Abstract
Nitrogen (N) rhizodeposition by cowpea (Vigna unguiculata (L.) Walp) is potentially a large N source in cropping systems of Sub-Saharan Africa. A field experiment was conducted to measure cowpea N rhizodeposition under the conditions of the Sudano-Sahelian zone using direct 15N labelling techniques to trace the amount of deposition and its transfer to associated and subsequent crops. Half of the total cowpea crop N was located below-ground at plant maturity, which exceeded 20 kg N ha−1 when intercropped with millet. Only 15% of the below-ground cowpea N was recovered in roots, while 85% was found in the rhizodeposited pools. The experiment demonstrated that direct below-ground N transfer occurred from cowpea to millet in intercrop at a rate of 2 kg N ha−1 over the growing season. Forty percent of the 25 kg below-ground N that the cowpea crop left at harvest were identifiable in the top 0.30 m soil in the beginning of the next planting season 7 months later; a pool still present at the end of that second season. Thus, the subsequent crop of millet (Pennisetum glaucum (L.) R. Br.) only recovered 2.5 kg N ha−1 from the below-ground cowpea pre-crop N during this growth season. The role and potential of cowpea as N provider has been underestimated in the past by ignoring the large proportion of N contained in its rhizodeposits. However, information is needed to determine how losses of the rhizodeposited N can be minimized to fully harness the potential of cowpea as N provider in agro-ecosystems of the region.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Crop production in the Sudano-Sahelian eco-zone is limited by low soil nutrient availability, especially nitrogen (N) and phosphorus (P). Soils of the region are characterized by low organic C, total and available P and N, and effective cation exchange capacity (ECEC) (Bationo et al. 2002). Cowpea is the main legume grown in the region where it is cultivated for grain, fodder and for its soil improvement effects (Sanginga et al. 2003). The main cropping system of the region consists of an intercrop of millet and cowpea; 90% of the millet fields in Niger are intercropped with cowpea (Bationo et al. 2004).
Cowpea is thought to improve soil fertility by supplying N to the soil via symbiotic nitrogen fixation (Alvey et al. 2001; Bado et al. 2006). Knowledge of cowpea symbiotic nitrogen fixation and inputs to soil is based solely on measurement of above-ground plant parts (Bado et al. 2006; Bagayoko et al. 1998). Despite the fact that forage shoot material is mostly removed from the field to feed livestock in the Sudano-Sahelian region (Bationo et al. 2004; Singh et al. 2003), improved cereal yields following cowpea monoculture or cowpea/millet mixtures in the rotation have been reported (Alvey et al. 2001; Bado et al. 2006; Bagayoko et al. 1996, 1998; Fussell and Serafini 1985; Samake et al. 2006). It is therefore necessary to measure the N contained in the roots and rhizodeposits of cowpea to better understand the N effects in rotation and in intercrop with cereals (Bationo et al. 2004).
Rhizodeposits form a large part of legume below-ground N and include roots exudates, fine roots and root necrosis products accrued in the soil during plant growth (Hertenberger and Wanek 2004). Fine roots, exudates and decomposed material are not recovered when roots excavation is performed. Physical recovery of roots thus substantially underestimates the role of the plants below-ground organs in nutrient cycling. Direct labelling methods using the stable isotope 15N have been developed and used to quantify N of plant origin and trace its fate in the soil (Khan et al. 2003; McNeill et al. 1997; Yasmin et al. 2006).
Studies carried out in the Guinean savannah of Nigeria indicated that below-ground N contribution from soybean is potentially a large source of N in agro-ecosystems of that region (Franke et al. 2008; Laberge et al. 2009). Similar findings were reported for legumes of temperate and Mediterranean regions (Gylfadóttir et al. 2007; Høgh-Jensen and Schjoerring 2001; Khan et al. 2003). To our knowledge, no information is available on rhizodeposition of cowpea and its potential transfer of N to millet in intercrop and rotation.
The objectives of this study were 1) to measure the contribution of rhizosphere processes to N fluxes during and following cowpea cultivation in the Sudano-Sahelian zone and 2) to measure below-ground N transfer to a millet crop grown in association or in rotation with cowpea. A third objective was to trace the fate of N accrued in cowpea roots and rhizodeposition in soil over a 1-year period. The hypotheses behind this study are 1) that N rhizodeposited by grain legumes is a substantial but hitherto ignored source of N in cropping systems of the Sudano-Sahelian zone, 2) that cowpea below-ground N contributes positively to the N supply of an associated or subsequent millet crop, and 3) that cowpea-derived below-ground N pools are highly degradable with a high potential to be taken up by plants or to be lost from the soil.
Materials and methods
Experimental site
The experiment was conducted in 2006 and 2007 at the ICRISAT Sahelian centre (ISC) in Sadore, Niger. The region is part of the Sudano-Sahelian agro-ecological zone, which is characterized by a mono-modal rainfall distribution (FAO 2004). Long-term (1983 to 2004) average annual precipitation at Sadore station was 542 mm. Soils at the experimental sites are arenosols (FAO 1988).
Rainfall, measured by a gauge at the experimental site, was close to the seasonal average in 2006 despite the season starting late (Fig. 1). Millet and cowpea were planted late in the second and third weeks of July, respectively. The season also started late in 2007 with precipitation of 415 mm well below the seasonal average of 542 mm (Fig. 2). The rainfall events were well distributed over the season and millet yield at the experimental site was little affected by the rain deficit in 2007.
