g kg−1, available nitrogen (AN) at 85.3 mg kg−1, available phosphorous (AP) at 8.6 mg kg−1, and available potassium (AK) at 147.5 mg kg−1 (see subsequent texts for the method of analyses). After the land reclamation and soil sampling, the project area was rented to farmers to grow tobaccos (Nicotiana) and vegetables.
The soil from each of these sites was sampled randomly in “S” layout according to plot area, and soil type and the central GPS coordinates were documented. The soil samples from 52 sites at 0–20, 20–40, and 40–60 cm soil layers were collected before the land reclamation in December 2013, and soil samples from 28 sites were collected in the approximate same place after the land reclamation in March 2014. The soil samples of each site had three replicates. Samples were used to analyze soil physical properties, including soil bulk density, soil porosity, soil moisture content and soil particle composition (clay, silt, sand), soil chemical properties including soil pH value, SOM, total nitrogen (TN), total phosphorous (TP), total potassium (TK), AN, AP, AK, cation exchange capacity (CEC), ratio of total C to total N (C:N), ratio of total N to total P (N:P), ratio of total C to total P (C:P), MBC and MBN, and soil biological properties, including the quantities of bacteria, fungi, and actinomycetes, total quantity of microbial biomass, and the activities of catalase enzyme, urease enzyme, and invertase enzyme.
2.3 Soil property measurement
Composite samples of the soils were collected with five sub-samples from each sampling site. The samples were divided into two parts—one part was air-dried and sieved and the other part was preserved in a refrigerator to measure biological properties. Soil bulk density was measured by the cutting-ring method, and soil porosity was calculated based on the soil bulk density (Yang et al. 2008). Soil moisture content was determined by the soil weight before and after the soil was oven-dried at 105 °C for 24 h (Yang et al. 2008). Soil particle composition was determined by the hydrometer method (Yang et al. 2008). Additionally, soil pH was measured using a glass electrode (PB-10, Sartorius, Germany) in a 1:2.5 (w:v) soil–water solution. Soil organic carbon (SOC) content was measured through K2Cr2O7 oxidation and FeSO4 titration (Yang et al. 2008; Huang et al. 2017). SOM was equal to 1.724 times of SOC (Yang et al. 2008). Total N was measured according to micro-Kjeldahl method (Cannon, Shanghai, China). Total P was measured using molybdenum blue colorimetric analysis by visible spectrophotometer (UV-1200, Shanghai, China) after Na2CO3 oxidation (Yang et al. 2008). Total K was measured using flame photometer (FP6400A, Shanghai, China) also after Na2CO3 oxidation (Yang et al. 2008). Available N was measured by alkaline hydrolysis diffusion method (Huang et al. 2017). Available P was extracted by a 0.5 mol L−1 NaHCO3 (pH 8.5) solution and then analysed using the same methods as that in TP. Available K was extracted by 1 mol L−1 NH4Ac (pH 7.0) and then analysed using the same methods as that in TK. Soil CEC was determined by the EDTA-ammonium acetate (pH 7.0) method (Yang et al. 2008). Soil MBC and MBN were extracted by 0.5 mol L−1 K2SO4 and then determined by K2Cr2O7 oxidation and FeSO4 titration for MBC and micro-Kjeldahl method for MBN. The quantities of soil culturable bacteria, fungi, and actinomycetes were cultured on beef extract + peptone + agar medium, Martin medium, and improved Gauss No. 1 medium, respectively, and counted using dilution plate surface coating method (Zhang et al. 2013a). The activities of the soil enzymes (catalase, urease, and invertase) were analysed according to the method of Guan et al. (1986). Soil catalase activity was measured using the hydrogen peroxide titration after the enzymatic reaction. Soil urease activity was measured colorimetrically using indophenol blue and expressed in mg NH4+-N g−1 soil for 24 h. Soil invertase activity was determined colorimetrically using 3,5-dinitrosalicylic acid and was expressed in mg glucose g−1 soil for 24 h.
2.4 Statistical analyses
The Pearson’s correlation analysis was used to assess correlations among soil properties before and after the land reclamation. Figures 1 and 2 were made by Arcgis10.2. Statistical calculations were performed with SPSS 22.0. One-way analysis of variance (ANOVA) was used to test the significance of the same soil layer during land reclamation. The data were checked for normality (Shapiro–Wilk test) and homogeneity (Levene’s test) of variance before ANOVA, and the data were found normally distributed with homogenous variances. Significant differences were determined by the least significant difference (LSD) at P < 5%. Linear regression analysis was performed among the soil C, N, and P stoichiometry and SOM at 0–20 cm depth before and after the land reclamation.
3 Results
3.1 Changes of the soil physical characteristics before and after the land reclamation
The soil texture was improved after the land reclamation (Table 1). The soil bulk density was 1.34 g cm−3 before the land reclamation and decreased by 24.1% after the land reclamation at 40–60 cm (P < 0.05), but it was not different at 0–20 and 20–40 cm. Soil porosity was increased by 19.3% after the land reclamation at 40–60 cm. Soil moisture content was increased by 9.2 and 14.2% at 20–40 and 40–60 cm, respectively, after land reclamation as compared to that before the land reclamation, but it was not significant at 0–20 cm. The clay content was also increased by 59.7 and 102.7% at 0–20 and 40–60 cm, respectively, and the medium silt content increased by 60.7% at 40–60 cm after the land reclamation. The medium sand content was decreased by 87.0 and 90.3% at 20–40 and 40–60 cm, respectively, after the land reclamation (P < 0.001), but it was not significant at 0–20 cm. The content of fine silt, coarse silt, and fine sand was not different at 0–20, 20–40, and 40–60 cm depths before and after the land reclamation. The soil particles became smaller and uniform in size and the soils were easier to retain water and fertility at 0–60 cm after the land reclamation.
3.2 Changes of the soil chemical properties before and after the land reclamation
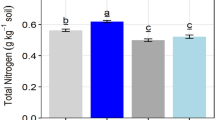
The pH was increased by 10.0, 8.8, and 6.5% at 0–20, 20–40, and 40–60 cm depths, respectively, as compared to that prior the land reclamation (P < 0.05) (Table 2). The SOM was decreased by 49.9% at 0–20 cm compared to that before land reclamation, but it was not significant at 20–60 cm. Soil TN was increased by 138.1% at 40–60 cm after the land reclamation, but it had no significance at 0–20 and 20–40 cm before and after the land reclamation. Soil AP was decreased significantly by 83.4 84.8, and 80.8% at 0–20, 20–40, and 40–60 cm, respectively, after the land reclamation as compared to that before the land reclamation, but soil TP was not different at 0–20, 20–40, and 40–60 cm depths before and after the land reclamation. Soil TK was decreased by 32.8% at 0–20 cm after the land reclamation, but it was not different at 20–40 and 40–60 cm. Soil AN and AP were not different but varied a lot in different sites at 0–20, 20–40, and 40–60 cm soil depths before and after the land reclamation. Soil CEC was increased by 115.6% at 40–60 cm after the land reclamation compared to that before the land reclamation. Soil MBC was decreased by 20.3 and 37.3% at 0–20 and 20–40 cm, respectively, after the land reclamation. Soil MBN was also decreased by 47.9% at 0–20 cm after the land reclamation, but it was not significant at 20–40 and 40–60 cm.
3.3 Changes of the soil stoichiometry before and after the land reclamation
The contents of soil organic C were significantly different among sites at 0–20 cm before the land reclamation (Fig. 3a) and were decreased significantly in most of the reclaimed sites after the land reclamation (Fig. 3b). The ratio of C to N was decreased by 55.2 and 65.5% at 20–40 and 40–60 cm, respectively, after the land reclamation (Table 2). The SOC was correlated with soil TN before and after the land reclamation at 0–20 cm (P < 0.01). Soil organic C was correlated with soil TP before the land reclamation (P < 0.05) (Fig. 3c), but the correlation between SOC and TP was not significant after the land reclamation (Fig. 3d). Similarly, soil TN was correlated with soil TP before the land reclamation at 0–20 cm (Fig. 3e), but the correlation between TN and TP was not significant (Fig. 3f). The ratio of N to P was increased by 105.9 and 179.8% at 20–40 and 40–60 cm, respectively, after the land reclamation compared to that before the land reclamation. Before the land reclamation, soil moisture content was positively correlated with N:P but not after the land reclamation (Table S1—ESM). After the land reclamation, the content of soil clay, coarse silt, and fine sand was correlated with C:P (P < 0.01).