Accumulated precipitation in the growing season of 2006. The dots represent the different field activities. pm: planting millet, pc: planting cowpea, w: weeding, t: thinning, l: labelling, hc: harvest and sampling of labeled cowpea plots, hf: harvest of the entire field. The cowpea beans were harvested throughout the season
The site had been under natural fallow for 4 years when the experiment began. The field was disc-harrowed once prior to planting in 2006. Soil preparation was finished by hand and the plant residues were removed from the field. Soil samples were taken to a depth of 0–0.30 m before planting in 2005 using a soil auger 0.10 m in diameter. Twelve samples were randomly taken over the field (1,740 m2) and combined to one composite sample for analyses. Soil particle size, pH (H2O, 1:2.5 soil to water ratio), organic C content (Walkley-Black method), total N (Macro-Kjeldahl method) and Bray-I extractable P (Table 1) were determined at ICRISAT Sadore’s analytical service lab. Soils of the experimental site are considered fertile with Bray-I extractable P values of 10 mg P kg−1 in comparison to most soils of South-West Niger with Bray-I extractable P values below 3 mg P kg−1 soil (Voortman et al. 2004).
Experimental set-up in 2006
Millet planted on 18th June 2006 failed to establish due to recurring drought periods and was replanted the 7th of July. Cowpea was planted 1 week later to minimize cowpea competition with millet following the normal practice in the area.
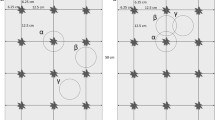
One row of millet planting holes, pockets, was alternated with one row of cowpea pockets throughout the field (Fig. 3). Cowpea and millet were planted in a regular grid pattern with 1 m between every pocket in a flat seed bed as is common practice in Western Niger. Fertilizer was applied at a rate of 1.1 g NH +4 and 0.9 g P2O5 per pocket at planting, a fertilization strategy known as micro-dosing (Hayashi et al. 2007). The plants were thinned to three millet plants and two cowpea plants per pocket 3 weeks after millet planting. The timeframe for the field and sampling operations are indicated in Fig. 1.
The millet variety used in this experiment was the improved cultivar IT-89 305, maturing in 95 days. The cowpea variety was the Kodé local variety, collected in the village of Kodé close to Niamey. It is an indeterminate creeping variety grown for its high production of residues.
The field was divided into 12 plots of each 7 × 7 m. The four plots at the four corners of the field were used to measure cowpea N fixation by the 15N isotope dilution method (IAEA 2004). The eight other plots (Fig. 3) were used to measure cowpea N rhizodeposition and cowpea N transfer to the associated millet crop using direct 15N labelling techniques (McNeill et al. 1997; Khan et al. 2003). Hand weeding was done 2 weeks and 8 weeks after millet planting. Crops were sprayed with an insecticide (Karate®, lambda-cyhalothrine, 150 ml a.i. ha−1) 3 and 9 weeks after millet planting.
Throughout 2006, shedded leaves and beans from the labelled cowpeas were collected in a tightly woven mosquito net fabric placed close to the ground around the labelled cowpea pockets. The captured leaves were removed frequently to avoid any enriched leaf material transferring into the soil.
Procedures for direct 15N labelling of cowpea
The central pockets of eight plots were labelled and used for the rhizodeposition study. A multiple direct 15N labelling strategy was used to enrich simultaneously the cowpea plants that these central pockets contained. The two cowpea plants were enriched in 15N by weekly feeding them each 1 ml of 0.5% 15N urea (99.6 atom% 15N) over 6 weeks. The procedures for labelling the plants followed the leaf-flap methods described by Khan et al. (2002): a V-shape leaf-flap was cut underwater using a scissor with the tip of the V centred on the mid-vein of one leaflet. The leaflet-flap was then rapidly inserted in a 5-ml plastic vial containing 1 ml of the urea solution. The vial was sealed using inert plastic material after ensuring that the leaf flap was in contact with the solution. To maintain the vial in position it was attached to a short wooden stick anchored in the soil. Leaflets chosen for labelling were selected from the lower part of the stem for ease of labelling operations.
The vials were left in place for 24 h to 48 h depending on the speed of solution uptake and were then removed. The labelled leaflets were removed with the vials after labelling to avoid contaminating the soil with 15N that might have remained in the leaflets. Labelling started on the fifth week after planting millet; the cowpea plants were 1-month old. The plants were all then pulse labelled once a week for 6 weeks.
Procedures for 15N of soil N pools to determine symbiotic nitrogen fixation
Symbiotic nitrogen fixation in the cowpea was measured using the 15N isotope dilution method (IAEA 2004) with millet used as a non-fixing reference plant. Four plots (7 × 7 m) were kept solely to measure N2-fixation. The same planting pattern was used as in the rest of the field but millet was planted on the same date as the cowpea. A solution of KNO3 (5.6 g, 98 atom% 15N in 20 L of water) was uniformly applied over an 8 m2 area in every plot with a watering can. Sucrose (18 g) was added to the solution to obtain a C: N ratio of approximately 10:1 leading to an initial immobilization of the 15N and to have a relatively constant enrichment of the plant available N throughout the season (Giller and Witty 1987; Witty and Roose 1984).