Relationships between soil organic carbon (SOC) and total nitrogen (TN) before (n = 52) (a) and after (n = 28) (b) land reclamation; relationships between SOC and total phosphorous (TP) before (c) and after (d) land reclamation; relationships between TN and TP before (e) and after (f) land reclamation
3.4 Changes of the soil biological characteristics before and after the land reclamation
Soil total microbial biomass at 0–20, 20–40, and 40–60 cm was not affected by the land reclamation (Table 3). However, the number of fungi was increased by 52.0% at 40–60 cm as compared to that before the land reclamation. The number of actinomycetes was also increased at 40–60 cm after the land reclamation (P < 0.05). The activities of catalase enzyme were decreased by 27.9, 21.3, and 15.4% at 0–20, 20–40, and 40–60 cm, respectively, after the land reclamation compared to that before the land reclamation. The activities of urease enzyme were decreased by 52.0% at 0–20 cm after the land reclamation. However, the activities of invertase enzyme were not different at 0–20, 20–40, and 40–60 cm soil depths before and after the land reclamation. The activities of urease enzyme were positively correlated with SOM and TN at 0–20 cm depth (Table S3—ESM). The activities of catalase enzyme and invertase enzyme were also positively correlated with pH at 0–20 cm.
4 Discussion
Soil physical characteristics were affected by the land reclamation activities. Lower soil bulk density and higher soil porosity were found at 40–60 cm after the land reclamation because the reclaimed soils were loosened deeply. The soil water retention was also increased at 20–40 and 40–60 cm after the land reclamation because the soil texture was changed in the land unknotting work. Soil particles became smaller and the soil texture became uniform after the land reclamation, which can contribute to preventing the nutrient leaching, without serious repercussions on gas exchanges and water drainage. Soil chemical properties, including pH, SOM, TN, AP, TK, MBC, and MBN, were also influenced by reclamation activities. The increased soil pH at 20–40 and 40–60 cm after land reclamation may be influenced by the type of bed rocks by reclamation activities (Indorante et al. 1992). The SOM was decreased at 0–20 cm after land reclamation in this study, and a similar decrease in SOC concentrations was observed after the land reclamation (Ganjegunte et al. 2009; Shrestha and Lal 2011). On the one hand, the soil disturbance caused by reclamation activities may result in the dilution of SOM, increase of oxidation, and acceleration of erosion during the reclamation (Stahl et al. 2002). On the other hand, the soil added to the surface during the land reclamation had a lower content of SOM as compared to the local soil which may also be one reason for the decrease of SOM after the land reclamation. Soil AP at 0–60 cm and TK, MBC, and MBN at 0–20 cm which were decreased after the land reclamation may also be affected by the soil disturbance resulted from reclamation activities. The linear regression analysis revealed that SOM was positively correlated with MBC and MBN at 0–20 cm (P < 0.05) (Table S4—ESM). So, the decrease of SOM contributed to the decrease of MBC and MBN at 0–20 cm after the land reclamation.
After the land reclamation, soil organic C had a significant linear relationship with TN at 0–20 cm (Fig. 3b), which suggests that the distribution of nitrogen fertilizers may increase the production of biomass, and, therefore, there is an increase in organic matter (Zhang et al. 2013b). This is because an increase in N facilitates the build-up of C in soils, increasing the overall rate of nutrient cycling (Gao et al. 2014). In this study, the range of C:N ratio was 11.1–20.9 at 0–60 cm before the land reclamation, which was consistent with the range of C:N ratio of 10.5–18.8 at 0–100 cm soil layer in China (Tian et al. 2010). The decrease of C:N at 20–40 and 40–60 cm after the land reclamation suggests that the SOM had a more rapid mineralization at 20–40 and 40–60 cm in reclaimed soils because the lower soil C:N easily contributed to the mineralization and humification of SOM (Wang and Yu 2008). The average N:P ratio was increased at 20–40 and 40–60 cm after the land reclamation, while increased N:P ratio in soil reflected the deficient P that would be limited for increasing microbial biomass and diversity (Ren et al. 2016).
Land reclamation helped increase the quantity of fungi and actinomycetes at 40–60 cm, but the quantity of total microbial biomass was not significant before and after the land reclamation in this study. The reason may be due to the soil that was loose at 40–60 cm after land reclamation, which helped the increase of fungi and actinomycetes. However, the total microbial biomass varied a lot in different sample sites and hence showed no significance during the land reclamation. The activities of catalase enzyme decreased at 0–60 cm and the activities of urease enzyme decreased at 0–20 cm after the land reclamation indicating the disturbance of reclamation activities affected the enzyme activities. The decrease of catalase enzyme at 20–60 cm after the land reclamation may be affected by the changes of soil pH as well because the correlation analysis revealed that the activities of catalase enzyme were correlated with pH in this study. The significant decrease of urease enzyme activities at 0–20 cm after the land reclamation may resulted from the decrease of SOM because the urease enzyme activities were positively correlated with soil soluble organic C and N in SOM (Yang et al. 2012; Liu et al. 2014).
5 Conclusions
Reclamation activities significantly affected physical, chemical, and biological properties of the reclaimed soils. The soil particles became smaller and the soil texture became uniform through the land reclamation. Water and fertilizer retention capacity increased, and it was beneficial to maintain soil fertility and promote crop growth. However, soil organic matter, AP, TK, MBC, MBN, and activities of urease enzyme at 0–20 cm decreased significantly after the land reclamation. The ratio of C to N was decreased but the ratio of N to P was increased at 20–60 cm. Land reclamation affected the activities of urease enzyme and catalase enzyme but not the total quantity of the microorganisms. Soil organic matter was positively correlated with TN, C:N ratio, and C:P ratio; so, the SOM may be the most important factor to affect the soil ecological stoichiometry and appropriate measures should be taken to increase C sequestration after the land reclamation.
References
Chatzistathis T, Koutsos T (2017) Olive mill wastewater as a source of organic matter, water and nutrients for restoration of degraded soils and for crops managed with sustainable systems. Agric Water Manag 190:55–64
Elser JJ, Dobberfuhl DR, Mackay NA, Schampel JH (1996) Organism size, life history, and N:P stoichiometry. Bioscience 46:674–684
Elser JJ, Sterner RW, Gorokhova E, Fagan WF, Markow TA, Cotner JB, Harrison JF, Hobbie SE, Odell GM, Weider LW (2000) Biological stoichiometry from genes to ecosystems. Ecol Lett 3:540–550
Elser JJ, Fagan WF, Kerkhoff AJ, Swenson NG, Enquist BJ (2010) Biological stoichiometry of plant production: metabolism, scaling and ecological response to global change. New Phytol 186:593–608
FAO (2006) World Reference Base for Soil Resources 2006, a framework for international classification, correlation and communication, FAO. World soil resources reports 103
Ganjegunte G, Wick A, Stahl P, Vance G (2009) Accumulation and composition of total organic carbon in reclaimed coal mine lands. Land Degrad Dev 20:156–175
Gao Y, He NP, Yu GR, Chen WL, Wang QF (2014) Long-term effects of different land use types on C, N, and P stoichiometry and storage in subtropical ecosystems: a case study in China. Ecol Eng 67:171–181
Guan SY, Zhang D, Zhang Z (1986) Soil enzyme and its research methods. Agriculture Press, Beijing (in Chinese)
Heuck C, Spohn M (2016) Carbon, nitrogen and phosphorus net mineralization in organic horizons of temperate forests: stoichiometry and relations to organic matter quality. Biogeochemistry 131:229–242
Howell DM, Mackenzie MD (2017) Using bioavailable nutrients and microbial dynamics to assess soil type and placement depth in reclamation. Appl Soil Ecol 116:87–95
Hu N, Li H, Tang Z, Li ZF, Li GC, Jiang Y, Hu XM, Lou YL (2016) Community size, activity and C:N stoichiometry of soil microorganisms following reforestation in a karst region. Eur J Soil Biol 73:77–83
Huang R, Lan ML, Liu J, Gao M (2017) Soil aggregate and organic carbon distribution at dry land soil and paddy soil: the role of different straws returning. Environ Sci Pollut Res 24:27942–27952
Indorante S, Grantham D, Dunker R, Darmody R (1992) Mapping and classification of minesoils: past, present, and future, prime farmland reclamation. Proceedings of National Symposium on Prime Farmland Reclamation, St. Louis, MO, pp 10–14
Iqbal J, Hu R, Feng M, Shan L, Malghani S, Ali IM (2010) Microbial biomass, and dissolved organic carbon and nitrogen strongly affect soil respiration in different land uses: a case study at Three Gorges Reservoir Area, South China. Agric Ecosyst Environ 137:294–307
Jürgenson E (2016) Land reform, land fragmentation and perspectives for future land consolidation in Estonia. Land Use Policy 57:34–43
Juwarkar AA, Mehrotraa K, Nair R, Wanjari T, Singh S, Chakrabarti T (2010) Carbon sequestration in reclaimed manganese mine land at Gumgaon, India. Environ Monit Assess 160:457–464
Lal R (2004) Soil carbon sequestration impacts on global climate change and food security. Science 304:1623–1627
Liu YM, Yang HY, Li XR, Xing ZS (2014) Effects of biological soil crusts on soil enzyme activities in revegetated areas of the Tengger Desert, China. Appl Soil Ecol 80:6–14
Long HL (2014) Land consolidation: an indispensable way of spatial restructuring in rural China. J Geogr Sci 24:211–225