Dry matter measurement of millet and cowpea at maturity
The dry matter of cowpea, beans and residues and the dry matter of millet, grain and residues were measured by harvesting three areas (15 × 15 m) at different locations throughout the field. Cowpea beans were harvested throughout the season when the pods were maturing. The harvested material was air dried in a greenhouse to determine its dry matter content. The final harvest of cowpea plants and millet took place on the 22nd of October.
Sampling for 15N determination
The whole above-ground direct 15N labelled cowpea plants were harvested on the 16th of October. One neighbouring pocket of millet was harvested to measure N transfer from cowpea to millet. One neighbouring pocket of cowpea was also harvested to measure N transfer from cowpea to cowpea. Soil and roots were also sampled on that date. Weeds growing within 0.45 m of the labelled cowpeas were collected throughout the season also to measure cowpea N transfer to weeds.
Sampling of soil and roots under and around the labelled cowpea plants took place in a two-step manner. First, to sample what is termed the ‘pockets soil’, a metal box (0.30 m × 0.30 m × 0.15 m depth) was inserted in the soil precisely over the pocket. Secondly, to sample what is considered the soil affected by the individual plants rooting, termed ‘rooting soil’, a circle with 0.45 m radius was traced on the soil with the box at its centre. All the soil and roots within this circle and outside of the box was dug out to a depth of 0.15 m and kept in large plastic pots. After the soil within the circle was removed, a metal flap was inserted under the box and the content of the box was transferred to a container. The whole procedure was repeated to sample between 0.15 m to 0.30 m depth.
The samples were dry-sieved through a 2-mm mesh and all visible roots collected. The sieved soil was thoroughly mixed and a 100 g sub-sample was taken and dried to constant weight at 60°C for 72 h to determine soil water content and soil dry weight. These soil samples were also the ones analysed for 15N content. Four soil-root samples were thus obtained around the labelled plants, two within the pocket soil at 0–0.15 m and 0.15–0.30 m depth, and two samples from the rooting soil, i.e. within the 0.45 m radius but outside the box at 0–0.15 m and 0.15–0.30 m depth (Fig. 3).
Five control cowpea plants and their root systems were sampled from the border rows to determine the natural 15N enrichment of their N pools. Five control samples of soil were also taken from the border at two different depths: 0–0.15 m and 0.15–0.30 m. Millet was harvested from five pockets in the borders to be used as control in the calculations of N transfer. Weeds growing in the border plots were also collected to determine their enrichment and use them as controls against enrichment of weeds collected close to the labelled cowpeas. Only the above-ground parts of the weeds were collected.
The 15N enriched area for symbiotic nitrogen fixation determination had at its centre a pocket with cowpea and two pockets of millet. These three pockets were harvested together at the end of the experiment to measure symbiotic nitrogen fixation. The whole millet and cowpea above-ground sections were sampled to determine their 15N enrichment.
Experimental set-up in 2007 to determine pre-crop effect
All above-ground plant residues had been removed from the field in 2006. The soils sampled for determination of cowpea below-ground N in 2006 were returned to their respective plots along with the roots of cowpea 2 days after sampling in October 2006. Subsequently, new millet was established in the field in 2007 to estimate the N pre-crop effect.
Millet planted on 1st of June 2007 failed to establish in the absence of subsequent rainfall but a second planting on the 1st of July established readily. The plants were thinned to two plants per pocket on the 24th of July. The field was weeded on the 24th of July and on the 10th of September. The field was harvested and sampled on the 16th of October 2007. The timing of the field operations is presented in Fig. 2 along with the accumulated precipitation in 2007.
Pockets of millet were grown directly over the pockets where cowpea was labelled in 2006 to determine transfer of cowpea below-ground N to millet from 1 year to the next. The millet plants growing over the labelled cowpea were harvested at maturity and treated as the millet intercropped in 2006; see above, to determine their biomass, N content, and 15N enrichment.
Decay patterns of below-ground cowpea N
A sub-experiment was initiated in the field alongside the main experiment in 2006. The objective of the sub-experiment was to follow the decay of cowpea below-ground N following cowpea harvest.
Twelve round free-draining plastic containers (0.35 m deep, 0.20 m radius) were placed 0.30 m deep in the soil, leaving the edge of them 5 cm above the soil surface. Once in place, the containers were filled with the soil displaced during their insertion. The soil was sieved to remove the large pieces of organic material and facilitate root sieving in the later stages of the experiment. Two cowpea plants were established in every container and these plants were treated and labelled in exactly the same way and at the same time as the cowpea grown in the field in the labelled plot in 2006; plants from two containers were unlabelled as control. The sampling followed the same procedures as the sampling in the field.
Following sampling, the roots and soil were returned to the containers in the field. The containers were left in place without plants and were sampled twice more to determine their soil 15N enrichment in June 2007 at planting of millet and in October 2007 after harvest of the millet crop. The soil samples were taken directly under the millet pocket of 2007, where cowpea had been grown in 2006 to trace the fate of the 15N labelled legume below-ground N.
15N determination of plant and soil samples
Grain, beans, residues, roots and weeds were dried to constant weight in a forced air oven at 60°C, for 72 h weighed and ground with a rotary mill (Wiley Mill, A.H., Thomas, Philadelphia, PE, USA) to pass a 0.5 mm sieve. Subsequently, soils, pods, residues and roots samples were analyzed for total N content and 15N enrichment by flash combustion on an elemental analyzer (EA1110, Carlo Erba, Milan, Italy) coupled in continuous flow mode with an isotope ratio mass spectrometer (Finnigan MAT, Bremen, Germany).
Calculations of 15N partitioning, rhizodeposition, transfer to intercropped millet and weeds and symbiotic nitrogen fixation
15N enrichment is expressed as δ 15N (‰) relative to the natural enrichment of atmospheric N (0.3663 atom%) as in Eq. 1.
The proportion of soil N derived from rhizodeposition (%Ndfr) was calculated from the ratio of enrichment of labelled soil within a given sampled volume over that of labelled roots within the same sampled volume. In the calculations, the enrichment of the labelled soil and roots was corrected against the enrichment of the same N pool in the controls (Schmidtke 2005) as in Eq. 2.
The quantity of soil N coming from rhizodeposition was obtained by multiplying the total soil N with%Ndfr. Values obtained for below-ground measurements around the pocket of the labelled cowpea were converted to per area unit basis by multiplying them by the ratio of the above-ground biomass of cowpea measured in the whole field over the biomass measured in the labelled pocket.
Nitrogen transfer from the labelled cowpea plants to the two neighbouring cowpea plants, the two neighbouring millet plants and weeds was measured by comparing 15N enrichment in the cowpea roots and in the receivers above-ground biomass. The average enrichment of the donor cowpea roots in the rooting soil, i.e. the 45 cm radius to a depth of 0.30 m, were used in this calculation. The proportion of N in the receiver plant was calculated using Eq. 3 as given by Giller et al. (1991).
The quantity of N transferred to the two neighbouring millet plants was obtained by multiplying the total receiver N with%N transfer.
To determine symbiotic nitrogen fixation, the 15N enrichment of the whole cowpea above-ground parts was compared to that of millet above-ground parts.%Ndfa was calculated by using Eq. 4.
The amount of N fixed by cowpea was determined using this value multiplied by total N accumulated in the cowpea crop and its rhizodeposits.
The pre-crop N in millet plants derived from the previous cowpea plants was determined using Eq. 5, where the δ15N enrichment of cowpea below-ground N is the average enrichment of the rhizodeposits and roots in the soil under the labelled pocket. The equation is adapted from previous equations on labelled fertilizer uptake (IAEA 2004).
The amount of N transferred to the subsequent crop was obtained by multiplying the millet N content with%Ndf cowpea BNG.
Data handling
Average biomass, N content and 15N content were calculated from the measurements taken from the eight labelled pockets. Results were presented with their corresponding mean standard errors. The same was done for pockets of millet and cowpea and for weeds with the data on transfer. Two-ways ANOVA were carried out using R (R Development Core Team 2007) with the replicates and organs as criteria to test whether the labelled cowpea plants were uniformly enriched in 15N following labelling.
Results
Millet and cowpea biomass, N content and cowpea symbiotic nitrogen fixation
Millet intercropped with cowpea in this experiment yielded above average for the region with a total biomass accumulation of 3.4 Mg of DM ha−1 of which 1.4 Mg of DM ha−1 was in the spikes The millet crop contained 50 kg N ha−1 of which 30 kg N ha−1 was located in the grain (Table 2).
Cowpea accumulated 1.0 Mg DM ha−1 in its above-ground organs of which 0.8 Mg DM ha−1 was in the harvested residues, the balance being in the grain and shedded leaves (Table 3). The low harvest index is attributed to the indeterminate growth of this variety and to insect attacks on flowers, mainly by Mylabris spp. The above-ground sections of cowpea accumulated 23 kg N ha−1 (Table 4).
Recoverable cowpea roots biomass in the rooting soil amounted to almost 300 kg DM ha−1 (Table 3). Approx. 30% of the root dry matter was located in the central 0.09 m2 core around the planting pocket soil and 90% was located in the upper 0.15 m soil layer of the rooting zone. The recoverable roots of cowpea contained 3 kg N ha−1 (Table 4). Cowpea derived on average 55% of its N from the atmosphere (data not shown).
Cowpea and soil 15N enrichment after leaf-labelling
Cowpea roots and the soil in their surroundings were sufficiently 15N enriched relatively to the controls to measure cowpea rhizodeposition. The 15N enrichment varied amongst the cowpea organs after labelling although this difference was not significant (P = 0.08). The 15N enrichment of the roots recovered in the rooting soil in the 0.15–0.30 m depth had lower enrichment than the roots closer to the surface or than the roots from the pocket soil (Table 3). The soil was similarly enriched in the samples taken within the 0.45 m radius of the pocket and within the pocket soil in the lower depth from 0.15–0.30 m. Soil 15N enrichment was higher in the pocket soil at 0–0.15 m depth compared to the other sampling locations, there were also more roots in the top 0–0.15 m depth (Table 3).
Cowpea rhizodeposition
More than half of the total cowpea N was located below-ground in roots and rhizodeposits. More than 85% of this below-ground cowpea derived N was found in the rhizodeposits. 72% of the N in roots and rhizodeposits were located in the top 0.15 m of soil. The rhizodeposits of the cowpea contained 21.5 kg N ha−1 (Table 4).
N transfer from labelled cowpea to millet, cowpea and weeds
Millet plants grown in proximity of the labelled cowpea had significantly higher 15N enrichment than the millet used as control (Table 5). On the other hand, the unlabelled cowpea plants growing in proximity of the labelled cowpea had similar enrichment to cowpea used as control, which implies that cowpea to cowpea N transfer is negligible and that millet is the main competitor for cowpea derived N from rhizodeposition.