Manickam N, Pathak A, Saini H, Mayilraj S, Shanker R (2010) Metabolic profiles and phylogenetic diversity of microbial communities from chlorinated pesticides contaminated sites of different geographical habitats of India. J Appl Microbiol 109:1458–1468
Mihara M (1996) Effects of agricultural land consolidation on erosion processes in semi-mountainous paddy fields of Japan. J Agric Eng Res 64:237–247
Moscatelli M, Fonck M, De Angelis P, Larbi H, Macuz A, Rambelli A, Grego S (2001) Mediterranean natural forest living at elevated carbon dioxide: soil biological properties and plant biomass growth. Soil Use Manag 17:195–202
Nyamadzawo G, Shukla M, Lal R (2008) Spatial variability of total soil carbon and nitrogen stocks for some reclaimed minesoils of Southeastern Ohio. Land Degrad Dev 19:275–288
Peng M, Jia HB, Wang QY (2017) The effect of land use on bacterial communities in saline–alkali soil. Curr Microbiol 74:325–333
Ren CJ, Zhao FZ, Kang D, Yang GH, Han XH, Tong XG, Feng YZ, Ren GX (2016) Linkages of C:N:P stoichiometry and bacterial community in soil following afforestation of former farmland. For Ecol Manag 376:59–66
Sağlam M (2015) Evaluation of the physicochemical properties of alluvial and colluvial soils formed under ustic moisture regime using multivariate geostatistical techniques. Arch Agron Soil Sci 61:943–957
Shrestha RK, Lal R (2011) Changes in physical and chemical properties of soil after surface mining and reclamation. Geoderma 161:168–176
Stahl PD, Perryman BL, Sharmasarkar S, Munn LC (2002) Topsoil stockpiling versus exposure to traffic: a case study on in situ uranium wellfields. Restor Ecol 10:129–137
Tian HQ, Chen GS, Zhang C, Melillo JM, Hall CAS (2010) Pattern and variation of C:N:P ratios in China’s soils: a synthesis of observational data. Biogeochemistry 98:139–151
Wang SQ, Yu WG (2008) Ecological stoichiometry characteristics of ecosystem carbon, nitrogen and phosphorus elements. Acta Ecol Sin 28:3937–3947 (in Chinese with English abstract)
Wang J, Dun YL, Guo YQ, Dou S (2014) Effects of land consolidation on improvement of salinity soil in Western Songnen Plain. Trans CSAE 30:266–275 (in Chinese with English abstract)
Yang JH, Wang C, Dai HL (2008) Soil agricultural chemical analysis and environmental monitoring. China Earth Press, Beijing (in Chinese)
Yang K, Zhu JJ, Yan QL, Zhang JX (2012) Soil enzyme activities as potential indicators of soluble organic nitrogen pools in forest ecosystems of Northeast China. Ann For Sci 69:795–803
Yu GM, Feng J, Che Y, Lin XW, Hu LM, Yang S (2010) The identification and assessment of ecological risks for land consolidation based on the anticipation of ecosystem stabilization: a case study in Hubei Province, China. Land Use Policy 27:293–303
Zhang XQ, Huang GQ, Bian XM, Zhao QG (2013a) Effects of nitrogen fertilization and root interaction on the agronomic traits of intercropped maize, and the quantity of microorganisms and activity of enzymes in the rhizosphere. Plant Soil 368:407–417
Zhang ZS, Song XL, Lu XG, Xue ZS (2013b) Ecological stoichiometry of carbon, nitrogen, and phosphorus in estuarine wetland soils: influences of vegetation coverage, plant communities, geomorphology, and seawalls. J Soils Sediments 13:1043–1051
Zheng FL, He XB, Gao XT, Zhang CE, Tang KL (2005) Effects of erosion patterns on nutrient loss following deforestation on the Loess Plateau of China. Agric Ecosyst Environ 108:85–97
Acknowledgments
We would like to thank Dr. Xinhua He and Dr. Chengsheng Ni for their constructive help during the manuscript writing. We would also like to thank anonymous reviewers and editors for their valuable suggestions and time that helped to substantially improve the manuscript. This study was supported by the Chongqing Postgraduate Research and Innovation Project (No. CYB18091), the National “Five-Year” Key Research and Development Program (2017YFD0800101), and the National Innovation Training Program (201910635077).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Claudio Bini
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 129 kb)
Rights and permissions
About this article
Cite this article
Qi, L., Zhou, P., Yang, L. et al. Effects of land reclamation on the physical, chemical, and microbial quantity and enzyme activity properties of degraded agricultural soils. J Soils Sediments 20, 973–981 (2020). https://doi.org/10.1007/s11368-019-02432-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-019-02432-1