The N in millet that was derived from cowpea was 2.7 and 4.4% of the N contained in spikes and leaves, respectively, which correspond to a net transfer of 1.4 and 0.7 kg N ha−1. Assuming a similar proportion of N transferred in the stems and roots would add approx. 1 kg from stems and 2 kg N from roots to the balance, reaching an amount transferred of approx. 5 kg N ha−1 (Table 5).
Subsequent millet utilisation of pre-crop cowpea derived N
The pure stands of millet grown in the field in 2007 produced high yields for the area with a biomass production of 3.9 Mg DM ha−1 and a grain production of 1.8 Mg DM ha−1 (Table 2), which is equivalent to a total of 61.5 kg N ha−1 (Table 6). Millet directly derived 4.3% of its N from the pre-crop N pools located below-ground of the cowpea grown in the same pocket the preceding growing season. This proportion equals 2.6 kg N ha−1 (Table 6).
The fate of cowpea derived N from cowpea harvest to subsequent millet crop seeding was monitored in the sub-experiment using soil cores. In the beginning of the planting season, June 2007, 40% of the excess 15N present in October 2006 remained in the pots. Sampling in June was done a few weeks after the first rain. By harvest time, October 2007, 1 year after the harvest of cowpea, 36% of the excess 15N from the rhizodeposits and roots remained in the pots (Table 7) with two-thirds still located in the top 0.15 m soil. As the sampled volume in the field study corresponded to the section of soil where samples were taken within the top 0.15 m of the soil cores, these figures strongly indicate that the residue N and the N deposited does not significantly move vertically through the soil profile. Losses may thus mainly occur during the dry season and the pools recoverable at the onset of the growth season appear relatively recalcitrant.
Discussion
The yield of millet in 2006 with 1.4 Mg DM ha−1 of grain were high compared to general yields of less than 1 Mg DM ha−1 common to the region (Table 2) (Voortman et al. 2004; Gandah et al. 2003; Fofana et al. 2008). This productivity reflected the high soil fertility at the site, including the long fallow period and the application of P using the micro-dose technique (Tabo et al. 2005; Hayashi et al. 2007). The cowpea variety also accumulated higher biomasses, 1 Mg DM ha−1, compared to typical yields of less than 0.4 Mg DM ha−1 in intercrop in the area (Table 3; Bielders et al. 2000). The results of this study thus represent the potential N contribution of cowpea to the farming systems of the Sudano-Sahelian eco-zone.
This is the first time that legume rhizodeposition and its subsequent fate has been quantified in the field using a 15N direct labelling techniques without constraining the roots of the plants (e.g. Høgh-Jensen and Schjoerring 2001; Khan et al. 2003). It is demonstrated that it is feasible to measure N rhizodeposition in this way as the roots and soils were sufficiently enriched (Table 3) to accurately measure N rhizodeposition, which is especially important when working with plants that develop large root systems.
The method used is based on the assumption that plant N pools will be equally labelled over time. This was attempted by labelling over 6 weeks during the period with the fastest growth. An incorporation of the tracer into the strongest sinks must be expected and this is also apparently the case in this study as shedded leaves and grains are more enriched than the largest N pool, i.e. residues. This may lead to an overestimation of rhizodeposition (Eq. 2). However, a weighted mean of the enrichment, calculated by weighing the enrichment by the N pool size of each organ, would be very close to the value of the enrichment of roots in the upper part of the pocket soil (Table 3), which underline the robustness of the method.
During the labelling, the leaf parts being directly labelled were removed to eliminate any potential contamination from the source. Shedded leaves were captured in a fine woven net and they are not expected to be any source of error due to the minor amount of N that may have entered the soil this way.
Transfer of N from cowpea to millet, cowpea and weeds
The current labelling technique has been used in the field to study N transfer between closely associated perennial forage species (Gylfadóttir et al. 2007; Høgh-Jensen and Schjoerring 2001; Høgh-Jensen et al. 2006) but our study is the first occasion in which it is taken to full field conditions to study N transfer between more distantly grown grain legumes and cereals. It was demonstrated that transfer contributes modestly to the overall N economy of the receiving crop; including root N it amounted to approx. 5 kg N ha−1 (Table 5), which must be seen in relation to the 50 kg N in the millet crop.
The high proportion of cowpea derived N in weeds growing closer to the donor plants and the modest proportion of transfer to neighbouring millet plants indicate that the plant-available cowpea derived N pools are available. However, they may largely be re-assimilated by the donor as a distance of 1 m from the receiver plants neighbouring the donor gives poor opportunities to access the cowpea derived N pools. Further, the delayed planting of cowpeas in a medium duration crop like the current, maturing in 95 days, may leave a relatively short time window for the transfer to take place. Consequently, values of 15% transfer from beans to maize obtained by Giller et al. (1991) in a greenhouse experiment may overestimate field conditions.
Pre-crop N transfer from intercropped cowpea to subsequent pure stand millet
One hypothesis tested in the current study was that rhizodeposition of legume N is potentially a large N input in the farming systems of the Sudano-Sahelian eco-zone. The current study confirmed this hypothesis as 50% of the total accumulated cowpea N was located below-ground, more than three quarters of which was found in the physically unrecoverable rhizodeposited fractions (Table 4). A review of grain legumes N rhizodeposition studies recently indicated that, on average, 33% of grain legumes N are found below-ground (Wichern et al. 2008) but this will obviously depend on growth conditions and legume species. Our findings indicate that previous studies quantifying the N contribution by cowpea in various cropping systems underestimate its N contribution by measuring only its above-ground N content (e.g. Eaglesham et al. 1982; Bado et al. 2006) or by underestimating its below-ground N contribution (e.g. Adjei-Nsiah et al. 2008).
Up to 95% of the rhizodeposited N was located in the soil top layer from 0–0.15 m (Table 4) which agrees with other studies (Gylfadóttir et al. 2007; Høgh-Jensen and Schjoerring 2001; Høgh-Jensen et al. 2006; Khan et al. 2003; Araujo et al. 2006).
Generally a cowpea pre-crop will increase the yield of subsequent millet crops compared to continuous millet (Bagayoko et al. 1998; Alvey et al. 2001; Samake et al. 2006) caused most likely by an improved soil N availability (Bagayoko et al. 2000; Shahandeh et al. 2004; Bado et al. 2006) and to an N sparing effect from growing N2-fixing legume crops (Giller 2002). Our results demonstrate that the uptake of cowpea derived below-ground N by a subsequent millet crop the following growth season was relatively small with a N uptake of less than 3 kg N ha, equivalent to around 5% of the total N uptake by millet.
The present study took place on a sandy soil typical for the Sahelian eco-zone. The early rain showers may flush such soils and pre-crop N effects are consequently expected to be lower (Shahandeh et al. 2004). In corresponding studies, the cereal crop recovered 10% and 34% of the pre-crop soybeans below-ground N in Brazil (Araujo et al. 2006) and Tanzania (Vesterager et al. 2007), respectively. A pool substitution effect (Jenkinson et al. 1985) is unlikely to be important due to the very low levels of N pools in the soil (Table 1).
Fate of cowpea below-ground N during a 1 year period after harvest
A large proportion of the cowpea N residues measured in October 2006, i.e. at harvest, had disappeared from the top-soil by June 2007, i.e. after the dry season (Table 7). Plant rhizodeposits are to a large extent made of rapidly degradable N compounds that can easily be mineralized or immobilized by the soil microbial population (Merbach et al. 1999; Hertenberger and Wanek 2004). N rhizodeposits are thus considered a rapidly cycling N flow with a high potential for losses on the sandy soils of the experiment with low activity clay and low organic matter content. Disappearance of the 15N from the experimental plots and pot experiment is likely to be the results of N leaching and/or volatilization.
The onset of the rains after a dry period brings a rapid N mineralization, followed by a decrease in the soil N mineralization rate during the rest of the rainy season (Bernhardt-Reversat 1982; Shahandeh et al. 2004). Substantial rainfall occurring prior to the establishment of a standing crop holds the potential for soil N losses. Measurements of the soil 15N content in June 2007 were taken after a few heavy showers and nitrate-N may already have been leached from the top soil layers. Low N use efficiencies have been reported under eastern African condition where heavy rain showers may leach N below the rooting zone (Semb and Robinson 1969; Vesterager et al. 2008).
Soil 15N content decreased comparatively little during the rainy season from June to October 2007 with the greater share of the most degradable pool of rhizodeposited N having already been lost from the system (Tables 5 and 6). The losses may be ascribed to a phenomenon known as the “Birch effect” (Birch 1964) where drying and rewetting of the soil as occurs at the beginning of the rainy season produces a disproportionately large and uniform release of C and N.
Utilisation efficiency of the pre-crop cowpea below-ground N by the first subsequent millet crop was small compared to N losses and the N remaining in the soil. More information is needed on how these N pools losses can be managed in the Sudano-Sahelian regions in order to grasp the full potential of N fixation by grain legumes.
Conclusion
Below-ground plant derived N was found to constitute 50% of the total cowpea N in intercrop in 2006. The rhizodeposited N pools were eight times larger than the N pool in the recoverable roots. Below-ground N transfer from cowpea to millet in intercrop was demonstrated under field conditions at significant levels of approx. 10 kg N ha−1. Most of the cowpea below-ground N had disappeared from the top-soil before planting of millet in 2007. Forty percent of the excess 15N remained in the soil in June 2007. The transfer from below-ground N of cowpea to millet in rotation was 5 kg N ha−1. The experiment showed that cowpea rhizodeposition is a potentially large input of N in cropping systems of the Sudano-Sahelian region.
References
Adjei-Nsiah S, Kuyper TW, Leeuwis C, Abekoe MK, Cobbinah J, Sakyi-Dawson O, Giller KE (2008) Farmers’ agronomic and social evaluation of productivity yield and N2-fixation in different cowpea varieties and their subsequent residual N effects on a succeeding maize crop. Nutr Cycl Agroecosys 80:199–209
Alvey S, Bagayoko M, Neumann G, Buerkert A (2001) Cereal/legume rotations affect chemical properties and biological activities in two West African soils. Plant Soil 231:45–54
Araujo ES, Boddey RM, Urquiaga S, Alves BJR (2006) Soybean below-ground N and its contribution to the N nutrition of a subsequent sorghum crop. Paper presented at 18th World Congress of Soil Science, Philadelphia, Pennsylvania, USA, 9–15 July 2006
Bado B, Bationo A, Cescas M (2006) Assessment of cowpea and groundnut contributions to soil fertility and succeeding sorghum yields in the Guinean savannah zone of Burkina Faso (West Africa). Biol Fertil Soils 43:171–176
Bagayoko M, Mason SC, Traore S, Eskridge KM (1996) Pearl millet/cowpea cropping systems yield and soil nutrient levels. Afr Crop Sci J 4:453–462
Bagayoko M, Mason SC, Traoré S (1998). The role of cowpea on pearl millet yield, N uptake and soil nutrient status in millet–cowpea rotation in Mali. In: Renard G, Neef A, Becker K, von Oppen M (eds) Soil fertility management in West African land-use systems, Margraf Verlag Weikersheim, Germany. pp 109–114
Bagayoko M, Buerkert A, Lung G, Bationo A, Romheld V (2000) Cereal/legume rotation effects on cereal growth in Sudano-Sahelian West Africa: soil mineral nitrogen, mycorrhizae and nematodes. Plant Soil 218:103–116
Bationo A, Ntare BR, Tarawali SA, Tabo R (2002) Soil fertility management and cowpea production in the semi-arid tropics. In: Fatokun CA, Tarawali SA, Singh BB, Kormawa PM, Tamò M (eds) Challenges and opportunities for enhancing sustainable cowpea production. Proceedings of the World Cowpea Conference III held at the International Institute of Tropical Agriculture (IITA), Ibadan, Nigeria, 4–8 September 2000. IITA, Ibadan, Nigeria. pp 301–318
Bationo A, Traore Z, Kimetu J, Bagayoko M, Bado V, Lompo M, Tabo R, Koala S (2004) Cropping systems in the Sudano-Sahelian zone: implications on soil fertility management. Centro Internacional de Agricultura Tropical (CIAT), Tropical Soil Biology and Fertility (TSBF), Nairobi. pp 34
Bernhardt-Reversat S (1982) Biogeochemical cycle of nitrogen in a semi-arid savanna. Oikos 38:321–332
Bielders CL, Michels K, Rajot JL (2000) On-farm evaluation of ridging and residue management practices to reduce wind erosion in Niger. Soil Sci Soc Am J 64:1776–1785
Birch HF (1964) Mineralization of plant nitrogen following alternate wet and dry conditions. Plant Soil 20:43–49
Eaglesham ARJ, Ayanaba A, Rao VR, Eskew DL (1982) Mineral N effects on cowpea and soybean crops in a Nigerian soil. 2. Amounts of N fixed and accrual to the soil. Plant Soil 68:183–192
FAO (1988) FAO Soil Map of the World, revised legend. World Soil Resources Report 60, Food and Agricultural Organization, Rome
FAO (2004) Sahel weather and crop situation. Report No 2. Global information and early warning system on food and agriculture. Food and Agricultural Organization, Rome
Fofana B, Wopereis M, Bationo A, Breman H, Mando A (2008) Millet nutrient use efficiency as affected by natural soil fertility, mineral fertilizer use and rainfall in the West African Sahel. Nutr Cycl Agroecosyst 81:25–36
Franke AC, Laberge C, Oyewole BD, Schulz S (2008) A comparison between legume technologies and fallow, and their effects on maize and soil traits, in two distinct environments of the West African savannah. Nutr Cycl Agroecosyst 82:117–135
Fussell LK, Serafini PG (1985) Associations de cultures dans les zones tropicales semi-arides d’Afrique de l’Ouest: stratégies de recherché antérieures et futures. In: Ohm HW, Nagy JG (eds) Technologies appropriées pour les paysans des zones semi-arides de l’Afrique de l’Ouest. Purdue University, West Lafayette, pp 254–278
Gandah M, Brouwer J, Hiernaux P, Van Duivenbooden N (2003) Fertility management and landscape position: farmers’ use of nutrient sources in western Niger and possible improvements. Nutr Cycl Agroecosyst 67:55–66
Giller KE (2002) Nitrogen fixation in tropical cropping systems. CABI Publishing, Wallingford, p 352
Giller KE, Witty JF (1987) Immobilized 15N-fertilizer sources improve the accuracy of field estimates of N2-fixation by isotope dilution. Soil Biol Biochem 19:459–463
Giller KE, Ormesher J, Awah FM (1991) Nitrogen transfer from Phaseolus bean to intercropped maize measured using 15N-enrichment and 15N-isotope dilution methods. Soil Biol Biochem 23:339–346
Gylfadóttir T, Helgadóttir Á, Høgh-Jensen H (2007) Consequences of including adapted white clover in northern European grassland: transfer and deposition of nitrogen. Plant Soil 297:93–104
Hayashi K, Abdoulaye T, Gerard B, Bationo A (2007) Evaluation of application timing in fertilizer micro-dosing technology on millet production in Niger, West Africa. Nutr Cycl Agroecosyst 80:257–265
Hertenberger G, Wanek W (2004) Evaluation of methods to measure differential 15N labelling of soil and rood N pools for studies of root exudation. Rapid Commun Mass Spectrom 18:2415–2425
Høgh-Jensen H, Schjoerring JK (2001) Rhizodeposition of nitrogen by red clover, white clover and ryegrass leys. Soil Biol Biochem 33:439–448
Høgh-Jensen H, Nielsen B, Thamsborg SM (2006) Productivity and quality, competition and facilitation of chicory in ryegrass/legume-based pastures under various nitrogen supply levels. Eur J Agron 24:247–256
IAEA (2004) Use of isotope and radiation methods in soil and water management and crop nutrition, an interactive CD. IAEA, Vienna
Jenkinson DS, Fox RH, Rayner JH (1985) Interactions between fertilizer nitrogen and soil nitrogen-the so-called “priming” effect. J Soil Sci 36:425–444
Khan DF, Peoples MB, Herridge DF (2002) Quantifying below-ground nitrogen of legumes - 1. Optimising procedures for 15N shoot-labelling. Plant Soil 245:327–334
Khan DF, Peoples MB, Schwenke GD, Felton WL, Chen DL, Herridge DF (2003) Effects of below-ground nitrogen on N balances of field-grown fababean, chickpea, and barley. Aust J Agric Res 54:333–340
Laberge G, Franke AC, Ambus P, Høgh-Jensen H (2009) Nitrogen rhizodeposition from soybean (Glycine max) and its impact on nutrient budgets in two contrasting environments of the Guinean savannah zone of Nigeria. Nutr Cycl Agroecosyst 84:49–58
McNeill AM, Zhu CY, Fillery IRP (1997) Use of in situ 15N-labeling to estimate the total below-ground nitrogen of pasture legumes in intact soil-plant systems. Aust J Agric Res 48:295–304
Merbach W, Mirus E, Knof G et al (1999) Release of carbon and nitrogen compounds by plant roots and their possible ecological importance. J Plant Nutr Soil Sci 162:373–383
R Development Core Team (2007) R: a language and environment for statistical computing. R Foundation for Statistical Computing. Available via http://www.R-project.org. Cited 2 April 07
Samake O, Stomph T, Kropff M, Smaling E (2006) Integrated pearl millet management in the Sahel: effects of legume rotation and fallow management on productivity and Striga Hermonthica infestation. Plant Soil 286:245–257
Sanginga N, Dashiell KE, Diels J et al (2003) Sustainable resource management coupled to resilient germplasm to provide new intensive cereal-grain-legume-livestock systems in the dry savanna. Agric Ecosyst Environ 100:305–314
Schmidtke K (2005) How to calculate nitrogen rhizodeposition: a case study in estimating N rhizodeposition in the pea (Pisum sativum L.) and grasspea (Lathyrus sativus L.) using a continuous 15N labelling split-root technique. Soil Biol Biochem 37:1893–1897
Semb G, Robinson JBD (1969) The natural nitrogen flush in different arable soils and climates in East Africa. E Afr Agric For J 34:350–370
Shahandeh H, Blanton-Knewtson SJ, Doumbia M, Hons FM, Hossner LR (2004) Nitrogen dynamics in tropical soils of Mali, West Africa. Biol Fertil Soils 39:258–268
Singh BB, Ajeigbe HA, Tarawali SA, Fernandez-Rivera S, Abubakar M (2003) Improving the production and utilization of cowpea as food and fodder. Field Crops Res 84:169–177
Tabo R, Bationo A, Diallo Maimouna K, Hassane O, Koala S (2005) Fertilizer micro-dosing for the prosperity of smallscale farmers in the Sahel. Final Report, ICRISAT, Niamey
Vesterager JM, Nielsen NE, Høgh-Jensen H (2007) Nitrogen budgets in crop sequences with phosphorus fertilisation with or without cowpea in the maize-based cropping systems of semi-arid eastern Africa. Afr J Agric Res 2:261–268
Vesterager JM, Nielsen NE, Høgh-Jensen H (2008) Effects of cropping history and phosphorus source on land and nitrogen uptake efficiency in sole or intercropped cowpea maize crops. Nutr Cycl Agroecosyst 80:61–73
Voortman RL, Brouwer J, Albersen PJ (2004) Characterization of spatial soil variability and its effect on millet yield on Sudano-Sahelian coversands in South-West Niger. Geoderma 121:65–82
Wichern F, Eberhardt E, Mayer J, Joergensen RG, Muller T (2008) Nitrogen rhizodeposition in agricultural crops: methods, estimates and future prospects. Soil Biol Biochem 40:30–48
Witty JF, Roose E (1984) Slow-release 15N fertilizer formulations to measure N2-fixation by isotope dilution. Soil Biol Biochem 16:657–661
Yasmin K, Cadisch G, Baggs EM (2006) Comparing 15N-labelling techniques for enriching above- and below-ground components of the plant-soil system. Soil Biol Biochem 38:397–400
Acknowledgments
The authors wish to thank Tahirou Saley and Amadou Mounkeila along with Chindo, Tindi and Issaka Kouarokoye for their skilled technical assistance. The contributions of Dr. Matsunaga and the JIRCAS program at ICRISAT who provided the cowpea seeds and the millet breeding program of ICRISAT who provided the millet seeds should also be acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Elizabeth M. Baggs.
Rights and permissions
About this article
Cite this article
Laberge, G., I. G. Haussmann, B., Ambus, P. et al. Cowpea N rhizodeposition and its below-ground transfer to a co-existing and to a subsequent millet crop on a sandy soil of the Sudano-Sahelian eco-zone. Plant Soil 340, 369–382 (2011). https://doi.org/10.1007/s11104-010-0609-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-010-0609-6